Abstract
Purpose of Review
Critically ill patients with acute respiratory distress syndrome (ARDS) may require sedation in their clinical care. The goals of sedation in ARDS patients are to improve patient comfort and tolerance of supportive and therapeutic measures without contributing to adverse outcomes. This review discusses the current evidence for sedation management in patients with ARDS.
Recent Findings
Deep sedation strategies should be avoided in the care of patients with ARDS because deep sedation has been associated with increased time on mechanical ventilation, longer ICU and hospital length of stay, and higher mortality in critically ill patients. Adoption of protocol-based, light-sedation strategies are preferred and improve patient outcomes. Although the optimal sedative agent for ARDS patients is unclear, benzodiazepines should be avoided due to associations with oversedation, delirium, prolonged intensive care unit and hospital length of stay, and increased mortality. Minimizing sedation in patients with ARDS facilitates early mobilization and early discharge from the intensive care unit, potentially aiding in recovery from critical illness. Strategies to optimize ventilation in ARDS patients, such as low-tidal volume ventilation and high positive end-expiratory pressure (PEEP) can be employed without deep sedation; however, deep sedation is required if patients receive neuromuscular blockade, which may benefit some ARDS patients. Knowledge gaps persist as to whether or not prone positioning and extracorporeal membrane oxygenation (ECMO) can be tolerated with light sedation.
Summary
Current evidence supports the use of protocol-based, light-sedation strategies in critically ill patients with ARDS. Further research into sedation management specifically in ARDS populations is needed.
Keywords: acute respiratory distress syndrome, sedation, outcomes
Introduction
Many patients with moderate or severe acute respiratory distress syndrome (ARDS) will require sedation and analgesia in the setting of mechanical ventilation. Sedation management is an important component of the care of critically ill patients and a modifiable factor influencing their outcomes. While sedation can improve comfort for critically ill patients, some sedation strategies can have negative consequences including prolonged duration of mechanical ventilation and increased risk of delirium. This review provides an overview of recent advances to minimize sedation and focuses on the benefits of limiting sedation, weaning strategies and protocols, and the relationship between sedation strategies and delirium.
Although very few trials of sedation management in the ICU limited enrollment to include only patients with ARDS, many of the key randomized trials of sedation management published in the last two decades included a substantial proportion of patients with ARDS (Table 1). We review herein the evidence from these trials to provide guidance in improving care delivered to critically ill patients with ARDS.
Table 1.
Enrollment of Patients with Acute Respiratory Distress Syndrome in Clinical Studies of Sedation
| Author | Year | Clinical Study | Patients with ARDS/Total Patients in Study (%) |
|---|---|---|---|
| Kress et al1 | 2000 | RCT of daily sedation interruption versus usual care | 35/150 (23.33%) |
| Pandharipande et al2 | 2007 | RCT of continuous dexmedetomidine versus midazolam | 39/103 (37.86%)* |
| Girard et al3 | 2008 | RCT of paired daily sedation interruption and spontaneous breathing trials versus usual care | 166/355 (49.55%)* |
| Mehta et al4 | 2012 | RCT of protocolized sedation versus protocolized sedation with daily sedation interruption | 23/65 (35.38%)** |
- Includes patients with ARDS and with sepsis
- Includes patients with ARDS and with pneumonia
Minimizing Sedation
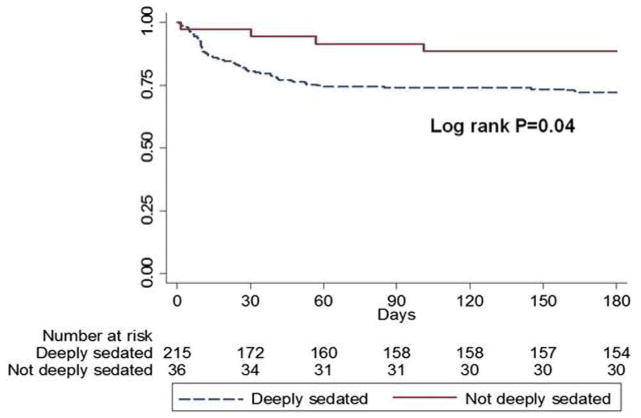
The goals of sedation in the intensive care unit are to keep the patient comfortable enough to tolerate treatment and, occasionally, to promote patient safety.5,6 In patients with ARDS, sedation is used to improve patient tolerance of mechanical ventilation, reduce discomfort, and, in some cases, to improve patient-ventilator synchrony.7 Sedation practices have changed dramatically in the past three decades. In the 1980s, deep sedation was common to provide comfort and limit memory of the critical illness. However, several clinical trials since the 1990s have shown that oversedation is common in the ICU and contributes to adverse outcomes.8 A prospective multi-center Australian cohort study demonstrated that deep sedation within the first 48 hours of ICU admission was predictive of delayed time to extubation and increased risk of in-hospital and 180-day mortality in a mixed ICU population. [Figure 1]9 A subsequent Brazilian prospective cohort study similarly associated early deep sedation with increased time on mechanical ventilation, risk of having a tracheostomy, and higher mortality, and furthermore demonstrated that the effects of deep sedation on mortality were independent of severity of ARDS illness.10
Figure 1.
