Abstract
Background
Management of recurrent prostate cancer (CaP) after radiotherapy (RT) is dependent on accurate localization of the site of recurrent disease.
Objective
To describe the anatomic patterns and clinical features associated with CaP recurrence following RT identified on advanced imaging.
Design, setting, and participants
Retrospective review of 184 patients with a rising prostate-specific antigen (PSA) after RT for CaP.
Intervention
C-11 choline positron emission tomography/computed tomography (CholPET).
Outcome measurements and statistical analysis
Recurrence patterns were classified as pelvic soft tissue only (as a surrogate for potentially salvageable disease) versus any extrapelvic disease, and clinical features were compared between patterns. Multivariable logistic regression was used to generate a predictive nomogram for extrapelvic recurrence. Discrimination was assessed with a c-index.
Results and limitations
Recurrence site was identified in 161 (87%) patients, with 95 (59%) sites histologically confirmed. Factors associated with the detection of recurrence included the difference between PSA nadir and PSA at CholPET (odds ratio: 1.30, p < 0.01) and National Comprehensive Cancer Network high-risk classification (odds ratio: 10.83, p = 0.03). One hundred (54.3%) patients recurred in the pelvic soft tissue only, while 61 (33%) had extrapelvic recurrence. Of 21 patients who underwent CholPET prior to meeting the Phoenix criteria of biochemical failure, 15 (71%) had recurrence identified on CholPET with 11 localized to the pelvis. On multivariable analysis, PSA at CholPET, time from RT, and National Comprehensive Cancer Network risk group were predictive of recurrence outside of the pelvis, and a nomogram was generated with a c-index of 0.79.
Conclusions
CholPET identified the site of recurrence in 87% of patients with a rising PSA after RT; most commonly within the pelvis in potentially salvageable locations. A predictive nomogram was generated, and pending external validation, this may aid in assessing the risk of disease beyond the pelvis. These findings underscore the importance of advanced imaging when considering management strategies for patients with a rising PSA following primary RT.
Patient summary
We identified anatomic patterns of recurrence in patients with a rising prostate-specific antigen after radiotherapy using C-11 choline positron emission tomography/computed tomography. Most recurrences were localized to the pelvis and we were able to generate a tool to aid in disease localization prior to evaluation with advanced imaging.
Keywords: Prostate Cancer, Radiation Therapy, Recurrence, PET/CT, Nomogram
1. Introduction
A rising serum prostate-specific antigen (PSA) after radiation therapy (RT) for prostate cancer (CaP) may be a harbinger of local, regional, or distant failure. While the Phoenix definition is the current standard to define biochemical recurrence (BCR) in patients with a rising PSA after primary RT [1], it represents a threshold value which has known limitations with respect to establishing disease recurrence and outcome [2]. Other biochemical metrics—such as nadir PSA, PSA velocity, and PSA doubling time—may provide additional prognostic information [3,4]; however, no biochemical threshold has been proven to localize recurrence and, with the exception of nadir PSA, all require longitudinal PSA measures. Given that local salvage treatment of recurrent CaP can result in cancer-specific survival of up to 70–83% at 10 yr [5], accurate localization of recurrence site is critically important for the optimal management of patients with a rising PSA after RT.
In order to accurately localize the site of disease recurrence, numerous advanced imaging modalities—those modalities beyond conventional computed tomography (CT) and bone scan— are being studied, including multi-parametric magnetic resonance imaging (MRI), MR-spectroscopy, MRI-lymphangiography, and numerous radio-isotopes for positron emission tomography (PET)/CT imaging [6,7]. A common conclusion drawn from these studies is that advanced imaging has the potential to alter the management of biochemically recurrent CaP. Given the inability of PSA to localize disease recurrence after primary RT and the promise of advanced imaging, we sought to: (1) describe our experience with C11 choline PET/CT (CholPET) in patients with a rising PSA after primary RT, including the description of features which are associated with finding recurrence at evaluation with CholPET, (2) define anatomic patterns of recurrence as identified on CholPET, and (3) evaluate potential clinical features which may improve localization of recurrence, thereby guiding the utilization of advanced imaging.
2. Materials and methods
After Institutional Review Board approval, patients who underwent CholPET for a rising PSA after primary RT for CaP between 2007 and 2015 were identified and retrospectively analyzed. The goal of the present analysis was to characterize recurrence among patients presenting with a rising PSA prior to the development of widespread disseminated disease or a castration-resistant state. Therefore patients were excluded if they had a PSA >20 ng/ml at CholPET, were actively managed with androgen deprivation therapy (ADT) at the time of CholPET, or had clinical evidence of castration-resistant CaP at the time of evaluation, defined by a rising PSA despite castrate testosterone levels or by treating physician diagnosis.
Treatment related variables included age at RT, pretreatment PSA, Gleason score, grade group, clinical stage, National Comprehensive Cancer Network (NCCN) risk-group [8], type of RT, target and dose of RT, and the use of hormone suppression during RT. Biochemical variables included PSA nadir, time to nadir from RT, PSA at CholPET, PSA doubling time at CholPET (in months), PSA velocity (in ng/ml/yr) at CholPET, and time to CholPET from RT. An additional calculated variable—ΔPSA—was generated from the difference between nadir PSA and PSA at evaluation with CholPET. When performed, the results of pelvic MRI obtained at the time of CholPET evaluation were abstracted and compared with the CholPET findings. Patients were further classified by BCR status as defined by the Phoenix criteria (PSA nadir + 2.0ng/ml) [1]. The primary outcome was the description of sites of recurrence following RT. Our technique for performing CholPET has been previously described [9], with 555–740 MBq of C11 choline administered prior to image acquisition. CholPET scans were classified as either positive or negative based on the presence of identified lesions by reviewing radiologists. True positive (diagnostic) scans were defined as biopsy confirmation of recurrent disease of an identified PET-avid lesion, progression of PET-avid lesions on follow-up imaging without treatment, or biochemical improvement with adjuvant therapy and/or a subsequent decrease in PET-avidity on follow-up imaging [10]. A PET-avid lesion which was biopsy confirmed negative was defined as a false positive. Negative (nondiagnostic) CholPET scans were defined as no evidence of PET avidity and no disease progression during follow-up (true negative) or absence of PET avidity but with subsequent identification of a site of recurrence within 1 yr (false negative). Patients who were identified with PET avid sites of recurrence but did not have follow-up at our institution following their CholPET were unable to be evaluated with respect to classification of findings as true or false positives and were excluded from analysis. Patterns of recurrence were classified as pelvic soft tissue including the prostate, seminal vesical, or pelvic lymph nodes, and any extrapelvic disease, inclusive of any osseous recurrence (pelvis or beyond).
