Abstract
Background
Kaposi sarcoma (KS) is an angioproliferative tumor caused by human herpesvirus 8 (HHV-8). Gastrointestinal (GI) involvement by KS is a rare endoscopic finding, scarcely characterized in the literature.
Objective
To characterize clinical and endoscopic features of patients with GI KS.
Methods
This is a single-center retrospective study of GI KS cases confirmed by immunohistochemistry in the last decade (2006–2015). The following variables were analyzed: demographic data; clinical data (extraintestinal involvement, symptoms, presence and stage of HIV infection, immunosuppressive therapy); endoscopic data; stage-stratified therapeutic approach; and mortality (at 3 and 6 months).
Results
Thirteen patients with GI KS were identified: 77% were men, the mean age was 55 years, and 62% of them were Native Africans. In most cases (n = 10, 77%), KS was associated with HIV. A total of 90% of the HIV patients had a CD4+ count of <200/μL (C3, CDC classification), and 80% of them had KS as the initial manifestation of HIV infection. Thirty percent of the cases had other AIDS-defining illnesses, and only 20% received antiretroviral therapy. In the remaining 3 patients (23%), KS was associated with immunosuppressive therapy. Most patients (85%) had cutaneous lesions and 15% lung involvement. In most cases (85%), the lesions were diagnosed in the upper digestive tract in asymptomatic patients (7 stomach; 2 stomach and duodenum; 2 esophagus). Colonic involvement occurred in 2 patients presenting with hematochezia. Nearly half of the patients had more than 3 endoscopic lesions and the most frequent morphologic type was polypoid/nodular (62%). Treatment was based on antiretroviral therapy or reduction of immunosuppression and in 39% of the patients on administration of doxorubicin. Survival at 3 and 6 months was 46 and 39%, respectively.
Conclusion
GI KS is mostly found in nontreated, stage 3, HIV patients, and particularly in men from areas where HHV-8 is endemic. Involvement of the upper digestive tract is often asymptomatic. The endoscopic appearance is variable and these patients have a poor prognosis.
Keywords: Gastrointestinal tract, Endoscopy, Kaposi sarcoma
Resumo
Introdução: O sarcoma de Kaposi (SK) é um tumor angioproliferativo causado pelo virus humano herpes 8 (HHV-8). O envolvimento do tracto gastrointestinal (GI) é um achado endoscópico raro e pouco caracterizado na literatura. Objectivo: Caracterizar do ponto de vista clínico e endoscópico os doentes com sarcoma de Kaposi gastrointestinal. Métodos: Estudo rectrospectivo, unicêntrico, dos casos de SK GI, confirmados por imunohistoquímica, na última década (2006–2015). Foram analisados dados demográficos, clínicos (envolvimento extraintestinal, sintomas, presença e estadio da infecção por VIH, terapêutica imunossupressora) e endoscópicos, a abordagem terapêutica e a mortalidade aos 3 e aos 6 meses. Resultados: Diagnosticados 13 doentes com SK GI: 77% género masculino; idade media 55 anos; 62% naturais de África. Na maioria dos casos (n = 10, 77%) o SK estava associado à infecção por VIH: 90% com CD4 <200/uL (C3, CDC); 80% com SK como manifestação inaugural desta infecção; 30% com outras doenças definidoras de SIDA; 20% sob terapêutica anti-retroviral. Nos outros 3 doentes (23%) o SK estava associado à imunossupressão. A maioria dos doentes (85%) apresentavam lesões cutâneas, e 15% envolvimento pulmonar. 85% apresentavam envolvimento assintomático do tubo digestivo alto (7 estômago; 2 estômago e duodeno; 2 esófago). Dois doentes apresentavam envolvimento cólico, que se manifestou por hematoquézia. Aproximadamente metade dos doentes tinham >3 lesões endoscópicas e o tipo morfológico mais frequente (62%) foi polipoide/nodular. A terapêutica baseou-se na instituição de terapêutica anti-retroviral, redução da imunossupressão e em 39% dos doentes na administração de doxirrubicina. A sobrevida aos 3 e 6 meses foi, respectivamente, de 46 e 39%. Conclusões: SK GI é mais frequente em doentes VIH+, categoria 3, não tratados, particularmente em homens de áreas onde o HHV-8 é endémico. O envolvimento do tubo digestivo alto é frequentemente assintomático. Os achados endoscópicos são variados e estes doentes têm mau prognóstico.
