Abstract
Successful dental and orthopedic implant outcomes are determined by the degree of osseointegration. Over the last 60 years, endosseous implants have evolved to stimulate osteogenesis without the need for exogenous biologics such as bone morphogenetic proteins. An understanding of the interaction between cells and the physical characteristics of their environments has led to development of bioactive implants. Implant surfaces that mimic the inherent chemistry, topography, and wettability of native bone have shown to provide cells in the osteoblast lineage with the structural cues to promote tissue regeneration and net new bone formation. Studies show that attachment, proliferation, differentiation, and local factor production are sensitive to these implant surface characteristics. This review focuses on how surface properties, including chemistry, topography, and hydrophilicity, modulate protein adsorption, cell behavior, biological reactions, and signaling pathways in peri-implant bone tissue, allowing the development of true biomimetics that promote osseointegration by providing an environment suitable for osteogenesis.
Keywords: : titanium, osteoblast, implant, mesenchymal stem cell, osteoclast, biomimetic
Introduction
Implants are used in orthopedics and dentistry to improve the quality of life for patients who suffer from chronic back pain, degenerative joints, or edentulism. Titanium (Ti) and its alloys, including commercially pure Ti (cpTi), titanium–aluminum–vanadium (Ti-6Al-4V), and titanium–zirconium (TiZr), are commonly used to restore structure and function of musculoskeletal tissues because of their biocompatibility and corrosion resistance. These attributes of Ti are due to its ability to form a passive oxide layer making it stable in biological systems. Since bone has a high composition of inorganic calcium phosphates, many have speculated that the passive oxide layer of Ti mimics the ceramic nature of bone. Ti and Ti alloy implants support stable osseointegration, the biological phenomenon providing a direct structural and functional connection between the surface of a load-bearing implant and living bone.
The modern use of metallic implants for orthopedic and dental applications has been evolving over the last 60 years.1–4 Early efforts in biomaterial design were primarily concerned with the biocompatibility of the material with the host tissue. Materials that limited the development of foreign body giant cells, provided no evidence of local toxicity, and mitigated fibrous encapsulation were deemed acceptable. Implants made from these materials were viewed as biologically inert fixtures, anchored in place either through the use of cement or by mechanical interlocking between the implant and the surrounding bone tissue. However, failure to achieve mechanical stability led to micromotion of the implant and localized inflammation, frequently ending with osteolysis and implant failure.
Over the last few decades, research has made it clear that implant materials should go beyond compatibility and actively promote bone formation. One approach has been to induce peri-implant bone formation through the use of osteoinductive agents such as demineralized bone matrix5,6 and bone morphogenetic protein-2 (BMP2).7,8 Alternative approaches have included the use of bone graft substitutes that are designed to be osteogenic through the use of a variety of factors, including platelet-rich plasma,9–11 platelet-derived growth factor,12,13 and insulin-like growth factor-1,14,15 as well as vasculogenic factors such as vascular endothelial growth factor (VEGF)14,16 and fibroblast growth factor-2 (FGF2).17,18 Regulatory approval to use these factors has limited their accessibility, but scientists continue to investigate better delivery techniques to improve their success, including incorporation into biodegradable polymer scaffolds19,20 and attachment to the implant through dip coating21 as well as chemical coupling.22
While these approaches are well intentioned, they are temporary solutions designed to stimulate initial bone formation. However, long-term stability of an implant requires solutions that not only result in net bone formation but also provide continued osteogenic signals during and after the remodeling phase. Physical modifications to the implant material surface, which will be present long after the exogenous bioactive agents are gone, provide the best options to achieve this.
Biomaterial Surface Properties as a Bone Biomimetic
In vivo, bone is formed by osteoblasts on a surface that has been preconditioned by osteoclasts. As the osteoclasts resorb bone mineral, they leave structural elements, which include collagen and other extracellular matrix constituents. In addition, they deposit proteins on the surface that are chemotactic for osteoprogenitor cells.23,24 The size and shape of the osteoclast result in the formation of an osteoclast resorption pit that has microscale, mesoscale, and nanoscale dimensions. Thus, native bone has physical features capable of altering the mechanical environment experienced by osteoblast lineage cells,25 and the inherent chemistry, roughness, and hydrophilicity of the bone surface can influence cell attachment and cell shape, which can dictate cell proliferation and differentiation.26–30
Implant fabrication
Traditionally, Ti implants are produced by machining Ti or alloyed rods. The machining process results in a smooth surface with some microscopic irregularities; however, these irregularities have not been shown to be clinically significant. Using this machined surface as a base, additive or subtractive postprocessing methods are employed to further alter the surface of the implant to generate a surface topography with features similar in size or larger than cells.
Additive layering of Ti particles onto existing machined surfaces is frequently used to create bulk structures from computer-aided designs. Ti plasma spraying applies layers of Ti particles onto the surface of machined rod implants using a plasma torch. Local melting typically occurs during this process, which can generate a patchwork surface containing irregular regions of depressions and protrusions, as well as relatively smooth areas. Bulk structures can also be produced by additive manufacturing reducing cost, time, and material waste. Metallic implants built bottom-up are capable of possessing irregular geometries. Selective laser melting and electron beam melting are two methods in which powder layers of micron-sized metallic particles are fused using a high-energy beam in a layer-by-layer manner following a computer-aided design.
Subtractive methods used to modify implant surfaces at the macro- and microscale generally employ a grit-blasting or acid-etching procedure. For example, fabrication of the clinically available SLA (sandblasted, large grit, and acid etched) implant (Institut Straumann AG, Basel, Switzerland) begins with a machined surface that is subjected to large-grit corundum (250–500 μm) sandblasting, followed by acid etching in a boiling mixture of HCl and H2SO4. A modified version of the SLA process is used to generate the modSLA surface. It has the same initial sandblasting and acid-etching procedures as SLA, but the process is carried out under nitrogen and the implant is stored in an aqueous solution to prevent exposure to air and contamination with ambient hydrocarbons. By retaining the original clean surface, this processing method generates an implant that is both rough and hydrophilic.
