Noncompacted myocardium (NCM) was first reported by Grant in 1926 as a heterogeneous myocardial disorder characterized by prominent ventricular trabeculation, intratrabecular recesses, and bilayered myocardium composed by a compacted and a noncompacted layer.1,2 It can occur isolated or associated with other cardiomyopathies, complex syndromes, metabolic disorders and congenital heart diseases, such as Ebstein's anomaly, left ventricular (LV) or right ventricular outflow tract obstruction, bicuspid aortic valve, cyanotic congenital heart diseases, and coronary artery anomalies. Although NCM usually affects the left ventricle, it can also affect both ventricles or the right ventricle alone.3
The etiology of LV noncompaction is uncertain, and several etiological bases have been implicated. It is believed to be due to pathogenic mechanisms resulting in a failure in the final phase of myocardial morphogenesis, or myocardial compaction. Increasing evidence has supported a genetic base by identifying mutation in the genes that encode sarcomeric, cytoskeletal and nuclear membrane proteins.4-6
Although considered rare by some authors, NCM incidence and prevalence are uncertain. Ritter et al.7 have reported a 0.05% prevalence in all echocardiographic exams of a large institution. Patients with heart failure (HF) have been reported to have a 4% prevalence of NCM.8
Currently, it is controversial whether NCM is a distinct cardiomyopathy or a morphological characteristic shared by different heart diseases. Thus, while the World Health Organization/International Society and Federation of Cardiology considers NCM an unclassified cardiomyopathy, the American Heart Association considers it a primary genetic cardiomyopathy.9,10 The most recent classification of cardiomyopathies proposed by the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases considers NCM an unclassified familial cardiomyopathy.11
The clinical presentation can occur at any age, being highly variable. Patients can be asymptomatic or have symptoms of severe HF, associated or not with lethal arrhythmias, sudden cardiac death and thromboembolic events.12
Many asymptomatic patients are identified incidentally by an echocardiography performed for assessment of cardiac murmur or for familial screening after identifying an index case.12
Symptoms of HF occur in more than half of the patients with NCM, LV dysfunction being reported in up to 84% of them. In addition, arrhythmias are common: atrial fibrillation can affect 25% of adult patients, and ventricular tachyarrhythmias, up to 47% of patients. The occurrence of thromboembolic events, such as stroke, transient ischemic attack, pulmonary embolism and mesenteric ischemia, ranges from 0 to 38%, according to studies published.3,13,14
Electrocardiographic abnormalities can be present in up to 90% of patients, being, however, unspecific. The most common findings include intraventricular conduction delay, LV hypertrophy, ventricular repolarization changes, and Wolff-Parkinson-White syndrome.3,13,14
The increasing advancement in imaging techniques, in addition to the increasing application of genetic tests for the diagnosis of NCM, significantly impacts on the understanding of the mechanisms involved in the NCM genesis and its clinical treatment.
Of the cardiac imaging techniques, echocardiography and cardiac magnetic resonance imaging (CMR) are the major diagnostic tools. Because of its large availability and easy access, in addition to no need for contrast agents, no radiation exposure, and mainly its low cost as compared to CMR, echocardiography is the first choice and most commonly used method for the diagnosis of NCM.8,12-14
Usually the diagnosis of NCM should be considered in the presence of a bilayered myocardium composed by one thinner epicardial layer and one thick endocardial layer with prominent trabeculations and deep intraventricular recesses. The trabeculations are mainly identified on two-dimensional (2D) mode, but can be evidenced on one-dimensional or M mode. Color Doppler imaging shows blood flow in those recesses in continuity with the left ventricle.8,12-15
Different echocardiographic criteria have been used to diagnose NCM, and the main ones used in clinical practice are as follows (Table 1):
Table 1.