Palavras Chave: Tracto gastrointestinal, Endoscopia, Sarcoma de Kaposi
Introduction
Kaposi sarcoma (KS) is a low-grade angioproliferative tumor, firstly described in 1872 by the Hungarian dermatologist Moritz Kohn Kaposi [1]. However, human herpesvirus 8 (HHV-8) was only recognized as the causative agent of this neoplasm more than 100 years later [2]. Since then, the DNA of this virus has been detected in more than 95% of AIDS- and non-AIDS-related KS lesions [3]. Nevertheless, additional unidentified co-factors seem to be needed for the clinical expression of HHV-8 infection [4].
Four clinical variants of KS have been described: (1) classic KS, which typically involves the lower extremities and rarely visceral organs, usually follows a benign course, and mostly occurs in elderly men of Mediterranean, Eastern European, and Ashkenazi Jewish descent [4]. (2) African/endemic KS predominantly affects men at younger ages, and its incidence tends to increase with age [4, 5]. (3) Immunosuppression-related KS, which occurs in organ transplant recipients and patients who are receiving immunosuppressive therapy. This form tends to be aggressive and frequently (∼50%) has visceral involvement [3, 6]. (4) AIDS-related KS that is the most common form of KS in the USA and Europe and the most common malignancy in patients with AIDS [3]. AIDS-associated KS is the most aggressive form, typically presenting with cutaneous lesions and with visceral involvement with disease progression [4, 6].
In AIDS-related KS, up to half of the patients with cutaneous lesions have visceral involvement which can affect the gastrointestinal (GI) tract, lungs and, less commonly, liver, spleen, kidney, and heart [6, 7]. The GI tract is the most commonly involved extracutaneous site, and GI lesions can be present even in the absence of cutaneous lesions [7, 8, 9, 10]. GI involvement by KS is a rare endoscopic finding, still scarcely characterized in the literature [7, 9, 10]. The objective of this study is to characterize the clinical and endoscopic features of KS involvement of the GI tract in a single Portuguese center over the last decade.
Patients and Methods
An electronic database search was performed, and all cases of GI KS diagnosed by histopathological and immunohistochemical examination of endoscopic biopsy material in a single Portuguese center (Centro Hospitalar de Lisboa Ocidental) over the last decade (from January 2006 to December 2015) were retrospectively analyzed.
The following variables were assessed: demographic data (gender, age, race), clinical data (extraintestinal organ involvement, presence, and clinical stage of HIV infection, presence of other AIDS-defining illnesses, use of highly active antiretroviral therapy [HAART], use of immunosuppressive therapy, or any other underlying disease), and endoscopic data (localization, number, and endoscopic appearance of the lesions) [9]. Stage-stratified therapeutic approach and mortality (at 3 and 6 months) were also assessed. HIV infection was staged according to the CDC classification (2007 revision). CD4 T-cell counting by flow cytometry was assessed, and it was performed less than 4 months before the diagnosis of KS in all cases.
