Abstract
The use of preservatives in eye drops (eyewashes) has known glory at the beginning, but the side effects that they have on the ocular surface have led to a decrease of their popularity. Lachrymal film dysfunction, ocular hyperemia, dotted keratitis or toxic keratopathy were reported and analyzed in terms of pathophysiological mechanism of the role played by preservatives in ophthalmic drops (eyewashes). This article reviews the most common preservatives and the existing alternatives for the maintenance of the eye sterile drops.
Keywords: preservatives, eye drops, ocular surface
Introduction
Multidose eye drops contain preservatives, which justify their long-term use and represent a risk for the ocular surface.
After the year 1960, following the occurrence of some severe eye infections after using multidose eye drops [1], the use of some preservatives has been imposed.
Many studies have shown contamination of multidose eye drops [2, 3]. The infection mode is either the ambient air or the touching of the dropper with the fingers, eyelids or eyelashes during the drip. According to Rahman, the containers’ contamination rate is 8.4% [3].
The most common microbial agents identified were Staphylococcus aureus coagulaso negative [4] and pseudomonas.
Kishnanet et al. described five cases of severe infections with Pseudomonas aeruginosa [5] secondary to the use of eye drops.
Over time, many preservatives have been used, but each one with its limitations, concretized in ocular surface damage.
A first class of preservatives used was the one from the group of quaternary ammonium compounds, of which the usual is the benzalkonium chloride (BAK) [6].
Benzalkonium chloride is a quaternary ammonium which is used in concentrations that vary between 0.005- 0.2%.
This is a mixture of alkyl benzyl dimethyl ammonium chloride and alkyl chains varying from C8H17 to C18H37, having the following structure (Fig. 1)
Fig. 1.
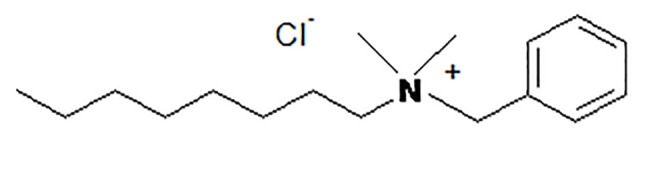
Benzalkonium chloride. The chemical structure
This preservative has highly effective bactericide and fungicide action, which is achieved by the rupture of the outer membrane of the microorganism, via the lowering of the surface tension; thereby, the DNA synthesis is affected at 37 degrees C [7].
Benzalkonium chloride (BAK) has been used for a long time in numerous eye drops composition. Also it is a cationic surfactant whose surface activity results in an improvement of transcorneal penetration of medicinal substances by increasing the space between the epithelial cells. This characteristic of the BAK can cause the solubilization of the lipophilic protective layer, determining the instability or rupture of the tear film. For this reason, benzalkonium chloride is not used in combination with local anesthetics [8, 9].
However, numerous studies that revealed the side effects of BAK, the impact this preservative has on the ocular surface have emerged. BAK toxicity depends on the amount administrated daily, the duration of the treatment and its concentration in the administered solution. At each administration of an eye drop containing benzalkonium chloride, its detergent effect disrupts the lipid layer of the tear film. This cannot be regenerated and can no longer protect the aqueous layer of the tear film, which evaporates easily. In these circumstances, the cornea is exposed and eye dryness occurs. In addition, benzalkonium chloride has a cellular toxicity on caliciform cells, entailing a reduction in the amount of mucin, an additional reason for disrupting the tear film.
Pissella et al. [10] demonstrated that using preservative-free eye drops is much better tolerated at the conjunctival cytology level. In his study conducted over one year of treatment for open-angle glaucoma, with timolol with preservative on a lot and preservative-free timolol on another lot it was noted that conjunctival inflammation markers (HLA_D membrane antigens and ICAM-1) are much higher in the group in which eye drops contained preservatives (BAK).
Albietz et al. [11] showed a significant decrease in conjunctival mucous cells. The degree of inflammation of the conjunctival epithelium is higher in patients treated with eye drops with preservatives than in patients treated with eye drops without preservatives.
The preservatives from the eye drops (BAK) often cause subclinical conjunctival inflammation characterized by inflammatory cell infiltration, epithelial hyperplasia and mucous cell loss [12].
