Abstract
Chronic anal fissure is a linear ulcer in the anal canal that has not cicatrized for more than 8–12 weeks of treatment. Most anal fissures are idiopathic and are located in the posterior midline. Squamous cell carcinoma of the anus commonly presents as bleeding and anal pain. It may also present as a mass, nonhealing ulcer, itching, discharge, fecal incontinence and fistulae. Not uncommonly, small and early cancers are misdiagnosed as benign anorectal disorders like anal fissures or hemorrhoids. The clinical suspicion of squamous cell carcinoma of the anus is of paramount importance in patients with nonhealing anal fissures, fissures in atypical positions or with indurated or ulcerated anal tags and in patients with risk factors for the development of anal squamous intraepithelial lesions that are precursors of invasive anal squamous cell carcinoma. The authors present 3 cases of squamous cell carcinoma of the anus initially misdiagnosed as benign chronic anal fissure.
Keywords: Anal canal, Anus neoplasms, Chronic disease, Fissure in ano
Resumo
A fissura anal crónica é uma úlcera linear presente no canal anal que não cicatriza após 8 a 12 semanas de tratamento. A maioria das fissuras anais são idiopáticas e localizadas na linha média posterior. O carcinoma epidermoide do canal anal apresenta-se frequentemente com proctalgia e hemorragia, podendo também manifestar-se como uma úlcera que não cicatriza, corrimento anal, incontinência fecal e fístula. Não raramente, os carcinomas pequenos e em estádios precoces podem ser diagnosticados incorretamente como patologia anorrectal benigna, como fissuras ou hemorróidas. A suspeição clínica de carcinoma epidermoide do canal anal é de enorme importância em fissuras anais que não cicatrizam, localizadas em posições atípicas, com marisca anal ulcerada ou endurecida e em doentes com fatores de risco para lesões escamosas intraepiteliais anais que são percursoras do carcinoma epidermoide anal. Os autores apresentam três casos de carcinoma epidermoide do canal anal inicialmente diagnosticados incorretamente como fissuras anais benignas.
Palavras Chave: Canal anal, Neoplasias do ânus, Doença crónica, Fissura anal
Introduction
Chronic anal fissure is a linear ulcer in the anal canal that has not cicatrized for more than 8–12 weeks of treatment [1]. The clinical hallmarks are postdefecatory pain and bleeding [1]. Most anal fissures are idiopathic and are located in the posterior midline. However, some fissures may be associated with systemic diseases, infections or malignancy [1, 2].
Squamous cell carcinoma of the anus (SCCA) commonly presents with bleeding and anal pain [3]. It may also present as a mass, itching, discharge, fecal incontinence, nonhealing ulcer, fissures in atypical positions, and fistulae [4]. Not uncommonly, small and early cancers are misdiagnosed as benign anorectal disorders like anal fissures or hemorrhoids [4]. The definitive diagnosis of anal cancer is made histologically, after biopsy of the lesions [3, 4].
The clinical suspicion of SCCA is of paramount importance in patients with nonhealing anal fissures, fissures in atypical positions or with indurated or ulcerated anal tags and in patients with risk factors for the development of anal squamous intraepithelial lesions that are precursors of invasive anal squamous cell carcinoma.
The authors present 3 cases of SCCA initially misdiagnosed as benign anal fissures that were referred to a proctology unit in a gastroenterologic department. Furthermore, the authors discuss the differential diagnosis of chronic anal fissures and suggest an algorithm for its evaluation and management.
Case Reports
Case 1
A 67-year-old woman presented with postdefecatory anal pain and rectal bleeding for almost 1 year. The primary care physician diagnosed an anal fissure and prescribed conservative measures (laxatives, anti-inflammatory/analgesics and healing ointments), but the symptoms did not improve. She was not a smoker and denied gynecologic diseases. She had a previous diagnosis of constipation and anal warts treated with cryotherapy. Digital rectal examination detected a depressed hard area in the anal canal with blood on the glove, despite a normal anus appearance. Anoscopy showed an ulcer in the anterior anal canal and biopsies were taken (Fig. 1). Histologic examination established the diagnosis of SCCA. Human immunodeficiency virus (HIV) serology was negative. Magnetic resonance imaging (MRI) and computerized tomography (CT) excluded nodal and distant metastasis and the patient was staged as T4N0M0. The patient started chemoradiation with no evidence of residual or recurrent disease after 24 months of follow-up. Anal function was preserved.
Fig. 1.
