Abstract
Rationale: Neovascular secondary glaucoma is a condition characterized by increased intraocular pressure due to the neovascularization occurring at the iridocorneal angle and iris, the most common complication of end-stage ischemic retina. The early diagnosis and treatment of this disease are important, because the functional prognosis is reserved.
Objective: Knowing and understanding the etiology and pathogenesis of neovascular secondary glaucoma.
Methods and results: Review of the angiogenesis theory to understand the etiology and pathogenesis of neovascular secondary glaucoma. VEGF is the most studied proangiogenic factor involved in the neovascular glaucoma pathogenesis. The 9 isoforms contain consensus signal sequences for extracellular secretion, all of them binding to a specific receptor subtype and stimulating tissue specific angiogenesis. VEGF and VEGF- m RNA levels are significantly increased in the ischemic retina. Diabetes mellitus (with diabetic retinopathy), central retinal vein thrombosis and repeated retinal detachments are diseases that cause neovascular glaucoma through ischemia.
Discussion: Correct evaluation of the iris neovascularization followed by a proper treatment is the most important in a case of secondary neovascular glaucoma. Repeated gonioscopy is indicated in cases with high risk of developing neovascular glaucoma. Close monitoring of a patient with high thromboembolic risk: valvular heart disease, open-heart surgery, other angioplasties.
Keywords: neovascular secondary glaucoma, iridocorneal angle and iris, ischemic retina, neovascularization, rubeosisiridis
Etiology and Pathogenesis
Iris neovascularization was described in 1868 by Bader, and, in 1879, Deutschmann showed the association between it and neovascular glaucoma. Nettleship highlighted in 1888 the relationship of neovasculature and diabetic retinopathy, and in 1906, he demonstrated the link between the central retinal vein thrombosis and iris neovascularization. Salbus named the iris neovascularization “rubeosisiridis diabetic”, but changed it to “rubeosisiridis” because of the existence of many etiologies.
The concept of an existing factor that spreads and stimulates the forming of new blood vessels was stated in 1948. From the original description, iris neovascularization and that of the anterior chamber have been described in a multitude of diseases, the majority (97%) being associated with changes that involve hypoxia and retinal ischemia. The rest of 3% are represented by inflammatory diseases - chronic uveitis and intraocular neoplasms [1]. The most frequent conditions associated with neovascular glaucoma are diabetic retinopathy, central retinal vein occlusion and ischemic ocular syndrome.
Retinal hypoxia has been observed in cases of rubeosisiridis and frequently in proliferative retinopathies. It is possible that a part of the oxygen from the aqueous humor diffuses posterior towards the hypoxic retina, thus resulting the iris hypoxia, through a compensatory mechanism. Therefore, this might explain the high risk of rubeosis in cases of neovascular glaucoma after surgery like vitrectomy and intracapsular lens extraction, in which the oxygen can better reach the ischemic retina through diffusion and lead to a quick and severe iris hypoxia.
The endothelial vascular cells have a crucial role in the angiogenesis process. They respond to a specific stimulus (tissular hypoxia) and secrete proangiogenic factors like: VEGF (vascular endothelial growth factor), bFGF (basic fibroblast growth factor), TNF (tumor necrosis factor), IGF (insulin growth factor) and PDGF (platelet derived growth factor). All these processes stimulate a chain reaction characterized by the activation, proliferation and migration of the endothelial cells that have one outcome: the formation of new blood vessels that are fragile and permeable.
The vascular theory claims that the forming of new blood vessels happens through branching from the existing vessels. The hypoxic tissue determines an increase of adenosine production, which binds to its specific cell receptors and increases the activity of VEGF. The hypoxia induction factor (HIF-1) is the primary regulator of oxygen homeostasis. The genes on which HIF-1 acts encode proteins that determine increased tissular oxygen release and mediate the adaptive responses in hypoxia. Activation of this factor is influenced by the intracellular oxygen level and by the transduction pathways of the stimulus of different growth factors.
VEGF is the most studied proangiogenic factor implicated in the neovascular glaucoma pathogenesis. The 9 isoforms VEGF contain consensus signal sequences for extracellular secretion, all of them binding to a specific receptor subtype and stimulating tissue specific angiogenesis. VEGF and VEGF- m RNA levels are significantly increased in the ischemic retina.
VEGF-A is most involved in vascular neogenesis. It belongs to the PDGF family (platelet-derived growth factor) and represents a glycopeptide of 45kDal that stimulates the proliferation, migration and proteolytic activity of endothelial cells. It serves in the survival of the endothelial cells by inhibiting apoptosis and capillary regression.
VEGF induces the production of NO (nitric oxide), resulting in vasodilatation and increased blood flow which precedes angiogenesis. VEGF also has a role in increasing the vascular permeability.