Observational cohort study by Shehabi et al9 demonstrating that deep sedation is associated with worse survival at 3 months in mechanically ventilated critically ill patients. (With permission from 9)
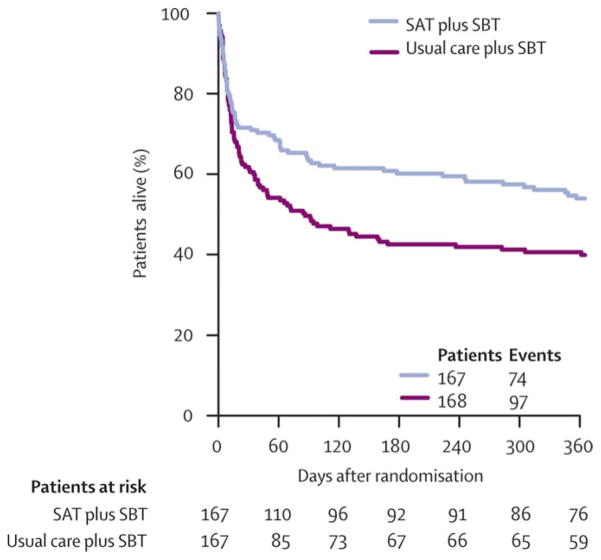
Strategies to minimize deep sedation have beneficial effects in critically ill patients. A landmark randomized trial in 1999 found that protocol-directed, nursing-led sedation decreased duration of mechanical ventilation, ICU and hospital length of stay, and need for tracheostomy when compared with usual care. 11 A second landmark trial in 2000 found that daily interruptions of continuous sedation reduced total amount of sedative delivered and subsequently reduced time on mechanical ventilation by more than 2 days and ICU length of stay by 3.5 days compared with usual care.1 Later, a multi-center randomized controlled trial compared paired sedation interruption and spontaneous breathing trials with usual care and demonstrated that the paired protocol minimized deep sedation and reduced one-year mortality in critically ill patients. [Figure 2]3 Opponents of minimizing sedation raised the possibility of increased risk of neuropsychological outcomes, but several studies have shown that patients who received lighter sedation do not experience these adverse effects.8,12,13 Furthermore, some protocols for critically ill patients utilize a “no sedation” approach using analgesics alone with no or intermittent sedative use, an approach which in small studies has decreased time on mechanical ventilation compared with usual care.14,15
Figure 2.
Randomized controlled clinical trial by Girard et al3 demonstrating that pairing spontaneous awakening trials (SAT) and spontaneous breathing breathing trials (SBT) in critically ill patients increased 1 year survival. (with permission from 3)
One of the key benefits to limiting sedation use in patients with ARDS may be improved ability to participate in early mobilization and rehabilitation.16 Early mobilization is particularly important in patients with ARDS as over 50% of survivors suffer from deficits in physical and cognitive function that persist for years beyond the inciting event.12,17–21 Several clinical trials have shown that early mobilization in both medical and surgical critically ill patients is safe and associated with increased ventilator-free days and improved physical function at hospital discharge.22–25 Early mobilization is limited by use of deep sedation and development of delirium, which can be minimized through the use of scale-based targeted light sedation is implemented early on.26
After reviewing this literature in 2013, the Society of Critical Care Medicine (SCCM)’s Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit recommended using a light rather than deep sedation strategy for critically ill patients.27 Similarly, new guidelines on liberation from mechanical ventilation published jointly by the American College of Chest Physicians and the American Thoracic Society recommend using a sedation protocol to minimize sedation of mechanically ventilated ICU patients.28
Balanced against the recommendation for light sedation is the occasional need for deep sedation during advanced therapies for severe ARDS. A number of studies have shown that deep sedation is not required for patients to tolerate the low tidal volume ventilation29–31 or high PEEP strategies32,33 that are often employed for management of ARDS. However, deep sedation is required in patients with ARDS who receive neuromuscular blockage to ensure patients do not consciously experience paralysis.34,35 Neuromuscular blockage is sometimes used in severe ARDS based on results of a multicenter trial in France that showed improved mortality with 48 hours of neuromuscular blockage in patients who have severe hypoxemia (PaO2/FiO2 ratio<150) early during their ARDS.36 A large, multicenter trial (NCT02509078) is now underway in the United States (US) to rigorously test whether early neuromuscular blockade results in better outcomes for patients with severe ARDS compared to current standard of care approaches, including light sedation. It is important to recognize that the French trial used a double-blind design, thus patients in the control arm also received deep sedation similar to patients in the intervention arm, an approach some have criticized as harmful to patients in the control arm. In contrast, the Prevention and Early Treatment of Acute Lung Injury (PETAL) investigators conducting the ongoing US trial are not blinded to the intervention and light sedation is recommended for patients in the control group.