Categorical variables were summarized using frequencies/percentages and continuous variables were summarized with medians and interquartile ranges. Missing data were summarized with frequencies and were excluded from subsequent logistic regression analyses. Univariable and multivariable logistic regression analyses were performed to identify clinical features associated with the likelihood of identifying recurrence at CholPET, reported with odds ratios and 95% confidence intervals.
The association of the site of recurrence (extrapelvic versus pelvic soft tissue) with clinical features was assessed using multivariable logistic regression with backward selection based on lowest Akaike information criterion correction for generation of a predictive nomogram. We chose backward selection based on the number of evaluable PSA metrics which are colinear in order to identify those metrics most associated with the outcome of interest. Components of the NCCN risk group (clinical stage, diagnostic PSA, and Gleason score) were not included as the risk group captured these components in an aggregate score and was evaluable on the majority of patients. Assessment of calibration and discrimination are summarized by a calibration plot and c-index, respectively. In order to assess the influence of the predictive model at informing decisions, a decision curve analysis was performed as previously described [11], comparing our model to commonly used PSA thresholds for the initiation of ADT (3 ng/ml and 10 ng/ml) and to PSA considered as a continuous adjustment [12,13]. All analyses were performed using SPSS 22.0 (IBM Corp, New York, NY, USA) or R version 3.2.3 (R-Foundation, Vienna, Austria) with two-sided p-values reported and significance considered at p <0.05.
3. Results
A total of 198 patients were identified with a rising PSA following primary RT and with a PSA <20ng/ml. Of these, 14 were missing confirmation of disease recurrence and thus excluded. Median age at RT was 65 (interquartile range [IQR]: 60–70) yr, with a median time to CholPET from RT of 68 (IQR: 39–104) mo. Median PSA at the time of CholPET was 5.7 (IQR: 3.4–8.9) ng/ml (Table 1). Of the 184 patients, 161 (87%; 95% confidence interval [CI]: 83–92%) had an identified site of recurrence. The sensitivity, specificity, positive predictive value, and negative predictive values for CholPET on a per patient basis in our cohort were 95% (95% CI: 91–98%), 73% (95% CI: 45–92%), 98% (95% CI: 94–99%), and 58% (95% CI: 33–80%), respectively. Compared with patients with a negative CholPET, positive CholPET findings were more often identified in patients with higher pretreatment PSA, NCCN risk-group, PSA level at CholPET, ΔPSA, PSA doubling time, and PSA velocity (Table 1). On multivariable logistic regression, ΔPSA (odds ratio: 1.30 per 1 ng/ml increase in ΔPSA) and NCCN high risk group (odds ratio: 10.83) were independently associated with CholPET positivity (Table 2). Notably, no patient with a PSA >10 ng/ml had a negative CholPET, and when restricted to patients with a PSA <10 ng/ml at CholPET, only the NCCN risk group was associated with positive scans (Supplementary Table 1).
Table 1.
Pretreatment and treatment characteristics of patients with a rising prostate-specific antigen (PSA) after primary radiotherapy (RT) for prostate cancer
| Whole cohort (n = 184) |
Positive scan (n = 161) |
Negative scan (n = 23) |
p value | |
|---|---|---|---|---|
| Age at RT | ||||
| Median | 65 | 65 | 64 | >0.9 |
| IQR | 60–70 | 60–70 | 57–73 | |
| PSA at diagnosis (ng/ml), n =169 | ||||
| Median | 7.8 | 8.1 | 6.0 | 0.01 |
| IQR | 5.6–10.5 | 5.7–11.7 | 4.7–8.2 | |
| Gleason pattern, n = 178 (%) | ||||
| ≤6 | 58 (33) | 47 (30) | 11 (48) | 0.1 |
| 7 | 82 (46) | 71 (46) | 11 (48) | |
| 8–10 | 38 (21) | 37 (24) | 1 (4) | |
| Grade group, n = 178 (%) | ||||
| 1 (≤3+3) | 59 (33) | 48 (31) | 11 (48) | 0.2 |
| 2 (3+4) | 52 (29) | 44 (28) | 8 (35) | |
| 3 (4+3) | 29 (16) | 26 (17) | 3 (13) | |
| 4 (8) | 19 (11) | 19 (12) | 0 (0) | |
| 5 (9 and 10) | 19 (11) | 18 (12) | 1 (4) | |
| Clinical stage, n = 124 (%) | ||||
| T1c | 63 (51) | 50 (48) | 13 (65) | 0.4 |
| T2a–c | 48 (39) | 43 (41) | 5 (25) | |
| T3a–b | 13 (10) | 11 (11) | 2 (10) | |