Benzalkonium chloride from eye drops is incriminated in the alteration of the tear film. Campagna et al. [13] had a study in which the rupture time of tear film (BUT) decreases to 7.9 seconds when using BAK. Replacing the eye drops with preservatives with others without preservatives allows a significant improvement of the lachrymal function by increasing the number of mucous cells and restoring the tear film rupture time.
Avisar et al. [14] analyzed the effects of the instillation of artificial tears without preservatives and reached the conclusion that these may restore the precorneal film, while bringing the tear film rupture time to values of 25 to 27 sec., compared to artificial tears containing BAK, where the time of rupture of the tear film may decrease under 15 sec.
BAK toxicity is manifested through apoptosis phenomena (free radical production) and/ or cellular necrosis, depending on the concentration [15].
A decrease in the conjunctival and corneal epithelium cell density and the change of their morphological appearance (metaplasia) has been observed [16].
The detergent effect is manifested by the loss of epithelial microvillar brush [17]. The extracellular space widens and the epithelium is disorganized. The alteration of the lipid layer of the tear film worsens the ocular dryness syndrome [18].
The side effects of BAK are inflammatory phenomena, often subclinical, with immediate or delayed hypersensitivity reactions. The most common ocular symptoms observed are, according to Pissela [19], discomfort after instillation, foreign body sensation, burning sensation, ocular dryness, lachrymation, eyelid pruritus, and ocular surface damage signs are redness of the eye, conjunctival follicles, superficial dotted keratitis, anterior blepharitis, meibomite, eyelid eczema.
The cytotoxicity of the benzalkonium chloride can be direct and indirect. The direct cytotoxicity is dose dependent and was described above. The indirect cytotoxicity, tied to the conjunctival and palpebral flora changing is less present in relation to the active principles of the eye drops (antibiotics, antivirals) than with the preservative itself.
BAK related immunological reactions are more frequently of the delayed type (type IV hypersensitivity) than of the immediate type (type I). The allergen is formed by binding the hapten with a high molecular weight of 1000 daltons contained in the eye drop, with a protein molecule of the subject. Therefore, it was envisaged that all the constituents of an eye drop have a molecular weight lower than the one mentioned [20]. The most common allergic manifestations are eczema and blepharitis.
Polyquad is a preservative derived from the benzalkonium chloride of the quaternary ammonium class. It was firstly used for the storage solutions of contact lenses. Today it is found in many eye drops such as artificial tears and antiglaucomatous. It is considered less toxic to the corneo-conjunctival surface [29].
However, Rosenthal et al. recognized that polyquad reduces the goblet cells number and affects the production of aqueous sequence of tear film [21].
Polyquad is a compound with high molecular weight, highly effective in preventing the microbial growth, especially of fungus, and seems to be better tolerated by patients [22].
Due to its large molecule (Fig.2 ) it is not absorbed into hydrogel lenses and then toxic and allergic reactions are rare [23].
Fig. 2.
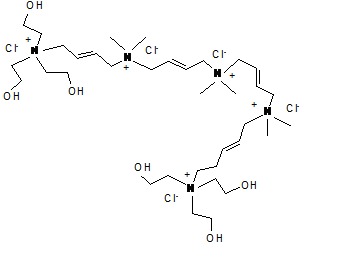
Polyquad. The chemical structure
Currently, polyquad is increasingly used as a preservative in ophthalmic solutions used in the treatment of glaucoma. Although it is a derivative of BAK, Polyquad has properties that distinguish it from the other preservatives. Bacterial cells attract Polyquad, but human corneal epithelial cells tend to reject the compound [24].
However, recent discoveries showed that polyquad has negative effects on the integrity of the cell membrane and induces cytotoxicity in the ocular surface cells [25, 26].The main disadvantage associated with this preservative is its tendency to reduce conjunctival caliciform cell density, thereby decreasing the tear film aqueous sequence production.Although Polyquad was proved far less toxic to the corneo-conjunctival surface than BAK [27], it was shown to cause superficial corneal epithelial damage [28].
These preservatives used in ophthalmic products are well tolerated in the eye when they are in normal concentrations and small doses. Ocular tolerance can be modified by the concentration of preservatives, frequency of instillation, the combination of preservatives, their chemical purity, the duration of treatment, the condition of the cornea, wearing contact lenses and using polymer in formulating ophthalmic preparations [29].
