Abstract
A general, efficient, and site-selective visible light-induced Pd-catalyzed remote desaturation of aliphatic alcohols into valuable allylic, homoallylic, and bis-homoallylic alcohols has been developed. This transformation operates via a hybrid Pd-radical mechanism, which synergistically combines the favorable features of radical approaches, such as a facile remote C–H HAT step, with that of transition-metal-catalyzed chemistry (selective β-hydrogen elimination step). This allows achieving superior degrees of regioselectivity and yields in the desaturation of alcohols compared to those obtained by the state-of-the-art desaturation methods. The HAT at unactivated C(sp3)–H sites is enabled by the easily installable/removable Si-auxiliaries. Formation of the key hybrid alkyl Pd-radical intermediates is efficiently induced by visible light from alkyl iodides and Pd(0) complexes. Notably, this method requires no exogenous photosensitizers or external oxidants.
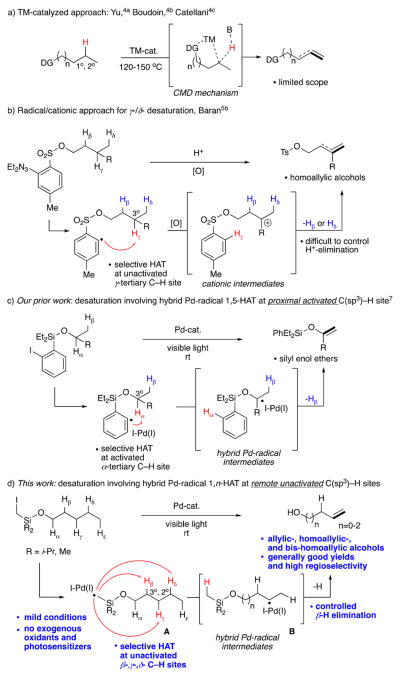
Alkenes are one of the most widely used functional groups in organic synthesis, broadly found in a wide range of bioactive molecules and natural products.1 Methods toward synthesis of these privileged motifs often involve nonuniversal strategies that require prefunctionalization of starting materials at the desired reaction site. Unfortunately, methods for synthesis of alkenes via direct desaturation of aliphatic chains are less explored. This is mainly attributed to the inherent difficulty of activating the kinetically inert C(sp3)–H bonds. Although, considerable progress has been made in converting unactivated C–H bonds into valuable C–C and C–heteroatom fragments via transition metal (TM) catalysis,2 a synthetically appealing selective site-controlled desaturation of aliphatic systems into privileged olefins still remains underdeveloped. Modern TM-catalyzed approaches typically operate via a direct transfer-hydrogenation process3 or a directed concerted metalation–deprotonation (CMD) pathway.4 Between the two pathways, the latter approach is an attractive option owing to its site-controlled capability (Scheme 1a). Nevertheless, this method suffers from low selectivity and efficiency, limited substrate scope, and harsh reaction conditions. Moreover, the site of functionalization is often restricted based on formation of a favorable TM-cyclic intermediate and is limited to activation of 1° and 2° γ-/δ -C–H bonds (Scheme 1a). To the best of our knowledge, there have been no reports on TM-catalyzed functionalization of unactivated 3° C–H bonds. Emerging radical strategies, however, have provided solutions for 3° C–H bond functionalization.5,6 Thus, in Baran’s pioneering work,5b he showcased a guided C–H desaturation of aliphatic systems involving a tether possessing an aryl radical hydrogen abstracting group, which, due to geometrical constraints, favors γ-C–H HAT at the 3° site (Scheme 1b). Owing to the nature of this transformation, the translocated radical undergoes oxidation with an external oxidant, followed by elimination into the alkene moiety. While groundbreaking, in some cases, this approach suffers from regioselectivity issues due to a nonselective proton loss step from the formed cationic intermediate. Overall, both TM-catalyzed and radical approaches are complementary but still have certain limitations. We have recently reported visible light-induced formation of a hybrid aryl Pd-radical intermediate capable of the HAT process and a subsequent β-hydrogen elimination step to furnish an alkene moiety. Due to the favorable 1,5-HAT process of the aryl hydrogen-atom-abstracting group of the silicon tether, the H atom abstraction occurred exclusively at the proximal α-C–H site, thus resulting in the α-/β-desaturation of silyl ethers into silyl enols (Scheme 1c).7 Herein, we report a general, efficient, selective, and mild visible light-induced8 Pd-catalyzed remote desaturation of aliphatic alcohols. This method involves an unprecedented, for alkyl hybrid Pd-radical species,9 HAT process at unactivated C(sp3)–H sites10 (Scheme 1d). The abstraction at β-, γ-, or δ-sites is enabled by employment of easily installable/removable Si-auxiliaries11 (A). The formed transposed hybrid species B furnishes a remote alkene moiety upon a controlled β-hydrogen elimination step, thus resulting in desaturated alcohols with degrees of regioselectivity, unseen, for radical methods.
