Abstract
Objective
In an automated dose dispensing (ADD) service, medicines are dispensed in unit-dose bags according to administration times. When the service is initiated, the patient’s medication list is reconciled and a prescription review is conducted. The service is expected to reduce drug use. The aim of this national controlled study was to investigate whether the ADD service with medication review reduces drug use among geriatric primary care patients.
Design, setting and patients
This is a nationwide cohort study with matched controls. The study group consisted of all primary care patients ≥65 years enrolled in the ADD service in Finland during 2007 (n = 2073). Control patients (n = 2073) were matched by gender, age, area of patient’s residence and number of the prescription drugs reimbursed. The data on all prescription drugs reimbursed during the 1 year periods before and after the ADD service enrollment were extracted from the Finnish National Prescription Register. Drug use was calculated as defined daily doses (DDD) per day.
Results
The studied 20 most used drugs covered 86% of all reimbursed drug use (in DDD) of the study group. The use of 11 out of these 20 active substances studied was reduced significantly (p < .001–.041) when the drug use was adjusted by the number of chronic diseases. Two of these drugs were hypnotics and six were cardiovascular system drugs.
Conclusions
Drug use was decreased after initiation of the ADD service in primary care patients ≥65 years compared to matched controls in this 1 year cohort study. Further studies should be conducted in order to explore the causality, assess the ADD service’s impact on drug use quality and costs, as well as impact of accompanied prescription review on positive outcomes.
Keywords: Automated dose dispensing, medication safety, drug use, prescription review, geriatric patients
1. Introduction
Achieving appropriate and safe medication use is challenging among elderly primary care patients [1,2]. Interventions, such as different types of medication reviews, have been developed to identify, solve and prevent drug-related problems among these patients [3–5]. In some countries community pharmacies provide an automated dose dispensing (ADD) service for primary care patients [6]. In this service, medicines are dispensed in unit-dose bags according to administration times [7]. The service is rather widely used, in 2011 in Sweden there were 190,000 patients using the service while in the Netherlands there were 360,000 users in 2011 [8,9]. At the end of 2016, the number of patients in Finland was 49,500 and it is increasing (unpublished data received from the Social Insurance Institution).
Although ADD is used quite commonly for geriatric patients with multiple morbidities and medications, little is known of ADD’s impact on patients medication use. A systematic review showed that no controlled studies have assessed its outcomes [6]. The systematic review showed that patients using the ADD service have more potentially inappropriate drugs in their regimens than patients using the standard dispensing procedure, which is in line with the idea of ADD performing as a preventive procedure for medication risks [6,10,11]. However, the impact of the ADD service on inappropriate drug use has not been evaluated, since the studies have applied cross-sectional study designs. A study from Norway revealed that ADD may improve medication safety in terms of reconciling medication records [12]. There is also some evidence that patients may benefit if their medications are reviewed as part of the ADD procedure [13]. The aim of this national controlled study was to investigate associations between the ADD service with medication review and drug use in the elderly. Our hypothesis was that introduction of the ADD service was followed by a reduction in drug use among geriatric primary care patients in Finland.
2. Methods
2.1. Study design
This study was a register-based cohort study with matched controls. The control patients were matched by gender, age, area of patient’s residence and number of prescription drugs used. These parameters were selected because these issues commonly affect drug use and confounding could be avoided by matching in a cohort study [14].
2.2. Intervention
In the ADD service, the patient’s regularly used medicines are packed by a machine in plastic unit-dose bags according to the time of administration [6,7]. Each unit-dose bag is labeled with the patient’s data, drug contents and administration time. When the ADD service is initiated for an individual patient, the patient’s medication list is reconciled and the medication is reviewed in collaboration with a physician and a community pharmacist [7]. When the data for this study were collected in 2007, there was one community pharmacy (Espoonlahti Pharmacy, Espoo, Finland) specialized in preparing unit-dose bags for national demand. Other community pharmacies could make a contract with it for procuring unit-dose bags for their customers.
2.3. Patients and data sources
The study group consisted of all primary care patients who were ≥65 years and were enrolled in the ADD service in 2007 in Finland and used the service at least 1 year after the start-up date. The patients enrolled in the ADD service were extracted from the customer register of Espoonlahti Pharmacy. For each patient in the study group, a control patient was selected individually from the registers of the Social Insurance Institution by the personnel of the institution in June 2011. They were matched by gender, age (at the end of the year), area of patient’s residence (hospital district) and the exact number of prescription drugs reimbursed during the period August–November in 2006. The number of prescription drugs reimbursed was calculated using the Anatomic Therapeutic classification (ATC) system’s 5th level [15]. The start-up date of the ADD service was used as an index date for both the control and study patients.