The effect of preservatives from the eye drops on the cornea is manifested directly through anatomical and physiological changes of the corneal epithelium, which affect the optical properties of the epithelial barrier function, and indirectly by changing the tear film, which results in wetting disorders of the eye [30].
Alternatives
Due to the adverse effects of the preservatives on users with chronic diseases, the pharmaceutical industry is oriented either towards the production of single dose vials or less toxic preservatives.
An interesting alternative to single-dose vials are the multidose devices fitted with a special filter. Whether the device contains a sterile, preservative-free ophthalmic solution, protected against microbial contamination through a 0.2 microns pore filter, or contains a preservative (e.g., BAK), which is retained by a sieve (adsorbent resin) at instillation. In both cases, eye drops delivered to the eye are without preservatives [29].
ABAK system is equipped with a one-way passage system through an antimicrobial membrane with a porosity of up to 0.2 μm to prevent contamination with microorganisms from the outside. In addition, the drops can be administered for up to eight weeks from the opening of the bottle and drops that can be eliminated one by one, having the same size regardless of the pressure of the patient on the bottle. The ABAK system is successfully used in the production of artificial tears and some antiglaucomatous elements.
References
- 1.Kallings L, Rigertz O, Silverstolpe I. Microbial contamination of medical preparations. Acta Pharm. Sue. 1966;3:199–213. [PubMed] [Google Scholar]
- 2.Furrer P, Mayer J, Gurny R. Ocular tolerance of preservatives and alternatives. Eur. J. Pharm.Biopharm. 2002;53(3):363–380. doi: 10.1016/s0939-6411(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 3.Raham MQ. Microbial contamination of preservative free eye drops in multiple application containers. Br. J. Ophthalmol. 2006;90(2):139–141. doi: 10.1136/bjo.2005.078386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris MG. Survival of contaminating bacteria in over the counter artificial tears. Jam. Optom. Assoc. 1996;67(11):676–680. [PubMed] [Google Scholar]
- 5.Krishnan S. Secondary pseudomonas infection of fungal keratitis following use of contaminated natamycin eye drops: a case series. Eye (Lond). . 2009;23(2):477–479. doi: 10.1038/eye.2008.290. [DOI] [PubMed] [Google Scholar]
- 6.Baudoin C. Detrimental effect of preservatives in eye drops: Implications for the treatment of glaucoma. Acta Ophthalmol. . 2008;86(7):16–26. doi: 10.1111/j.1755-3768.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- 7.Eun H, Chung JH, Jung SY, Cho KY, Kim KH. A comparative study of cytotoxicity of skin irritants on cultured keratinocytes. Br. J. Dermatol . 1994:24–130. doi: 10.1111/j.1365-2133.1994.tb06877.x. [DOI] [PubMed] [Google Scholar]
- 8.Popovici I, Lupuliasa D. Tehnologie farmaceutica. Iasi: Polirom; 1997. [Google Scholar]
- 9.Abelson MB, Udell IJ. Principles and Practice of Ophthalmology. Philadelphia: W.B. Saunders; 2000. [Google Scholar]
- 10.Pisella PJ, Lala E, Brignole F, Baudoin C. Retentissement conjonctival des conservateurs: etude comparative de collyres betabloquants conserves et non conserves chez des patients glaucomateux. J. Fr. Ophtalmol. 2003;26:675–679. [PubMed] [Google Scholar]
- 11.Albietz JM, Bruce AS. The conjunctival epithelium in dry eye subtypes effect of preserved and- non preserved topical treatments. Curr Eye Res. 2001;22:8–18. doi: 10.1076/ceyr.22.1.8.6977. [DOI] [PubMed] [Google Scholar]
- 12.Baudouin C. 10 anos de revolucion sin conservantes . Laboratoiros Theainovacion. 32(7) [Google Scholar]