Scheme 1.
Desaturations Methods in Aliphatic Systems
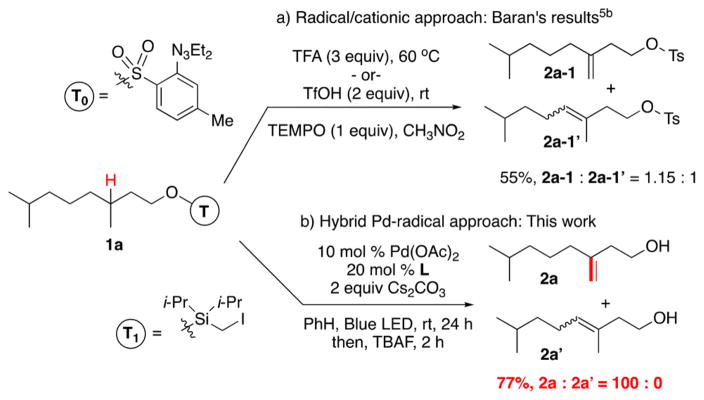
Based on our recent work on the formation of silyl methyl hybrid-Pd radicals from iodomethylsilanes,12 we hypothesized that if this moiety (T1, Scheme 2) could be engaged in a 1,6-HAT event,13,14 it would subsequently enable γ-/δ-desaturation toward important homoallylic alcohols.15 Furthermore, based on the usually selective β-hydride elimination step in the hybrid Pd-radical mechanism,9b,12 it was anticipated that the alkenol product would be formed with high degrees of regiocontrol. Accordingly, we were eager to validate this premise on desaturation of challenging alcohol derivative 1a, which under conditions of the reported radical/cationic protocol5b resulted in a nearly equal mixture of regioisomeric alkenols (2a-1, 2a-1′, Scheme 2a). We were pleased to find that exposure of T1-tethered alcohol 1a-T1 to our optimized16 photocatalytic conditions resulted in γ-/δ-desaturation product 2a in 77% yield as a single regioisomer, thus fully supporting the above hypothesis! Notably, this outcome represents the first remote Pd-catalyzed 3 °C–H functionalization event under mild and neutral conditions. Finally, it deserves mentioning that this photochemical transformation does not require the employment of exogenous photosensitizers17 or external oxidants.
Scheme 2.