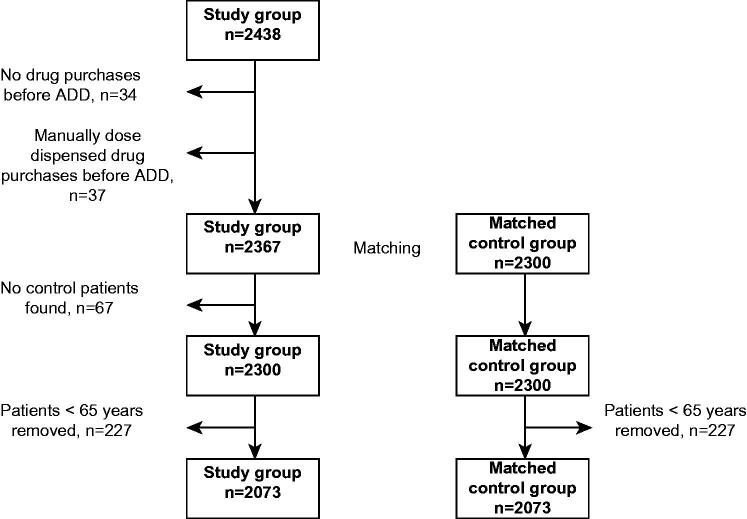
The data on all prescriptions reimbursed during the 1 year periods before and after initiation of the ADD service were extracted from the Finnish National Prescription Register for each patient in the study and control groups [16]. This register contains information on all reimbursed prescriptions for outpatients. Every patient has a personal identification (ID) number that was used to link the data from the customer register of Espoonlahti Pharmacy with the prescription data. Patients who had no drug purchases in the register before the ADD service was initiated (n = 34) were omitted since this might indicate, that they have been living in an institution and their drug use might have been quite different compared to patients living at home. Also patients who had manually dose dispensed drug purchases before the ADD service was initiated (n = 37) were omitted since our aim was to study drug use in automatic dose dispensing (Figure 1). If a matched control patient was not found, the patient was removed from the study group (n = 67). As the aim was to study the elderly population, patients younger than 65 years were excluded from the study and control groups.
Figure 1.
Patients selection process.
The data on all chronic diseases for the study and control patients were extracted from the Special Reimbursement Entitlement Register, which is also maintained by the Social Insurance Institution [17].
2.4. Outcome measures and definitions
Drug use was calculated as defined daily doses per day (DDD/day) by active substance derived from ATC fifth level [15,18]. Drug use was calculated separately for each patient in the study and control groups during the 1 year periods before and after initiation of the ADD service. For each patient, the first and last purchase dates of each drug (by ATC code) were recognized. The number of days between these two time points was counted. The sum of the DDDs was counted from the first purchase point until the second last purchase point. The number of DDDs of the last purchase point was not counted in the sum since we could not predict the duration of drug use because the following purchase date was not known. The sum of the DDDs was divided by the sum of the days to obtain DDD/day values.
A patient was assumed to be a new drug user if he/she had no purchases of a certain drug in the 1 year period before ADD but there was at least one purchase in the 1 year period after the ADD service was initiated. If the patient had no purchases of a certain drug for 1 year after the ADD service was initiated but there was at least one purchase during the 1 year period before ADD, the drug use was assumed as discontinued.
The 20 most used drugs (in DDDs) in the 2 year study period were chosen for the analysis. These 20 drugs covered 86% of all reimbursed drug use (in DDD) of the study group.
2.5. Statistical analysis
The mean drug use in DDD/day was tested with the general linear model, using repeated measures analysis. The p values for group, time and time*group effects were calculated. The group effect compares the drug use of the study and control groups (not taking into account the initiation of the ADD service). The time effect compares the drug use before and after the initiation of the ADD service (not taking into account the study and control groups). The time*group takes account both of these aspects. The number of chronic diseases was used as a covariate in the analysis. After fitting the model, outliers in the values of the drug use were checked individually from the original register data. In 10 cases, the values of the drug use were removed from the data, due to an apparent error in the original register data. The differences between the proportions of the patients who started and discontinued the drug use in the study and control groups were tested with the Pearson’s chi-squared test.