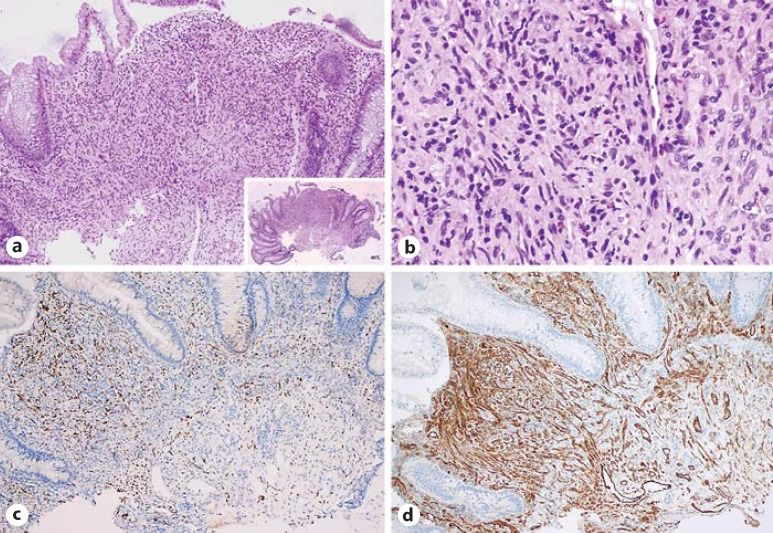
In histopathological and immunohistochemical analysis, hematoxylin and eosin staining, investigation of HHV-8 LNA-1, and immunohistochemistry for CD31 and CD34 were performed (Fig. 1). Descriptive statistics were used to summarize the analyzed data.
Fig. 1.
a Biopsy of a colonic KS lesion in a kidney transplant recipient. HE. ×100. b Detail of the spindle-shaped tumor cells closely associated with small slit-like vessels. HE. ×400. c Immunohistochemistry stain for HHV-8 (positive, dotty nuclear stain, ×100). d Immunohistochemistry stain for CD31, highlighting blood vessels (membranous stain, ×100).
Results
Thirteen cases of GI KS were identified. The majority of patients were men (77%) and were of African descent (62%). The mean age was 55 years. Table 1 summarizes the patient demographics, clinical and endoscopic features, therapy and mortality at 6 months in GI KS.
Table 1.
Patients' demographic, clinical, and endoscopic features, therapy, and mortality at 6 months in GI KS
| Age, years | Gender | Race | AIDS-related KS (CDC classification, antiretroviral therapy) | IS-related KS (therapy) | Symptoms | Cutaneous lesions | Other organ involvement | GI tract involvement | GI lesions, n | Morphology of GI lesions | Biopsies, n | Therapy | Outcome at 6 months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 63 | female | Native African | yes (C2, no HAART) | no | yes | no | esophagus | >3 | depressed | 2 | HAART | alive | |
| 54 | male | Caucasian | yes (C3, no HAART) | no | yes | no | esophagus | ≤3 | flat | 4 | − | dead | |
| 59 | male | Caucasian | yes (C3, no HAART) | no | yes | ascites | stomach | >3 | flat | 2 | HAART | alive | |
| 39 | male | Caucasian | yes (C3, no HAART) | no | yes | ascites | stomach | ≤3 | polypoid | 7 | − | dead | |
| 46 | male | Native African | yes (C3, no HAART) | no | yes | lung | stomach | ≤3 | polypoid | 4 | HAART, doxorubicin | alive | |
| 79 | male | Caucasian | yes (C3, no HAART) | no | no | no | stomach | ≤3 | polypoid | 2 | HAART | dead | |
| 73 | male | Native African | no | kidney transplant (sirolimus, MMF, PDN) | no | yes | no | stomach, duodenum | ≤3 | polypoid | 4 | reduction of IS, doxorubicin | dead |
| 61 | female | Native African | yes (C3, HAART for 2 years) | no | yes | lung | stomach | >3 | polypoid | 7 | doxorubicin | dead | |
| 30 | male | Native African | yes (C3, no HAART) | no | yes | no | stomach | >3 | polypoid | 2 | − | dead | |
| 58 | female | Native African | yes (C3, HAART for 10 years) | no | yes | no | stomach | >3 | flat | 5 | doxorubicin | dead | |
| 38 | male | Caucasian | yes (C3, no HAART) | no | yes | no | stomach, duodenum | >3 | flat and polypoid | 4 | HAART, doxorubicin | alive | |
| 56 | male | Native African | no | kidney transplant (tacrolimus, MMF, PDN) | hematochezia | yes | no | colon | ≤3 | flat | 4 | reduction of IS | dead |
| 58 | male | Native African | no | ulcerative colitis (PDN 60 mg/intradermally) | hematochezia | no | no | colon | >3 | polypoid | 4 | reduction of IS | alive |
GI, gastrointestinal; HAART, highly active antiretroviral therapy; IS, immunosuppression; KS, Kaposi sarcoma; MMF, mycophenolic acid; PDN, prednisone.