Management of sedation for patients who receive other interventions in ARDS, such as prone positioning and extracorporeal membrane oxygenation (ECMO), remains unclear. For instance, compared to ARDS patients who do not receive ECMO, patients receiving ECMO may need higher sedation to tolerate the invasiveness of the procedure, to compensate for sedatives consumed by the ECMO circuit itself,37 and because patients who receive ECMO tend to be younger and have higher illness severity.38,39 Whether higher sedation is actually necessary for these patients is not known. Minimizing sedation in these patients should remain an important goal to minimize delirium, increase patient mobilization, and optimize patient recovery.40
Weaning Sedation
Two early landmark trials in sedation management demonstrated a benefit in both daily sedation awakening and in paired sedation interruption and spontaneous breathing trials in decreasing overall sedation requirements, decreasing time on mechanical ventilation, and decreasing ICU length of stay in critically ill patients when compared to usual care.1,3 Recent meta-analyses examining the effects of daily sedation interruption trials have not demonstrated the same magnitude of benefit in reducing time on mechanical ventilation or ICU length of stay.41,42 There are several possible explanations for these findings. First, studies suggested that the benefit of daily sedation interruption is only realized when the overall amount of sedatives received by critically ill patients is reduced, and not all trials achieved this goal in patients randomized to daily interruption of sedatives.4 For example, patients in the daily interruption of sedatives group of the SLEAP trial received more midazolam and fentanyl boluses than those managed without daily interruption of sedatives, and the trial found no difference in outcomes between groups. Second, the benefits of daily interruption of sedatives noted in early trials may not be observed in more recent trials due to changes in sedation practices over time affecting the management of sedation in the control arm. For instance, guidelines now recommend light sedation goals and advocate for the use of validated sedation scales, such as the Richmond Agitation-Sedation Scale, which help limit sedative and analgesia use. Thus the standard of care has changed over time and may have led to use of lighter sedation in the control arm in more recent clinical trials compared to older studies.27,43–46 Finally, isolating the effects of daily sedation interruption trials in clinical practice is challenging as daily awakening trials are now frequently bundled with other interventions to improve the quality of care delivered to critically ill patients. An example of a bundled quality care intervention includes the ABCDE Bundle (Awakening and Breathing Coordination of daily sedation and ventilator removal trials; Choice of sedative or analgesic exposure; Delirium monitoring and management; and Early mobility and exercise).47–49 The use of these evidence-based bundles, which starts with decreasing sedation use, has been associated with decreased delirium, decreased time on mechanical ventilation, and decreased ICU length of stay.50, 51 Thus, whether minimized via the use of daily interruption of sedatives or other protocolized approaches to minimizing sedation, one thing is clear—light (or even no) sedation results in better outcomes for mechanically ventilated ICU patients, including those with ARDS.