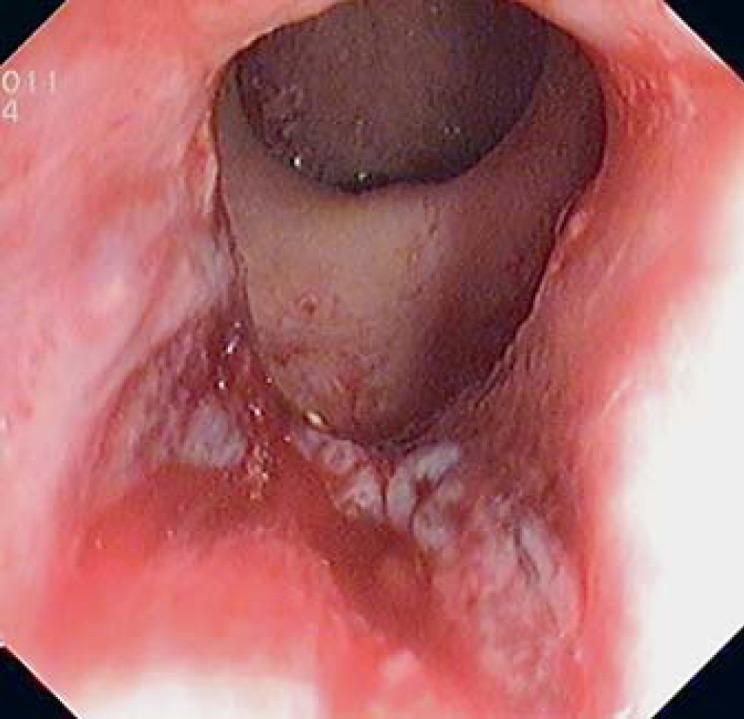
Anoscopy was performed with the patient lying in the knee-chest position and showed an irregular ulcer that occupied 25% of anal canal circumference.
Case 2
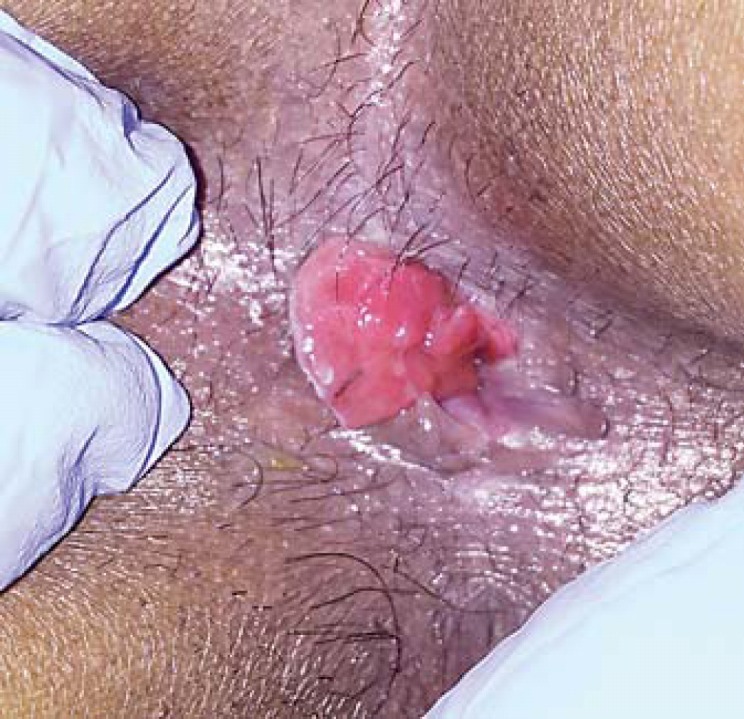
A 52-year-old male was referred with intense and progressive anal pain that precluded him from remaining in the sitting position and limited his mobility, with a major impact on his quality of life. He had an 18-month history of anal fissure which did not respond to conservative measures (laxatives, anti-inflammatory, topical anesthetics and healing ointments). He was a smoker with no past medical history and denied receptive anal intercourse. He had refused colonoscopy in the past. The anal observation was only possible under general anesthesia and revealed an ulcer in the posterior anal margin with hard and raised edges (Fig. 2). Biopsies were taken and histology confirmed the diagnosis of SCCA. HIV serology was negative. The tumor was classified as T2N0M0 after MRI and CT. The patient started chemoradiation with complete response and symptom remission. No evidence of recurrent disease was reported at 12 months of follow-up.
Fig. 2.
Observation was made with the patient in the left lateral position. An excavated ulcer of 22 mm was identified in the posterior anal margin.
