Abstract
Objective
Faecal immunochemical tests (FITs) are used to screen for colorectal cancer (CRC) and as diagnostic aids in symptomatic patients. However, the number of samples per FIT varies. It is unclear if there is any advantage to analyse multiple-sample FITs in symptomatic patients.
Design and setting
This is a post hoc analysis of a retrospective study that included all cases of CRC and adenomas with high-grade dysplasia (HGD) between 2005 and 2009 in the county of Jämtland, Sweden.
Subjects
All patients with CRC and adenomas with HGD that initially presented with symptoms to primary care and delivered FITs.
Main outcome measure
The likelihood of a positive FIT in cases of CRC and adenomas with HGD; when analysing one, two or three samples.
Results
Of 195 patients, 160 delivered three-sample FITs. Using the 139 cases in which at least one sample was positive, the likelihood of detecting a positive sample upon analysis of only one of the three samples was 0.91 (95% CI: 0.85–0.95), indicating that 13 positive cases may have been missed.
Conclusion
Use of a one-sample FIT instead of a three-sample FIT as a diagnostic aid may result in the missing of one tenth of symptomatic CRCs and adenomas with HGD.
Keywords: Colorectal neoplasms, faecal immunochemical test, number of faecal samples, occult blood, primary health care, symptomatic patients.
Introduction
Colorectal cancer (CRC) is the second most common cancer in women and the third most common in men worldwide [1]. Adenomas with high-grade dysplasia (HGD) have an increased risk of developing into cancer [2]. Faecal occult blood tests (FOBTs) are used in many countries to screen for CRC [3]. FOBTs are also used as diagnostic aids for symptomatic patients in several countries, for example in secondary care after a negative sigmoidoscopy or, as in Sweden in unselected patients in primary care [4,5]. Despite lack of evidence and guidelines, FOBTs are frequently used in Sweden when doctors feel clinical uncertainty [5].
The older guaiac faecal occult blood tests (gFOBTs) are being replaced with the newer faecal immunochemical tests (FITs), which are more sensitive and specific [6]. FITs can be qualitative with a fixed cut-off level for the amount of faecal haemoglobin, or quantitative with the possibility of setting different cut-offs. Convenient qualitative point of care (POC) tests have been in use for many years. There is some minor evidence to support the use of FITs in symptomatic patients in primary care [7]. Studies concerning patients already referred to secondary care have also suggested that FITs can be useful in the evaluation of symptomatic patients [8,9]. Knowledge of the optimal cut-off level and the optimal number of samples per test are lacking.
While screening with gFOBTs often analyses six-sample tests, screening with FITs often analyses one-sample tests [3]. However, studies have shown that two-sample and three-sample FITs detect more cases of CRC than one-sample FITs [10–12]. On the other hand, a recent study suggested that a one-sample FIT was preferable to a two-sample FIT for screening [13].
Our aim was to evaluate the advantage, if any, of analysing more than one sample per FIT in symptomatic patients.
Method
This is a post hoc analysis of a retrospective study that included all patients diagnosed with CRC and adenomas with HGD from 2005 to 2009 in the county of Jämtland, Sweden. Detailed information about materials and methods has been previously published [14]. In brief, information of all patients diagnosed with CRC and adenomas with HGD in the county were received from the regional Cancer Registry. The county’s electronic health records, including all primary and secondary care data for all patients, were searched for all FITs for the patients, beginning two year before the diagnosis. Two years was chosen as this is the recommended screening interval in Europe [15]. This analysis included all patients who initially presented to primary care and there delivered FITs.
It was customary to order and analyse three-sample FITs in all health centres except one; this centre was excluded in this analysis. The samples were taken from three consecutive stools and the time from the consultation to the third sample taken rarely exceeded one week. There was no screening program for CRC.
The samples were analysed by trained laboratory staff at health centres. Actim Fecal Blood (Oy Medix Biochemica Ab), an immunochemical, qualitative and visually read dipstick test, was used for the analysis. The sensitivity was 50 ng haemoglobin/ml of faecal solution. With an expected mass of 10–20 mg faeces in each sample, and a volume of the buffering solution of 10 ml, this corresponded to 25–50 µg/g faeces according to the manufacturer [16]. Data regarding all FITs that were analysed within two years prior to clinical diagnosis of CRC and adenomas with HGD was registered.
