Abstract
Introduction
Extended survival outcomes from improved treatments for patients with cancer come with an increased risk of developing a metachronous second malignancy (MSM). We evaluated the incidence of MSM after successful treatment of SCLC and compared survival between SCLC patients who developed MSM and those who did not.
Methods
Selection criteria were a diagnosis of limited-stage SCLC and receipt of ≥45 Gy radiotherapy and chemotherapy at a single institution in 1985–2012. MSM was defined as a tumor of a different histologic type than the primary that appeared more than 2 years after the diagnosis of SCLC.
Results
Of 704 patients identified, 32 were excluded for lack of follow-up, 48 for having SCLC as MSM after treatment of another type of cancer, 37 for non-melanoma skin cancer as MSM, and 46 for MSM within 2 years after SCLC diagnosis; of the remaining 541 patients, 346 had recurrent SCLC, 180 had no second malignancy and no recurrence, and 15 (2.8%) had MSM (13 in lung [8 adenocarcinoma, 5 squamous cell carcinoma], 1 sarcoma, 1 acute myeloid leukemia). All 15 patients with MSM achieved complete response to the SCLC treatment. Overall survival was longer for patients with MSM than for patients with no other malignancies and no recurrence, with 10-year rates of 61.9% (95% confidence interval [CI] 30.0%–82.6%) and 29.9% (95% CI 21.5%–38.6%), respectively (p=0.03).
Conclusions
Long-time survivors after treatment for SCLC should be made aware of the risk of MSM and the necessity of follow-up.
Keywords: limited-stage small cell lung cancer, metachronous second malignancy, radiochemotherapy, survival
INTRODUCTION
Survival outcomes after treatment for many types of cancer have improved because of the ability to detect disease early and because of improvements in treatment modalities and supportive care. Extended survival outcomes after the treatment of a first cancer unfortunately come with an increased risk of developing a metachronous second malignancy (MSM) owing to environmental factors, cancer-specific treatments, or genetic predisposition.1
Small cell lung cancer (SCLC) is an aggressive form of lung cancer with notoriously poor survival2,3; overall survival (OS) rates have not improved substantially over the past 3 decades.4,5 Some patients diagnosed with limited-stage SCLC have lived long enough to manifest MSM owing to improvements in diagnostic imaging, radiation planning and delivery, and chemotherapy.6 The survival rate for patients with limited-stage SCLC at 2 years has improved slightly from about 47%7 to 53%–56% over the past 15 years,8,9 suggesting that patients with limited-stage SCLC, especially those whose disease responded well to treatment, should be monitored for the appearance of a second malignancy for a long time. However, because of the relative rarity of SCLC, the incidence of MSM after successful treatment is unknown.
In this study, we hypothesized that patients with limited-stage SCLC who develop MSM will survive longer than patients who do not develop other malignancies or recurrent SCLC because the longer survival time after successful treatment of SCLC gives such patients more chance of developing MSM. Our primary objective was to evaluate the incidence and types of MSM that appeared after successful treatment of SCLC; our secondary objective was to compare survival between patients who developed MSM and those who did not.
METHODS
We reviewed a large, single-institution database of patients with lung cancer to identify those who had received chemotherapy and radiotherapy to ≥45 Gy to the thorax for limited-stage SCLC from 1985 through 2012. MSM was defined as a second type of primary cancer appearing more than 2 years after the diagnosis of SCLC.10 To avoid surveillance bias, we excluded patients who developed SCLC as MSM after treatment of other cancers, those who developed second malignancies within 2 years of the SCLC diagnosis, and those who developed non-melanoma skin cancer as a second malignancy (Fig. 1).
Figure 1.