All tests were carried out with SPSS version 18.0 for Mac (IBM SPSS, Armonk, NY). A difference was considered statistically significant if the p value was less than .05.
2.6. Ethical approval
The University of Helsinki Viikki Campus Ethics Committee approved the study protocol.
3. Results
3.1. Characteristics of the study and control groups
The total number of patients in both the study and control groups was 2073 (Figure 1, Table 1). A majority of the patients in both groups were female (73%). Most of the patients (85%) were >75 years of age, the mean age being 82 (SD 6.9) years. The mean number of reimbursed prescription drugs used during the 4 month period before the study period was 6.5 (SD 3.5).
Table 1.
Matching criteria of the study (n = 2073) and control (n = 2073) groups. The composition of both groups is presented in the same column, since the groups were similar.
| Variable | Study and control % (n) |
|---|---|
| Gender | |
| Female | 73 (1522) |
| Male | 27 (551) |
| Age, yearsa | |
| Median (years) | 81.9 |
| Mean (years) | 82 (SD 6.9) |
| 65–74 | 15 (311) |
| 75–84 | 48 (988) |
| 85–94 | 35 (731) |
| 95–103 | 2 (43) |
| Patients’ area of residence | |
| Capital area | 37 (776) |
| Western Finland | 5 (112) |
| Central Finland | 18 (379) |
| Eastern Finland | 19 (392) |
| Northern Finland | 20 (414) |
| Number of reimbursed prescription drugs used before automated dose dispensingb | |
| Median (n) | 6 |
| Mean (n) | 6.5 (SD 3.5) |
in late 2007.
during 4 months (August–November 2006).
The study and control groups were different in terms of chronic diseases (Table 2). The proportions of the patients suffering from Alzheimer’s disease, diabetes, severe psychotic or other severe mental disorders, Parkinson’s disease and epilepsy were higher in the study than in the control group. The contrasting relationship prevailed for dyslipidemia, glaucoma and chronic asthma or other chronic obstructive pulmonary diseases.
Table 2.
Prevalence of diagnosed chronic diseases in the study (n = 2073) and control (n = 2073) groups.
| Diagnosed disease (in late 2006) | Study % (n) | Control % (n) |
|---|---|---|
| Chronic heart or cardiovascular disease | 59.9 (1242) | 62.4 (1293) |
| Alzheimer’s disease | 24.4 (506) | 6.5 (134) |
| Diabetes mellitus | 19.2 (399) | 16.6 (344) |
| Dyslipidemia | 10.0 (207) | 12.7 (263) |
| Severe psychosis and other severe mental disorders | 9.4 (195) | 2.4 (50) |
| Glaucoma | 9.2 (190) | 12.5 (260) |
| Chronic asthma or other chronic obstructive pulmonary diseases | 9.2 (190) | 11.9 (247) |
| Thyroid insufficiency | 6.4 (132) | 6.8 (141) |
| Disseminated connective tissue diseases, rheumatoid arthritis, and comparable conditions | 5.6 (117) | 5.0 (103) |
| Parkinson’s disease | 4.0 (82) | 1.6 (33) |
| Epilepsy | 3.0 (62) | 1.3 (27) |
| Cancer | 3.0 (62) | 3.9 (81) |
| Other diseases | 7.2 (149) | 6.8 (140) |
3.2. Drug use
In 11 of the 20 most-used active substances studied, the reduction in drug use in the study group was statistically significant when both the time and group effects were taken into account in the analysis, and the drug use was adjusted by the number of chronic diseases (Table 3). Two of these substances were hypnotics (temazepam and zopiclone), six were drugs for cardiovascular diseases (simvastatin, ramipril, amlodipine, isosorbide mononitrate, bisoprolol and metoprolol) and the others were donepezil (used for Alzheimer’s disease), paracetamol (used for pain) and metformin (used for diabetes).
Table 3.