Hydrophilicity
Hydrophilicity is related to the wettability of the implant surface and is generally regarded as a result of surface chemistry.31 The hydrophilicity of an osteoclast resorption pit surface is not known, making it difficult to mimic this property, but hydrophilic surfaces are known to promote an environment conducive for bone formation.32,33 This has been attributed to rapid spreading of serum on the surface providing an overall coating of bioactive factors34 that can influence early cell adhesion,35 proliferation,36 and differentiation30,37 by modulating the bonding strength, total amount, and conformation of adsorbed proteins.38 One method commonly used to retain surface hydrophilicity of a biomaterial is to reduce atmospheric hydrocarbon contamination.39
Surface topography
Surface topography and roughness also influence the biological response of osteoblast lineage cells.40 Bone formation occurs in structurally complex areas generated after osteoclast-mediated bone resorption.41 This surface topography has been shown to provide osteoblasts and mesenchymal stem cells (MSCs) with structural cues to promote tissue regeneration and net new bone formation.42,43 As a result, many bioactive implants are designed to mimic the hierarchical structure of bone by incorporating features at the micro-, meso-, and nanoscales.
Ensuring successful implant outcomes for patients requires a comprehensive understanding of how cells interact with the physical features of their environments. This review focuses on how surface properties, including chemistry, topography, and hydrophilicity, modulate protein adsorption, cell behavior, biological reactions, and signaling pathways in peri-implant bone tissue, allowing for the development of true biomimetics that promote osseointegration by providing an environment suitable for osteogenesis.
Lessons from Bone Remodeling
Bone remodeling is a continuous process that provides the mechanism for adaptation to mechanical stress, repair of microdamage, and replacement of primary bone during osseointegration of implanted materials. Due to its versatility, bone remodeling is a highly controlled process capable of occurring in different parts of the skeleton at different times. Its very nature highlights the importance of locally generated and regulated factors and environmental cues to ensure the appropriate balance among bone-resorbing osteoclasts, bone-forming osteoblasts, and their precursors. At the cellular level, osseointegration is a cascade of coordinated and sequentially organized events sharing many of the same mechanisms as normal bone formation and remodeling.
Osteoclasts resorb calcified cartilage and model bone during skeletal growth. In adult bone, osteoclasts are responsible for remodeling. In vivo bone formation and remodeling occur on surfaces that have been prepared by osteoclasts, whether during bone remodeling or during bone development and long-bone growth. It is unclear as to how resorption sites are selected, but the process is initiated by retraction of bone-lining cells to uncover the osteoid. After removal of the osteoid, the osteoclast attaches to the mineralized surface and forms a sealing zone. Beneath the osteoclast, the formation of a closed compartment called the resorption lacuna occurs where the osteoclast membrane is folded forming the ruffled border. The osteoclast is able to release hydrogen ions through the ruffled border through activity of carbonic anhydrase, thereby reducing the pH and dissolving the mineralized portion of the extracellular matrix.44–46 Other enzymes such as lysosomal cysteine proteinases and matrix metalloproteinases are then secreted to degrade the organic matrix.47,48
The rate and extent of osteoclast resorption are controlled through a variety of mechanisms, including release of active transforming growth factor beta-1 (TGFβ1);43 production of osteoprotegerin (OPG) by neighboring cells,49 which acts as a decoy receptor for receptor activator of NFκB ligand (RANKL);50 and cross talk with neighboring osteoblasts through ephs/ephrins51 and semaphorins.52 In addition, pH within the ruffled border increases as hydroxyapatite is dissolved, releasing hydroxyl ions. Thus, they are unable to completely degrade all of the extracellular matrix components leaving debris such as collagen and hydroxyapatite fragments in the wake of their resorption. As a result, resorption pits ranging from 30 to 100 μm in diameter are left behind. Furthermore, the residual components provide a unique surface with a particular chemistry and complex hierarchical structure that contains micro-, meso-, and nanofeatures.
The resorption pit created by the osteoclast has been shown to be chemotactic for osteoprogenitor cells,41 which migrate onto the surface and differentiate into mature osteoblasts. These cells produce growth factors such as BMPs together with their regulatory apparatus, which induce osteoblast differentiation of MSCs and osteoprogenitor cells. The secretory osteoblasts produce osteoid consisting of extracellular matrix proteins such as type I collagen as well as osteocalcin and osteonectin. Mineralization of the osteoid is then promoted through the regulation of local concentrations of calcium and phosphate and tailoring of the extracellular matrix components.
The topography and biochemical information of the osteoclast resorption pit provides signals necessary to initiate formation of new bone by osteoblasts. This is supported by studies investigating the response of human MG63 osteoblast-like cells to the surface of bone wafers.53,54 When cultured on bone wafers that were preconditioned by osteoclasts, these cells preferentially colonized the resorption pits; cell number was reduced compared with cells grown on tissue culture polystyrene (TCPS), but alkaline phosphatase activity increased and osteocalcin production was increased. In addition, production of prostaglandin E2 (PGE2) and TGFβ1 was elevated, both of which were shown in other studies to be necessary for osteoblast differentiation and matrix synthesis.55,56 The effect of the bone surface was enhanced the longer it was conditioned by osteoclasts (Fig. 1).
FIG. 1.
The growth of MG63 osteoblast-like cells on bone wafers pretreated with osteoclasts decreases cell proliferation, but increases production of local factors that promote osteogenesis. Bone wafers were treated for 0, 10, and 20 days with osteoclasts and compared with tissue culture plastic. *p < 0.05 vs. plastic; #p < 0.05 vs. bone wafers not pretreated with osteoclasts; •p < 0.05 vs. bone wafers treated with osteoclasts for 10 days. Adapted from a study by Boyan et al.53 PGE2, prostaglandin E2; TGFβ1, transforming growth factor beta-1.