Type of Sedation
The 2013 SCCM Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit suggest using a non-benzodiazepine agent for sedation of mechanically ventilated ICU patients except when a benzodiazepine is indicated due to seizure, alcohol or benzodiazepine withdrawal, or to ensure deep sedation and amnesia (e.g., during paralysis). Recent studies have confirmed that the use of benzodiazepines for sedation in critically ill patients is associated with increased delirium, increased hospital length of stay, increased time on mechanical ventilation, and increased intensive care unit hospitalization.2,52–54 A recent propensity-based analysis also suggested that benzodiazepine use in critically ill patients is associated with increased mortality, further strengthening the argument for relying on non-benzodiazepine agents, such as propofol or dexmedetomidine.55 Benzodiazepines may still be considered in rare cases when patients develop side effects from other sedatives or when they have chronically been using benzodiazepines in the outpatient setting, but even in these cases an intermittent sedative approach can be considered and benzodiazepine use should be limited when possible.56 While evidence for type of sedation for critically ill patients specifically with ARDS remains limited, results from these recent studies support starting initially with a non-benzodiazepine sedative agent.
Risks of Delirium
Delirium complicates up to 70%–80% percent of cases of ARDS and has been identified as an independent risk factor for increased hospital and ICU length of stay, increased mortality, and increased long-term cognitive impairment in survivors of critical illness.21, 57–63 The mechanisms linking delirium to adverse outcomes in ARDS remain unclear,64,65 and several recent studies have explored the potential role of sedation as an iatrogenic contributing factor.
A recent cohort study of adult ICU patients admitted with respiratory failure or shock prospectively assessed inpatient delirium and sedative use and examined risk factors associated with long-term cognitive impairment in survivors of critical illness. After adjusting for duration of delirium, which was found to be associated with development of long-term cognitive impairments in this study, sedative use was not an independent risk factor for long-term cognitive deficits.21 Given that heavy sedation, especially with benzodiazepines, has been consistently found to increase delirium risk, these findings do not rule out the possibility that heavy sedation plays a role in adverse long-term cognitive outcomes. Instead, they suggest that the patients with ARDS who are at highest risk for sedative-associated long-term cognitive impairment are those who develop persistent delirium when receiving sedatives. For reasons that have yet to be elucidated, some patients may be at higher risk for sedative-associated delirium than others. The SCCM guidelines recommend monitoring all ICU patients for delirium using either the Confusion Assessment Method for the ICU66, 67 or the Intensive Care Delirium Screening Checklist,68 both of which have been validated in mechanically ventilated ICU patients, including those with ARDS. When delirium is identified, one change in management to consider is that of sedative choice.
One recent study suggested that 10% of mechanically ventilated ICU patients develop rapidly reversible delirium in the setting of sedation, whereas a larger proportion develop delirium that persists after cessation of sedatives. This study categorized patients into one of four groups: (1) no delirium, (2) rapidly reversible delirium associated with sedative use which resolved when sedation was held, (3) persistent delirium where delirium did not improve when sedation was weaned, and (4) mixed delirium, which had characteristics of both rapidly reversible delirium and persistent delirium. Persistent and mixed delirium were associated with significantly worse ICU outcomes, whereas rapidly reversible delirium associated with sedation was associated with outcomes similar to those that had no delirium. Of note, benzodiazepine use was minimal in this study, and the rapidly reversible form of delirium was uncommon, such that this small study was underpowered to detect differences in outcomes in this group.69
Optimal management of critically ill patients with delirium remains unclear but focuses on correcting the underlying illness and minimizing medications that could contribute to delirium.70 Two recent randomized, placebo-controlled trials showed that, in critically ill patients with agitation, dexmetetomidine reduces delirium and decreases time to successful extubation.71,72 Additionally, a meta-analysis compared the efficacy of dexmedetomidine to other sedative agents and showed that dexmedetomidine is associated with less delirium.73 The role of antipsychotic medications, common in the treatment of hospitalized patients with delirium that are not in the ICU, remains unclear as few placebo-controlled, randomized clinical trials have been performed to assess the effects of typical or atypical antipsychotics in critical care settings.74–76 A recent clinical trial exploring the role of haloperidol to prevent and treat the occurrence of delirium found that the regular use of haloperidol did not affect number of days with delirium or survival in critically ill patients.77 The results of these clinical trials emphasize the need for further research into the mechanisms and management of delirium in critically ill patients but do not suggest that the deleterious effects of delirium are influenced by sedative use.