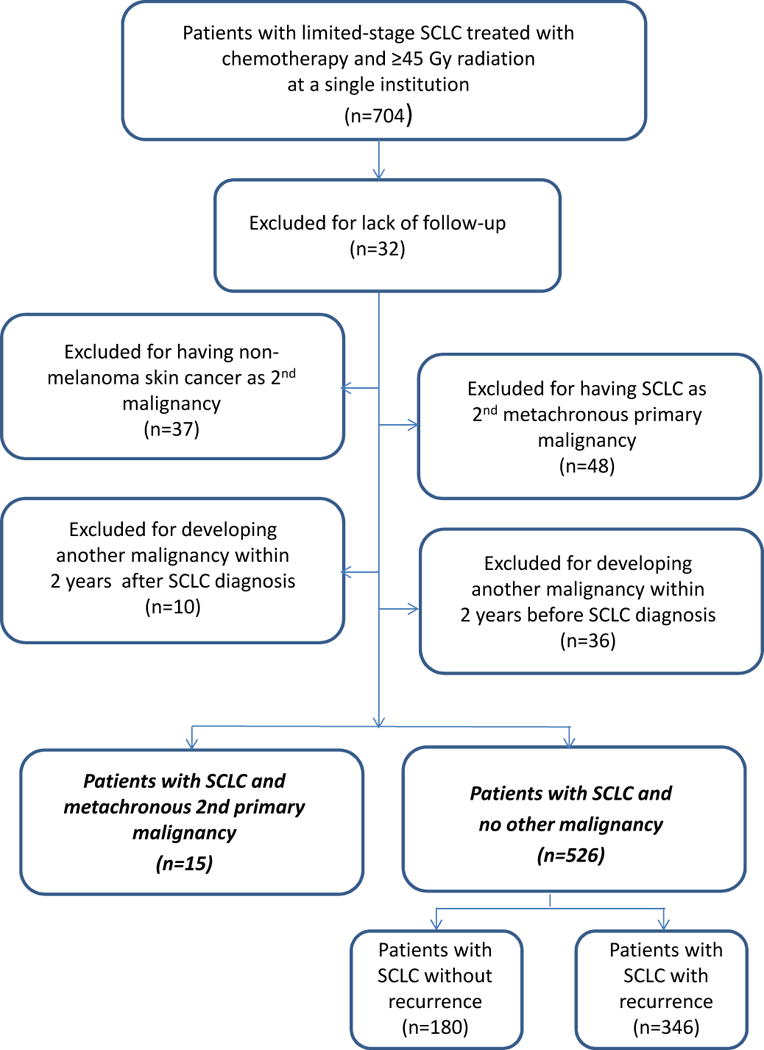
Patient selection process. SCLC, small cell lung cancer.
Diagnosis, treatment, and follow-up guidelines did not change appreciably over the course of the study, with the exceptions noted here. Routine use of positron emission tomography (PET) for disease staging began in 2000. Also, before 2000, thoracic radiation therapy was delivered either with 2-dimensional anteroposterior/posteroanterior fields followed by oblique fields or as non-conformal 3-dimensional therapy; beginning in 2000, thoracic radiation was delivered as 3-dimensional conformal or intensity-modulated radiation therapy. Throughout the study period, the first follow-up visit took place 4–6 weeks after the completion of thoracic radiotherapy and included computed tomography (CT) of the chest and upper abdomen with and without contrast in addition to complete blood count with differentials, blood urea nitrogen, creatinine, and electrolytes; liver function tests; and, after 1998, PET-CT if the CT scans showed evidence of recurrence. After the completion of 4 cycles of etoposide with cisplatin or carboplatin, magnetic resonance images of the brain were obtained. Patients who had at that time a complete response or a good partial response, they were offered prophylactic cranial irradiation (25 Gy in 15 fractions). Thereafter, patients were followed every 3 months for 2 years with CT scans, blood tests, and liver function tests, every 6 months for another 2 years, and then once a year indefinitely until death.
Statistical analysis
Continuous variables were summarized by descriptive statistics such as means, standard deviations, medians, and ranges. Categorical variables were tabulated by frequency and percentage. OS was measured from the date of SCLC diagnosis until the date of death or last follow-up. OS was calculated by using Kaplan-Meier estimators, and the log-rank test was used to compare the Kaplan-Meier curves. Statistical significance was defined as p<0.05. Sex, age, year of diagnosis (≥2000 vs. before 2000), race, smoking history and intensity, and treatment for SCLC (radiation dose, receipt and timing of chemotherapy [concurrent vs. sequential or induction], and receipt pf prophylactic cranial irradiation) were evaluated as potential predictors of MSM development by using Fisher’s exact test and the Wilcoxon rank-sum test.