| NCCN risk group, n = 170 (%) | ||||
| Low risk | 42 (25) | 33 (22) | 9 (41) | 0.02 |
| Intermediate risk | 82 (48) | 70 (47) | 12 (55) | |
| High/very high risk | 46 (27) | 45 (30) | 1 (5) | |
| Type of therapy, n = 183 (%) | ||||
| EBRT alone | 104 (57) | 92 (58) | 12 (52) | 0.7 |
| BT as part of therapy | 79 (43) | 68 (43) | 11 (48) | |
| HT during RT, n = 179 (%) | 55 (31) | 49 (30) | 6 (33) | 0.8 |
| Dose of RT, n = 89 (Gy) | ||||
| Median | 75.6 | 75.8 | 75.6 | 0.1 |
| IQR | 75.0–80.0 | 75.2–83.7 | 72.9–75.6 | |
| Target (n = 169) | ||||
| Prostate | 137 (81) | 125 (82) | 12 (75) | 0.7 |
| Prostate + SV | 19 (11) | 17 (11) | 2 (13) | |
| Prostate + SV + pelvic nodes | 13 (8) | 11 (7) | 2 (13) | |
| PSA nadir, ng/ml (n = 178) | ||||
| Median | 0.5 | 0.5 | 0.4 | 0.4 |
| IQR | 0.2–1.1 | 0.2–1.2 | 0.2–0.9 | |
| PSA at CholPET scan (ng/ml) | ||||
| Median | 5.7 | 6.3 | 2.9 | <0.01 |
| IQR | 3.4–8.9 | 3.9–9.6 | 2.2–7.1 | |
| ΔPSA, ng/ml (n = 178)a | ||||
| Median | 5.1 | 5.4 | 2.6 | <0.01 |
| IQR | 2.9–7.9 | 3.2–8.2 | 1.9–5.2 | |
| Time to CholPET from RT (mo) | ||||
| Median | 68 | 67 | 70 | 0.7 |
| IQR | 39–104 | 37–104 | 44–101 | |
| Time to CholPET from nadir PSA, mo (n = 172) |
||||
| Median | 47 | 43 | 50 | 0.4 |
| IQR | 25–77 | 24–74 | 30–79 | |
| PSA doubling time, mo (n = 151) | ||||
| Median | 11 | 10 | 15 | 0.01 |
| IQR | 6–20 | 5–20 | 11–25 | |
| PSA velocity, ng/ml/yr (n = 169) | ||||
| Median | 1.3 | 1.4 | 0.7 | 0.01 |
| IQR | 0.6–2.9 | 0.6–3.1 | 0.3–1.8 |
BT = brachytherapy; CholPET = C-11 choline PET/CT; EBRT = external beam radiation therapy; HT = hormonal therapy; IQR = interquartile range; NCCN = national comprehensive cancer network; PET/CT = positron emission tomography/computed tomography; SV = seminal vesical.
Difference between PSA at C11 choline PET/CT and PSA nadir.
Table 2.
Univariable and multivariable logistic regression assessing associations with C-11 choline positron emission tomography/computed tomography positivity
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Factor | OR | 95% CI | p value | OR | 95% CI | p value |
| Age (per yr) | 1.00 | 0.94–1.06 | 0.9 | |||
| PSA at diagnosis (per 1 ng/ml) | 1.23 | 1.03–1.46 | 0.02 | |||
| Gleason score (ref. 6) | ||||||
| 7 | 1.51 | 0.61–3.77 | 0.4 | |||
| 8–10 | 8.66 | 1.07–70.15 | 0.04 | |||
| Clinical stage (ref. T1) | ||||||
| cT2a–c | 2.24 | 0.74–6.78 | 0.2 | |||
| cT3–4 | 1.43 | 0.28–7.27 | 0.7 | |||
| NCCN risk group (ref. low risk) | ||||||
| Intermediate risk | 1.59 | 0.61–4.15 | 0.3 | 1.27 | 0.45–3.58 | 0.7 |
| High risk | 12.27 | 1.48–101.66 | 0.02 | 10.83 | 1.26–93.06 | 0.03 |
| EBRT (ref. BT-containing) | 1.24 | 0.52–2.98 | 0.6 | |||
| HT during RT | 0.80 | 0.31–2.47 | 0.8 | |||
| Radiation field (ref. prostate only) | ||||||
| Prostate + SV | 0.82 | 0.17–3.96 | 0.8 | |||
| Prostate + SV + LN | 0.53 | 0.11–2.67 | 0.4 | |||
| PSA nadir (per 1 ng/ml) | 1.08 | 0.67–1.72 | 0.8 | |||
| PSA at CholPET scan (per 1 ng/dl) | 1.29 | 1.08–1.53 | <0.01 | |||
| ΔPSAa (per 1 ng/ml) | 1.30 | 1.08–1.56 | <0.01 | 1.30 | 1.08–1.57 | <0.01 |
| PSA doubling time (per mo) | 1.00 | 0.99–1.01 | 0.8 | |||
| PSA velocity (per 1 ng/ml/yr) | 1.48 | 1.01–2.18 | 0.04 | |||
BT = brachytherapy; CholPET = C-11 choline positron emission tomography/computed tomography; CI = confidence interval; EBRT = external beam radiotherapy; HT = hormone therapy; LN = lymph node; NCCN = national comprehensive cancer network; OR = odds ratio; PSA = prostate-specific antigen; ref. = reference; RT = radiation therapy; SV = seminal vesicle.
Difference between PSA at C11 choline PET/CT and PSA nadir.
Of the 161 patients with a positive CholPET, 95 (59.0%) had histologic confirmation at a median of 80 (IQR: 48–112) mo after RT (Table 3). Additionally, 111 patients underwent multi-parametric MRI, of whom 82 (76%; 95% CI: 68–84%) had findings concordant with the CholPET. In total, 100/184 (54%; 95% CI: 47–61%) patients had recurrence localized to the pelvic soft tissue, compared with 61/184 (33%; 95% CI: 26–40%) patients who had either extrapelvic metastatic and/or pelvic osseous disease. Table 4 summarizes the clinical characteristics associated with the pattern of recurrent disease.