Statistics
In order to have a balanced data set with sufficient number of cases, only the cases with three analysed samples were used. Because the order in which each patient had collected the samples was unknown, it was not possible to directly estimate the effect of analysis of one, two or three samples. For cases where one or two samples out of three were positive, the order between the three samples was randomised. The randomisation was repeated 10,000 times, and the likelihood of receiving at least one positive sample when analysing one, two or three samples was estimated. This was done using data for all cases, as well as using data only for cases where at least one of the samples showed a positive result. Confidence intervals were estimated according to Jeffrey [17].
Ethical approval was obtained from the Regional Ethical Review Board, Umeå (2010-358-31M).
Results
Of 323 patients that initially presented to primary care, 195 patients were included in the studied group (Figure 1). A total of 160 patients delivered exactly three samples, 139 of these had at least one positive sample. The number of positive samples for each FIT is presented in Table 1. Twenty-one patients did not deliver all three ordered samples. All samples of the FIT were negative in 25 (12.8%) patients.
Figure 1.
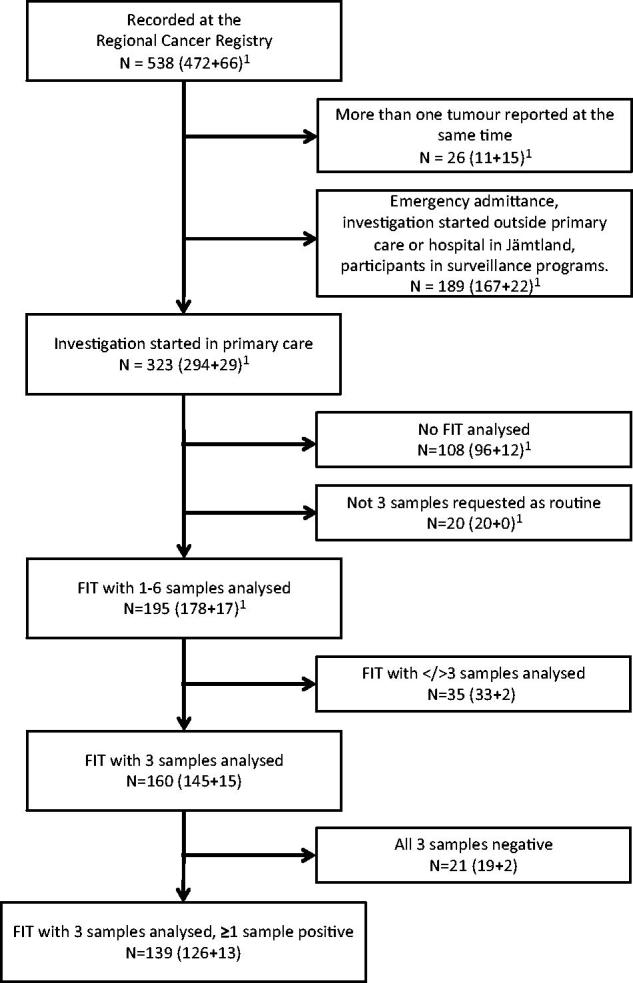
Selection of the study group, starting with 538 patients. 1(colorectal cancer + adenomas with high-grade dysplasia). FIT: faecal immunochemical test.
Table 1.
The number of positive samples for each faecal immunochemical test (FIT) in symptomatic patients diagnosed with colorectal cancer or adenomas with high grade dysplasia (HGD).
| Number of positive samples.Total (adenomas HGD). |
||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| FITs with 1 sample | N = 8 | 2 | 6 | |||||
| FITs with 2 samples | N = 13 | 1 | 2 | 10 (1) | ||||
| FITs with 3 samples | N = 160 | 21 (2) | 12 (4) | 15 (2) | 112 (7) | |||
| FITs with 4 samples | N = 4 | 1 | 1 | 1 | 0 | 1 | ||
| FITs with 5 samples | N = 2 | 0 | 0 | 0 | 0 | 0 | 2 (1) | |
| FITs with 6 samples | N = 8 | 0 | 0 | 1 | 1 | 1 | 0 | 5 |
| Total | N = 195 | 25 (2) | 21 (4) | 27 (3) | 113 (7) | 2 | 2 (1) | 5 |
Using all 160 cases with exactly three analysed samples, the likelihood of obtaining a positive sample when analysing one of the three samples was 0.79 (95% CI: 0.72–0.85), compared to 0.87 (95% CI: 0.81–0.91) when analysing all three samples. Using only the 139 cases (with three analysed samples) where at least one sample was positive, the likelihood of finding a positive sample when analysing one of the samples was 0.91 (95% CI: 0.85–0.95) (Table 2). These results indicate that 13 positive cases may have been overlooked in the second scenario.