- 13.Campagna P, Macri A, Rolando M, Calabria G. Chronic topical eye preservative –free beta-blocker therapy effect on the ocular surface in glaucomatous patients. Acta Ophthalmol. Scand. Suppl. . 1997:224–253. doi: 10.1111/j.1600-0420.1997.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 14.Avisar R, Creter D, Levinsky H, Savir H. Comparative study of tear substitutes and their immediate effect on the precorneal tear film. Isr. J. Med. Sci. 1997;33:194–197. [PubMed] [Google Scholar]
- 15.De Saint Jean M, Brignole F, Bringuier AF, Bauchet A, Feldmann G, Baudouin C. Effects of Benzalkonium chloride on growth and survival of conjunctival cells. Invest. Ophtalmol. vis Sci. . 1999;40:619–630. [PubMed] [Google Scholar]
- 16.Mietz H, Niesen U, Krieglstein GK. The effect of preservatives and antiglaucomatous medication on histopathology of the conjunctiva. Graefes Arch. Clin. Exp. Ophthalmol. . 1994;232:561–565. doi: 10.1007/BF00182000. [DOI] [PubMed] [Google Scholar]
- 17.Berdy GJ, Abelson MB, Smith LM, George MA. Preservative free artificial tear preparations. Assessement of corneal epithelial effects. Arch. Ophthalmol. . 1992;110:528–532. doi: 10.1001/archopht.1992.01080160106043. [DOI] [PubMed] [Google Scholar]
- 18.Badouin C, De Lunardo C. Short-term comparative study of topical; 2% carteol with and without benzalkonium chloride in healthy volunteers. Br. J. Ophthalmol. 1998;82:39–42. doi: 10.1136/bjo.82.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 2002;86:418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertrand–Cuignet H, Le Blond E, Melacaze M, Narduzzi J. Comment limiter les effets deleteres des conservateurs de certains collyres? . Les chaiers d’Ophtalmologie. 2008;123:15–17. [Google Scholar]
- 21.Rosenthal R, Henry C, Stone R, Stone R, Schlech B. Anatomy of a regimen: consideration of multipurpose solutions during non-compliant use. Cont. Lens Anterior Eye. 2003;26:17–26. doi: 10.1016/S1367-0484(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 22.Chibret H. Conservateurs et preparations ophtalmiques: realites et perspectives. Ann. Pharm. Fr. 1997;55:228–231. [PubMed] [Google Scholar]
- 23.Bartlett JD. Clinical Ocular Pharmacology. https://books.google.ro/books?isbn [Google Scholar]
- 24.Rosenthal R, Henry C, Stone R, Schlech B. Anatomy of a regimen: consideration of multipurpose solutions during non-compliant use. Cont. Lens Anterior Eye. 2003;26:17–26. doi: 10.1016/S1367-0484(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 25.Choy CK, Cho P, Boost MV. Cytotoxicity and effects on metabolism of contact lens care solutions on human corneal epithelium cells. . Clin. Exp. Optom. 2012;95:198–206. doi: 10.1111/j.1444-0938.2011.00687.x. [DOI] [PubMed] [Google Scholar]
- 26.Choy CK, Cho P, Boost MV, Benzie IF. Do multipurpose solutions damage porcine corneal epithelial cells? Optom. Vis. Sci. . 2009;86E:447–453. doi: 10.1097/OPX.0b013e31819fa422. [DOI] [PubMed] [Google Scholar]
- 27.Labbe A, Pauly A, Liang H, Brignole-Baudouin F, Martin C, Warnet JM, Baudouin C. Comparison of toxicological profiles of benzalkonium chloride and polyquaternium-1: an experimental study. J. Ocul. Pharmacol. Ther. . 2006;22:267–278. doi: 10.1089/jop.2006.22.267. [DOI] [PubMed] [Google Scholar]
- 28.Lopez B, Ubel J. Quantitative evaluation of the corneal epithelial barrier: effect of artificial tears and preservatives. Curr. Eye Res. . 1991;10:645–656. doi: 10.3109/02713689109013856. [DOI] [PubMed] [Google Scholar]
- 29.Furrer P, Mayer JM, Gurny R. Ocular tolerance of preservatives and alternatives. Review article. Europ. Jo. of Pharm. and Biopharm. . 2002;53:263–280. doi: 10.1016/s0939-6411(01)00246-6. [DOI] [PubMed] [Google Scholar]
- 30.Pfister RR, Burstein N. The effects of ophthalmic drugs, vehicles, study. Invest. Ophthalmol. Vis. Sci. . 1976;15:246–259. [PubMed] [Google Scholar]