VEGF has two receptors: Flt (fms - like tyrosine kinase = VEGFR-1) and Flk (fetal liver kinase = VEGFR-2). Both are trans membrane tyrosine-kinase type receptors.
VEGFR-1 is involved in cellular differentiation and VEGFR-2 in endothelial cell proliferation. These receptors are not specific to endothelial cells and can be found in the membrane of other cells like trophoblasts, pulmonary fibroblasts, pancreatic duct cells, small cell cancers [2].
The angiogenesis process starts with the forming of small gaps between the endothelial cells of the capillary walls, which leads to increased permeability for plasmatic proteins and fibrinogen. The fibrinogen converts to fibrin resulting in a temporary matrix for the new blood vessel. The endothelial cells organize to form the “vascular bud” and express integrins. These cells advance from the main vessel to the angiogenic stimulus. Proliferation of the cells from the “bud” will determine the development of the vascular lumen, resulting in a thin capillary wall with few pericytes, but which can start to secrete the basal membrane components. In this stage, the suppression of VEGF or blocking of the VEGF receptors will stop the vascular growth and lead to the regression of the newly formed vessel.
Increased levels of VEGF have been found in the aqueous humor of patients with neovascular glaucoma, particularly in diabetic patients. Experimental studies on primates have shown that injection of human recombinant factor VEGF (in doses comparable with those found in patients with ocular neovascularization) is enough to produce iris neovascularization and neovascular glaucoma.
In most tissue, vascularization is maintained in a repose state by the delicate balance between the proangiogenic and antiangiogenic factors. Regarding the ocular structures, it seems that the formation of new blood vessels is determined by the balance between the angiogenic factor VEGF and the antiangiogenic factor PEDF (pigment epithelium - derived factor). PEDF is frequently secreted and has a strong inhibitory angiogenic effect as well as a neuroprotective effect. This theory is supported by studies, which state that increased levels of VEGF and decreased levels of PEDF have been found in the vitreous body of patients with proliferative diabetic retinopathy [3,4].
The formation of the new blood vessels is accompanied by the proliferation of a fibro-cellular support, a fibro-vascular membrane, which represents its contractile structure and also a field of migration for the newly recruited cells. The membrane contains inflammatory cells, macrophages, type B lymphocytes, auxiliary and suppressor T lymphocytes. The adherence and contractile capacity is caused by the presence of cells rich in actin and fibronectin and by the extracellular tissue, especially at the perivascular regions. The fibro-vascular membrane is rich in type I and III collagen [5, 6].
From the causes that can determine secondary neovascular glaucoma, the following can be listed:
1. Vascular ocular diseases: diabetic retinopathy; obstruction of the central retinal artery; coats disease; eales disease; retinal hemangioma;primary hyperplastic persistent vitreous; retinopathy of prematurity.
2. Extra-ocular vascular diseases: Carotid occlusive diseases;Carotid-cavernous fistula;Ligation of the carotid artery;Giant cell arteritis (Horton arteritis);Takayasu disease
3. Other ocular disorders: Rhegmatogenous retinal detachment;Chronic uveitis;Retinal-vitreous degeneration.
4. Ocular neoplasia: Iris: melanoma, hemangioma, metastatic lesions;Ciliary body: melanoma;Choroid: melanoma;Conjunctiva: squamous cell carcinoma;Retina: retinoblastoma, large cell lymphoma
5. After surgery involving: Cataract; Vitrectomy;Surgery for retinal detachment.
Clinical Matters
A correct evaluation of the iris neovascularization followed by a proper treatment is the most important in a case of secondary neovascular glaucoma.
The clinical examination requires a slit-lamp evaluation and a rigorous gonioscopy.
A slit-lamp evaluation shows the presence of thin newly formed capillary, tortuous, randomly oriented on the surface of the iris, near the pupillary margin. The existence of these new blood vessels is more obvious in patients with light colored iris. In these patients, the neovascularization can be shown through anterior pole angiofluorography. These newly formed blood vessels must be differentiated from the normal vessels that exist in the angle: the radial vessels from the ciliary trunk; the radial iris vessels from the circular ciliary band.
Newly formed blood vessels have the following characteristics:
- they are individualized vascular trunks, formed at the base of the iris, crossing over the ciliary trunk and then branching;
- gonio-angiography shows their ciliary body origin and the connection with the neovascular network at the iris periphery.
Fig. 1.
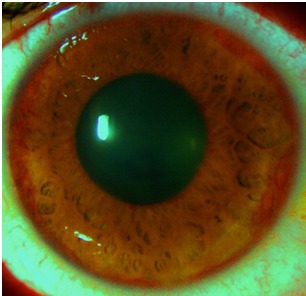
Neovascularization of the iris- part 1
Fig. 2.