| Criterion | Chin et al.2 | Jenni et al.16 | Stöllberger et al.17 |
|---|---|---|---|
| Number of patients | 8 | 7 | 104 |
| Phase of the cardiac cycle | End-diastole | End-systole | Trabeculations assessed at end-diastole; NCM and CM assessed at end-systole |
| View | Short axis parasternal views and/or apical views | Short axis parasternal | Conventional and modified views |
| NCM/CM ratio | - | >2 | - |
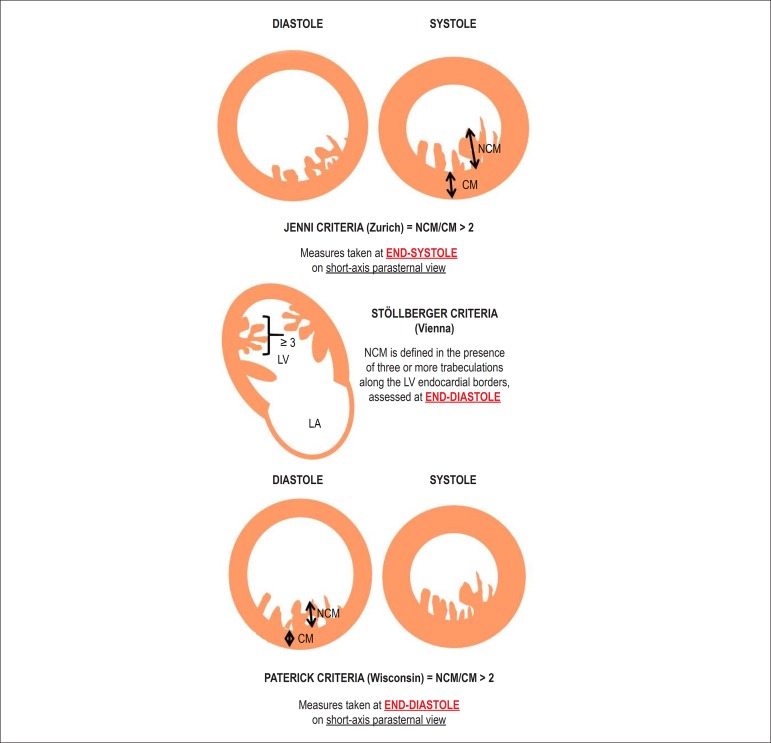
Jenni et al.16 consider for the diagnosis of NCM the presence of two myocardial layers, a thin compacted one (compacted myocardium - CM), and another thicker, noncompacted layer (NCM), with deep endomyocardial recesses filled with blood flow on color Doppler, in the absence of other cardiac abnormalities. A NCM/CM ratio > 2 is considered diagnostic. The measures should be acquired at end-systole on short axis parasternal view16 (Figure 1).
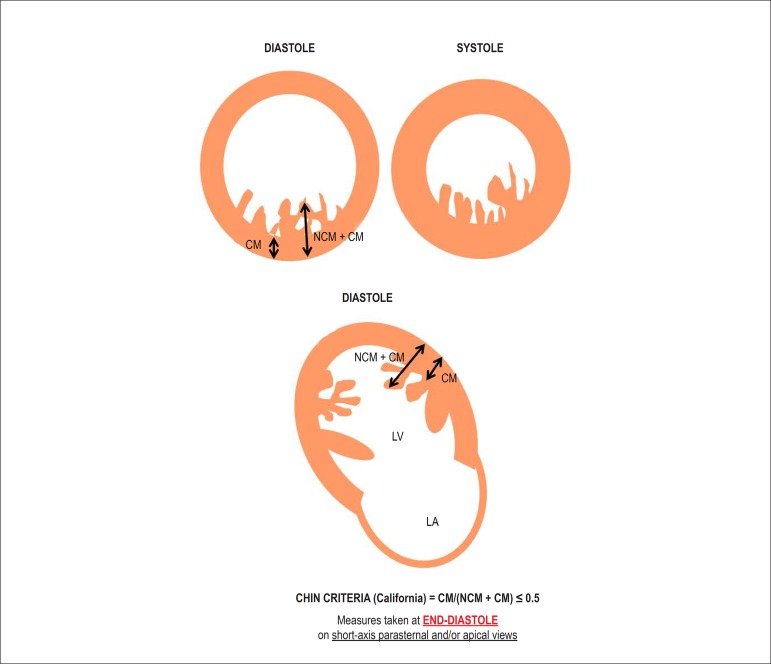
Chin et al.2 define NCM as the presence of excessively prominent ventricular trabeculations and progressively increased total thickness of the myocardial wall from the mitral valve and towards the apical region, characterized by CM/(NCM + CM) ≤ 0.5, assessed at end-diastole on short-axis parasternal views and/or apical views17 (Figure 2).