RESULTS
We identified 704 patients with limited-stage SCLC from 1985 through 2012. Of those 704 patients, we excluded 163, 32 for lack of follow-up information, 48 who had SCLC as MSM, 37 with non-melanoma skin cancer as a second malignancy, and 46 with a second malignancy appearing either within 2 years before SCLC diagnosis (n=36) or within 2 years after SCLC diagnosis (n=10). Among the 541 patients analyzed, 15 patients (2.8%) developed MSM; 180 did not develop a second malignancy or recurrent SCLC; and the remaining 346 had recurrent SCLC. The subjects of the current analysis were the first two groups, that is, those who developed MSM and those who did not. (Fig. 1). The median follow-up time was 91.4 months (range 38.3–207.7 months) for patients with MSM and 38.3 months (range 0.2–255.3 months) for patients with no other malignancies and no recurrence.
Figure 2 shows survival curves for patients who developed MSM and those who did not develop a second malignancy or recurrent SCLC. The estimated OS rates were higher among patients who developed MSM than among patients who did not develop other malignancies, with 10-year rates of 61.9% (95% confidence interval [CI] 30.0%–82.6%) and 29.9% (95% CI 21.5%–38.6%), respectively (log-rank p=0.03).
Figure 2.
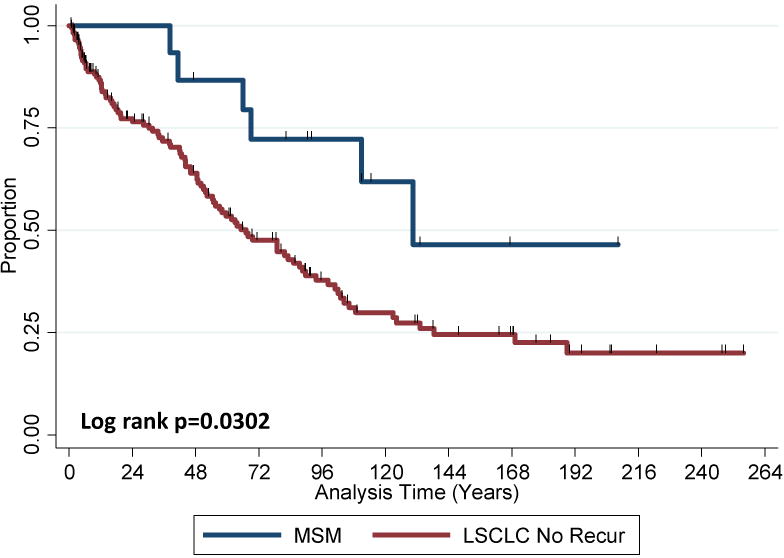
Overall survival (OS) in patients with metachronous second malignancies (MSM) and in patients with no other malignancies and without recurrence of limited-stage small cell lung cancer (LSCLC No Recur). OS was better among patients with MSM (log-rank P=0.0302).
Patient and treatment characteristics stratified by MSM or no MSM are shown in Table 1. No differences were found between groups in any of the characteristics analyzed.
Table 1.