Immune Effects of Sedation
The benefits of limiting sedation may extend beyond avoiding oversedation, facilitating early mobilization, and reducing the duration of mechanical ventilation, ICU stay, and delirium. Sedative agents have been suggested to have broad immune effects. For example, studies in rats have shown that propofol may impair neutrophil phagocytosis and lipopolysaccharide-induced macrophage Th1 cytokine response.78,79 Similarly, dexmedetomidine may have anti-inflammatory effects and improve macrophage function.80, 81 Some of these immune effects may be beneficial in patients with sepsis, the most common cause of ARDS. There is increasing recognition that sepsis is a heterogeneous condition and includes patients who have increased inflammation and are immunosuppressed.82,83 Thus, understanding the immunomodulatory effects of sedative agents on ARDS will be challenging and will be an important area for future studies.
Conclusion
Sedation management is an important component in the care of critically ill patients with ARDS. The goals of sedation should be to reduce discomfort and improve patient tolerance of mechanical ventilation and other advanced therapies for ARDS, while avoiding deep sedation when possible. Sedation protocols should prioritize non-benzodiazepine regimens and utilize daily sedation weaning and/or protocol based sedation algorithms to target light levels of sedation titrated using validated scales. Improved sedation management in critically ill patients is associated with decreased delirium and improved early mobilization which may help improve outcomes in patients with ARDS. Further research into the management of sedation and mechanisms by which specific sedation strategies would improve outcomes, specifically in patients with ARDS, is needed.
Key Phrases.
This review highlights recent advances in sedation management in critically ill patients with the acute respiratory distress syndrome (ARDS).
Current evidence supports the use of protocol-based, light-sedation strategies; many therapeutic interventions for ARDS, including high PEEP and low-tidal volume ventilation, can be achieved without deep sedation.
Further research in sedation use specifically in ARDS populations is needed.
Benefits of minimizing sedation in ARDS patients include decreasing delirium, facilitating early mobilization, and reducing ICU and hospital length of stay, and potentially improving mortality and long term recovery.
Acknowledgments
Financial Support or Sponsorship:
Faraaz Shah, Timothy Girard, and Sachin Yende are supported by grants from the National Institutes of Health.
Footnotes
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the Department of Veterans Affairs or the United States Government.
Conflicts of Interest:
None.
References
- 1.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. The New England journal of medicine. 2000 May 18;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 2.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. Jama. 2007 Dec 12;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 3.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008 Jan 12;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 4.Mehta S, Burry L, Cook D, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. Jama. 2012 Nov 21;308(19):1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 5.Weinert CR, Calvin AD. Epidemiology of sedation and sedation adequacy for mechanically ventilated patients in a medical and surgical intensive care unit. Critical care medicine. 2007 Feb;35(2):393–401. doi: 10.1097/01.CCM.0000254339.18639.1D. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Critical care medicine. 2002 Jan;30(1):119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Mehta S, Burry L, Fischer S, et al. Canadian survey of the use of sedatives, analgesics, and neuromuscular blocking agents in critically ill patients. Critical care medicine. 2006 Feb;34(2):374–380. doi: 10.1097/01.ccm.0000196830.61965.f1. [DOI] [PubMed] [Google Scholar]
- 8.Treggiari MM, Romand JA, Yanez ND, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Critical care medicine. 2009 Sep;37(9):2527–2534. doi: 10.1097/CCM.0b013e3181a5689f. [DOI] [PubMed] [Google Scholar]
- 9.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. American journal of respiratory and critical care medicine. 2012 Oct 15;186(8):724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka LM, Azevedo LC, Park M, et al. Early sedation and clinical outcomes of mechanically ventilated patients: a prospective multicenter cohort study. Critical care. 2014;18(4):R156. doi: 10.1186/cc13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Critical care medicine. 1999 Dec;27(12):2609–2615. doi: 10.1097/00003246-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Jackson JC, Girard TD, Gordon SM, et al. Long-term cognitive and psychological outcomes in the awakening and breathing controlled trial. American journal of respiratory and critical care medicine. 2010 Jul 15;182(2):183–191. doi: 10.1164/rccm.200903-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. American journal of respiratory and critical care medicine. 2003 Dec 15;168(12):1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 14.Devabhakthuni S, Armahizer MJ, Dasta JF, Kane-Gill SL. Analgosedation: a paradigm shift in intensive care unit sedation practice. The Annals of pharmacotherapy. 2012 Apr;46(4):530–540. doi: 10.1345/aph.1Q525. [DOI] [PubMed] [Google Scholar]