Case 3
A 38-year-old woman presented with an 8-month history of intermittent anal pain, rectal bleeding and anal mass. She did not seek medical help. Her past medical history included Fanconi anemia and tobacco use. She had regular gynecologic surveillance and past cervical abnormal cytology that regressed without treatment.
Anal inspection and palpation revealed an anal fissure located in the right lateral position (9 h, with the patient lying in the knee-chest position) with a painful, indurated and ulcerated anal tag and anal sphincter hypertonicity (Fig. 3). HIV serology was negative. Treatment with topic diltiazem was started. Biopsies of the anal tag were taken but the result was inconclusive and an excisional biopsy was performed. Histology analysis demonstrated a poorly differentiated SCCA with positive margins. The patient refused chemoradiation because she was planning to get pregnant. There was no evidence of nodal or distant metastasis and the patient was submitted to local re-excision. R0 resection could not be achieved. Local recurrent disease was observed at 4 months of follow-up and was confirmed with pelvic MRI, along with nodal metastasis. Abdominal tomography excluded distant metastasis (T2N1M0). The patient was then followed in another center, where radiotherapy was started, with complete response and without recurrence of the tumor at 8 months of follow-up.
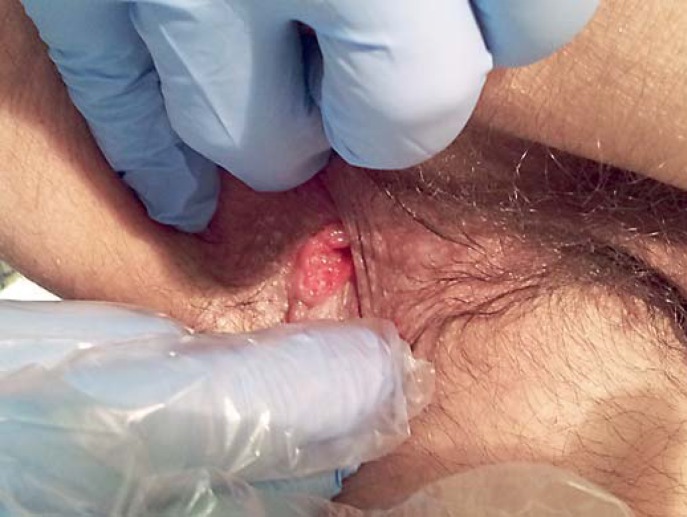
Fig. 3.
Anal inspection and palpation was performed with the patient lying in the knee-chest position and showed a chronic fissure located in the right lateral position on the anal margin with a painful, indurated and ulcerated perianal skin tag.
Discussion
Most anal fissures are idiopathic and are located in the posterior midline, but they can also occur in the anterior midline [1]. Fissures in lateral positions should raise suspicion for associated diseases such as Crohn's disease, venereal infection, trauma, tuberculosis, HIV infection, dermatologic disorders, chemotherapy and malignancy [1, 2]. Table 1 enumerates the differential diagnosis that should be considered in patients with fissures in lateral positions or nonhealing/atypical chronic anal fissures.
Table 1.
| Chronic idiopathic anal fissure |
| Sexually transmitted disease: herpes, syphilis, gonorrhea, granuloma inguinale, chancroid, chlamydia, candidiasis |
| Other infections: tuberculosis, Streptococcus spp. |
| Ulcerative colitis or Crohn's disease |
| Malignancy: squamous cell carcinoma, adenocarcinoma, melanoma or other skin tumors, Paget's disease, histiocytosis, anal metastasis, leukemia |
| Dermatosis: anal eczema, Lichen sclerosus, Lichen planus, Behçet disease |
| Chemotherapy |
| Idiopathic anogenital ulceration in HIV |
| Trauma |
Anal cancer involves the anus, anal canal and/or anorectum. Most primary cancers of the anal canal are of squamous cell histology [3]. Other less common anal cancers include adenocarcinomas, small cell carcinoma, undifferentiated cancers, and melanomas [3]. SCCA is a rare disease accounting for 1–2% of digestive tract tumors [4]. The annual incidence is 1 in 100,000, higher in women and is increasing. Five-year survival varies between 44 and 66% [4].