Biomimetic Implant Design
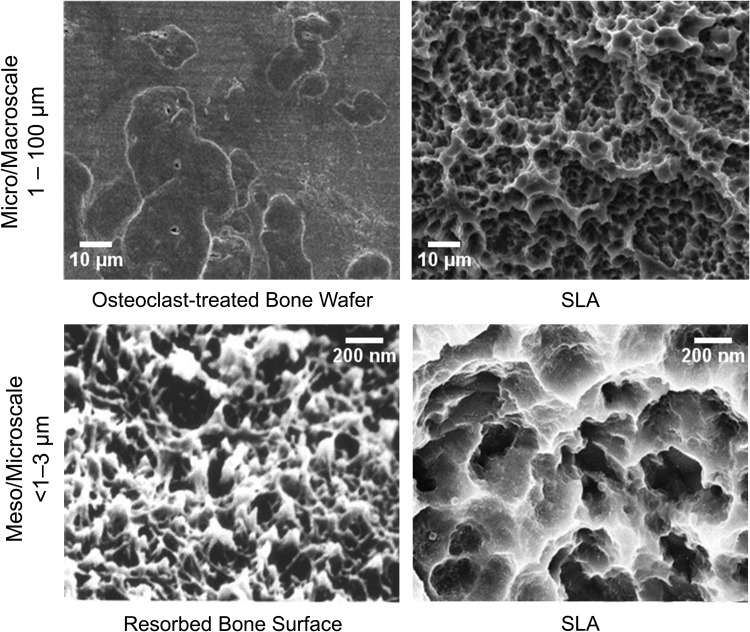
Based on the research from our group and others,40 biologically inspired dental and orthopedic implant surfaces are now being produced that mimic topographical features of an osteoclast resorption pit (Fig. 2). Studies using model Ti surfaces fabricated using photolithography and electromicromachining show that 30–100-μm diameter craters are preferentially colonized by osteoblasts. These craters are similar in size to osteoclast resorption pits. Furthermore, the osteoblasts are able to detect differences in submicron and nanoscale features within the craters.57,58 Recent studies comparing osteoblastic differentiation of MSCs cultured on Ti-6Al-4V substrates with similar microscale roughness, but different mesoscale and nanoscale properties, indicate that production of osteogenic proteins, including BMP2, as well as other regulatory factors that favor an osteogenic environment is favored by specific kurtosis and skewness of these features.59
FIG. 2.
Scanning electron micrograph of an osteoclast-resorbed bone surface and a Ti surface that has been sandblasted with large-grit corundum and acid etched (SLA). SLA, sandblasted, large grit, and acid etched.
Morphological studies of the osteoclast resorption pit indicate that it is roughly of the same dimensions of the craters generated on Ti, Ti-6Al-4V, and TiZr implants by grit blasting. Chemical etching generates micro-, meso-, and nanoscale features depending on the type of material, the properties of the grit used, and the etching protocol. In addition, thermal, hydrothermal, or chemical treatments have been implemented to restructure the passive oxide layer that forms on the surface of Ti implants.60–62 Reorganization of the oxide layer leads to a disordered nanostructure orientation that has been shown to be more favorable compared with a highly ordered pattern as it more closely mimics the random resorption patterns created by osteoclasts.63
Mechanisms
In vitro studies confirm the bioactivity of these biomimetic implant surfaces. As discussed in greater detail hereunder, MSCs exhibit rapid osteoblast differentiation when cultured on Ti, Ti-6Al-4V, and TiZr surfaces that have been grit blasted and acid etched.29,64,65 Osteoblast differentiation markers, including increased alkaline phosphatase-specific activity and production of osteocalcin, OPG, and osteopontin, are increased compared with smooth surface. Whereas osteoblast differentiation on TCPS can take as long as 21 days in cultures grown in osteogenic media, it occurs within 4 days on the Ti substrates and no osteogenic factors are required. The stimulatory effect of the topographical features of the substrate is enhanced when the hydrophilicity of the surface is retained postprocessing.30 The change in phenotypic markers is accompanied by a change in cell shape,28 suggesting that the physical environment elicits some of its effects through mechanical signals. This transition from a multipotent MSC to a committed osteoblast is dependent on a change in integrin expression from predominantly α5β1, which binds fibronectin, to α2β1 and α1β2, which bind collagen type 1.66 Knockdown of β1, α1, or α2 blocks differentiation. The effect of the surface microstructure is mediated by α2β1, whereas surface chemistry is mediated by α1β1.67
The biomimetic surface not only regulates osteoblast differentiation but also its effects on the cells have consequences for MSCs and osteoprogenitor cells distal from the implant surface. Osteoblasts grown on the surface generate factors that simulate osteoblast differentiation of MSCs grown on TCPS, demonstrated in coculture experiments.37 These factors include production of BMPs as well as production of anti-inflammatory mediators68 and factors that regulate osteoclast activity. Thus, cells on the surface can generate a microenvironment that favors net new bone formation during the bone-modeling phase of osseointegration.
Inflammation
Although the process of osseointegration shares many similarities with bone remodeling, including an overall goal of net new bone formation, initial events are very different (Fig. 3). Osseointegration begins with surgical trauma, followed by the insertion of an implant. It has been shown that implant insertion modifies the nature of bone healing.69 After implant insertion, blood vessels rupture and release proteins, ions, sugars, and lipids, important for blood clot formation (Fig. 3A). Following formation of the blood clot, initial inflammatory response is controlled through immunomodulatory cells such as neutrophils, dendritic cells, and macrophages (Fig. 3B). These immune cells produce chemokines that clean and disinfect the microenvironment, guide MSC recruitment or additional immune cells, and initiate tissue repair.
FIG. 3.
Schematic illustrating the steps of implant osseointegration. Osseointegration involves formation of a fibrin clot (A), recruitment of monocytes and macrophages and migration of MSCs along the clot (B), osteoblastic differentiation of MSCs and woven bone formation, as well as vasculogenesis (C), and ultimately, osteoclast-mediated bone remodeling (D), leading to mature lamellar bone (E). MSCs, mesenchymal stem cells.