- 15.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010 Feb 6;375(9713):475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 16.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Critical care medicine. 2013 Jun;41(6):1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 17.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. The New England journal of medicine. 2003 Feb 20;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 18.Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive care medicine. 2016 May;42(5):725–738. doi: 10.1007/s00134-016-4321-8. [DOI] [PubMed] [Google Scholar]
- 19.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama. 2010 Oct 27;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilcox ME, Herridge MS. Long-term outcomes in patients surviving acute respiratory distress syndrome. Seminars in respiratory and critical care medicine. 2010 Feb;31(1):55–65. doi: 10.1055/s-0029-1246285. [DOI] [PubMed] [Google Scholar]
- 21.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. The New England journal of medicine. 2013 Oct 3;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayambu G, Boots R, Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive care medicine. 2015 May;41(5):865–874. doi: 10.1007/s00134-015-3763-8. [DOI] [PubMed] [Google Scholar]
- 23.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Archives of physical medicine and rehabilitation. 2010 Apr;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009 May 30;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016 Oct 1;388(10052):1377–1388. doi: 10.1016/S0140-6736(16)31637-3. [DOI] [PubMed] [Google Scholar]
- 26.Jolley SE, Caldwell E, Hough CL. Factors associated with receipt of physical therapy consultation in patients requiring prolonged mechanical ventilation. Dimensions of critical care nursing : DCCN. 2014 May-Jun;33(3):160–167. doi: 10.1097/DCC.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Critical care medicine. 2013 Jan;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- **28.Schmidt GA, Girard TD, Kress JP, et al. Official Executive Summary of an American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. American journal of respiratory and critical care medicine. 2016 Oct 20; doi: 10.1164/rccm.201610-2076ST. Annotation: Updated guidelines from the American College of Chest Physicians and the American Thoracic Society on best practice recommendations to improve liberation from mechanical ventilation, of which sedation management is an important component. [DOI] [PubMed] [Google Scholar]
- 29.Serpa Neto A, Simonis FD, Barbas CS, et al. Association between tidal volume size, duration of ventilation, and sedation needs in patients without acute respiratory distress syndrome: an individual patient data meta-analysis. Intensive care medicine. 2014 Jul;40(7):950–957. doi: 10.1007/s00134-014-3318-4. [DOI] [PubMed] [Google Scholar]
- 30.Kahn JM, Andersson L, Karir V, Polissar NL, Neff MJ, Rubenfeld GD. Low tidal volume ventilation does not increase sedation use in patients with acute lung injury. Critical care medicine. 2005 Apr;33(4):766–771. doi: 10.1097/01.ccm.0000157786.41506.24. [DOI] [PubMed] [Google Scholar]
- 31.Wolthuis EK, Veelo DP, Choi G, et al. Mechanical ventilation with lower tidal volumes does not influence the prescription of opioids or sedatives. Critical care. 2007;11(4):R77. doi: 10.1186/cc5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arroliga AC, Thompson BT, Ancukiewicz M, et al. Use of sedatives, opioids, and neuromuscular blocking agents in patients with acute lung injury and acute respiratory distress syndrome. Critical care medicine. 2008 Apr;36(4):1083–1088. doi: 10.1097/CCM.0B013E3181653895. [DOI] [PubMed] [Google Scholar]
- 33.Mehta S, Cook DJ, Skrobik Y, et al. A ventilator strategy combining low tidal volume ventilation, recruitment maneuvers, and high positive end-expiratory pressure does not increase sedative, opioid, or neuromuscular blocker use in adults with acute respiratory distress syndrome and may improve patient comfort. Annals of intensive care. 2014;4:33. doi: 10.1186/s13613-014-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bein T, Grasso S, Moerer O, et al. The standard of care of patients with ARDS: ventilatory settings and rescue therapies for refractory hypoxemia. Intensive care medicine. 2016 May;42(5):699–711. doi: 10.1007/s00134-016-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kredel M, Bierbaum D, Lotz C, Kustermann J, Roewer N, Muellenbach RM. Therapy of acute respiratory distress syndrome : Survey of German ARDS centers and scientific evidence. Der Anaesthesist. 2015 Apr;64(4):277–285. doi: 10.1007/s00101-015-0010-1. [DOI] [PubMed] [Google Scholar]