Drug use (DDD/day) adjusted by the number of chronic diseases in the study and control groups before and after the automated dose dispensing (ADD) service was initiated. The 20 most-used (in DDDs) drugs among the study group were chosen for the analysis.
| Active substance (ATC code) | Study group (n = 2073) |
Control group (n = 2073) |
p values |
||||
|---|---|---|---|---|---|---|---|
| Before ADD Mean | After ADD Mean | Before ADD Mean | After ADD Mean | Group effect | Time effect | Time*group effect | |
| Cardiovascular system | |||||||
| Furosemide (C03CA01) | 1.69 | 1.67 | 1.45 | 1.57 | .121 | .488 | .060 |
| Ramipril (C09AA05) | 2.21 | 2.04 | 2.42 | 2.58 | .003 | .922 | .003 |
| Enalapril (C09AA02) | 1.32 | 1.22 | 1.53 | 1.50 | .005 | .528 | .188 |
| Bisoprolol (C07AB07) | 0.53 | 0.42 | 0.57 | 0.56 | <.001 | .002 | <.001 |
| Metoprolol (C07AB02) | 0.55 | 0.48 | 0.57 | 0.57 | .035 | .386 | <.001 |
| Amlodipine (C08CA01) | 1.40 | 1.16 | 1.27 | 1.32 | .782 | .010 | <.001 |
| Isosorbide mononitrate (C01DA14) | 0.81 | 0.67 | 0.79 | 0.79 | .057 | .186 | <.001 |
| Simvastatin (C10AA01) | 1.27 | 1.14 | 1.20 | 1.28 | .511 | .097 | <.001 |
| Nervous system | |||||||
| Zopiclone (N05CF01) | 1.01 | 0.95 | 0.97 | 0.99 | .981 | .382 | .014 |
| Temazepam (N05CD07) | 1.02 | 0.82 | 1.04 | 1.03 | .015 | .032 | <.001 |
| Citalopram (N06AB04) | 0.88 | 0.86 | 0.89 | 0.90 | .623 | .449 | .624 |
| Mirtazapine (N06AX11) | 0.85 | 0.76 | 0.71 | 0.73 | .068 | .411 | .060 |
| Donepezil (N06DA02) | 1.15 | 1.11 | 1.12 | 1.21 | .475 | .045 | .021 |
| Memantine (N06DX01) | 0.87 | 0.87 | 0.87 | 0.91 | .327 | .072 | .307 |
| Paracetamol (N02BE01) | 0.43 | 0.42 | 0.37 | 0.41 | .032 | .528 | .041 |
| Alimentary tract and metabolism | |||||||
| Lactulose (A06AD11) | 2.22 | 2.10 | 1.76 | 1.76 | .044 | .072 | .593 |
| Glimepiride (A10BB12) | 1.46 | 1.37 | 1.62 | 1.63 | .033 | .366 | .158 |
| Metformin (A10BA02) | 0.85 | 0.81 | 0.81 | 0.84 | .861 | .855 | .002 |
| Calcium combinations (A12AX) | 0.94 | 0.82 | 0.88 | 0.83 | .697 | .151 | .545 |
| Pantoprazole (A02BC02) | 0.76 | 0.67 | 0.67 | 0.65 | .064 | .012 | .059 |
ATC: anatomic therapeutic classification; ADD: automated dose dispensing; DDD: defined daily dose.
3.3. Patients who started and discontinued drug use
There were more starts and discontinuations in the study group than in the control group during the follow-up period (Table 4). The zopiclone, temazepam and calcium combinations were more actively started and discontinued in the study group. Glimepiride and metoprolol were more actively started, while isosorbide mononitrate was more actively discontinued in the study group.
Table 4.
Proportions of patients who started and discontinued drug use in the study and control groups. The 20 most-used (in DDDs) drugs among the study group were chosen for the analysis.