Comparison Study
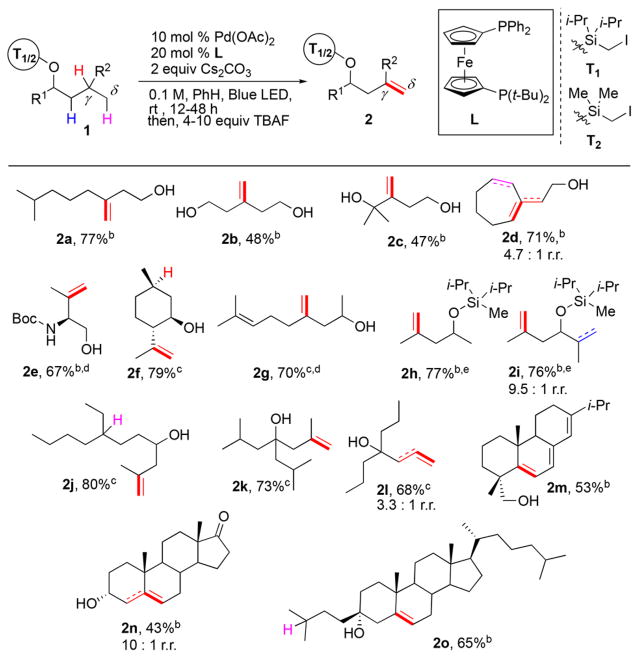
Next, the generality of the γ-/δ-desaturation of aliphatic alcohols toward homoallylic alcohols was investigated (Table 1). After completion of the desaturation reaction, except for the cases with volatile low-molecular weight alkenols, the silyl-based tethers were, in one pot, routinely removed by treatment with TBAF. It was found that various primary alcohols, possessing functional groups such as alcohols (1b, c) and amides (1e), underwent smooth γ-/δ-desaturation to generate their respective homoallylic derivatives (2b, c, e) in good yields. Then, desaturation of bulkier secondary alcohols was tested (1f–1j). For these cases, due to the relative ease of installation, we opted to employ sterically less hindered dimethyl-(iodomethyl)silane tether T2 instead of bulkier T1. Desaturation of naturally occurring (−)-menthol (1f), possessing two equally distant sites of functionalization, selectively furnished chiral building block (−)-isopulegol (2f) in 79% yield due to a favorable transition state for an HAT event at the isopropyl group.18,19 Endogenous alkene moiety 1g did not compromise the reaction, as diene 2g was formed efficiently. Desaturation of 1h proceeded uneventfully, producing alkene 2h in good yield. Notably, substrates 1i and 1j, which, along with Hγ, possess competitive Hβ and Hδ sites of abstraction, reacted selectively at the γ-C–H sites, thus producing 2i and 2j in good yields and high regioselectivity. Based on these, as well as on the results of a direct competition between Hβ and Hδ sites (vide infra, 2z using T1) and the kinetic studies,16 the following reactivity preference order for silyl tethers T1 and T2 for HAT in substrates possessing 3 °C–H sites with similar BDEs20 was revealed: 1,6 HAT of Hγ > 1,5 HAT of Hβ > 1,7 HAT of H. Tertiary alcohols were also compatible with our desaturation protocol (2k, l). Importantly, HAT at challenging 2° C–H bonds of tertiary alcohol 1l was also achieved, producing 2l in good yield. Furthermore, this method proved competent for desaturation of complex natural products and derivatives. Thus, remote desaturation of abietol (1m) generated its dehydrogenated analog 2m in moderate yield. Moreover, γ-/δ-desaturation of secondary cis-androsterone (1n) and tertiary cholestanol derivative (1o) worked well, furnishing 2n and 2o in reasonable yields.
Table 1.
γ-/δ-Desaturation of Alcoholsa
Isolated yields; r.r. = regioisomeric ratio.
T1 was used.
T2 was used.
Contains minor amount of hydrodehalogenation byproduct.
The desilylation step (TBAF) was omitted.