In most cases (n = 10, 77%), KS was associated with HIV. Ninety percent of these patients (9/10) were classified as stage C3, according to the CDC classification (CD4+ T-cell count <200/μL), and only 1 patient was classified as stage C2 (200 < CD4+ cells <499/μL). In 80% of the AIDS patients, KS was the initial manifestation of HIV infection and 30% had simultaneously other AIDS-defining illnesses (cytomegalovirus retinitis, systemic mycobacteriosis, and pneumocystis pneumonia). Only 20% of the AIDS patients (2/10) were under antiretroviral therapy at the time of KS diagnosis (both on HAART for more than 1 year).
Only 3 patients (23%) had GI KS unrelated to HIV. In those 3 patients, KS was associated with immunosuppressive therapy: 2 kidney transplant recipients (taking mycophenolic acid, steroids, and tacrolimus/sirolimus) and 1 patient with ulcerative colitis on corticosteroid therapy. The interval between the initiation of immunosuppressive therapy and the diagnosis of GI KS in these patients was 4–12 months.
Most patients (85%, 11/13) had cutaneous lesions, 15% (2/13) had pulmonary involvement, and 15% (2/13) had malignant ascites - as confirmed by the isolation of HHV-8 in the ascitic fluid by polymerase chain reaction.
In most patients (85%, 11/13), GI KS was asymptomatic and diagnosed by upper GI endoscopy performed for staging of cutaneous or oropharyngeal KS: 2 cases involving the esophagus (Fig. 2); 7 cases involving the stomach (Fig. 3); and 2 cases involving both the duodenum (Fig. 4) and stomach. Colonoscopy was performed in 36% of patients with upper GI KS (4/11) and was unremarkable in all of them. Colonic involvement by KS was established only in 2 patients presenting with hematochezia, both under immunosuppressive treatment (a kidney transplant recipient and a patient with steroid-dependent ulcerative colitis) (Fig. 5).
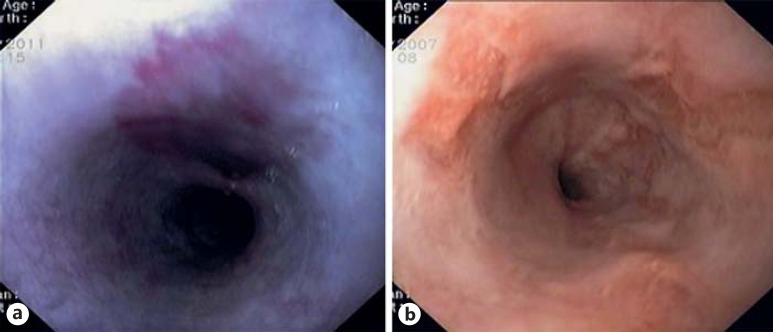
Fig. 2.
Esophageal flat macular purplish KS lesion (a) and several depressed KS lesions in the lower esophagus (b).