Table 2.
The likelihood of receiving at least one positive sample of a three-sample faecal immunochemical test (FIT), with different numbers of samples analysed, in patients with colorectal cancer or adenomas with high-grade dysplasia and 95% confidence intervals.
| Using all cases (n = 160) | Using only positive cases (n = 139) | |
|---|---|---|
| One sample analysed | 0.79 (0.72–0.85) | 0.91 (0.85–0.95) |
| Two samples analysed | 0.84 (0.78–0.89) | 0.97 (0.93–0.99) |
| Three samples analysed | 0.87 (0.81–0.91) | 1.00 (0.98–1.00) |
Discussion
To our knowledge, this is the first evaluation of the effectiveness of different numbers of samples for a POC FIT used for diagnostic purposes in primary care in symptomatic patients with CRC or adenomas with HDG.
It is important to rule out serious conditions such as CRC with a high degree of safety. This study showed that using one-sample FITs instead of three-sample FITs, in 139 cases with CRC or adenomas with HGD where at least one of three faecal samples was positive, would potentially have resulted in the missing of 13 of the 139 cases.
However, only relying on FITs seems not to be sufficient. Also with three samples, 21 out of 160 patients had negative FITs and would not have been detected. Potentially, adding presence of anaemia to the FIT result could be of help in diagnosing CRC, as we have previously indicated [7].
A one-sample FIT with a very low cut-off level may be an alternative to multiple-sample FITs, however, resulting in a low specificity [18,19]. Also, it can only be used with quantitative FITs and thus is less practical for use at primary care health centres. Furthermore, with the use of a one-sample FIT the risk of not detecting CRCs with intermittent bleeding may remain.
This study has several limitations. It is retrospective and it has a rather small number of patients. FITs were not analysed in all patients with CRC or adenomas with HGD, and presumably not in those regarded to have a definite need for referral. The majority of patients delivered a three-sample FIT, but not all. However, negative FITs were found in patients delivering one-sample or two-sample FITs as well as in patients delivering more than three samples, which indicates that there was no major bias in the selection of patients with three-sample FITs. The interpretation of the FIT relies on the staff visually reading the test. Potentially this could influence the results even though the study well reflects the clinical situation. It is also worth remembering that with other FITs brands, using other cut-offs, perhaps another sensitivity of the test would have been demonstrated.
Qualitative FITs are visually read and can be less exact than quantitative FITs, as test results may be affected by variations between different readers. Further studies are needed to determine the optimal cut-off level for qualitative and quantitative FITs, respectively, when used as diagnostic aids in primary care. Potentially, an increased chance of at least one false-positive test result could be a consequence of multiple-sample testing, and thus, a larger study is needed to clarify the adequate number of samples and the clinical situations where FITs could help the clinician. However, the study reflects current practices in that the FITs were ordered at the discretion of primary care physicians when they presumably needed a diagnostic aid.
In conclusion, this study shows that the use of a one-sample POC FIT instead of a three-sample POC FIT as a diagnostic aid in primary care may result in the missing of one-tenth of symptomatic CRCs and adenomas with HGD.
Funding Statement
This work was supported by unrestricted grants from the Region Jämtland Härjedalen, the Regional Cancer Centre North, the Cancer Research Foundation in Northern Sweden and the Northern County Councils (Visare Norr).
Disclosure statement
The authors report no conflicts of interest.
Notes on contributors
Cecilia Högberg, MD, PhD, is a specialist in family medicine, Krokom Health Centre, Region Jämtland Härjedalen and Umeå University, Sweden.
Lars Söderström, BSc, is a senior statistician, Unit of Research, Education, and Development, Östersund Hospital, Region Jämtland Härjedalen, Sweden.
Mikael Lilja, MD, PhD, is a specialist in family medicine, Unit of Research, Education, and Development, Östersund Hospital, Region Jämtland Härjedalen and Umeå University, Sweden.