Table 3.
Characterization of positive C-11 choline positron emission tomography/computed tomography (PET/CT) scans
| N or median | % or IQRa | |
|---|---|---|
| Total | 161 | |
| Histologic confirmation | 95 | 59 |
| Sites of confirmationb | ||
| Prostate | 71 | 44 |
| Seminal vesical | 5 | 3 |
| Pelvic lymph node(s) | 15 | 9 |
| Distant | 10 | 6 |
| Retroperitoneal node(s) | 4 | 2 |
| Mediastinal node(s) | 3 | 2 |
| Osseous site(s) | 1 | 1 |
| Other | 2 | 1 |
| Method of confirmation | ||
| Transrectal biopsy | 64 | 38 |
| Surgical excision | 16 | 10 |
| CT-guided biopsy | 9 | 5 |
| Transbronchial biopsy | 4 | 2 |
| Time to biopsy (mo) | 80 | 48–112 |
| Multi-parametric MRI positive (n =108) | 82 | 76 |
| No. of lesions on CholPET | ||
| Median (IQR) | 1 | 1–2 |
| 1 | 83 | 52 |
| 2 | 39 | 25 |
| 3 | 23 | 14 |
| 4 | 7 | 4 |
| 5+ | 7 | 4 |
| Locations of lesions | ||
| Prostate | 105 | 66 |
| Seminal vesical | 17 | 11 |
| Perirectal lymph nodes | 3 | 2 |
| Presacral lymph nodes | 2 | 1 |
| Pelvic lymph nodes | 47 | 29 |
| Common iliac lymph nodes | 23 | 14 |
| Retroperitoneal lymph nodes | 29 | 18 |
| Distant lymph nodes | 9 | 6 |
| Pelvic bones | 14 | 9 |
| Vertebral column | 10 | 6 |
| Ribs, sternum, scapula | 6 | 4 |
| Appendical skeleton | 2 | 1 |
| Lung | 2 | 1 |
| Skull | 1 | 1 |
| Patterns of recurrence | ||
| Prostate/seminal vesical only | 74 | 46 |
| Pelvic soft tissue onlyc | 100 | 62 |
| Extrapelvic | 61 | 38 |
| Lymphotropic | 29 | 18 |
| Osseous | 6 | 4 |
CholPET = C-11 choline PET/CT; IQR = interquartile range; MRI = magnetic resonance imaging.
Percentage represents percentage of those patients with a positive scan (n =167).
Sum of sites is greater than 95 as some patients had multiple sites confirmed.
Pelvic soft tissue only = prostate, seminal vesical, perirectal, presacral, or pelvic lymph node only.
Table 4.
Clinical and biochemical features associated with pelvic soft tissue versus extrapelvic and/or osseous recurrence as identified by C-11 choline positron emission tomography/computed tomography (CholPET) scans
| Pelvic soft tissue | Extrapelvic/osseous | p value | |
|---|---|---|---|
| Total | 100 | 61 | |
| Median age at RT (IQR) | 65 (60–70) | 65 (60–70) | 0.6 |
| Median PSA at diagnosis, ng/ml (IQR) | 7.5 (5.7–10.0) | 8.9 (5.8–14.0) | 0.1 |
| Total Gleason score (IQR) | 7 (6–7) | 7 (7–8) | <0.01 |
| Primary Gleason score (IQR) | 3 (3–4) | 4 (3–4) | <0.01 |
| Gleason score (%) | |||
| ≤6 | 34 (35) | 13 (22) | <0.01 |
| 7 | 47 (49) | 24 (41) | |
| 8–10 | 15 (16) | 22 (37) | |
| Grade group (%) | |||
| 1 (≤3+3) | 35 (37) | 13 (22) | <0.01 |
| 2 (3+4) | 33 (34) | 11 (19) | |
| 3 (4+3) | 13 (14) | 13 (22) | |
| 4 (8) | 9 (9) | 10 (17) | |
| 5 (9 and 10) | 6 (6) | 12 (20) | |
| Clinical stage (%) | |||
| T1c | 34 (52) | 16 (41) | 0.04 |
| T2a–c | 28 (43) | 15 (38) | |
| T3a–b | 3 (5) | 8 (21) | |
| NCCN risk group (%) | |||
| Low risk | 25 (28) | 8 (14) | 0.03 |
| Intermediate risk | 45 (49) | 25 (44) | |
| High/very high risk | 21 (23) | 24 (42) | |
| Type of therapy (%) | |||
| EBRT | 57 (57) | 35 (58) | 1.0 |
| BT or combination with BT | 43 (43) | 25 (42) | |
| HT during RT (%) | 26 (26) | 23 (38) | 0.7 |
| Target (%) | |||
| Prostate | 84 (87) | 41 (73) | 0.1 |
| Prostate + SV | 7 (7) | 10 (18) | |
| Prostate + SV + pelvic lymph nodes | 6 (6) | 5 (9) | |
| PSA nadir, ng/ml (IQR) | 0.5 (0.3–1.2) | 0.5 (0.1–1.2) | 0.6 |
| PSA at CholPET scan, ng/ml (IQR) | 5.3 (3.6–8.2) | 8.0 (4.7–12.4) | <0.01 |
| ΔPSAa, ng/ml (IQR) | 4.5 (3.0–7.4) | 6.9 (3.9–10.7) | <0.01 |
| Time to CholPET from RT, mo (IQR) | 77 (49–116) | 50 (26–90) | <0.01 |
| Time to CholPET from nadir, mo (IQR) | 51 (29–79) | 32 (15–67) | <0.01 |
| PSA doubling time, mo (IQR) | 13 (8–26) | 6 (4–13) | <0.01 |
| PSA velocity, ng/ml/yr (IQR) | 1.2 (0.5–2.2) | 2.7 (1.0–5.6) | <0.01 |
BT = brachytherapy; EBRT = external beam radiation therapy; HT = hormonal therapy; IQR = interquartile range; NCCN = national comprehensive cancer network; PSA = prostate-specific antigen; RT = radiation therapy; SV = seminal vesical.