| Active substance (ATC-code) | Started drug usea |
Discontinued drug useb |
||||
|---|---|---|---|---|---|---|
| Study % (n) | Control % (n) | p valuec | Study % (n) | Control % (n) | p valuec | |
| Cardiovascular system | ||||||
| Furosemide (C03CA01) | 17.7 (160) | 18.8 (120) | .581 | 5.1 (40) | 8.9 (51) | .005 |
| Ramipril (C09AA05) | 17.5 (48) | 20.9 (48) | .330 | 9.9 (25) | 13.7 (29) | .202 |
| Enalapril (C09AA02) | 9.9 (18) | 13.9 (28) | .225 | 11.4 (21) | 13.9 (28) | .447 |
| Bisoprolol (C07AB07) | 13.1 (85) | 13.8 (95) | .720 | 6.2 (37) | 6.8 (43) | .677 |
| Metoprolol (C07AB02) | 8.5 (35) | 4.2 (14) | .017 | 6.2 (25) | 9.8 (35) | .067 |
| Amlodipine (C08CA01) | 18.6 (49) | 19.6 (55) | .765 | 14.1 (35) | 13.5 (35) | .846 |
| Isosorbide mononitrate (C01DA14) | 11.9 (48) | 10.1(45) | .397 | 9.6 (38) | 5.4 (23) | .021 |
| Simvastatin (C10AA01) | 12.1 (56) | 15.7 (88) | .103 | 6.5 (28) | 9.6 (50) | .080 |
| Nervous system | ||||||
| Zopiclone (N05CF01) | 22.1 (94) | 14.9 (57) | .008 | 25.6 (114) | 18.1 (72) | .009 |
| Temazepam (N05CD07) | 22.9 (73) | 11.2 (25) | <.001 | 23.6 (76) | 13.1 (30) | .002 |
| Citalopram (N06AB04) | 17.2 (59) | 21.8 (29) | .246 | 19.3 (68) | 22.4 (30) | .451 |
| Mirtazapine (N06AX11) | 24.6 (73) | 31.4 (38) | .152 | 15.2 (40) | 25.2 (28) | .021 |
| Donepezil (N06DA02) | 17.0 (39) | 29.5 (18) | .029 | 10.0 (21) | 6.5 (3) | .469 |
| Memantine (N06DX01) | 30.6 (75) | 31.3 (21) | .909 | 4.0 (7) | 2.1 (1) | .548 |
| Paracetamol (N02BE01) | 39.0 (316) | 43.1 (169) | .174 | 29.5 (207) | 35.0 (120) | .074 |
| Alimentary tract and metabolism | ||||||
| Lactulose (A06AD11) | 40.6 (162) | 40.2 (49) | .931 | 28.4 (94) | 38.1 (45) | .049 |
| Glimepiride (A10BB12) | 13.1 (26) | 6.1 (11) | .014 | 19.2 (41) | 13.8 (27) | .143 |
| Metformin (A10BA02) | 10.0 (27) | 14.0 (38) | .159 | 12.6 (35) | 9.7 (25) | .274 |
| Calcium combinations (A12AX) | 59.8 (297) | 38.6 (134) | <.001 | 41.0 (139) | 20.8 (56) | <.001 |
| Pantoprazole (A02BC02) | 28.2 (104) | 25.7 (67) | .485 | 23.4 (81) | 29.5 (81) | .088 |
ATC: anatomic therapeutic classification; DDD: defined daily dose.
Drug use was considered as started if patient did not fill any prescriptions for 1 year before ADD but filled at least one prescription for 1 year after ADD was initiated.
Drug use was considered discontinued if a patient did not fill any prescriptions for 1 year after ADD initiation but filled at least one prescription for 1 year before ADD was initiated.
Chi-squared test.
4. Discussion
To our knowledge, this is the first nationwide controlled study on the influence of ADD on drug use in primary care patients. The study findings suggest that ADD decreased drug use in a 1 year observation period. The decrease was found in more than half of the top 20 active substances used. Two of these drugs, temazepam and zopiclone, are potentially inappropriate hypnotics for geriatric patients [19]. ADD service patients also had more starts and discontinuations in their drug use than matched control patients.
The decrease in drug use may be related to two of the ADD service’s characteristics, a prescription review conducted and reduced amount of the drug wastage. First, the ADD procedure in Finland includes a prescription review for each patient before the enrollment. At minimum, doses, duplications and drug–drug interactions are checked during the prescription review [7]. As a consequence, this may lead to reduction in drug use, as suggested by our findings. If the medication review is properly conducted, it should also lead to qualitative changes in the individual patient’s medications in those cases with potentially inappropriate medications. In our study, the changes in drug use quality were indicated by the fact that hypnotic use was more often started and discontinued in the ADD service group. The daily doses of zopiclone and temazepam were also reduced. They are both medicines that should be avoided or at least their use should be limited to a minimum in older people, due to their short-term and long-term adverse effects [19,20]. Still, they are quite commonly used among older primary care patients.