In vivo studies indicate that inflammation is necessary for the osseointegration of metallic implants; however, inflammation must occur in a coordinated series of events. Without proper resolution of the inflammatory response, certain adverse reactions can occur, including chronic inflammation, fibrous encapsulation, osteolysis, and implant rejection. Cultures of dendritic cells on TCPS, smooth Ti, or microrough Ti substrates exhibit proinflammatory maturation, showing that microroughness alone is not capable of modulating the immune response of dendritic cells.70 Microrough Ti also drove macrophages toward a proinflammatory M1 state.71 However, retaining the hydrophilic nature of microtextured Ti by preventing hydrocarbon deposition prevented the maturation of the dendritic cell phenotype70 and drove macrophages toward an anti-inflammatory M2 state.71 Moreover, a comparison of Ti and TiZr implants with an identical microroughness demonstrated increased production of anti-inflammatory cytokines while simultaneously decreasing proinflammatory cytokines.72 This suggests that surface chemistry is an important immune response mediator for macrophages and dendritic cells with an overall goal to increase factors that promote early stages of bone formation.
Stem cells
Formation of the blood clot is an important step in implant osseointegration as it serves as a provisional matrix and scaffold for the migration of MSCs to the implant surface (Fig. 3B). Surface design that facilitates this contact osteogenesis has been shown to control the fundamental processes that drive the osteoblastic differentiation of MSCs.29,37 Differentiating MSCs and mature osteoblasts produce factors that create an osteogenic microenvironment, promote angiogenesis, reduce inflammation, and regulate osteoclast-mediated bone resorption. A cell-rich immature bone (woven bone) forms in direct contact with the surface of the Ti implant (Fig. 3C). Following the formation of the woven bone, osteoclasts begin to form (Fig. 3D). Their formation marks the beginning of the bone-modeling phase of osseointegration and with it comes the progressive removal of the woven bone, creating space for new lamellar bone formation and replacing passive primary stability with active secondary fixation through biological bonding (Fig. 3E).
It has been understood for some time that macro- and microrough implants outperform their smooth counterparts. Cells grown on TCPS or smooth Ti surfaces tend to attach, spread, and proliferate, whereas cells grown on microrough surfaces tend to proliferate less, but show increased markers of osteoblastic differentiation. There exists an increasing body of evidence implicating a favorable cell response to submicron and nanoscale features. Osteoblasts and MSCs have been consistently shown to produce higher messenger RNA (mRNA) and protein levels of osteoblast markers, such as osterix, alkaline phosphatase, and osteocalcin. Furthermore, morphological evaluations demonstrate cells to exhibit more filopodia extensions, cytoskeletal alignment, and adhesion73–75 when grown on substrates with nanoscale features compared with smooth controls.
In addition to regulating osteoblast attachment, morphology, and differentiation, surface roughness also modulates local production of angiogenic factors.76 Adequate vascular supply in peri-implant tissues is imperative for the clinical success of implanted materials. When osteoblast lineage cells are cultured on smooth Ti, VEGF-A, FGF2, and endothelial growth factor are increased compared with cells cultured on TCPS. A further increase is observed in cultures grown on microrough Ti substrates. Moreover, microrough Ti substrates facilitate increased endothelial tubule formation assessed using both Matrigel® and fibrin gel angiogenesis assays,76 suggesting that implant surface roughness may directly enhance neovascularization. This hypothesis is supported by an in vivo study in elderly mice, showing increased vasculogenesis when microtextured hydrophilic implants were placed in the femoral bone medullary canal compared with smooth surfaced Ti implants.77
Biomimetic Implants Regulate Osteogenesis Through Alternative Pathways
One way a material's surface can influence the response of cells is through differential adsorption of proteins onto the surface.38 In turn, the protein profile that adsorbs to the surface can influence integrin-mediated cell attachment through which cells interact with their underlying substrate. Integrin signaling, therefore, provides cells with information regarding the surrounding microenvironment. MSCs and osteoblasts express mRNAs for a number of integrin subunits, including α1, α2, α5, αv, β1, and β3. Typically, when cultured on TCPS, MSCs and osteoblasts primarily express the α5β1 integrin complex, but shift to α1β1 and α2β1 when grown on microstructured substrates.78 When integrin signaling is inhibited either by silencing the α2 or α1 subunit or the β1 subunit, osteoblast differentiation is inhibited. Cells silenced for α2β1 fail to undergo changes in cell shape associated with the shift from proliferation to a columnar morphology associated with a secretory osteoblast.28 Interestingly, inhibition of α5β1 signaling results in reduced cell attachment to the surface and reduced proliferation, suggesting its importance for initial cell attachment.27
The binding of integrins to their underlying substrate initiates a signaling cascade, resulting in new gene expression and protein synthesis. On TCPS, MSC differentiation occurs through the canonical Wnt3A pathway, causing an accumulation of β-catenin in the cytoplasm and its translocation into the nucleus to serve as a transcriptional activator.79 Our studies have demonstrated that surface-mediated MSC differentiation downregulates genes associated with the Wnt3A pathway while upregulating genes of the noncanonical calcium-dependent Wnt5a pathway.80 Moreover, increased expression of ITGA1 and ITGA2 is associated with Wnt5a, not Wnt3a, as surface-mediated regulation of integrin expression is absent in WNT5A knockdown MSCs.
Many of the factors produced by osteoblasts cultured on microrough biomimetic Ti surfaces regulate bone remodeling by favoring osteogenesis over osteoclastic resorption, promoting net new bone formation (Fig. 4). MSCs and osteoblasts also exhibit surface-dependent changes in physiology, resulting in altered autocrine and paracrine regulation in adjacent tissues. Although PGE2 stimulates osteoclastic activity at high levels, it is required at low levels for osteoblast activity.81,82 Osteoblast production of PGE1 and PGE2 is markedly increased when cells are cultured on microrough surfaces.83 Blocking the production of prostaglandins by the cyclooxygenase (Cox) inhibitor, indomethacin, also blocks the increase in other osteoblastic markers when cells are cultured on these surfaces, suggesting that they are necessary for enhanced osteogenesis.84
FIG. 4.
Cell signaling mechanisms among osteoblast lineage cells and osteoclasts involved in osseointegration lead to release of local regulatory factors in the peri-implant space. 1,25(OH)2D3, 1α,25-Dihydroxy vitamin D3; OPG, osteoprotegerin; RANKL, receptor activator of NFκB ligand.