Difference between PSA at C11 choline PET/CT and PSA nadir.
Twenty-one (11%) patients underwent CholPET prior to meeting Phoenix criteria for BCR, of whom 15 had an identified site of recurrence (Supplementary Table 2). Median PSA at CholPET in the 21 patients was 1.9 ng/ml, with a median ΔPSA of 1.4 (IQR: 0.6–1.7) ng/ml. In total, nine of the 15 recurrences were histologically confirmed, at a median of 82 (IQR: 60–90) mo from RT. Notably, 11 of the 15 had recurrences localized to the pelvis, with an additional four having extrapelvic metastatic disease. No clinical feature was associated with the pattern of recurrence in this subgroup of patients.
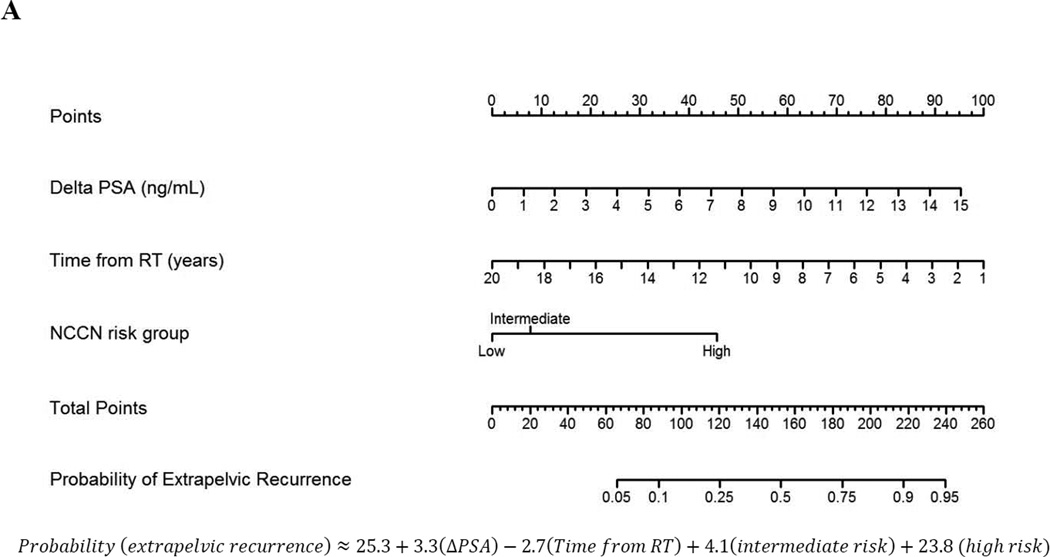
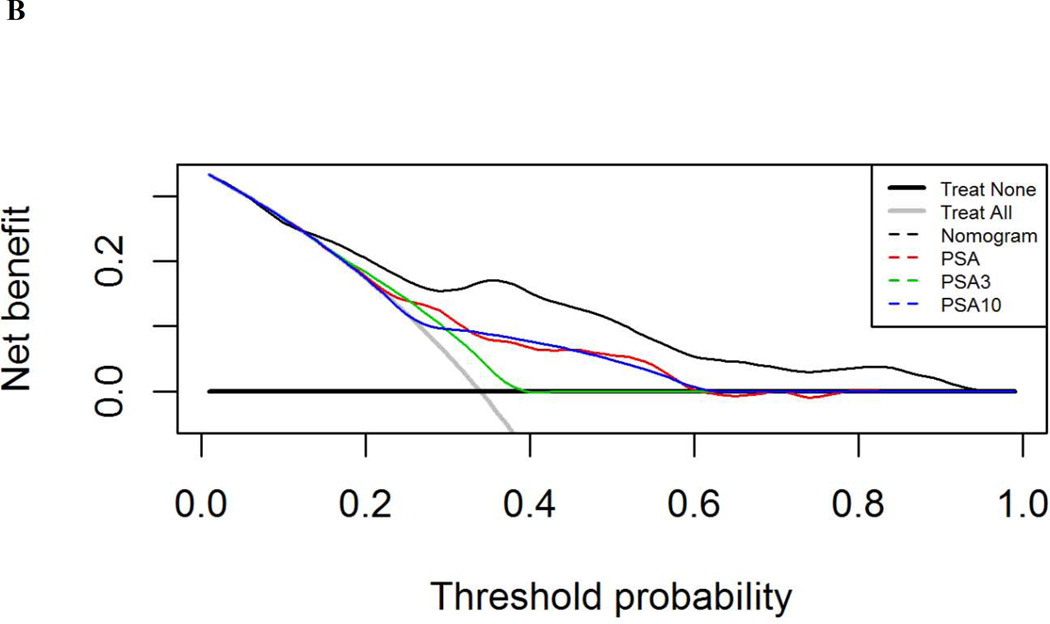
On multivariable logistic regression, ΔPSA and NCCN risk group were associated with extrapelvic recurrence (Table 5). A nomogram was generated (Fig. 1A, Supplementary Fig. 1) from this model, with a c-index of 0.79 (95% CI: 0.72–0.86). When restricted to patients with histologically confirmed recurrence, model performance improved, with a c-index of 0.84. Using a decision curve analysis, the model was superior to PSA thresholds and assuming all patients had extrapelvic disease at evaluation beginning at a threshold probability of 13% (Fig. 1B).