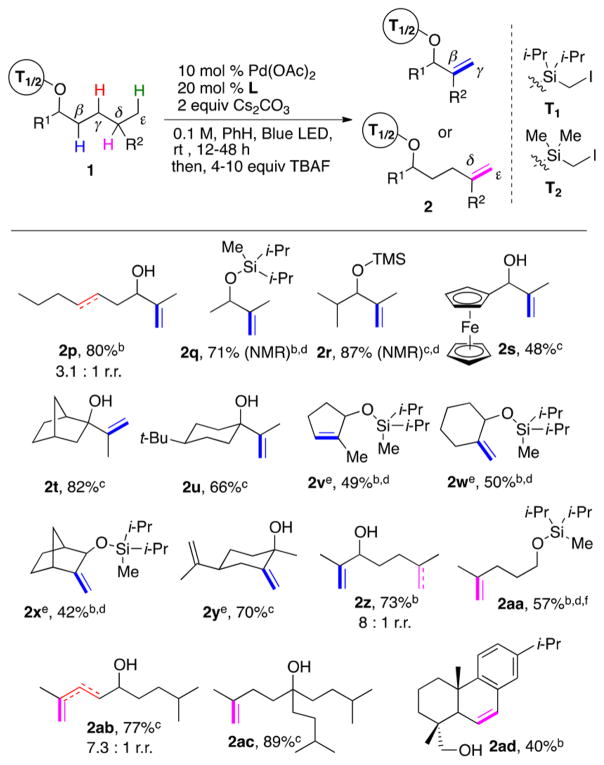
After developing an efficient method for γ-/δ-desaturation of aliphatic alcohols into homoallylic alcohols, we aimed at extending this protocol toward formation of valuable allylic alcohols21 via the β-/γ-desaturation process involving 1,5 HAT (Table 2). Delightfully, it was found that secondary and tertiary alcohols bearing an isopropyl unit underwent selective desaturation to generate the corresponding alcohols in a highly efficient manner (2p–2u). Subjecting five-membered cycloalkane 1v to the reaction conditions unexpectedly resulted in the formation of the thermodynamically more stable endo-isomer 2v. In contrast, six-membered β-methyl cycloalkanes produced the kinetic exo-methylene products 2w–x in reasonable yields. Complex limonene derivative 1y underwent β-/γ-desaturation producing exo-alkene 2y in 70% yield. Expectedly, based on the Si-tether reactivity preference for HAT (vide supra), desaturation of ambident substrate 1z, possessing competing β-/γ- and δ-/ε-desaturation sites, preferably occurred at the former site, producing allylic alcohol 2z in good yield. Finally, we examined the boundaries of this methodology toward δ-/ε-desaturation of alcohols. Gratifyingly, primary (1aa), secondary (1ab), and tertiary alcohols (1ac) all produced the δ-C–H functionalized products in moderate to excellent yields! Likewise, desaturation of complex derivative dehydroabietol 1ad resulted in δ-/ε-desaturation product 2ad in moderate yield.
Table 2.
β-/γ- and δ-/ε-Desaturation of Alcoholsa
Isolated yields; r.r. = regioisomeric ratio.
T1 was used.
T2 was used.
The desilylation step (TBAF) was omitted.
For 1v–1y, the trans precursor was used.
Contains minor amount of hydrodehalogenation byproduct.
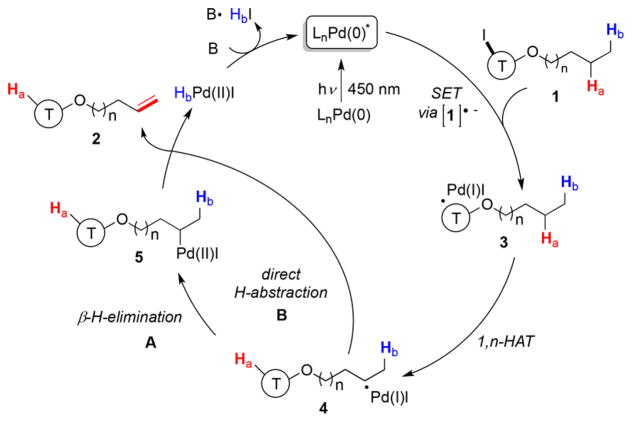
The results of performed radical trap-, radical clock-, and isotope labeling studies16 supported the radical nature of this transformation. The UV–vis analysis revealed that the Pd(0) complex is the photoabsorbing species. Moreover, the Stern–Volmer studies supported quenching of the excited state of the Pd(0) catalyst by alkyl iodide 1f.16 Based on the aforementioned studies, the following mechanism is proposed (Scheme 3). Visible light induction of the in situ generated Pd(0) complex produces the active photoexcited Pd(0) complex, which engages in an SET process with alkyl iodide 1 to form alkyl hybrid intermediate 3. Next, the formed radical species undergoes a rate-limiting 1,n-HAT event (for n = 5, kH/kD = 3.516) to generate translocated alkyl hybrid Pd-radical species 4. The latter, either via a recombination of 4 with the putative Pd(I) intermediate followed by a β-hydride elimination from 5 (path A) or via a direct abstraction of β-hydrogen atom22 by Pd(I) species (4 → 2, path B),23 produces the alkene 224 and regenerates the Pd catalyst.