Although it is important to stimulate osteoblast differentiation and maturation, it is also critical to control osteoclastic remodeling. One mechanism is mediated by TGFβ1-dependent regulation of OPG production.85 TGFβ1 enhances the proliferation of MSCs and osteoblasts and stimulates extracellular matrix production of proteins such as type I collagen. Latent TGFβ1 and latent TGFβ-binding protein are synthesized and stored in the extracellular matrix. Most of the TGFβ1 produced by osteoblasts cultured on Ti is in latent form,83 and the amount incorporated into the matrix is increased on rougher surfaces. Once the bone-modeling phase of osseointegration begins (Fig. 3D), the osteoclasts degrade the newly synthesized matrix releasing the stored latent TGFβ1 and convert it into active TGFβ1. Once activated, TGFβ1 acts on osteoclasts and downregulates their activity, in part, by regulating production of OPG.85
OPG serves as a decoy receptor for RANKL. By binding to RANKL, it prevents osteoclast differentiation by preventing its interaction with RANK on the osteoclast precursor surface, an interaction required for the fusion and subsequent maturation of differentiated osteoclasts. A feedback mechanism is in place that allows osteoblasts to produce soluble RANKL to deplete excess OPG if the regulatory stimulus favors new osteoclast formation. Microrough Ti substrates facilitate increased production of OPG by osteoblasts; however, levels of RANKL do not change. Thus, the net effect is bone formation without bone resorption.
Local regulatory factor production has also been shown to be sensitive to microstructured Ti implants. Recently, it was shown that BMP2 signaling is tightly regulated during surface-mediated osteogenesis and that modulation of these signals can enhance or inhibit this process.86 Autocrine and paracrine actions of BMPs are known to be involved in cell proliferation, differentiation, and apoptosis.87 Among these proteins, BMPs 2, 4, and 7 have shown to be the most important in bone formation and healing (Fig. 5). Modulation of these osteogenic factors by implant surfaces provides molecular evidence of the increased osteoblastogenesis seen in vitro and increased healing times seen clinically.
FIG. 5.
Ti surfaces modulate the mRNA levels and protein production of BMP ligands in MSCs cultured on microstructured Ti surfaces. The surfaces used were able to distinguish among the basic surface features such as smooth PT, microrough/hydrophobic SLA, and microrough/hydrophilic modSLA. *p < 0.05 vs. TCPS; #p < 0.05 vs. PT; $p < 0.05 vs. SLA. Substrates were provided by Institut Straumann AG. Adapted from a study by Olivares-Navarrete et al.86 BMP, bone morphogenetic protein; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; mRNA, messenger RNA; PT, pretreatment; TCPS, tissue culture polystyrene.
MSCs cultured on microstructured Ti substrates display temporal upregulation of BMPs with increases of BMP2 and BMP4 occurring as early as 4 days,86 suggesting that they are early regulators of surface-mediated osteogenesis. Their presence could also serve as the impetus for the commitment of MSCs to the osteoblast lineage when cultured on microstructured Ti as they are known to regulate embryonic skeletal development. Furthermore, they also provide a mechanism for the differentiation of MSCs distal to the implant surface as they are capable of multidirectional signaling. When MSCs were silenced for BMP2, production of osteoblastic markers decreased when cultured on microstructured Ti.86 In contrast, increased production was observed when exogenous BMP2 was added to wild-type MSCs. Interestingly, OPG levels decreased, suggesting that BMP2 effects may also impact bone remodeling.
Although BMP2 has been used to induce bone formation clinically, adverse effects, including osteolysis, bone resorption, swelling, and seroma formation, are concerning.88,89 Many of these adverse effects are attributed to the proinflammatory environment stimulated by high doses of BMP2 as well as its ability to induce apoptosis.68,87 This can be particularly problematic during the acute inflammatory response triggered in the early periods after injury associated with implantation. MSCs cultured on microrough Ti produce lower levels of proinflammatory IL6 and IL8 and higher levels of the anti-inflammatory IL10 compared with MSCs on smooth Ti or TCPS.68 While they produce BMPs locally, MSCs also produce factors involved in their regulation, including Noggin,26 and they express BMP2 receptors.8 This is important because the endogenous supply of BMP2 is modulated in a physiologically relevant way, and cells are able to respond to BMP2 when it is available. However, the effects of surface microtopography were reversed or blocked by the addition of exogenous BMP2. This led to increased proinflammatory IL6 and IL8 and decreased anti-inflammatory IL10.86 Mitigating inflammatory costimulation is one of the main advantages of substrate-induced activation of endogenous BMP2 compared with the addition of exogenous BMP2.
Limitations and Future Directions
Current research in the field of dental and orthopedic implantology tends to focus on development of modification techniques. However, limitations in the field of implantology are due to knowledge gaps in bone biology and how implant surface features alter the dynamics of wound healing. As research into these areas progresses, the future of implantology could see patient-specific implant designs that modulate different pathways, promoting healthy bone formation despite health issues the patient may present.
Summary
By targeting the surface of the substrate, major enhancements in the performance of dental and orthopedic implants have been achieved. A thorough understanding of how cells interact with the physical features of their environments has led to the development of implants that are both biocompatible and bioactive. Implant surface features are now designed to mimic the inherent surface roughness, surface chemistry, and surface hydrophilicity of native bone. Furthermore, methods to recreate the complex hierarchical features of an osteoclast resorption pit are now widely used to induce combinations of macroscale, microscale, mesoscale, and nanoscale topography. Thus, by mimicking the surface structure present on native bone tissue, contemporary dental and orthopedic implants can be designed to achieve rapid and optimal osseointegration.
Acknowledgments
The authors acknowledge the support of the ITI Foundation (Waldenburg, Switzerland); Institut Straumann AG (Basel, Switzerland); Titan Spine LLC (Mequon, Wisconsin, USA); AB Dental (Ashdod, Israel); and the National Institutes of Health (AR052102 and T32 GM08433). In addition, the authors acknowledge Caroline Bivens for her artistic contributions (Figs. 3 and 4) to the present work.
Disclosure Statement
B.D.B. is an unpaid consultant for Institut Straumann AG and a paid consultant for TitanSpine LLC. Z.S. is a paid consultant for AB Dental.