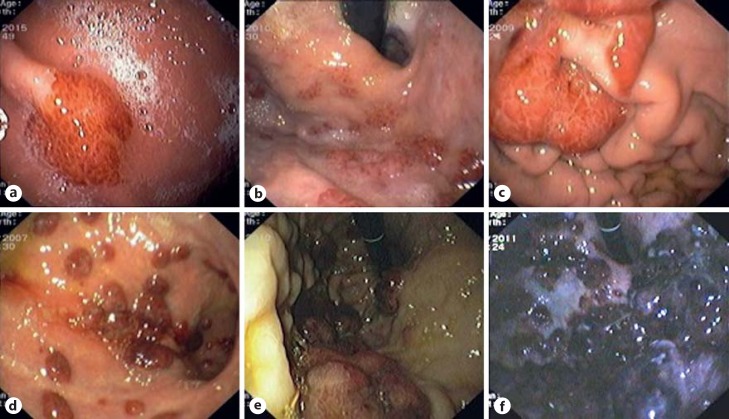
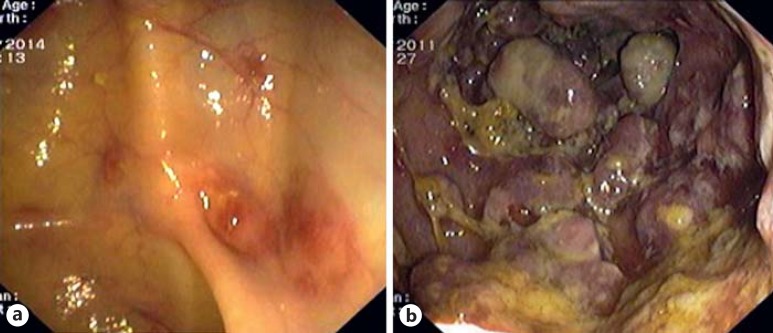
Fig. 3.
Gastric flat maculopapular KS lesions (a, b) and gastric polypoid/nodular KS lesions (c-f).
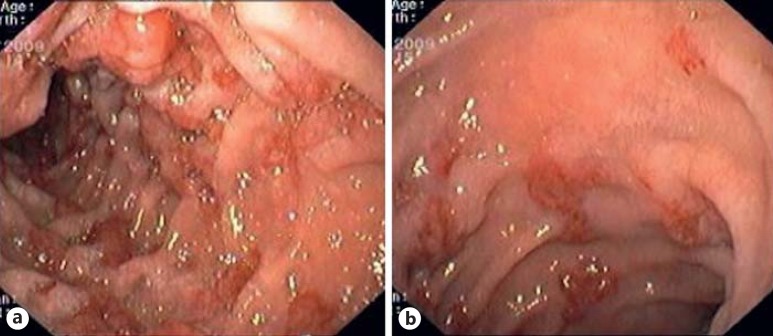
Fig. 4.
a, b Duodenal flat maculopapular KS lesions.
Fig. 5.
Colonic flat maculopapular KS lesions (a) and colonic polypoid “pseudotumoral” KS lesions (b).
Endoscopically, nearly half of the patients (54%) had more than 3 lesions. GI KS was morphologically characterized by reddish or purplish patchy lesions, with different sizes and shapes (Fig. 2, 3, 4, 5). The most prevalent morphologic type was polypoid/nodular (62%), most frequently noted in the stomach (Fig. 3c, d, e, f). Flat maculopapular lesions, characterized by a slight elevation of the mucosal surface, were found in the esophagus (Fig. 2a), stomach (Fig. 3a, b), duodenum (Fig. 4), and colon (Fig. 5a). In 1 case of colonic KS, polypoid lesions were larger (>2 cm), darker in color and confluent, with a “pseudotumoral” morphology (Fig. 5b). There was 1 case of KS presenting as slightly depressed reddish lesions in the esophagus (Fig. 2a). In this series of immunohistochemical documented GI KS, the average number of biopsies obtained by endoscopy was 4 (range, 2–7).
Treatment was based on the institution of anti-retroviral therapy or reduction of immunosuppression and in 39% (5/13) of the patients on the administration of doxorubicin.
Survival at 3 and 6 months after GI KS diagnosis was only 46 and 39%, respectively. Survival at 6 months was 40% among AIDS-related KS patients. In AIDS-unrelated KS patients, both kidney transplant recipients died shortly after the diagnosis and the ulcerative colitis patient had a favorable outcome after corticosteroid therapy withdrawal (with complete regression of the colonic pseudotumoral KS lesions). Patients died due to advanced KS, infectious diseases (namely AIDS-related KS, other AIDS-defining conditions), or both.