Scheme 3.
Proposed Mechanism
In summary, we have developed a room temperature, auxiliary-enabled visible light-induced Pd-catalyzed remote β-/γ-, γ-/δ-, and δ-/ε-desaturation of alcohols. The hybrid Pd-radical nature of this protocol enabled efficient functionalization of unactivated C–H sites. Moreover, the desaturation products were formed with superior degrees of regioselectivity compared to the state-of-the-art radical/cationic methods due to a better-controlled Pd-involved β-hydrogen elimination step. Overall, this transformation represents the first practical catalytic desaturation of aliphatic alcohols. We believe that this approach addresses the shortcomings of prior art and provides a new avenue for targeted aliphatic C–H functionalization under photoinduced transition metal catalysis.
Supplementary Material
Acknowledgments
This research was supported by the National Science Foundation (CHE-1663779) and the National Institutes of Health (GM120281). We also thank Ms. Jenny S. Martinez for help with graphical image.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.7b08459.
Experimental procedures and compound characterization data (PDF)
References
- 1.Larock RC. Comprehensive Organic Transformations. 2. Wiley; New York: 1999. [Google Scholar]
- 2.(a) He J, Wasa M, Chan KSL, Shao Q, Yu JQ. Chem Rev. 2017;117:8754. doi: 10.1021/acs.chemrev.6b00622. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Daugulis O, Roane J, Tran LD. Acc Chem Res. 2015;48:1053. doi: 10.1021/ar5004626. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem - Eur J. 2010;16:2654. doi: 10.1002/chem.200902374. [DOI] [PubMed] [Google Scholar]; (d) Topczewski JJ, Cabrera PJ, Saper NI, Sanford MS. Nature. 2016;531:220. doi: 10.1038/nature16957. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) McNally A, Haffemayer B, Collins BSL, Gaunt MJ. Nature. 2014;510:129. doi: 10.1038/nature13389. [DOI] [PubMed] [Google Scholar]; (f) Davies HML, Beckwith REJ. Chem Rev. 2003;103:2861. doi: 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]; (g) White MC. Science. 2012;335:807. doi: 10.1126/science.1207661. [DOI] [PubMed] [Google Scholar]; (h) Howell JM, Feng K, Clark JR, Trzepkowski LJ, White MC. J Am Chem Soc. 2015;137:14590. doi: 10.1021/jacs.5b10299. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Gensch T, Hopkinson MN, Glorius F, Wencel-Delord J. Chem Soc Rev. 2016;45:2900. doi: 10.1039/c6cs00075d. [DOI] [PubMed] [Google Scholar]
- 3.Choi J, MacArthur AHR, Brookhart M, Goldman AS. Chem Rev. 2011;111:1761. doi: 10.1021/cr1003503. [DOI] [PubMed] [Google Scholar]
- 4.(a) Giri R, Maugel N, Foxman BM, Yu JQ. Organometallics. 2008;27:1667. [Google Scholar]; (b) Baudoin O, Herrbach A, Gueritte F. Angew Chem, Int Ed. 2003;42:5736. doi: 10.1002/anie.200352461. [DOI] [PubMed] [Google Scholar]; (c) Motti E, Catellani M. Adv Synth Catal. 2008;350:565. [Google Scholar]