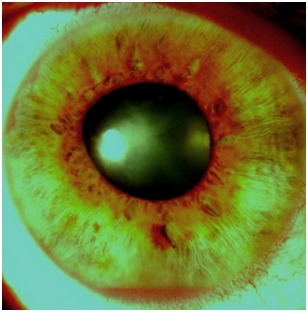
Neovascularization of the iris- part 2
Neovascularization typically progresses from the pupillary margin to the angle, but angle neovascularization without pupillary involvement can be possible.
Fig. 3.
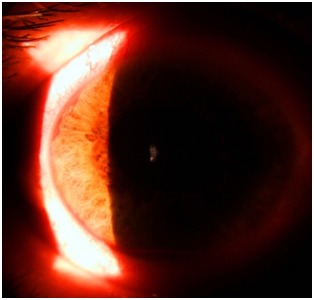
Angle neovascularization
Fig. 4.
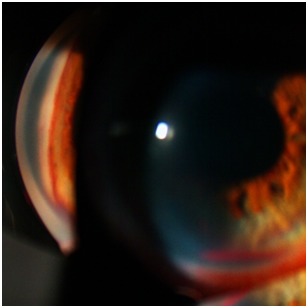
Angle neovascularization
Newly formed blood vessels move over the camerular angle towards the ciliary body and the scleral spur and then towards the trabecular meshwork which becomes reddish. Repeated gonioscopy is indicated in cases with high risk of developing neovascular glaucoma.
From the clinical point of view, the course of neovascular glaucoma comprises 3 stages:
Pre glaucoma or the “rubeosisiridis” stage: The pre glaucomatous stage is characterized by a normal intraocular pressure associated with iris neovascularization.
Open angle glaucoma: The second stage is characterized by an increased intraocular pressure. Gonioscopy shows a fibro-vascular membrane in the camerular angle. At this stage, there is an increased risk of bleeding, the open angle glaucoma could complicate with hyphema.
Closed angle glaucoma: The closed angle glaucoma stage is characterized by the contraction of the fibro-vascular membrane, which pulls the iris periphery over the trabecular meshwork and thus causes a variable closing of the camerular angle because of synechiae.
Uvealectropion appears frequently and results from the radial traction at the surface of the iris, traction that also involves the pigmented back layer of the iris around the pupillary margin. Gonioscopy shows the formation of anterior synechiae through the fibro-vascular membrane.
The patients complain of pain, conjunctival and episcleral hyperemia, a severe decrease in visual acuity.
In spite of advancements in the early diagnosis and treatment of this disease, the functional prognosis is reserved. To avoid the development of neovascular glaucoma one should:
Inform the patient of the risks that follow from the mistreatment of the chronic disease that he/ she has: diabetes, arterial hypertension, atherosclerosis.
Close monitoring of a patient with high thromboembolic risk: valvular heart disease, open-heart surgery, other angioplasties.
In patients with type 2 diabetes, insulin treatment should be considered for the protection of the other eye. For patients who have come in late stages at the doctor, or have been poorly treated, an assessment should be made regarding the mechanism of central retinal vein occlusion and, depending on the result, long term treatment should be considered, modulated (according to the lab results) with vitamin k antagonists, NSAIDs, anti platelet drugs and treatment of infectious or vascular disease.
If there are signs of pre thrombosis, one should insist on revaluating the treatment in patients with arterial hypertension: giving a thrombolytic agent and eliminating some drugs with high thrombogenic risk (Estrogens and diuretics - Furosemid).
It is also very important to perform angiofluorography in all these cases for an early highlighting of a susceptible area (a pre ischemic area, with low perfusion). Diabetes mellitus (with diabetic retinopathy), central retinal vein thrombosis and repeated retinal detachments are diseases that cause neovascular glaucoma through ischemia.
References
- 1.Haefliger IO, Zschaner A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide cyclic guanosin monophosphat pathway. Invest.Opth.Vis.Sci. . 1994 [PubMed] [Google Scholar]
- 2.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 3.Evans K, Wishart PK, Galliard JN. Neovascular complication after central vein occlusion. The Eye. 1993;7 doi: 10.1038/eye.1993.113. [DOI] [PubMed] [Google Scholar]
- 4.Leonard AL, Daniel MA. Mechanisms and Management. Elsevier; 2010. Ocular Diseases. [Google Scholar]
- 5.Bandello F, Battaglia Parodi M. Anti-VEGF Developments in Ophthalmoly. 2010 [Google Scholar]
- 6.Kaiser PK. Antivascular endothelial growth factor agents and their development: therapeutic implication in ocular diseases. Am J Ophthalmology. 2006;142:660–668. doi: 10.1016/j.ajo.2006.05.061. [DOI] [PubMed] [Google Scholar]