Discussion
Even though the number of new HIV infections has decreased consistently since 2000, Portugal is still one of the European countries with the highest incidence of this infection [11]. Considering that 40–51% of HIV patients with cutaneous lesions will develop visceral involvement, and that the GI tract is the most common extracutaneous site involved, endoscopists need to be alert for this diagnosis [7, 8]. GI KS is typically asymptomatic, as was the case of all patients with upper GI tract involvement by KS in the present series. The absence of clinical manifestations of GI involvement by KS and its high frequency (particularly regarding the upper GI tract) brings out the question if all patients with cutaneous KS should be submitted to an upper endoscopy. Nagata et al. [12] suggested endoscopy in all patients with cutaneous lesions, CD4+ T-cell count <100/μL, and men who have sex with other men. The present series highlights the relevance of epidemiologic data regarding GI involvement by KS (as 62% of patients were Native Africans), and endoscopy should probably be considered in Native African men with cutaneous KS and HIV infection at an advanced stage. Only about one third of our patients with upper GI tract involvement performed a colonoscopy, and colonic lesions were not identified in any of them. However, in a series by Friedman et al. [13], up to one fifth of the patients simultaneously had involvement of the upper and lower GI tract. It is important to determine whether a given patient should also be submitted to colonoscopy. The identification of predictive factors of upper and lower GI tract involvement would be helpful in managing endoscopic exams in these patients, especially nowadays, in the era of HAART, which has dramatically modified the course of the HIV infection.
GI involvement by immunosuppression-associated KS is poorly characterized in the literature. Controversial data suggests that iatrogenic KS could be more frequent among kidney recipients than in other solid organ recipients. However, some studies do not confirm these data, and the causes of this discrepancy are not known [4]. Among kidney recipients, KS is also more frequent in patients from areas where HHV-8 is endemic [14]. The incidence risk of KS among solid organ recipients in the USA is 0.2% (vs. 0.0002% in the general population) [4]. The poor prognosis of these patients (in the present series, both patients died shortly after the diagnosis) can be related to high levels of immunosuppression and comorbidities that are frequent in patients with chronic renal impairment [4]. Immunosuppression-associated KS typically occurs briefly after the start of immunosuppression, usually within the first 24 months, as was the case in our 2 patients [4]. Reports of colonic KS in ulcerative colitis are rare. In these patients, the diagnosis of colonic KS could be challenging, as in our patient, who presented with a pseudotumoral, nontypical appearance [15]. Therapeutic approach of immunosuppression-associated KS involving the colon is not well defined in the literature and, even though some of these patients have been treated with proctocolectomy [15], the withdrawal of immunosuppression could be enough to induce remission, as was the case in our patient.
In the present series, involvement of the upper GI tract by KS was clinically silent, a similar finding reported in other series in the literature [7, 8]. However, with tumor growth, patients can present abdominal pain, nausea, vomiting, anemia, digestive bleeding and, in rare cases, mechanical obstruction, intussusception, and perforation [16]. Endoscopic appearance of KS lesions is variable as ulcerated, flat, polypoid/nodular, and volcano-like lesions can be found [6]. Sometimes, these lesions can mimic more frequent benign (peptic) or malignant lesions [6]. As in other series, the most frequent morphologic type was polypoid/nodular, particularly noted in the stomach, which is the most commonly involved segment of the GI tract [6, 9].