- 36.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. The New England journal of medicine. 2010 Sep 16;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- *37.Nigoghossian CD, Dzierba AL, Etheridge J, et al. Effect of Extracorporeal Membrane Oxygenation Use on Sedative Requirements in Patients with Severe Acute Respiratory Distress Syndrome. Pharmacotherapy. 2016 Jun;36(6):607–616. doi: 10.1002/phar.1760. Annotation: Retrospective cohort study of sedative requirements in patients with severe acute respiratory distress syndrome managed with and without ECMO. [DOI] [PubMed] [Google Scholar]
- 38.Olafson K, Ramsey CD, Ariano RE, et al. Sedation and analgesia usage in severe pandemic H1N1 (2009) infection: a comparison to respiratory failure secondary to other infectious pneumonias. The Annals of pharmacotherapy. 2012 Jan;46(1):9–20. doi: 10.1345/aph.1Q446. [DOI] [PubMed] [Google Scholar]
- 39.Buscher H, Vaidiyanathan S, Al-Soufi S, et al. Sedation practice in veno-venous extracorporeal membrane oxygenation: an international survey. ASAIO journal. 2013 Nov-Dec;59(6):636–641. doi: 10.1097/MAT.0b013e3182a84558. [DOI] [PubMed] [Google Scholar]
- 40.Sen A, Callisen HE, Alwardt CM, et al. Adult venovenous extracorporeal membrane oxygenation for severe respiratory failure: Current status and future perspectives. Annals of cardiac anaesthesia. 2016 Jan-Mar;19(1):97–111. doi: 10.4103/0971-9784.173027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Augustes R, Ho KM. Meta-analysis of randomised controlled trials on daily sedation interruption for critically ill adult patients. Anaesthesia and intensive care. 2011 May;39(3):401–409. doi: 10.1177/0310057X1103900310. [DOI] [PubMed] [Google Scholar]
- 42.Burry L, Rose L, McCullagh IJ, Fergusson DA, Ferguson ND, Mehta S. Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation. The Cochrane database of systematic reviews. 2014;(7):CD009176. doi: 10.1002/14651858.CD009176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Critical care medicine. 2013 Feb;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 44.Carrasco G. Instruments for monitoring intensive care unit sedation. Critical care. 2000;4(4):217–225. doi: 10.1186/cc697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australian intensive care unit. Critical care medicine. 2008 May;36(5):1444–1450. doi: 10.1097/CCM.0b013e318168f82d. [DOI] [PubMed] [Google Scholar]
- 46.Elliott R, McKinley S, Aitken LM, Hendrikz J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian intensive care unit. Intensive care medicine. 2006 Oct;32(10):1506–1514. doi: 10.1007/s00134-006-0309-0. [DOI] [PubMed] [Google Scholar]
- 47.Balas MC, Vasilevskis EE, Burke WJ, et al. Critical care nurses’ role in implementing the “ABCDE bundle” into practice. Critical care nurse. 2012 Apr;32(2):35–38. 40–37. doi: 10.4037/ccn2012229. quiz 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kram SL, DiBartolo MC, Hinderer K, Jones RA. Implementation of the ABCDE Bundle to Improve Patient Outcomes in the Intensive Care Unit in a Rural Community Hospital. Dimensions of critical care nursing : DCCN. 2015 Sep-Oct;34(5):250–258. doi: 10.1097/DCC.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 49.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Current opinion in critical care. 2011 Feb;17(1):43–49. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 50.Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Critical care medicine. 2014 May;42(5):1024–1036. doi: 10.1097/CCM.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bassett R, Adams KM, Danesh V, et al. Rethinking critical care: decreasing sedation, increasing delirium monitoring, and increasing patient mobility. Joint Commission journal on quality and patient safety/Joint Commission Resources. 2015 Feb;41(2):62–74. doi: 10.1016/s1553-7250(15)41010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. Jama. 2012 Mar 21;307(11):1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 53.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006 Jan;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. Jama. 2009 Feb 4;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 55.Lonardo NW, Mone MC, Nirula R, et al. Propofol is associated with favorable outcomes compared with benzodiazepines in ventilated intensive care unit patients. American journal of respiratory and critical care medicine. 2014 Jun 1;189(11):1383–1394. doi: 10.1164/rccm.201312-2291OC. [DOI] [PubMed] [Google Scholar]