Table 5.
Multivariable logistic regression model predicting extrapelvic recurrence
| c-index: 0.79 (95%: CI 0.72–0.86) | OR | 95% CI | p value |
|---|---|---|---|
| ΔPSAa (ng/ml) | 1.24 | 1.13–1.38 | <0.01 |
| Time from RT (yr) | 0.84 | 0.73–0.94 | <0.01 |
| NCCN risk group (ref. low risk) | |||
| Intermediate risk | 1.30 | 0.47–3.80 | 0.6 |
| High risk | 4.71 | 1.58–15.41 | <0.01 |
CI = confidence interval; NCCN: national comprehensive cancer network; OR = odds ratio; PSA: prostate-specific antigen.
Difference between PSA at C-11 choline positron emission tomography/computed tomography and PSA nadir.
Fig. 1.
Nomogram analysis (A) calculating the probability of identifying extrapelvic and/or osseous disease on C-11 choline positron emission tomography/computed tomography at time of evaluation with a rising prostate-specific antigen (PSA) after primary radiotherapy (RT). Points are assigned by drawing a vertical line from each variable (ΔPSA, time from RT, and National Comprehensive Cancer Network [NCCN] risk group) to the “Points” line and adding the cumulative points. A line is then drawn down from the “Total Points” line at the corresponding point value. Where this line intersects with the “Probability of Extrapelvic Recurrence” line corresponds to the estimated probability of extrapelvic disease. (B) Decision-curve analysis comparing the net-benefit of using the nomogram (black dashed line) depicted above to the strategy of using PSA as a continuous predictor (red dashed line), a PSA cut-off of 3 ng/ml (green dashed line), or a PSA cut-off of 10 ng/ml (blue dashed line)
4. Discussion
Herein we report our experience with CholPET for the evaluation of patients with a rising PSA after primary RT for CaP. We found that CholPET had a high sensitivity (95%) and specificity (73%) for the detection of recurrence, with ΔPSA and NCCN risk groups associated with identification of a recurrence site at CholPET, which may aid in defining who should be referred for advanced imaging with CholPET. Furthermore, we identified the anatomic patterns of recurrence after RT, noting that most patients (54%) in our series recurred within pelvic soft tissue. A nomogram based on information routinely available at evaluation prior to CholPET was generated to aid in the localization of recurrence site with a c-index of 0.79.
Prior research has documented similar diagnostic characteristics for CholPET as our findings reported here. In a meta-analysis evaluating the role of choline (C11 and F18) radio-isotopes in PET/CT for prostate cancer, the pooled sensitivity and specificity when evaluating recurrence after a primary treatment was 85% (95% CI: 79–89%) and 88% (95% CI: 73–95%), respectively [14]. Similarly, in Giovacchini et al’s [10] analysis of 358 patients undergoing CholPET after prostatectomy, the detection rate was 82% at a PSA >3 ng/ml, compared with a detection rate of 93% using the same cut-off in our cohort here. However, a previous report from our institution assessing all patients with BCR after a primary curative intervention for CaP, found different detection rates within PSA thresholds (Supplementary Fig. 2), likely a result of our inclusion of only postprimary RT patients [9].
Additionally, our finding that most patients recurred within the pelvis is corroborated by a prior report on 474 patients with a clinically detectable recurrence after RT as evaluated using standard imaging, which identified 55% of patients with recurrence in the pelvis at 8-yr post-RT [15]. However, our finding of a similar frequency for the detection of local recurrence occurred at only 6-yr post-RT, an important distinction given that anatomic patterns of recurrence are associated with survival [15], and theoretically intervention prior to the development of more aggressive anatomic phenotypes may improve patient outcome. Conversely, the largest reported series of CholPET for the evaluation of biochemically recurrent CaP—after any prior treatment—found that local recurrence was detected in only 22.1% of their 4426 CholPETs performed, with an overall detection rate of only 52.8% [16]. This discrepancy versus the 87% detection rate in our series, may be partially explained by our higher dosages of C11 choline (555–740 MBq) compared with those used in that series (370–555 MBq), which may yield a higher photon flux and an increased signal-to-noise ratio.
Our results have some important implications. Currently, ADT represents the primary management strategy for patients with a rising PSA after RT, with over 90% of such patients receiving ADT in the CaPSURE database [17]. This approach is predicated on the assumption that recurrence after RT is systemic, and patients may be subjected to lifelong continuous or intermittent ADT, many of whom subsequently fail [13,17]. Furthermore, ADT can adversely affect quality of life [18], and is associated with the risk of osteoporosis and cardiovascular mortality [19]. As such, efforts to minimize the utilization of ADT and treat salvageable recurrences with definitive therapy are important to consider. While biochemical thresholds have been suggested—such as 3 ng/ml as used in the landmark intermittent ADT trial [13] or 10 ng/ml as identified by Canadian urologists [12]—to identify candidates for initiation of ADT, PSA cutoff points alone fail to localize disease. Specifically, in our cohort, 83 of 149 (56%) patients evaluated at a PSA ≥3 ng/ml had potentially salvageable recurrences localized to the pelvis.