- 5.(a) Breslow R, Baldwin S, Flechtner T, Kalicky P, Liu S, Washburn W. J Am Chem Soc. 1973;95:3251. doi: 10.1021/ja00791a031. [DOI] [PubMed] [Google Scholar]; (b) Voica AF, Mendoza A, Gutekunst WR, Fraga JO, Baran PS. Nat Chem. 2012;4:629. doi: 10.1038/nchem.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hollister KA, Conner ES, Spell ML, Deveaux K, Maneval L, Beal MW, Ragains JR. Angew Chem, Int Ed. 2015;54:7837. doi: 10.1002/anie.201500880. [DOI] [PubMed] [Google Scholar]
- 6.For moderately selective desaturation of alkyl peroxides via HAT at δ-C–H site employing stoichiometric amounts of transition metals, see: Cekovio Z, Dimitrijevic L, Djokic G, Srnic T. Tetrahedron. 1979;35:2021.
- 7.Parasram M, Chuentragool P, Sarkar D, Gevorgyan V. J Am Chem Soc. 2016;138:6340. doi: 10.1021/jacs.6b01628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For a review on exogenous photosensitizer-free, visible light-induced TM-catalyzed transformations, see: Parasram M, Gevorgyan V. Chem Soc Rev. 2017 doi: 10.1039/C7CS00226B.
- 9.For a review, see: Liu Q, Dong X, Li J, Xiao J, Dong Y, Liu H. ACS Catal. 2015;5:6111.See also: Bloome KS, McMahen RL, Alexanian EJJ. J Am Chem Soc. 2011;133:20146. doi: 10.1021/ja2091883.Bonney KJ, Proutiere F, Schoenebeck F. Chem Sci. 2013;4:4434.Venning ARO, Kwiatkowski MR, Peña JER, Lainhart BC, Guruparan AA, Alexanian EJJ. J Am Chem Soc. 2017;139:11595. doi: 10.1021/jacs.7b06794.For a review on UV-induced Pd-catalyzed transformations involving alkyl iodides, see: Sumino S, Fusano A, Fukuyama T, Ryu I. Acc Chem Res. 2014;47:1563. doi: 10.1021/ar500035q.
- 10.For alkyl hybrid Pd-radical intermediates capable of HAT of activated C–H bonds, see: Peacock DM, Roos CB, Hartwig JF. ACS Cent Sci. 2016;2:647. doi: 10.1021/acscentsci.6b00187.
- 11.For a review, see: Parasram M, Gevorgyan V. Acc Chem Res. 2017;50:2038. doi: 10.1021/acs.accounts.7b00306.
- 12.(a) Parasram M, Iaroshenko VO, Gevorgyan V. J Am Chem Soc. 2014;136:17926. doi: 10.1021/ja5104525. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kurandina D, Parasram M, Gevorgyan V. Angew Chem, Int Ed. doi: 10.1002/anie.201706554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For a review featuring examples beyond 1,5-HAT, see: Nechab M, Mondal S, Bertrand MP. Chem - Eur J. 2014;20:16034. doi: 10.1002/chem.201403951.
- 14.Generally, 1,5-HAT is conformationally favored (six-membered transition state) for carbon-based radicals; see ref 13. However, due to the longer bond length of C–Si bonds, silyl methyl radicals are predisposed to adopt transition states beyond that of analogous carbon-based radicals. Thus, for T1 and T2 presented in this work, 1,6-HAT (seven-membered transition state) is the most preferred process; see also: Koreeda M, Hamann LG. J Am Chem Soc. 1990;112:8175.Wilt JW, Lusztyk J, Peeran M, Ingold KU. J Am Chem Soc. 1988;110:281.