Stöllberger et al.17 define NCM as the presence of three or more trabeculations along the LV endocardial borders, different from the papillary muscles, false tendons and aberrant muscle bands, which move synchronized with the CM. In that study, the trabeculations were better visualized at end-diastole, while the bilayered myocardium was better assessed at end-systole18 (Figure 1).
Recently, Paterick et al.13 (Wisconsin) have proposed the diagnosis of NCM as a ratio NCM/CM > 2, with measures taken at end-diastole on short-axis parasternal view. This criterion requires clinical validation13 (Figure 1).
Figure 1.
Criteria proposed for the diagnosis of noncompacted myocardium. NCM: noncompacted myocardium; CM: compacted myocardium; LV: left ventricle; LA: left atrium.
Figure 2.
Criteria proposed by Chin for the diagnosis of noncompacted myocardium. NCM: noncompacted myocardium; CM: compacted myocardium; LV: left ventricle; LA: left atrium.
Critical analysis
The diagnostic criteria described by Jenni et al.16 and Chin et al.2 are based on the measurement of NCM and CM thicknesses. However, the criteria differ regarding the cardiac cycle point at which the measurements should be taken. Chin et al.2 propose the measurements of NCM and CM thickness be performed at end-diastole, while Jenni et al. propose them at end-systole.16
The criteria proposed by Jenni et al.16 used an NCM/CM ratio > 2 at end-systole, generating higher specificity and lower sensitivity as compared to the criteria by Chin et al.,2 who use the CM/(NCM + CM) ratio ≤ 0.5. However, the increase in sensitivity is due to a decrease in specificity, as compared to the criteria by Jenni et al.16
A recent study has assessed the accuracy of the echocardiographic criteria described by Chin et al.,2 Jenni et al.16 and Stöllberger et al.17 for the diagnosis of NCM in patients with HF as compared to a control group of normal individuals. The size and the number of the trabeculations identified on apical view at end-diastole were assessed, as were the measurements of the NCM layer thickness on short-axis parasternal view at end-systole. Only concordant cases assessed by two reviewers were considered positive.18
In that study, the percentages of patients meeting the diagnostic criteria for NCM were as follows: Chin et al.2 criteria, 79%; Jenni et al.16 criteria, 64%; and Stöllberger et al.17 criteria, 53%. In that study, the Chin et al. criteria had higher sensitivity, however with a higher percentage of false-positive diagnoses. The correlation between the three echocardiographic criteria applied was weak, with only 30% of patients meeting all three criteria. All individuals of the control group had preserved ventricular dimensions and systolic function. Five control group individuals (4 black and 1 white) met at least one criterion for the diagnosis of NCM. This result emphasizes the limitation of the echocardiographic criteria to diagnose NCM, particularly in black individuals, leading to an excessive diagnosis of NCM.18 In that study, if the control group was formed by individuals with HF, those results might have been even more discrepant.
The criterion proposed by Paterick et al.13 showed good correlation with the CMR findings, and, according to those authors, that criterion provided more accurate measurements of NCM and CM layer thickness. However, those criteria have not been validated, requiring additional confirmation and comparison with other populations with cardiac structural disease before they are adopted as a feasible diagnostic option.13
Despite the increasingly frequent diagnosis of NCM, the echocardiographic criteria applied for that purpose are based on studies with limited numbers of patients and different methodologies. The point of the cardiac cycle at which the measurements of NCM and CM thickness are taken influences directly the relationship between the two layers assessed. Myocardial thickness is maximal at systole, and minimal at diastole, which directly affects the ratio between NCM and CM. In addition, the echocardiographic view on which those measurements are taken should be considered. Most criteria suggest that the measurements be taken on short-axis parasternal view; however, in daily clinical practice, measurements are more often taken on apical 4- and 2-chamber views. Finally, there is no consensus on the ratio between NCM and CM to be adopted as the diagnostic criterion, because of the lack of uniformity accepted for diagnosis.