Patient and SCLC treatment characteristics
| Patients with MSM (n=15) |
Patients without MSM or Recurrence (n=180) |
P Value | |
|---|---|---|---|
| Patient Characteristics | |||
| Sex | 0.191 | ||
| Male | 5 | 93 | |
| Female | 10 | 87 | |
| Age at diagnosis, years | 0.918 | ||
| Median (range) | 63.0 (43–78) | 62.0 (34–90) | |
| Year of diagnosis | 1.000 | ||
| <2000 | 5 | 65 | |
| ≥2000 | 10 | 115 | |
| Race | 0.158 | ||
| White | 13 | 141 | |
| Black | 1 | 25 | |
| Hispanic | 0 | 10 | |
| Asian | 0 | 4 | |
| Other | 1 | 0 | |
| Smoking pack-years | 0.21 | ||
| Median (range) | 56.0 (20–160) | 50.0 (0–168) | |
| Smoking status | 1.000 | ||
| Never-smoker | 0 | 4 | |
| Former smoker | 9 | 102 | |
| Current smoker | 6 | 72 | |
| Unknown | 0 | 2 | |
| Treatment Characteristics | |||
| Chemotherapy | 0.843 | ||
| Concurrent chemo-RT | 11 | 122 | |
| Induction chemo followed by concurrent chemo-RT | 2 | 36 | |
| Induction chemo followed by sequential RT | 2 | 22 | |
| Radiation dose, Gy | |||
| Median (range) | 45 (45–70) | 45 (45–70) | 0.532 |
| Mean | 52.4 | 49.6 | 0.1619 |
| Radiation dose, Gy, for pts treated <2000* | 0.757 | ||
| Median (range) | 45 (45–56) | 45 (45–66) | |
| Mean | 49.2 | 47.2 | |
| No. of cases | 5 | 65 | |
| Radiation dose, Gy, for pts treated ≥2000** | 0.811 | ||
| Median (range) | 53.1 (45–70) | 45 (45–70) | |
| Mean | 54.0 | 51.0 | |
| No. of cases | 10 | 115 | |
| PCI | |||
| Yes | 9 | 101 | 1.000 |
| No | 6 | 79 |
Abbreviations: MSM, metachronous second malignancy; pts, patients; chemo, chemotherapy; RT, radiation therapy; PCI, prophylactic cranial irradiation
delivered as either 2-dimensional anteroposterior/posteroanterior fields followed by oblique fields or non-conformal 3-dimensional radiation therapy
delivered as 3-dimensional conformal or intensity-modulated radiation therapy
Table 2 shows the characteristics of the 15 patients who developed MSM. The median time from the diagnosis of SCLC to the diagnosis of MSM was 91.4 months (range 35.6–157.3 months). The most common MSM histotypes were adenocarcinoma (n=8) and squamous cell carcinoma (n=5), followed by sarcoma (n=1) and acute myeloid leukemia (AML) (n=1); 13 of the 15 MSMs appeared in the lung. All 15 patients had achieved complete response after SCLC treatment. Among the 15 patients with MSM, the MSM was AML in 1 and limited-stage cancers in the other 14.
Table 2.
Characteristics of the 15 patients who developed metachronous second primary malignancies
| Pt. No | Age (Sex) | Race | Pack-years of Smoking |
Primary SCLC Site |
Radiation Dose + Chemo Schedule |
PCI | Response to Initial Therapy |
Second Tumor Histology |
Second Tumor Site |
Second Tumor Therapy |
Time from Primary SCLC Diagnosis to MSM, mo. |
OS Time, mo. |
Status At Last Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 (F) | White | 60.0 | Right | 56.0 Gy + ind+seq | Y | CR | Sq | Rt upper lung | Chemo | 87.0 | 89.9 | Alive |
| 2 | 65 (M) | Other | 160.0 | Right | 55.0 Gy + ind+con | N | CR | Sq | Lt lung | RT | 55.0 | 207.7 | Alive |
| 3 | 66 (F) | White | 70.5 | Left | 61.2 Gy + con | Y | CR | Sq | Lt lower lung | Surgery | 47.5 | 114.5 | Alive |
| 4 | 55 (F) | Black | 37.0 | Left | 61.2 Gy + ind+con | N | CR | Sq | Rt Lower lung | RT | 104.2 | 111.0 | Alive |
| 5 | 63 (M) | White | 94.0 | Right | 70.0 Gy + con | Y | CR | Sq | Lt lung | RT | 59.5 | 81.8 | Alive |
| 6 | 62 (F) | White | 25.0 | Right | 45.0 Gy + ind+seq | N | CR | Adeno | Rt upper lung | Surgery+Chemo | 84.6 | 132.5 | Alive |
| 7 | 51 (M) | White | 20.0 | Left | 61.2 Gy + con | Y | CR | Adeno | Lt lower lung | Surgery | 116.2 | 130.5 | Dead |
| 8 | 75 (F) | White | 45.0 | Right | 45.0 Gy + con | N | CR | Adeno | Lt upper lung | RT | 52.5 | 66.1 | Dead |
| 9 | 78 (F) | White | 50.0 | Right | 45.0 Gy +con | Y | CR | Adeno | Lt lung | RT | 45.9 | 46.7 | Alive |
| 10 | 74 (F) | White | 112.0 | Right | 45.0 Gy + con | N | CR | Adeno | Lt lower lung | None | 36.7 | 38.3 | Dead |
| 11 | 65 (F) | White | 120.0 | Left | 45.0 Gy + con | Y | CR | Adeno | Rt upper lung | RT | 35.8 | 69.0 | Dead |