Integrating medication reviews as part of the initiation of ADD is also supported by the fact that patients using ADD may have more inappropriate drugs in their regimens than patients using the standard dispensing procedure [10,11]. This can be considered as a natural consequence of the fact that the ADD service is planned to aid in managing medications for patients with multiple medications and chronic diseases. Thus, those enrolled in the service have more complex diseases and treatments [6]. This tendency was also found in our study. However, as this is a register-based study, it is difficult to interpret the exact effect of the decrease in drug use on, e.g. patient functional ability or quality of life. Further studies should explore ADD’s impact on drug use quality or patient welfare in more detail. Special focus should be on assessing the usefulness of medication reviews as part of the ADD service during the initiation phase and later.
Another reason for the reduced drug use in the ADD group may be reduced drug wastage, compared with the standard dispensing procedure, because in ADD, drugs are dispensed for a period of 14 days. Normally in Finland, drugs are dispensed for 3 months in packages of 30 or 100 tablets. If a drug is discontinued for a patient having the ADD service, only a maximum of 2 weeks’ drug supply is wasted. In the standard dispensing procedure, the wastage could be up to 3 months’ supply, i.e. six times more.
Starts and discontinuations observed in drug use may partly be artefacts, rather than actual events. These are related to the reimbursement system, since it does not cover all medicines and package sizes. In ADD, reimbursed medicines are favored. If non-reimbursed medicines are dispensed before the ADD service initiation and reimbursable medicines after it, this would appear to indicate a change (initiation) in the register data. Thus, the register data would be lacking some of the data needed to evaluate the impact of the ADD service on the appropriateness of the drug use. In future studies, the impact of ADD on medication costs should also be estimated.
4.1. Strengths and limitations of the study
The main strength of this nationwide study is the controlled study design that was applied. Patients’ gender, age, area of residence and number of drugs dispensed were used as matching criteria for the study and control groups. Moreover, the number of patients’ diseases was controlled in the statistical analysis. Another strength is that the data were collected from the register data that cover all reimbursed prescription drug purchases for primary care patients living in Finland. All permanent residents of Finland are entitled to have their drug costs refunded. The reimbursement system remained the same during the study period of 2006–2008. Thus, drug use changes or changes in patient proportions starting and discontinuing drug use could not be explained by fundamental changes in the reimbursement system.
The register data used in this study were routinely collected for administrative purposes, and thus, they do not necessarily represent the actual drug use in primary care. The data do not include drug use in institutions, over-the-counter drugs and drugs that are not reimbursed, e.g. small packages of some medicines. The fact that only reimbursed products were included in the register could have resembled an increase in drug use, especially in the study group, since reimbursed products are favored in ADD. However, this study found that drug use decreased in the study group.
An important issue that should be remembered when interpreting the results of this study is that the patients using the ADD service were a highly selected patient group. Despite the matching, the prevalence of chronic diseases was higher in the study than in the control group. This may be explained by the fact that ADD patients suffer more often from severe central nervous system diseases, leading to complicated drug combinations [10,11]. Therefore, drug consumption could be expected to be higher in the study than in the control group, however, drug consumption was decreased in the study group. In the future studies exclusion of the Alzheimer’s disease patients and patients suffering severe mental diseases should be considered since drug use in this patient group might be quite different compared patients’ not suffering these diseases. This exclusion might add reliability of the results regarding drug use. The patients of the study and control groups might also be quite different as users of the healthcare services since there were more chronic diseases and starts or discontinuations in their drug use among patients using the ADD service.
A strict matching and exclusion criteria were applied in the study. For each patient in the study group one control patient was chosen according matching criteria. The study group was a selected patient group and thus controls for all patients were not found. If a control patient was not found the patient from the study group was removed. This fact might cause selection bias in the results. Strict exclusion criteria were also applied in this study. This caused 15% reduction in the study population. In the future, it would be useful to study ambulatory care ADD service in a randomized controlled trial setting. However, the observational design applied in this study gives an important contribution to the body of the ADD research [6]. By matching it was possible to enhance equal distribution of the variables that might possibly confound the results regarding the drug use [14].
5. Conclusions
Drug use was decreased after initiation of the ADD service with medication review in primary care patients ≥65 years compared to matched controls in this 1 year cohort study. Further studies should be conducted in order to explore the causality, assess the ADD service’s impact on drug use quality and costs, as well as impact of accompanied prescription review on positive outcomes.
Acknowledgments
Pharmaservice, the dose-dispensing unit of the Espoonlahti Pharmacy, provided the patients’ identification data for this study.