Biopsy is the gold standard for the diagnosis of both cutaneous and GI KS [17]. Histologically, the lesions have typical features and, with sufficient sampling, a high diagnostic suspicion can arise from observation of morphology in hematoxylin and eosin alone [18]. When morphology alone is not sufficient to reach a final diagnosis, immunohistochemical studies can be performed on the tissue sample [18]. Historically, CD31, CD34, ERG, and von Willebrand factor were used, as they are typically expressed by KS tumor cells [19]; however, these same markers can also be expressed by, among others, angiosarcoma cells (primary or metastatic to the GI tract), which could culminate in diagnostic difficulties, given that their morphologies can sometimes overlap [18, 20]. From 2002 onwards, an antibody with affinity to latent nuclear antigen-1 (LAN-1), a protein encoded by HHV-8, has been available and has shown excellent sensitivity and specificity in the detection of KS [20, 21, 22]. The tumor originates in the submucosa and can ulcerate the overlying mucosa; when this happens, diagnosis is usually straightforward on histology. However, the lesion can lie deeper in the wall of the bowel. In this situation, biopsies should be done at the ulcerated zone, if present, and should sample the submucosa [23]. False negatives have been found to occur most often in KS of the esophagus as well as in lesions in the patch and plaque phases [23]. Since we only analyzed the patients with a histologic diagnosis of KS, and this diagnosis can be challenging and have false negatives, we may not have identified all patients. Thus, this has probably contributed to the relatively small size of our series of this rare disease.
KS therapy depends on the clinical variant and organs involved. In patients with visceral involvement and AIDS-associated KS, systemic chemotherapy (preferably with liposomal doxorubicin) combined with HAART may be indicated. In immunosuppression-associated KS, withdrawal or reduction of immunosuppressants is advised [6]. Showing visceral involvement (namely in asymptomatic patients), endoscopic examinations could be useful for staging, assisting in therapeutic decision-making and for evaluating therapy outcomes.
The prognosis of GI KS is poor, both in immunosuppression-related and AIDS-related forms, with a 6-month survival of 40%. Involvement of the GI tract by KS is often asymptomatic, has multiple endoscopic appearances, and can be present at diagnosis in HIV patients, particularly in the C3 stage (even in the absence of cutaneous lesions). Thus, a high diagnostic suspicion is needed in this setting.
Statement of Ethics
This study did not require informed consent nor review/approval by the appropriate ethics committee.
Disclosure Statement
All authors have approved the manuscript and agree with its submission. All authors have nothing to disclose and there are no funding to declare.
Author Contributions
Joana Carmo, Susana Marques, and Daniel Pinto wrote the manuscript. Miguel Bispo and Cristina Chagas revised the paper.
References
- 1.Oriel JD. Moritz Kaposi (1837–1902) Int J STD AIDS. 1997;8:715–717. doi: 10.1258/0956462971918968. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpes-virus like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 3.Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027–1038. doi: 10.1056/NEJM200004063421407. [DOI] [PubMed] [Google Scholar]
- 4.Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88:500–517. [PubMed] [Google Scholar]
- 5.Schulz TF. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8): epidemiology and pathogenesis. J Antimicrob Chemother. 2000;45:15–27. doi: 10.1093/jac/45.suppl_4.15. [DOI] [PubMed] [Google Scholar]
- 6.Lee AJ, Brenner L, Mourad B, Monteiro C, Vega KJ, Munoz JC. Gastrointestinal Kaposi's sarcoma: case report and review of the literature. World J Gastrointest Pharmacol Ther. 2015;6:89–95. doi: 10.4292/wjgpt.v6.i3.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parente F, Cernuschi M, Orlando G, Rizzardini G, Lazzarin A, Bianchi Porro G. Kaposi's sarcoma and AIDS: frequency of gastrointestinal involvement and its effect on survival: a prospective study in a heterogeneous population. Scand J Gastroenterol. 1991;26:1007–1012. doi: 10.3109/00365529109003949. [DOI] [PubMed] [Google Scholar]