| 12 | 43 (F) | White | 56.0 | Right | 45.0 Gy + con | N | CR | Adeno | Lt lung | Chemo | 157.3 | 166.7 | Alive |
| 13 | 52 (F) | White | 80.0 | Right | 61.2 Gy + con | Y | CR | Adeno | Lt lung | RT | 80.9 | 111.0 | Dead |
| 14 | 49 (M) | White | 52.5 | Right | 45.0 Gy + con | Y | CR | Sarcoma | T3-T6 | RT | 87.7 | 91.4 | Alive |
| 15a | 72 (M) | White | 40.0 | Left | 45.0 Gy + con | Y | CR | AML (M4) | Unknown | Chemo | 35.6 | 41.3 | Dead |
Abbreviations: Pt, patient; PCI, prophylactic cranial irradiation; OS, overall survival (from diagnosis of small cell lung cancer [SCLC]); F, female, M, male; CR, complete response, Rt, right; Lt, left; Sq, squamous cell carcinoma; Adeno, adenocarcinoma; AML, acute myeloid leukemia; ind, induction; seq, sequential; con, concurrent; RT, radiation; chemo, chemotherapy; Y, yes; N, no.
Stage and site of second malignancy were unknown.
Second malignancy in patients 1–14 was limited/early stage.
DISCUSSION
Our major findings were that the incidence of MSM after treatment for limited-stage SCLC was 2.8% and that survival time after SCLC diagnosis, for patients with MSM, was sufficiently long to allow the development of MSM compared with patients who did not develop other malignancies or recurrent SCLC, as we had hypothesized. To the best of our knowledge, this is the first report of the incidence of MSM and survival for patients successfully treated for primary limited-stage SCLC. The rate of MSMs for all types of cancer in the U.S. population was approximately 0.56% in 2006,11 indicating that the risk of MSM after treatment of limited-stage SCLC is higher.
In 14 of the 15 patients in the current study who developed MSM, the MSM was detected at a limited stage and thus was potentially curable. Our findings suggest that early detection and treatment of MSM may be necessary to improve prognosis among long-term survivors of SCLC.
Another challenge is how to detect MSM after treatment of limited-stage SCLC. The National Lung Screening Trial showed that using low-dose chest helical CT to screen people at high risk of developing lung cancer led to a 20% reduction in lung cancer deaths compared with using chest radiography for screening (95% CI 6.8–26.7, P=0.004).12 Moreover, the NELSON trial showed that even small nodules detected by screening had a high probability of being malignant.13 Evaluation with PET can help to identify patients with low-risk, early-stage Hodgkin lymphoma who can be cured with less intensive treatment.14 Screening guidelines recommend that asymptomatic cancer survivors at high risk of developing MSM be screened at the same intervals as those for screening the general population.15 In the current study, 13 of the 15 patients developed MSM in a lung, indicating that MSM after SCLC treatment can occur in lung as a tumor of different histologic type, results similar to those reported in a review by Johnson more than 15 years ago.10 Taken together, these results suggest that low-dose chest helical CT may be useful to screen for MSM among patients who received radiochemotherapy for SCLC.
Although some evidence exists to suggest that receipt of radiotherapy to the chest increases the risk of developing secondary lung cancer,16,17 it remains unclear whether reducing the radiation dose for SCLC would increase the risk of recurrence or reduce the risk of MSM. The proven improvement in outcomes after concurrent radiotherapy led to its being established as standard therapy for limited-stage SCLC,18 and a previous study by us indicated that completing thoracic radiotherapy within 6 weeks of concurrent chemotherapy led to improved survival outcomes.19 Also, the type of chemotherapy and the radiation dose used for the initial treatment of SCLC in the current study did not seem to affect the incidence of MSM (p=0.84 and p=0.532). Thus at present, the therapeutic strategy need not change for patients with limited-stage SCLC who receive radiochemotherapy, but such patients should be followed carefully even after a good response to SCLC treatment.