References
- 1.GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. [cited 2017 May 9]. Available from: http://globocan.iarc.fr/Default.aspx. [Google Scholar]
- 2.Podolsky DK, Camilleri M, Fitz JG, et al. editors. Yamada's textbook of gastroenterology. 6th ed. London (UK): Wiley; 2015. [Google Scholar]
- 3.Schreuders EH, Ruco A, Rabeneck L, et al. . Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–1649. [DOI] [PubMed] [Google Scholar]
- 4.Sundhetsstyrelsen (Denmark) Pakkeforløb for kraeft i tyk-og endetarm (Cancer pathways. National integrated cancer pathway for colorectal cancer). Copenhagen: 2016. [cited 2017 May 9]. Available from: http://sundhedsstyrelsen.dk/da/sygdom-og-behandling/kraeft/pakkeforloeb/beskrivelser. [Google Scholar]
- 5.Högberg C, Samuelsson E, Lilja M, et al. . Could it be colorectal cancer? General practitioners' use of the faecal occult blood test and decision making – a qualitative study. BMC Fam Pract. 2015;16:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison JE, Fraser CG, Halloran SP, et al. . Population screening for colorectal cancer means getting FIT: the past, present, and future of colorectal cancer screening using the fecal immunochemical test for hemoglobin (FIT). Gut Liver. 2014;8:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Högberg C, Karling P, Rutegård J, et al. . Diagnosing colorectal cancer and inflammatory bowel disease in primary care: the usefulness of tests for faecal haemoglobin, faecal calprotectin, anaemia, and iron deficiency. A prospective study. Scand J Gastroenterol. 2017;52:69–75. [DOI] [PubMed] [Google Scholar]
- 8.Cubiella J, Salve M, Diaz-Ondina M, et al. . Diagnostic accuracy of the faecal immunochemical test for colorectal cancer in symptomatic patients: comparison with NICE and SIGN referral criteria. Colorectal Dis. 2014;16:O273–O282. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Alonso L, Rodriguez-Moranta F, Ruiz-Cerulla A, et al. . An urgent referral strategy for symptomatic patients with suspected colorectal cancer based on a quantitative immunochemical faecal occult blood test. Dig Liver Dis. 2015;47:797–804. [DOI] [PubMed] [Google Scholar]
- 10.Park DI, Ryu S, Kim YH, et al. . Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010;105:2017–2025. [DOI] [PubMed] [Google Scholar]
- 11.Raginel T, Puvinel J, Ferrand O, et al. . A population-based comparison of immunochemical fecal occult blood tests for colorectal cancer screening. Gastroenterology. 2013;144:918–925. [DOI] [PubMed] [Google Scholar]
- 12.Wu D, Luo HQ, Zhou WX, et al. . The performance of three-sample qualitative immunochemical fecal test to detect colorectal adenoma and cancer in gastrointestinal outpatients: an observational study. PLoS One. 2014;9:e106648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapidzic A, van Roon AH, van Leerdam ME, et al. . Attendance and diagnostic yield of repeated two-sample faecal immunochemical test screening for colorectal cancer. Gut. 2017;66:118–123. [DOI] [PubMed] [Google Scholar]
- 14.Högberg C, Karling P, Rutegård J, et al. . Immunochemical faecal occult blood tests in primary care and the risk of delay in the diagnosis of colorectal cancer. Scand J Prim Health Care. 2013;31:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Publication Office of the European Union. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Luxembourg (Europe): Publication Office of the European Union; [Internet]. 2011. Available from: http://bookshop.europa.eu. [Google Scholar]
- 16.Oy Medix Biochemica Ab (Finland) Espoo: 2016. [cited 2017 May 9]. Available from: http://www.medixbiochemica.com. [Google Scholar]
- 17.Brown LD, Cai TT, DasGupta A.. Interval estimation for a binomial proportion. Statist Sci. 2001;16:101–133. [Google Scholar]
- 18.Auge JM, Fraser CG, Rodriguez C, et al. . Clinical utility of one versus two faecal immunochemical test samples in the detection of advanced colorectal neoplasia in symptomatic patients. Clin Chem Lab Med. 2016;54:125–132. [DOI] [PubMed] [Google Scholar]
- 19.Mowat C, Digby J, Strachan JA, et al. . Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. 2016;65:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]