- 8.Stebbing J, Sanitt A, Nelson M, Powles T, Gazzard B, Bower M. A prognostic index for AIDS-associated Kaposi's sarcoma in the era of highly active antiretroviral therapy. Lancet. 2006;367:1495–1502. doi: 10.1016/S0140-6736(06)68649-2. [DOI] [PubMed] [Google Scholar]
- 9.Rezende RE, Kahwage RL, Costa TV, Machado AA, Brunaldi MO, Kemp R, et al. Upper gastrointestinal Kaposi's sarcoma in HIV-infected patients: ten years of endoscopy observation at a single Brazilian center. Inter J Infect Dis. 2015;39:110–115. doi: 10.1016/j.ijid.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed N, Nelson RS, Goldstein HM, Sinkovics JG. Kaposi's sarcoma of the stomach and duodenum: endoscopic and roentgenologic correlations. Gastrointest Endosc. 1975;21:149–152. doi: 10.1016/s0016-5107(75)73833-6. [DOI] [PubMed] [Google Scholar]
- 11.Diniz A, Duarte R, Caldeira C, Bettencourt J, Gomes M, Oliveira O, et al. Portugal - infecção VIH/SIDA e tuberculose em números - 2013. Lisboa: Direcão-Geral da Saúde; 2013. [Google Scholar]
- 12.Nagata N, Shimbo T, Yazaki H, Asayama N, Akiyama J, Teruya K, et al. Predictive clinical factors in the diagnosis of gastrointestinal Kaposi's sarcoma and its endoscopic severity. PLoS ONE. 2012;7:e46967. doi: 10.1371/journal.pone.0046967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman SL, Wright TL, Altman DF. Gastrointestinal Kaposi's sarcoma in patients with acquired immunodeficiency syndrome. Endoscopic and autopsy findings. Gastroenterology. 1985;89:102–108. doi: 10.1016/0016-5085(85)90750-4. [DOI] [PubMed] [Google Scholar]
- 14.Harwood AR, Osoba D, Hofstadter SL, Goldstein MB, Cadella CJ, Holecek MG, et al. Kaposi's sarcoma in recipients of renal transplants. Am J Med. 1979;67:759–765. doi: 10.1016/0002-9343(79)90731-9. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Peláez M, Fernández-García M, Gutiérrez-Corral N, Francisco R, Riestra S, Carcía-Pravia C, et al. Kaposi's sarcoma: an opportunistic infection by human herpesvirus-8 in ulcerative colitis. J Crohns Colitis. 2010;4:586–590. doi: 10.1016/j.crohns.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Balachandra B, Tunitsky E, Dawood S, Hings I, Marcus VA. Classic Kaposi's sarcoma presenting first with gastrointestinal tract involvement in a HIV-negative Inuit male - a case report and review of the literature. Pathol Res Pract. 2006;202:623–626. doi: 10.1016/j.prp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Bogaert LJ. Clinicopathological proficiency in the diagnosis of Kaposi's sarcoma. ISRN AIDS. 2012;2012:565463. doi: 10.5402/2012/565463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odze RD, Goldblum JR. Odze and Goldblum Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas. ed 3. Philadelphia: Elsevier/Saunders; 2015. [Google Scholar]
- 19.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens - evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol. 1994;7:82–90. [PubMed] [Google Scholar]
- 20.Patel RM, Goldblum JR, His ED. Immunohistochemical detection of human herpes virus-8 latent nuclear antigen-1 is useful in the diagnosis of Kaposi sarcoma. Mod Pathol. 2004;17:456–460. doi: 10.1038/modpathol.3800061. [DOI] [PubMed] [Google Scholar]
- 21.Courville P, Simon F, Le Pessot F, Tallet Y, Debab Y, Métayer J. Detection of HHV8 latent nuclear antigen by immunohistochemistry. A new tool for differentiating Kaposi's sarcoma from its mimics. Ann Pathol. 2002;22:267–276. [PubMed] [Google Scholar]
- 22.Cheuk W, Wong KO, Wong CS, Dinkel JE, Ben-Dor D, Chan JK. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004;121:335–342. doi: 10.1309/B8TC-0LBV-H8XY-5MFV. [DOI] [PubMed] [Google Scholar]
- 23.Nagata N, Sekine K, Igari T, Hamada Y, Yazaki H, Ohmagari N, et al. False-negative results of endoscopic biopsy in the diagnosis of gastrointestinal Kaposi's sarcoma in HIV-infected patients. Patholog Res Int. 2012;2012:854146. doi: 10.1155/2012/854146. [DOI] [PMC free article] [PubMed] [Google Scholar]