Many patients with SCLC have a history of continuous smoking and correspondingly high smoking intensity (number of smoking pack-years), which might be expected to be correlate with the development of MSM, perhaps through affecting immune function and mutational burden. The number of pack-years of smoking and smoking status did not seem to affect the incidence of MSM in this study (p=0.21 and p=1.00, respectively). A recent review article considered cigarette smoking to be a “double-edged sword” in that it can both exacerbate pathologic immune responses and attenuate the normal defensive function of the immune system.20
Previous studies have also shown an association between second cancer risk among survivors of SCLC and smoking20 and the high mutation burden associated with SCLC that may also be influenced by smoking.21 Specifically, 75%–90% of patients with SCLC have mutations in TP53,22–24 which often result in aggressive and highly complex disease presentations and may be conducive to the development of second malignancy regardless of previous therapies. The recent advent of immune checkpoint inhibitors has revolutionized cancer therapy, and the combination of immune checkpoint inhibitors and radiation therapy has shown some benefit in preclinical and clinical studies of other types of cancer.25,26 The PD1 inhibitor pembrolizumab has had promising activity in patients with metastatic SCLC with high numbers of somatic mutations, suggesting that genomic alternations in SCLC may serve as potential therapeutic targets.27,28 Results of a phase I study investigating pembrolizumab with radiochemotherapy for limited-stage SCLC (NCT02402920) are expected to be available shortly. More comprehensive molecular analysis may be needed to further understand the factors associated with immunosuppression and mutation burden that may facilitate the development of MSM after limited-stage SCLC.
Our study had several limitations. First, the number of patients who developed MSM was small. Second, information on radiation fields and patients’ genetic backgrounds and lifestyle factors such as alcohol or dietary consumption were not available for analysis but may have contributed to the risk of MSM.1 Several genomic aberrations have been identified in SCLC, including retinoblastoma 1 gene (RB1),21,29,30 TP53,21–24 and overexpression of poly (ADP)-ribose polymerase 1.31 Genomic analysis of patients who develop MSM may be helpful for developing more effective targeted treatment strategies.
In conclusion, we recommend that patients treated for LS-SCLC be made aware of the need for long-term follow-up and the importance of early detection and treatment of MSM. Despite many efforts to clarify the biology of SCLC and therapeutic strategies for it, therapeutic advances in SCLC have remained limited.21 Smoking status, mutation status, and immune status all seem to be important in SCLC and may affect the choice of treatment for it,32,33 but whether these factors are associated with the development of second malignancies remains unknown at this time. Once effective treatment for limited-stage SCLC is discovered, the risk of MSM will become still more important as the numbers of long-term survivors and their survival times increase after successful treatment of limited-stage SCLC. Further analysis is warranted to establish the optimal strategies for diagnosing or treating MSM in such cases.
Acknowledgments
The authors appreciate the assistance of Christine F. Wogan, MS, ELS, of MD Anderson Cancer Center’s Division of Radiation Oncology, for scientific editing.
Funding: This research was supported in part by Cancer Center Support (Core) Grant CA016672 from the US National Cancer Institute, National Institutes of Health, to The University of Texas MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests: none declared.