- 15.Carreira EM, Kvaerno L. Classics in stereoselective synthesis. Wiley-VCH; Weinheim, Germany: 2009. p. 153. [Google Scholar]
- 16.See Supporting Information for details.
- 17.For reviews, see: Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322. doi: 10.1021/cr300503r.Skubi KL, Blum TR, Yoon TP. Chem Rev. 2016;116:10035. doi: 10.1021/acs.chemrev.6b00018.Romero NA, Nicewicz DA. Chem Rev. 2016;116:10075. doi: 10.1021/acs.chemrev.6b00057.Staveness D, Bosque I, Stephenson CRJ. Acc Chem Res. 2016;49:2295. doi: 10.1021/acs.accounts.6b00270.Tellis JC, Kelly CB, Primer DN, Jouffroy M, Patel NR, Molander GA. Acc Chem Res. 2016;49:1429. doi: 10.1021/acs.accounts.6b00214.Meggers E. Chem Commun. 2015;51:3290. doi: 10.1039/c4cc09268f.Xie J, Jin H, Hashmi ASK. Chem Soc Rev. 2017;46:5193. doi: 10.1039/c7cs00339k.For selected examples, see: Shu W, Nevado C. Angew Chem, Int Ed. 2017;56:1881. doi: 10.1002/anie.201609885.Shu W, Genoux A, Li Z, Nevado C. Angew Chem, Int Ed. 2017;56:10521. doi: 10.1002/anie.201704068.Hu XQ, Chen JR, Xiao WJ. Angew Chem, Int Ed. 2017;56:1960. doi: 10.1002/anie.201611463.Zhang J, Li Y, Zhang F, Hu C, Chen Y. Angew Chem, Int Ed. 2016;55:1872. doi: 10.1002/anie.201510014.Wang C, Harms K, Meggers E. Angew Chem, Int Ed. 2016;55:13495. doi: 10.1002/anie.201607305.Chu JCK, Rovis T. Nature. 2016;539:272. doi: 10.1038/nature19810.Choi GJ, Zhu Q, Miller DC, Gu CJ, Knowles RR. Nature. 2016;539:268. doi: 10.1038/nature19811.
- 18.An analogous stereochemical outcome was observed upon desaturation of (−)-menthol under Baran’s protocol; see ref 5b.
- 19.The stereoselectivity of the HAT process was investigated using cis- and trans-stereoisomers of 1w-T2. For the cis-isomer, for which a quasi-liner TS required for an HAT event is unlikely, the reaction produced the hydrodehalogenation byproduct only. Conversely, the trans-isomer underwent HAT to produce 43% of the desaturation product. See SI for details.
- 20.Blanksby SJ, Ellison GB. Acc Chem Res. 2003;36:255. doi: 10.1021/ar020230d. [DOI] [PubMed] [Google Scholar]
- 21.Lumbroso A, Cooke ML, Breit B. Angew Chem, Int Ed. 2013;52:1890. doi: 10.1002/anie.201204579. [DOI] [PubMed] [Google Scholar]
- 22.For BDEs of C–H bonds adjacent to radical centers, see: Zhang X-M. J Org Chem. 1998;63:1872. doi: 10.1021/jo981129i.
- 23.(a) Kuo JL, Hartung J, Han A, Norton JR. J Am Chem Soc. 2015;137:1036. doi: 10.1021/ja511883b. [DOI] [PubMed] [Google Scholar]; (b) Hu Y, Shaw AP, Estes DP, Norton JR. Chem Rev. 2016;116:8427. doi: 10.1021/acs.chemrev.5b00532. [DOI] [PubMed] [Google Scholar]
- 24.The endgame, Pd-assisted H-atom elimination, occurs predominantly at the sterically less hindered site. For discussion via path A, see ref 12a.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.