In addition, some studies have shown a considerable number of young athletes meeting the NCM diagnostic criteria, emphasizing the lack of specificity of the current diagnostic criteria when applied to highly-trained athletes.19
Although infrequent, NCM has been reported in the right ventricle. However, in such cases, the diagnostic criteria are even more restricted as compared to those applied to the left ventricle on echocardiography, because of the limitation of the right ventricle echocardiographic analysis due to its complex geometry, which cannot be contemplated on only one echocardiographic view.20,21
Thus, despite the increasing knowledge on NCM by echocardiography professionals, the diagnostic bases of the criteria applied are frail. Therefore, studies with a larger number of patients diagnosed with NCM are required, in addition to uniformization of the views used for the measurements, and the identification of the most suitable point in the cardiac cycle for that purpose. Such studies should ideally compare healthy individuals and patients with HF, because some "normal" patients can meet the echocardiographic criteria for the diagnosis of NCM, with no apparent clinical finding and benign prognosis, considering that the prevalence of NCM seems higher in patients with HF. In addition, the definition of the diagnostic criteria should take into consideration the particularities of specific populations, such as highly-trained athletes and black individuals, as well as the right ventricular morphological characteristics.
Conclusions
Echocardiography is the first choice and most commonly used cardiac imaging method for the diagnosis of NCM. Higher knowledge and understanding of NCM is the first step to increase diagnostic accuracy in echocardiography laboratories. However, the echocardiographic criteria used so far for that purpose are highly varied and have been based on studies with a reduced number of patients.
Advanced techniques, such as three-dimensional echocardiography, use of contrast agents to better define the endocardial borders, mainly in the apical region of patients with limited acoustic window, as well the analysis of myocardial strain by use of speckle tracking, are promising methods, with potential to increase the diagnostic accuracy of echocardiography in patients with NCM. The use of such techniques in clinical practice has increased in past years; however, the improvement of imaging methods requires study and constant redefinition of the echocardiographic criteria for the diagnosis of NCM.22-25
The high prevalence of NCM in low-risk populations, such as athletes and normal black individuals, suggests that the increase in LV trabeculations and recesses can represent a pattern of response to the chronic increment of preload. Thus, because of the current limitations for the diagnosis of NCM, integration of clinical and electrocardiographic assessments, as well as a multimodality approach with echocardiography and CMR, is suggested.26-28
In addition to the multimodality imaging approach, future perspectives include changes that suit different ethnicities and functional assessment based on multicenter and international collaboration, incorporating genetic data for a more accurate diagnosis of NCM.26-28
Footnotes
Author contributions
Conception and design of the research: Hotta VT;Writing of the manuscript: Hotta VT, Tendolo SC; Critical revision of the manuscript for intellectual content: Hotta VT, Rodrigues ACT, Fernandes F, Mady C; Perform clinical follow-up of patients with NCM: Nastari L.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Grant RT. An unusual anomaly of the coronary vessels in the malformed heart of a child. Heart. 1926;13:273–283. [Google Scholar]
- 2.Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 3.Towbin JA, Lorts A, Jefferies JL. Left ventricular noncompaction cardiomyopathy. Lancet. 2015;386(9995):813–825. doi: 10.1016/S0140-6736(14)61282-4. [DOI] [PubMed] [Google Scholar]
- 4.Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol. 2014;64(17):1840–1850. doi: 10.1016/j.jacc.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oechslin E, Jenni R. Left ventricular noncompaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J. 2011;32(12):1446–1456. doi: 10.1093/eurheartj/ehq508. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Pavia P, De la Pompa JL. Left ventricular noncompaction: a genetic cardiomyopathy looking for diagnostic criteria. J Am Coll Cardiol. 2014;64(19):1981–1983. doi: 10.1016/j.jacc.2014.08.034. Erratum in: J Am Coll Cardiol. 2015;65(5):519. [DOI] [PubMed] [Google Scholar]
- 7.Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, Jenni R. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72(1):26–31. doi: 10.1016/S0025-6196(11)64725-3. [DOI] [PubMed] [Google Scholar]