In order to inform decision making prior to implementing ADT, here we report a nomogram for determining the likelihood of finding extrapelvic recurrence with CholPET. This nomogram is based solely on information available at the time of evaluation and without requiring repeated PSA measures to ascertain biochemical kinetics [20]. Our finding of improved discrimination when utilizing absolute PSA values as opposed to kinetics is similar to the analysis reported by Eiber and colleagues [21] with 68Ga prostate-specific membrane antigen scanning, whereby PSA velocity and PSA doubling time were not associated with diagnostic PET scans. Furthermore, using a decision curve analysis, this nomogram performed better than using PSA threshold metrics alone (Fig. 1C) beginning at a threshold probability of 13%, below which all models performed similarly. This is an important point, as most patients and providers would require greater than 13% probability of extrapelvic disease prior to initiating systemic therapy when effective alternative therapies exist. Furthermore, using a net reduction approach, the advantage of using our model over PSA thresholds alone is equivalent to a reduction in the use of ADT in between one and 16 patients per 100 patients presenting with a rising PSA following RT (Supplementary Fig. 3) if salvage is to be considered. These data and analyses demonstrate a high sensitivity of CholPET in the identification of extrapelvic recurrence following primary RT. Thus, through the use of this nomogram, it may be feasible to inform decisions on which patients should undergo further pelvic imaging and/or confirmatory biopsy if local salvage treatments are being considered [14] and conversely to initiate upfront ADT for those with a high probability of extrapelvic disease.
We acknowledge certain limitations in our findings. This study includes data from a heavily selected population of patients, and as such the generalizability of the diagnostic characteristics of CholPET is limited. While our study includes a relatively high rate of biopsy confirmation, similar to all studies of this type we included patients without histologic confirmation of recurrence, using previously defined clinical measures [10] which may have influenced our findings. In an attempt to control for any bias introduced through the inclusion of patients without histologically confirmed recurrences, a separate subgroup analysis of those patients with histologic confirmation was performed. This resulted in an increase in the performance of our model (c-index 0.84 vs 0.79). Furthermore, we acknowledge there are other advanced imaging modalities available—such as 68Ga prostate-specific membrane antigen scanning and 18-F fluorocyclobutane-1-carboxylic acid PET/CT [22–25]—which were not assessed here. Nevertheless, we have found CholPET to be very useful in identifying patients eligible for local or regional salvage treatments, or those to be considered for systemic therapy only.
We limited our data analysis to disease localization only, with local soft tissue recurrence in the pelvis being used as a surrogate for potentially salvageable disease, despite not all patients undergoing surgical, ablative, or radiation salvage. While not all patients’ local recurrence in our sample met the recommended criteria for local salvage, as has been previously described [5], it has been suggested that for trials assessing salvage ablative therapy, the only firm criteria for entry is histologic confirmation of local relapse [26]—a feature which a majority of patients in our series met compared to existing CholPET studies [10,16]. Additionally, we are not advocating specific salvage modalities, as choice of salvage therapy is dependent on prior radiation fields and patient/provider preference. Rather, our assertion is that there is growing evidence of the role of salvage therapy for select patients with recurrent CaP [27,28]. Furthermore, we were unable to include survival data as it was beyond the scope of the present analysis and limited by the short follow-up duration. Finally, while we have attempted to comprehensively evaluate all potential covariates which may influence disease recurrence and localization, the tertiary nature of our practice limited the ascertainment of certain measures, such as duration of hormonal therapy and subsequent testosterone recovery, radiation fields, and total radiation dosimetry. Despite these limitations, we have reported here, what is to our knowledge, the largest single center experience with advanced imaging in the evaluation and detailed analysis of patients with a rising PSA following primary RT.
5. Conclusions
In patients with a rising PSA after RT for CaP, C11 Choline PET/CT identified a site of recurrence in a large majority of patients, with local pelvic recurrence representing the most common site. A predictive nomogram for the identification of extrapelvic recurrence, using the difference between nadir PSA and PSA at evaluation, time from completion of RT to evaluation, and NCCN risk group was developed, which after additional validation may prove useful in clinical decision making. Based on the findings of this study, C11 choline PET/CT is a useful means to enhance staging and treatment selection in primary RT patients experiencing a post-treatment rising PSA who are being evaluated for local salvage and/or systemic therapies.
Supplementary Material
Take Home Message.
Using C-11 choline positron emission tomography/computed tomography we were able to identify the sites of recurrence in most patients presenting with a rising prostate-specific antigen following primary radiotherapy. Using these data we generated a predictive model for the identification of recurrence outside of the pelvis which, pending validation, may aid in the treatment of patients with a rising prostate-specific antigen following radiotherapy.
Acknowledgments
Funding/Support and role of the sponsor: This study was made possible in part by support from the National Institute of Health/National Cancer Institute, grant 1R01 CA200551 (Sean S. Park, Kenneth R. Olivier, Val J. Lowe, and Eugene D. Kwon). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. Additionally, this work was made possible in part through the generous support of the Richard M. Schulze Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Brian J. Davis had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Parker, Davis, Park, Choo, Kwon.
Acquisition of data: Parker.
Analysis and interpretation of data: Parker, Moriera, Harmsen, Davis.
Drafting of the manuscript: Parker, Davis.
Critical revision of the manuscript for important intellectual content: Parker, Davis, Park, Olivier, Choo, Nathan, Lowe, Welch, Evans, Harmsen, Zaid, Sobol, Moriera, Haloi, Tollefson, Gettman, Boorjian, Mynderse, Karnes, Kwon.
Statistical analysis: Parker, Harmsen, Moriera.
Obtaining funding: Park, Olivier, Lowe, Kwon.
Administrative, technical, or material support: None.
Supervision: Davis.
Other: None.
Financial disclosures: Brian J. Davis certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Davis serves as a consultant to UpToDate Inc., owns stock interest in Pfizer Inc., receives research funding from Takeda UK LLC, and receives Honoria from the American Society of Radiation Oncology and the American College of Radiation Oncology. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences, and receives research support from GE Healthcare, Seimens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (HIA and NCI). Boorjian serves on the scientific advisory board of Astellas-Medivation. Mynderse (and Mayo Clinic) have an Institutional Master Research Collaborative Agreement with Phillips Healthcare/Invivo Corporation which involves software and hardware loans for the treatment of prostate cancer. Karnes receives research funding and royalties (intellectual property rights) from Genome Dx Inc.