- 56.Pisani MA, Murphy TE, Araujo KL, Slattum P, Van Ness PH, Inouye SK. Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Critical care medicine. 2009 Jan;37(1):177–183. doi: 10.1097/CCM.0b013e318192fcf9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. Jama. 2004 Apr 14;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 58.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Critical care medicine. 2010 Jul;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Critical care medicine. 2004 Nov;32(11):2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 60.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. American journal of respiratory and critical care medicine. 2012 Jun 15;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. American journal of respiratory and critical care medicine. 2009 Dec 1;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Critical care medicine. 2010 Dec;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 63.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Critical care medicine. 2014 Feb;42(2):369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hsieh SJ, Soto GJ, Hope AA, Ponea A, Gong MN. The association between acute respiratory distress syndrome, delirium, and in-hospital mortality in intensive care unit patients. American journal of respiratory and critical care medicine. 2015 Jan 1;191(1):71–78. doi: 10.1164/rccm.201409-1690OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girard TD, Ware LB, Bernard GR, et al. Associations of markers of inflammation and coagulation with delirium during critical illness. Intensive care medicine. 2012 Dec;38(12):1965–1973. doi: 10.1007/s00134-012-2678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Critical care medicine. 2001 Jul;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 67.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) Jama. 2001 Dec 5;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 68.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive care medicine. 2001 May;27(5):859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 69.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. American journal of respiratory and critical care medicine. 2014 Mar 15;189(6):658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 70.Brummel NE, Girard TD. Preventing delirium in the intensive care unit. Critical care clinics. 2013 Jan;29(1):51–65. doi: 10.1016/j.ccc.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *71.Reade MC, Eastwood GM, Bellomo R, et al. Effect of Dexmedetomidine Added to Standard Care on Ventilator-Free Time in Patients With Agitated Delirium: A Randomized Clinical Trial. Jama. 2016 Apr 12;315(14):1460–1468. doi: 10.1001/jama.2016.2707. Annotation: Recent placebo-controlled randomized clinical trial examining the effects of dexmedetomidine on delirium and time to extubation in agitated critically-ill patients on mechanical ventilation. [DOI] [PubMed] [Google Scholar]
- 72.Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016 Oct 15;388(10054):1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 73.Pasin L, Landoni G, Nardelli P, et al. Dexmedetomidine reduces the risk of delirium, agitation and confusion in critically Ill patients: a meta-analysis of randomized controlled trials. Journal of cardiothoracic and vascular anesthesia. 2014 Dec;28(6):1459–1466. doi: 10.1053/j.jvca.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 74.Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Critical care medicine. 2010 Feb;38(2):419–427. doi: 10.1097/CCM.0b013e3181b9e302. [DOI] [PubMed] [Google Scholar]
- 75.Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic Medication for Prevention and Treatment of Delirium in Hospitalized Adults: A Systematic Review and Meta-Analysis. Journal of the American Geriatrics Society. 2016 Apr;64(4):705–714. doi: 10.1111/jgs.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santos E, Cardoso D, Apostolo J, Neves H, Cunha M, Rodrigues M. Effectiveness of haloperidol prophylaxis in critically ill patients with a high risk for delirium: a systematic review of quantitative evidence protocol. JBI database of systematic reviews and implementation reports. 2015;13(7):83–92. doi: 10.11124/jbisrir-2015-2301. [DOI] [PubMed] [Google Scholar]
- 77.Page VJ, Ely EW, Gates S, et al. Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. The Lancet. Respiratory medicine. 2013 Sep;1(7):515–523. doi: 10.1016/S2213-2600(13)70166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S, Bao H, Han L, Liu L. Effects of propofol on early and late cytokines in lipopolysaccharide-induced septic shock in rats. Journal of biomedical research. 2010 Sep;24(5):389–394. doi: 10.1016/S1674-8301(10)60052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Liu C, Wang G. Propofol Protects Rats and Human Alveolar Epithelial Cells Against Lipopolysaccharide-Induced Acute Lung Injury via Inhibiting HMGB1 Expression. Inflammation. 2016 Jun;39(3):1004–1016. doi: 10.1007/s10753-016-0330-6. [DOI] [PubMed] [Google Scholar]
- 80.Li B, Li Y, Tian S, et al. Anti-inflammatory Effects of Perioperative Dexmedetomidine Administered as an Adjunct to General Anesthesia: A Meta-analysis. Scientific reports. 2015 Jul 21;5:12342. doi: 10.1038/srep12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, Wang Z, Wang Y, Zhou G, Li H. The effect of dexmedetomidine on inflammatory response of septic rats. BMC anesthesiology. 2015 May 01;15:68. doi: 10.1186/s12871-015-0042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. Jama. 2011 Dec 21;306(23):2594–2605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. The Lancet. Infectious diseases. 2013 Mar;13(3):260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]