- 8.Frischknecht BS, Attenhofer Jost CH, Oechslin EN, Seifert B, Hoigne P, Roos M, et al. Validation of noncompaction criteria in dilated cardiomyopathy, and valvular and hypertensive heart disease. J Am Soc Echocardiogr. 2005;18(8):865–872. doi: 10.1016/j.echo.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93(5):841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. American Heart Association. Council on Clinical Cardiology, Heart Failure and Transplantation Committee. Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups. Council on Epidemiology and Prevention Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 11.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29(2):270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 12.Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet. 2015;386(9995):813–825. doi: 10.1016/S0140-6736(14)61282-4. [DOI] [PubMed] [Google Scholar]
- 13.Paterick TE, Umland MM, Jan MF, Ammar KA, Kramer C, Khandheria BK, et al. Left ventricular noncompaction: a 25-year odyssey. J Am Soc Echocardiogr. 2012;25(4):363–375. doi: 10.1016/j.echo.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Carrilho-Ferreira P, Almeida AG, Pinto FJ. Non-compaction cardiomyopathy: prevalence, prognosis, pathoetiology, genetics, and risk of cardioembolism. Curr Heart Fail Rep. 2014;11:393–403. doi: 10.1007/s11897-014-0227-3. [DOI] [PubMed] [Google Scholar]
- 15.Dawson DK, McLernon DJ, Raj VJ, Maceira AM, Prasad S, Frenneaux MP, et al. Cardiovascular magnetic resonance determinants of left ventricular noncompaction. Am J Cardiol. 2014;114(3):456–462. doi: 10.1016/j.amjcard.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86(6):666–671. doi: 10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stöllberger C, Gerecke B, Finsterer J, Engberding R. Refinement of echocardiographic criteria for left ventricular noncompaction. Int J Cardiol. 2013;165(3):463–467. doi: 10.1016/j.ijcard.2011.08.845. [DOI] [PubMed] [Google Scholar]
- 18.Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, McKenna WJ, et al. Diagnosis of left-ventricular non-compaction in patients with left ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur Heart J. 2008;29(1):89–95. doi: 10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 19.Gati S, Chandra N, Bennett RL, Reed M, Kervio G, Panoulas VF, et al. Increased left ventricular trabeculation in highly trained athletes: do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes? Heart. 2013;99(6):401–408. doi: 10.1136/heartjnl-2012-303418. [DOI] [PubMed] [Google Scholar]
- 20.Gomathi SB, Makadia N, Ajit SM. An unusual case of isolated non-compacted right ventricular myocardium. Eur J Echocardiogr. 2008;9(3):424–425. doi: 10.1093/ejechocard/jen016. [DOI] [PubMed] [Google Scholar]
- 21.Markiewicz-Loskot G, Moric-Janiszewska E, Loskot M, Szydlowski L, Weglarz L, Hollek A. Isolated ventricular non-compaction: clinical study and genetic review. Europace. 2006;8(12):1064–1067. doi: 10.1093/europace/eul125. [DOI] [PubMed] [Google Scholar]
- 22.Haland TF, Saberniak J, Leren IS, Edvardsen T, Haugaa KH. Echocardiographic comparison between left ventricular non-compaction and hypertrophic cardiomyopathy. Int J Cardiol. 2016;228:900–905. doi: 10.1016/j.ijcard.2016.11.162. [DOI] [PubMed] [Google Scholar]
- 23.Niemann M, Liu D, Hu K, Cikes M, Beer M, Herrmann S, et al. Echocardiographic quantification of regional deformation helps to distinguish isolated left ventricular non-compaction from dilated cardiomyopathy. Eur J Heart Fail. 2012;14(2):155–161. doi: 10.1093/eurjhf/hfr164. [DOI] [PubMed] [Google Scholar]
- 24.Bellavia D, Michelena HI, Martinez M, Pellikka PA, Bruce CJ, Connolly HM, et al. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart. 2010;96(6):440–447. doi: 10.1136/hrt.2009.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarando F, Coisne D, Galli E, Rousseau C, Viera F, Bosseau C, et al. Left ventricular non-compaction and idiopathic dilated cardiomyopathy: the significant diagnostic value of longitudinal strain. Int J Cardiovasc Imaging. 2017;33(1):83–95. doi: 10.1007/s10554-016-0980-3. [DOI] [PubMed] [Google Scholar]
- 26.Grothoff M, Pachowsky M, Hoffmann J, Posch M, Klaassen S, Lehmkuhl L, et al. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol. 2012;22(12):2699–2709. doi: 10.1007/s00330-012-2554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng H, Zhao S, Jiang S, Lu M, Yan C, Ling J, et al. Comparison of cardiac magnetic resonance imaging features of isolated left ventricular non-compaction in adults versus dilated cardiomyopathy in adults. Clin Radiol. 2011;66(9):853–860. doi: 10.1016/j.crad.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Gati S, Rajani R, Carr-White GS, Chambers JB. Adult left ventricular noncompaction: reappraisal of current diagnostic imaging modalities. JACC Cardiovasc Imaging. 2014;7(12):1266–1275. doi: 10.1016/j.jcmg.2014.09.005. [DOI] [PubMed] [Google Scholar]