References
- 1.Bobbio A. The first endosseous alloplastic implant in the history of man. Bull Hist Dent 20, 1, 1972 [PubMed] [Google Scholar]
- 2.Branemark P.I., Hansson B.O., Adell R., Breine U., Lindstrom J., Hallen O., and Ohman A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg Suppl 16, 1, 1977 [PubMed] [Google Scholar]
- 3.Lang B.R., and Chiappa A.A. Mandibular implants: a new method of attachment. J Prosthet Dent 22, 261, 1969 [DOI] [PubMed] [Google Scholar]
- 4.Sykaras N., Iacopino A.M., Marker V.A., Triplett R.G., and Woody R.D. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants 15, 675, 2000 [PubMed] [Google Scholar]
- 5.Bencharit S., Allen R.K., and Whitley D., 3rd. Utilization of demineralized bone matrix to restore missing buccal bone during single implant placement: clinical report. J Oral Implantol 42, 490, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Irinakis T. Efficacy of injectable demineralized bone matrix as graft material during sinus elevation surgery with simultaneous implant placement in the posterior maxilla: clinical evaluation of 49 sinuses. J Oral Maxillofac Surg 69, 134, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Bishop G.B., and Einhorn T.A. Current and future clinical applications of bone morphogenetic proteins in orthopaedic trauma surgery. Int Orthop 31, 721, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan M., and Cao X. BMP signaling in skeletal development. Biochem Biophys Res Commun 328, 651, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Bae J.H., Kim Y.K., and Myung S.K. Effects of platelet-rich plasma on sinus bone graft: meta-analysis. J Periodontol 82, 660, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Butterfield K.J., Bennett J., Gronowicz G., and Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg 63, 370, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Pocaterra A., Caruso S., Bernardi S., Scagnoli L., Continenza M.A., and Gatto R. Effectiveness of platelet-rich plasma as an adjunctive material to bone graft: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Oral Maxillofac Surg 45, 1027, 2016 [DOI] [PubMed] [Google Scholar]
- 12.DiGiovanni C.W., Lin S.S., Baumhauer J.F., Daniels T., Younger A., Glazebrook M., Anderson J., Anderson R., Evangelista P., Lynch S.E., North American Orthopedic F., and Ankle Study G. Recombinant human platelet-derived growth factor-BB and beta-tricalcium phosphate (rhPDGF-BB/beta-TCP): an alternative to autogenous bone graft. J Bone Joint Surg Am 95, 1184, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Nevins M., Nevins M.L., Karimbux N., Kim S.W., Schupbach P., and Kim D.M. The combination of purified recombinant human platelet-derived growth factor-BB and equine particulate bone graft for periodontal regeneration. J Periodontol 83, 565, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Devi R., and Dixit J. Clinical evaluation of insulin like growth factor-I and vascular endothelial growth factor with alloplastic bone graft material in the management of human two wall intra-osseous defects. J Clin Diagn Res 10, ZC41, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueno T., Mizukawa N., and Sugahara T. Experimental study of bone formation from autogenous periosteal graft following insulin-like growth factor I administration. J Craniomaxillofac Surg 27, 308, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Rabie A.B., and Lu M. Basic fibroblast growth factor up-regulates the expression of vascular endothelial growth factor during healing of allogeneic bone graft. Arch Oral Biol 49, 1025, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Alam S., Ueki K., Marukawa K., Ohara T., Hase T., Takazakura D., and Nakagawa K. Expression of bone morphogenetic protein 2 and fibroblast growth factor 2 during bone regeneration using different implant materials as an onlay bone graft in rabbit mandibles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103, 16, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Nakasa T., Ishida O., Sunagawa T., Nakamae A., Yasunaga Y., Agung M., and Ochi M. Prefabrication of vascularized bone graft using a combination of fibroblast growth factor-2 and vascular bundle implantation into a novel interconnected porous calcium hydroxyapatite ceramic. J Biomed Mater Res A 75, 350, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Holland T.A., and Mikos A.G. Biodegradable polymeric scaffolds. Improvements in bone tissue engineering through controlled drug delivery. Adv Biochem Eng Biotechnol 102, 161, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Liu X., and Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng 32, 477, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Goodman S.B., Yao Z., Keeney M., and Yang F. The future of biologic coatings for orthopaedic implants. Biomaterials 34, 3174, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nanci A., Wuest J.D., Peru L., Brunet P., Sharma V., Zalzal S., and McKee M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J Biomed Mater Res 40, 324, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Glowacki J., Rey C., Glimcher M.J., Cox K.A., and Lian J. A role for osteocalcin in osteoclast differentiation. J Cell Biochem 45, 292, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Ritter N.M., Farach-Carson M.C., and Butler W.T. Evidence for the formation of a complex between osteopontin and osteocalcin. J Bone Miner Res 7, 877, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Thompson W.R., Rubin C.T., and Rubin J. Mechanical regulation of signaling pathways in bone. Gene 503, 179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abe E. Function of BMPs and BMP antagonists in adult bone. Ann N Y Acad Sci 1068, 41, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Keselowsky B.G., Wang L., Schwartz Z., Garcia A.J., and Boyan B.D. Integrin alpha(5) controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. J Biomed Mater Res A 80, 700, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Lai M., Hermann C.D., Cheng A., Olivares-Navarrete R., Gittens R.A., Bird M.M., Walker M., Cai Y., Cai K., Sandhage K.H., Schwartz Z., and Boyan B.D. Role of alpha2beta1 integrins in mediating cell shape on microtextured titanium surfaces. J Biomed Mater Res A 103, 564, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotz E.M., Olivares-Navarrete R., Berner S., Boyan B.D., and Schwartz Z. Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces. J Biomed Mater Res A 104, 3137, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Zhao G., Schwartz Z., Wieland M., Rupp F., Geis-Gerstorfer J., Cochran D.L., and Boyan B.D. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A 74, 49, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Gittens R.A., Scheideler L., Rupp F., Hyzy S.L., Geis-Gerstorfer J., Schwartz Z., and Boyan B.D. A review on the wettability of dental implant surfaces II: biological and clinical aspects. Acta Biomater 10, 2907, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bornstein M.M., Valderrama P., Jones A.A., Wilson T.G., Seibl R., and Cochran D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: a histomorphometric study in canine mandibles. Clin Oral Implants Res 19, 233, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Eriksson C., Nygren H., and Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 25, 4759, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Huang Q., Lin L., Yang Y., Hu R., Vogler E.A., and Lin C. Role of trapped air in the formation of cell-and-protein micropatterns on superhydrophobic/superhydrophilic microtemplated surfaces. Biomaterials 33, 8213, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Jimbo R., Sawase T., Baba K., Kurogi T., Shibata Y., and Atsuta M. Enhanced initial cell responses to chemically modified anodized titanium. Clin Implant Dent Relat Res 10, 55, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Sawase T., Jimbo R., Baba K., Shibata Y., Ikeda T., and Atsuta M. Photo-induced hydrophilicity enhances initial cell behavior and early bone apposition. Clin Oral Implants Res 19, 491, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Olivares-Navarrete R., Hyzy S.L., Hutton D.L., Erdman C.P., Wieland M., Boyan B.D., and Schwartz Z. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials 31, 2728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson C.J., Clegg R.E., Leavesley D.I., and Pearcy M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng 11, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Wennerberg A., Galli S., and Albrektsson T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin Cosmet Investig Dent 3, 59, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gittens R.A., Olivares-Navarrete R., Schwartz Z., and Boyan B.D. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater 10, 3363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puzas J.E., Lewis G., Hsu J., Reynolds P.R., Rosier R.N., OKeefe R.J., Hicks D.G., Cushing J., and Martinez D.A. Osteoblasts preferentially adhere to sites of prior bone resorption. J Bone Miner Res 12, S241, 1997 [Google Scholar]
- 42.Bachle M., and Kohal R.J. A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clin Oral Implan Res 15, 683, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Boyan B.D., Lossdorfer S., Wang L., Zhao G., Lohmann C.H., Cochran D.L., and Schwartz Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater 6, 22, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Baron R., Neff L., Louvard D., and Courtoy P.J. Cell-mediated extracellular acidification and bone-resorption—evidence for a low Ph in resorbing lacunae and localization of a 100-Kd lysosomal membrane-protein at the osteoclast ruffled border. J Cell Biol 101, 2210, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fallon M.D. Alterations in the Ph of osteoclast resorbing fluid reflects changes in bone degradative activity. Calcified Tissue Int 36, 458, 1984 [Google Scholar]
- 46.Minkin C., and Jennings J.M. Carbonic-anhydrase and bone remodeling—sulfonamide inhibition of bone resorption in organ-culture. Science 176, 1031, 1972 [DOI] [PubMed] [Google Scholar]
- 47.Everts V., Korper W., Hoeben K.A., Jansen I.D., Bromme D., Cleutjens K.B., Heeneman S., Peters C., Reinheckel T., Saftig P., and Beertsen W. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: differences between calvaria and long bone. J Bone Miner Res 21, 1399, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto S., and Sakamoto M. Biochemical and immunohistochemical studies on collagenase in resorbing bone in tissue culture. A novel hypothesis for the mechanism of bone resorption. J Periodontal Res 17, 523, 1982 [DOI] [PubMed] [Google Scholar]
- 49.Blair J.M., Zhou H., Seibel M.J., and Dunstan C.R. Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol 3, 41, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology 142, 5050, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Matsuo K., and Otaki N. Bone cell interactions through Eph/ephrin: bone modeling, remodeling and associated diseases. Cell Adh Migr 6, 148, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang S., and Kumanogoh A. Semaphorins in bone development, homeostasis, and disease. Semin Cell Dev Biol 24, 163, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Boyan B.D., Schwartz Z., Lohmann C.H., Sylvia V.L., Cochran D.L., Dean D.D., and Puzas J.E. Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res 21, 638, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Schwartz Z., Lohmann C.H., Wieland M., Cochran D.L., Dean D.D., Textor M., Bonewald L.F., and Boyan B.D. Osteoblast proliferation and differentiation on dentin slices are modulated by pretreatment of the surface with tetracycline or osteoclasts. J Periodontol 71, 586, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Kieswetter K., Schwartz Z., Cochran D., Gomez R., Dean D.D., and Boyan B.D. Substrate surface-roughness affects growth-factor and prostaglandin production by Mg63 cells. J Bone Miner Res 10, S338, 1995 [Google Scholar]
- 56.Mustafa K., Rubinstein J., Lopez B.S., and Arvidson K. Production of transforming growth factor beta1 and prostaglandin E2 by osteoblast-like cells cultured on titanium surfaces blasted with TiO2 particles. Clin Oral Implants Res 14, 50, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Boyde A., Ali N.N., and Jones S.J. Optical and scanning electron microscopy in the single osteoclast resorption assay. Scan Electron Microsc (Pt 3), 1259, 1985 [PubMed] [Google Scholar]
- 58.Everts V., Delaisse J.M., Korper W., Niehof A., Vaes G., and Beertsen W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J Cell Physiol 150, 221, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Hansson K.N., and Hansson S. Skewness and kurtosis: important parameters in the characterization of dental implant surface Roughness—A computer simulation. ISRN Mater Sci 2011, 1, 2011 [Google Scholar]