The anal canal as well as the genital canal may display normal metaplastic change and abnormal dysplastic change related to infection with human papillomavirus (HPV) [4, 5]. Anal squamous intraepithelial lesions are HPV-associated lesions and may be further classified as low-grade anal intraepithelial neoplasia (grade 1, AIN1) and high-grade anal intraepithelial neoplasia (grades 2 and 3, AIN2 and AIN3) [4, 5]. Lesions AIN1 may spontaneously regress or progress to AIN2 or AIN3. AIN2 and AIN3 rarely regress, and are considered to be the true precursor of invasive SCCA [4, 5]. In these lesions, ablative treatment should be considered.
The risk factors for the development of anal squamous intraepithelial lesions include anal HPV infection, high-risk sexual behavior (receptive anal intercourse), HIV infection (especially with lower CD4 levels), history of genital warts, current cigarette smoking, history of cervical cancer, vulvar cancer, high-grade cervical intraepithelial neoplasia or vulvar intraepithelial neoplasia, and iatrogenic immunosuppression, such as following solid organ transplantation [6, 7, 8, 9, 10, 11]. It is appropriate to screen populations at increased risk of anal cancer: HIV-positive men and women, men who have sex with men, iatrogenic immunosuppression (solid organ transplant recipients or long-term oral corticosteroids), women with a history of high-grade cervical, vulvar, vaginal dysplasia or cancer and individuals with a history of anal warts [4, 12]. However, the advantage of screening in these high-risk populations is yet to be demonstrated [4]. The goal of screening is to identify individuals with an abnormal cytology who should undergo high-resolution anoscopy to assess the anal canal and map areas with visual markers consistent with AIN2 and AIN3 by biopsy and histopathologic evaluation [4, 5, 12]. In lesions AIN2 and AIN3, ablative treatment should be considered to prevent SCCA.
Risk factors were not always present or were omitted by the patients. The past history of anal warts in patient 1 raises the suspicion of HPV infection. Patient 2 was a smoker and denied other risk factors. Fanconi anemia, present in patient 3, is a very rare genetic disease that was associated with a high risk of cancer, including SCCA [13]. Additionally, patient 3 was a smoker and has a previous cervical abnormality, which could account for an increased SCCA risk. In patients with risk factors for the development of anal squamous intraepithelial lesions, screening was recommended due to the increased risk of anal cancer. However, no screening of anal squamous intraepithelial lesions was performed in these 3 patients.
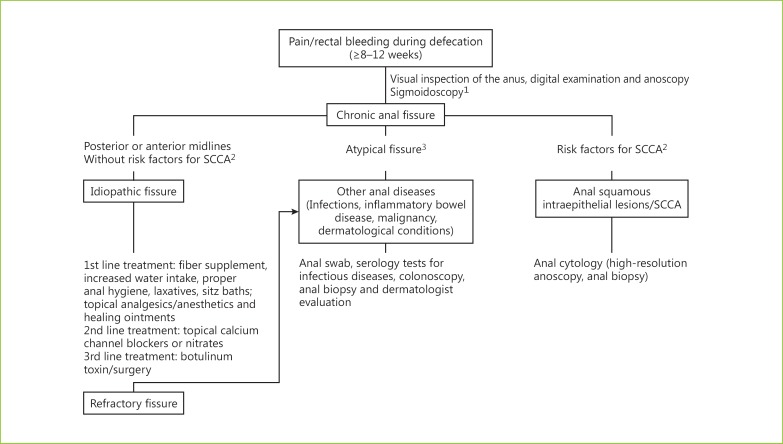
The clinical presentation of SCCA varies from bleeding and anal pain resembling anal fissures (patients 1 and 2) to a small perianal mass similar to an anal tag (patient 3) and, in advanced cases, extensive ulcerative and suspicious lesions. Early SCCA stages (patients 1–3) are frequently misdiagnosed and, therefore, treated as anorectal benign diseases [4]. Skin tags are frequent in patients with hemorrhoids and usually are not submitted to histologic analysis. Biopsies could be performed if they were ulcerated, tender or indurated. The histologic examination was indicated in patients with anal fissures in atypical positions or with chronic nonhealing anal fissures [2]. Rigid anoscopy under anesthesia may be appropriate to facilitate biopsy; however, the best approach to take biopsies of a suspicious anal fissure is not defined. In Figure 4, a suggested algorithm for the evaluation and management of chronic anal fissure is presented.
Fig. 4.