References
- 1.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Denham JW, Steigler A, Wilcox C, et al. Why are pretreatment prostate-specific antigen levels and biochemical recurrence poor predictors of prostate cancer survival? Cancer. 2009;115:4477–4487. doi: 10.1002/cncr.24484. [DOI] [PubMed] [Google Scholar]
- 3.Morris LM, Izard MA, Wan WY. Does prostate-specific antigen nadir predict longer-term outcomes of prostate cancer after neoadjuvant and adjuvant androgen deprivation therapy in conjunction with brachytherapy? Brachytherapy. 2015;14:322–328. doi: 10.1016/j.brachy.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Uchio E, Aslan M, Ko J, Wells CK, Radhakrishnan K, Concato J. Velocity and doubling time of prostate-specific antigen: Mathematics can matter. J Investig Med. 2016;64:400–404. doi: 10.1136/jim-2015-000008. [DOI] [PubMed] [Google Scholar]
- 5.Chade DC, Eastham J, Graefen M, et al. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: A systematic review of the literature. Eur Urol. 2012;61:961–971. doi: 10.1016/j.eururo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Crehange G, Roach M, 3rd, Martin E, et al. Salvage reirradiation for locoregional failure after radiation therapy for prostate cancer: Who, when, where and how? Cancer Radiother. 2014;18:524–534. doi: 10.1016/j.canrad.2014.07.153. [DOI] [PubMed] [Google Scholar]
- 7.Greco F, Cadeddu JA, Gill IS, et al. Current perspectives in the use of molecular imaging to target surgical treatments for genitourinary cancers. Eur Urol. 2014;65:947–964. doi: 10.1016/j.eururo.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell CR, Lowe VJ, Rangel LJ, Hung JC, Kwon ED, Karnes RJ. Operational characteristics of (11)c-choline positron emission tomography/computerized tomography for prostate cancer with biochemical recurrence after initial treatment. J Urol. 2013;189:1308–1313. doi: 10.1016/j.juro.2012.10.069. [DOI] [PubMed] [Google Scholar]
- 10.Giovacchini G, Picchio M, Coradeschi E, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging. 2010;37:301–309. doi: 10.1007/s00259-009-1253-3. [DOI] [PubMed] [Google Scholar]
- 11.Vickers AJ, Elkin EB. Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loblaw DA, Pickles T, Cheung PC, Lukka H, Faria S, Klotz L. Hormone use after radiotherapy failure: A survey of Canadian uro-oncology specialists. Can Urol Assoc J. 2009;3:460–464. doi: 10.5489/cuaj.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367:895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The role of 11C–choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64:106–117. doi: 10.1016/j.eururo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Zumsteg ZS, Spratt DE, Romesser PB, et al. Anatomical patterns of recurrence following biochemical relapse in the dose escalation era of external beam radiotherapy for prostate cancer. J Urol. 2015;194:1624–1630. doi: 10.1016/j.juro.2015.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graziani T, Ceci F, Castellucci P, et al. C-Choline PET/CT for restaging prostate cancer. Results from 4426 scans in a single-centre patient series. Eur J Nucl Med Mol Imaging. In press doi: 10.1007/s00259-016-3428-z. http://dx.doi.org/10.1007/s00259-016-3428-z. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal PK, Sadetsky N, Konety BR, Resnick MI, Carroll PR. Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 18.Alibhai SM, Breunis H, Timilshina N, et al. Long-term impact of androgen-deprivation therapy on physical function and quality of life. Cancer. 2015;121:2350–2357. doi: 10.1002/cncr.29355. [DOI] [PubMed] [Google Scholar]
- 19.Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–2399. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- 20.Zelefsky MJ, Ben-Porat L, Scher HI, et al. Outcome predictors for the increasing PSA state after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol. 2005;23:826–831. doi: 10.1200/JCO.2005.02.111. [DOI] [PubMed] [Google Scholar]
- 21.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 22.Evangelista L, Briganti A, Fanti S, et al. New clinical indications for F/C-choline, new tracers for positron emission tomography and a promising hybrid device for prostate cancer staging: A systematic review of the literature. Eur Urol. 2016;70:161–175. doi: 10.1016/j.eururo.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 23.Koo PJ, David Crawford E. (1)(8)F-NaF PET/CT and (1)(1)C-Choline PET/CT for the initial detection of metastatic disease in prostate cancer: Overview and potential utilization. Oncology (Williston Park) 2014;28:1057–1062. 1064–1065. [PubMed] [Google Scholar]
- 24.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 25.Nanni C, Zanoni L, Pultrone C, et al. F-FACBC (anti1-amino-3-F-fluorocyclobutane-1-carboxylic acid) versus C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging. 2016;43:1601–1610. doi: 10.1007/s00259-016-3329-1. [DOI] [PubMed] [Google Scholar]
- 26.van den Bos W, Muller BG, de Bruin DM, et al. Salvage ablative therapy in prostate cancer: international multidisciplinary consensus on trial design. Urol Oncol. 2015;33:29. doi: 10.1016/j.urolonc.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ploussard G, Almeras C, Briganti A, et al. Management of node only recurrence after primary local treatment for prostate cancer: A systematic review of the literature. J Urol. 2015;194:983–988. doi: 10.1016/j.juro.2015.04.103. [DOI] [PubMed] [Google Scholar]
- 28.Abdollah F, Briganti A, Montorsi F, et al. Contemporary role of salvage lymphadenectomy in patients with recurrence following radical prostatectomy. Eur Urol. 2015;67:839–849. doi: 10.1016/j.eururo.2014.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.