- 60.Gittens R.A., McLachlan T., Olivares-Navarrete R., Cai Y., Berner S., Tannenbaum R., Schwartz Z., Sandhage K.H., and Boyan B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 32, 3395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gittens R.A., Olivares-Navarrete R., McLachlan T., Cai Y., Hyzy S.L., Schneider J.M., Schwartz Z., Sandhage K.H., and Boyan B.D. Differential responses of osteoblast lineage cells to nanotopographically-modified, microroughened titanium-aluminum-vanadium alloy surfaces. Biomaterials 33, 8986, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wennerberg A., Svanborg L.M., Berner S., and Andersson M. Spontaneously formed nanostructures on titanium surfaces. Clin Oral Implants Res 24, 203, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Dalby M.J., Gadegaard N., Tare R., Andar A., Riehle M.O., Herzyk P., Wilkinson C.D., and Oreffo R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 6, 997, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Hyzy S.L., Cheng A., Cohen D.J., Yatzkaier G., Whitehead A.J., Clohessy R.M., Gittens R.A., Boyan B.D., and Schwartz Z. Novel hydrophilic nanostructured microtexture on direct metal laser sintered Ti-6Al-4V surfaces enhances osteoblast response in vitro and osseointegration in a rabbit model. J Biomed Mater Res A 104, 2086, 2016 [DOI] [PubMed] [Google Scholar]
- 65.Lotz E.M., Olivares-Navarrete R., Hyzy S.L., Berner S., Schwartz Z., and Boyan B.D. Comparable responses of osteoblast lineage cells to microstructured hydrophilic titanium-zirconium and microstructured hydrophilic titanium. Clin Oral Implants Res 28, e51, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Moursi A.M., Globus R.K., and Damsky C.H. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. J Cell Sci 110 (Pt 18), 2187, 1997 [DOI] [PubMed] [Google Scholar]
- 67.Park J.H., Wasilewski C.E., Almodovar N., Olivares-Navarrete R., Boyan B.D., Tannenbaum R., and Schwartz Z. The responses to surface wettability gradients induced by chitosan nanofilms on microtextured titanium mediated by specific integrin receptors. Biomaterials 33, 7386, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hyzy S.L., Olivares-Navarrete R., Hutton D.L., Tan C., Boyan B.D., and Schwartz Z. Microstructured titanium regulates interleukin production by osteoblasts, an effect modulated by exogenous BMP-2. Acta Biomater 9, 5821, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salvi G.E., Bosshardt D.D., Lang N.P., Abrahamsson I., Berglundh T., Lindhe J., Ivanovski S., and Donos N. Temporal sequence of hard and soft tissue healing around titanium dental implants. Periodontol 2000 68, 135, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Kou P.M., Schwartz Z., Boyan B.D., and Babensee J.E. Dendritic cell responses to surface properties of clinical titanium surfaces. Acta Biomater 7, 1354, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hotchkiss K.M., Reddy G.B., Hyzy S.L., Schwartz Z., Boyan B.D., and Olivares-Navarrete R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater 31, 425, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hotchkiss K.M., Ayad N.B., Hyzy S.L., Boyan B.D., and Olivares-Navarrete R. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin Oral Implants Res 28, 414, 2016 [DOI] [PubMed] [Google Scholar]
- 73.Biggs M.J., Richards R.G., Gadegaard N., Wilkinson C.D., and Dalby M.J. The effects of nanoscale pits on primary human osteoblast adhesion formation and cellular spreading. J Mater Sci Mater Med 18, 399, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Khang D., Lu J., Yao C., Haberstroh K.M., and Webster T.J. The role of nanometer and sub-micron surface features on vascular and bone cell adhesion on titanium. Biomaterials 29, 970, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Webster T.J., and Ejiofor J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V, and CoCrMo. Biomaterials 25, 4731, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Raines A.L., Olivares-Navarrete R., Wieland M., Cochran D.L., Schwartz Z., and Boyan B.D. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials 31, 4909, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olivares-Navarrete R., Raines A.L., Hyzy S.L., Park J.H., Hutton D.L., Cochran D.L., Boyan B.D., and Schwartz Z. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Miner Res 27, 1773, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olivares-Navarrete R., Raz P., Zhao G., Chen J., Wieland M., Cochran D.L., Chaudhri R.A., Ornoy A., Boyan B.D., and Schwartz Z. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A 105, 15767, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olivares-Navarrete R., Hyzy S.L., Hutton D.L., Dunn G.R., Appert C., Boyan B.D., and Schwartz Z. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater 7, 2740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olivares-Navarrete R., Hyzy S.L., Park J.H., Dunn G.R., Haithcock D.A., Wasilewski C.E., Boyan B.D., and Schwartz Z. Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop. Biomaterials 32, 6399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lohmann C.H., Dean D.D., Bonewald L.F., Schwartz Z., and Boyan B.D. Nitric oxide and prostaglandin E2 production in response to ultra-high molecular weight polyethylene particles depends on osteoblast maturation state. J Bone Joint Surg Am 84-A, 411, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Soskolne W.A., Cohen S., Sennerby L., Wennerberg A., and Shapira L. The effect of titanium surface roughness on the adhesion of monocytes and their secretion of TNF-alpha and PGE2. Clin Oral Implants Res 13, 86, 2002 [DOI] [PubMed] [Google Scholar]
- 83.Kieswetter K., Schwartz Z., Hummert T.W., Cochran D.L., Simpson J., Dean D.D., and Boyan B.D. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res 32, 55, 1996 [DOI] [PubMed] [Google Scholar]
- 84.Sisk M.A., Lohmann C.H., Cochran D.L., Sylvia V.L., Simpson J.P., Dean D.D., Boyan B.D., and Schwartz Z. Inhibition of cyclooxygenase by indomethacin modulates osteoblast response to titanium surface roughness in a time-dependent manner. Clin Oral Implants Res 12, 52, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Schwartz Z., Olivares-Navarrete R., Wieland M., Cochran D.L., and Boyan B.D. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials 30, 3390, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olivares-Navarrete R., Hyzy S.L., Haithcock D.A., Cundiff C.A., Schwartz Z., and Boyan B.D. Coordinated regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by endogenous bone morphogenetic proteins. Bone 73, 208, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hyzy S.L., Olivares-Navarrete R., Schwartz Z., and Boyan B.D. BMP2 induces osteoblast apoptosis in a maturation state and noggin-dependent manner. J Cell Biochem 113, 3236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oetgen M.E., and Richards B.S. Complications associated with the use of bone morphogenetic protein in pediatric patients. J Pediatr Orthop 30, 192, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Smoljanovic T., Cimic M., and Bojanic I. Aggressive end plate decortication as a cause of osteolysis after rhBMP-2 use in cervical spine interbody fusion. Spine J 10, 187, 2010 [DOI] [PubMed] [Google Scholar]