Suggested algorithm for the evaluation and management of chronic anal fissure. 1 Sigmoidoscopy to rule out other sources of bleeding. In patients over the age of 50 years or with a suggestive family history of colon cancer, a colonoscopy is recommended for the evaluation of the entire colon. 2 The risk factors for the development of SCCA include anal HPV infection, high-risk sexual behavior, HIV infection, history of genital warts, current cigarette smoking, high-grade cervical/vulvar intraepithelial neoplasia, cervical/vulvar neoplasia and iatrogenic immunosuppression. 3 Atypical fissure includes fissures in lateral positions, nonlinear/excavated/indurated ulcer in the anal canal, associated with discharges of pus or bloody fluid, or fissures associated with ulcerated and indurated skin tag.
A multidisciplinary approach is mandatory for the optimal management of SCCA [3, 4]. The primary aim of treatment is to achieve cure with locoregional control and preservation of anal function [3, 4]. Chemoradiation remains the standard of care leading to complete tumor regression in 80–90% of patients [3, 14].
SCCA is underrecognized due to the perceived rarity of the disease and because the inspection of the anal and perianal region is frequently neglected [14]. The diagnosis is delayed, not only due to medical misdiagnosis but also because in the beginning of symptoms patients defer medical attention and refuse observation (patient 3).
This article highlights the importance of anal inspection and palpation in all patients with proctologic symptoms, even in the primary care setting. The inspection of the anal and perianal region during routine endoscopic examinations, although not the gold standard for anal canal examination, can account for an early diagnosis. The clinical suspicion of SCCA may increase in patients with risk factors and is mandatory in patients with refractory or atypical chronic anal fissures.
Statement of Ethics
This study did not require informed consent nor approval by the appropriate ethics committee.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141–1157. doi: 10.1038/ajg.2014.190. (Quiz) 1058. [DOI] [PubMed] [Google Scholar]
- 2.Beaty JS, Shashidharan M. Anal fissure. Clin Colon Rectal Surg. 2016;29:30–37. doi: 10.1055/s-0035-1570390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Anal carcinoma (version 2.2016). 2016.
- 4.Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol. 2014;40:1165–1176. doi: 10.1016/j.ejso.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Darragh TM, Colgan TJ, Thomas Cox J, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32:76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 6.Palefsky JM, Holly EA, Ralston ML, et al. Anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual and bisexual men: prevalence and risk factors. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:320–326. doi: 10.1097/00042560-199804010-00005. [DOI] [PubMed] [Google Scholar]
- 7.Moscicki AB, Hills NK, Shiboski S, et al. Risk factors for abnormal anal cytology in young heterosexual women. Cancer Epidemiol Biomarkers Prev. 1999;8:173–178. [PubMed] [Google Scholar]
- 8.Palefsky JM, Holly EA, Ralston ML, Arthur SP, Hogeboom CJ, Darragh TM. Anal cytological abnormalities and anal HPV infection in men with Centers for Disease Control group IV HIV disease. Genitourin Med. 1997;73:174–180. doi: 10.1136/sti.73.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melbye M, Sprogel P. Aetiological parallel between anal cancer and cervical cancer. Lancet. 1991;338:657–659. doi: 10.1016/0140-6736(91)91233-k. [DOI] [PubMed] [Google Scholar]
- 10.Tramujas da Costa e Silva I, de Lima Ferreira LC, Santos Gimenez F, et al. High-resolution anoscopy in the diagnosis of anal cancer precursor lesions in renal graft recipients. Ann Surg Oncol. 2008;15:1470–1475. doi: 10.1245/s10434-007-9750-8. [DOI] [PubMed] [Google Scholar]
- 11.Ogunbiyi OA, Scholefield JH, Raftery AT, et al. Prevalence of anal human papillomavirus infection and intraepithelial neoplasia in renal allograft recipients. Br J Surg. 1994;81:365–367. doi: 10.1002/bjs.1800810313. [DOI] [PubMed] [Google Scholar]
- 12.Chiao EY, Giordano TP, Palefsky JM, Tyring S, El Serag H. Screening HIV-infected individuals for anal cancer precursor lesions: a systematic review. Clin Infect Dis. 2006;43:223–233. doi: 10.1086/505219. [DOI] [PubMed] [Google Scholar]
- 13.van Zeeburg HJ, Snijders PJ, Wu T, et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2008;100:1649–1653. doi: 10.1093/jnci/djn366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shridhar R, Shibata D, Chan E, Thomas CR. Anal cancer: current standards in care and recent changes in practice. CA Cancer J Clin. 2015;65:139–162. doi: 10.3322/caac.21259. [DOI] [PubMed] [Google Scholar]
- 15.Weyandt GH. Acute and chronic anal ulcers. Hautarzt. 2010;61:27–32. doi: 10.1007/s00105-009-1811-4. [DOI] [PubMed] [Google Scholar]