References
- 1.Travis LB. Therapy-associated solid tumors. Acta Oncol. 2002;41:323–333. doi: 10.1080/028418602760169361. [DOI] [PubMed] [Google Scholar]
- 2.Kato Y, Ferguson TB, Bennett DE, Burford TH. Oat cell carcinoma of the lung. A review of 138 cases. Cancer. 1969;23:517–524. doi: 10.1002/1097-0142(196903)23:3<517::aid-cncr2820230301>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute. SEER Cancer Statistics Review. 1975–2012 [online] 2015 http://seer.cancer.gov/csr/1975_2012/
- 5.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–672. doi: 10.1002/cncr.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer. 2002;37:271–276. doi: 10.1016/s0169-5002(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 7.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 8.Faivre-Finn C, Snee M, Ashcroft L, et al. CONVERT: An international randomized trial of concurrent chemo-radiotherapy (cCTRT) comparing twice-daily (BD) and once-daily (OD) radiotherapy schedules in patients with limited stage small cell lung cancer (LS-SCLC) and good performance status (PS) (abstract) J Clin Oncol. 2016;34(suppl) doi: 10.1136/bmjopen-2015-009849. Abstract 8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X, Allen PK, Welsh J, Lin SH, Cox JD, Komaki RU. Immediately concurrent chemoradiation therapy is associated with improved 2-year overall survival rates in patients with limited stage small-cell lung cancer (abstract) Int J Radiat Oncol Biol Phys. 2016;96(2 Suppl 1):E454–455. [Google Scholar]
- 10.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst. 1998;90:1335–1345. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 11.Travis LB, Rabkin CS, Brown LM, et al. Cancer survivorship—genetic susceptibility and second primary cancers: research strategies and recommendations. J Natl Cancer Inst. 2006;98:15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 12.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter JE, Heuvelmans MA, de Jong PA, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomized controlled NELSON trial. Lancet Oncol. 2016;17(7):907–916. doi: 10.1016/S1470-2045(16)30069-9. [DOI] [PubMed] [Google Scholar]
- 14.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;372:1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ. Surveillance for recurrence and second cancers: Guidelines and caveats; 2016 Cancer Survivorship Symposium; San Francisco, CA. General Session 3. Presented January 15 2016. [Google Scholar]
- 16.Travis LB, Demark Wahnefried W, Allan JM, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10:289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 17.Semenova EA, Nagel R, Berns A. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev. 2015;29:1447–1462. doi: 10.1101/gad.263145.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada M, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol. 2002;20:3054–3060. doi: 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 19.Komaki R, Allen PK, Wei X, Welsh JW, Lin SH, Cox JD. Completing thoracic radiotherapy with concurrent chemotherapy within 6 weeks is important for reducing distant disease in patients with limited-stage small cell lung cancer (abstract) Int J Radiat Oncol Biol Phys. 2016;96(2 Suppl 1):S31. [Google Scholar]
- 20.Qui F, Liang CL, Liu H, Zeng YQ, Hou S, Huang S, Lai X, Dai Z. Impacts of cigarette smoking on immune responses: up and down or upside down? Oncotarget. 2017;8(1):268–284. doi: 10.18632/oncotarget.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker MA, Murray N, Shaw EG, et al. Second primary cancers related to smoking and treatment of small-cell lung cancer. Lung Cancer Working Cadre. J Natl Cancer Inst. 1997;89:1782–1788. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 21.Bunn PA, Jr, Minna JD, Augustyn A, et al. Small cell lung cancer: can recent advances in biology and molecular biology be translated into improved outcomes? J Thorac Oncol. 2016;11:453–474. doi: 10.1016/j.jtho.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller CW, Simon K, Aslo A, et al. p53 mutations in human lung tumors. Cancer Res. 1992;52:1695–1698. [PubMed] [Google Scholar]
- 23.Takahashi T, Takahashi T, Suzuki H, et al. The p53 gene is very frequently mutated in small-cell lung cancer with a distinct nucleotide substitution pattern. Oncogene. 1991;6:1775–1778. [PubMed] [Google Scholar]
- 24.D’Amico D, Carbone D, Mitsudomi T, et al. High frequency of somatically acquired p53 mutations in small-cell lung cancer cell lines and tumors. Oncogene. 1992;7:339–346. [PubMed] [Google Scholar]
- 25.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2:831–838. doi: 10.1158/2326-6066.CIR-14-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. 2016 Sep 20; doi: 10.1158/1078-0432.CCR-16-1432. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-related genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–1110. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helin K, Holm K, Niebuhr A, et al. Loss of the retinoblastoma protein-related p130 protein in small cell lung carcinoma. Proc Natl Acad Sci U S A. 1997;94:6933–6938. doi: 10.1073/pnas.94.13.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaye FJ. RB and cyclin dependent kinase pathways: defining a distinction between RB and p16 loss in lung cancer. Oncogene. 2002;21:6908–6914. doi: 10.1038/sj.onc.1205834. [DOI] [PubMed] [Google Scholar]
- 31.Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietanza MC, Byers LA, Minna JD, Rudin CM. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res. 2015;21(10):2244–2255. doi: 10.1158/1078-0432.CCR-14-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24(28):4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]