Funding Statement
This study was funded by two grants received from the University Pharmacy of the University of Helsinki and the Association of Finnish Pharmacies.
Disclosure statement
Juha Sinnemäki was employed as a pharmacist in a privately own community pharmacy at the time the study was conducted. The pharmacy provided the ADD service for primary care patients. He is a former employee of the Association of Finnish Pharmacies. Marja Airaksinen is a former Board Member of the University Pharmacy of the University of Helsinki. The University Pharmacy also provides the ADD service for primary care patients. Both Maria Valaste and Leena K Saastamoinen are employed researchers at the Social Insurance Institution. The Institution reimburses the automated dose dispensing service fee according to explicit criteria.
Notes on contributors
Juha Sinnemäki, MSc (pharm.), PhD student, Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki, Helsinki, Finland.
Marja Airaksinen, PhD (pharm.), Professor, Division of Pharmacology and Pharmacotherapy, Faculty of Pharmacy, University of Helsinki, Helsinki, Finland.
Maria Valaste, PhD, senior researcher, Research Section, The Social Insurance Institution, Helsinki, Finland.
Leena K. Saastamoinen, PhD (pharm.), adjunct professor, senior researcher, Research Section, The Social Insurance Institution, Helsinki, Finland.
References
- 1.Fialová D, Topinková E, Gambassi G, et al. . Potentially inappropriate medication use among elderly home care patients in Europe. JAMA. 2005;293:1348–1358. [DOI] [PubMed] [Google Scholar]
- 2.Leikola S, Dimitrow M, Lyles A, et al. . Potentially inappropriate medication use among Finnish non-institutionalized people aged ≥65 years. A register-based, cross-sectional, national study. Drugs Aging. 2011;28:227–236. [DOI] [PubMed] [Google Scholar]
- 3.American Pharmacist Association; National Association of Chain Drug Stores Foundation Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0). J Am Pharm Assoc. 2008;48:341–353. [DOI] [PubMed] [Google Scholar]
- 4.Pharmaceutical Society of Australia Guidelines for pharmacist providing Home Medicines Review (HMR) services. 2016. [Internet]; [cited 2016 Mar 5]. Available from: http://www.psa.org.au/download/practice-guidelines/home-medicines-review-services.pdf.
- 5.Leikola S, Tuomainen L, Peura S, et al. . Comprehensive Medication Review – evidence-base of a collaborative procedure involving pharmacists. Int J Clin Pharm. 2012;34:510–514. [DOI] [PubMed] [Google Scholar]
- 6.Sinnemäki J, Sihvo S, Isojärvi J, et al. . Automated dose dispensing service for primary healthcare patients: a systematic review. Syst Rev. 2013;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinnemäki J, Saatamoinen LK, Hannula S, et al. . Starting an automated dose dispensing service provided by community pharmacies in Finland. Int J Clin Pharm. 2014;36:345–351. [DOI] [PubMed] [Google Scholar]
- 8.Apoteket AB. Annual Report. 2011. [Internet]; [cited 5 March 2013]. Available from: http://www.apoteket.se/privatpersoner/om/Documents/Apoteket%20AR-11%20eng_120629.pdf
- 9.Kwint H, Stolk G, Faber A, et al. . Medication adherance and knowledge of older patients with and without multidose drug dispensing. Age Ageing. 2013;42:620–626. [DOI] [PubMed] [Google Scholar]
- 10.Johnell K, Fastbom J.. Multi-dose drug dispensing and inappropriate drug use: a nationwide register-based study of over 700,000 elderly. Scand J Prim Health Care. 2008;26:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sjöberg C, Edward C, Fastbom J, et al. . Association between multi-dose drug dispensing and quality of drug treatment – a register-based study. PLoS One. 2011;6:e26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wekre LJ, Spigset O, Sletvold O, et al. . Multidose drug dispensing and discrepancies between medication records. Qual Saf Health Care. 2010;19:1–4. [DOI] [PubMed] [Google Scholar]
- 13.Kwint HF, Faber A, Gussekloo J, et al. . Effects of medication review on drug-related problems in patients using automated drug-dispensing systems: a pragmatic randomized controlled study. Drugs Aging. 2011;28:305–314. [DOI] [PubMed] [Google Scholar]
- 14.de Graaf MA, Jager KJ, Zoccali C, et al. . Matching, an appealing method to avoid confounding? Nephron Clin Pract. 2011;118:315–318. [DOI] [PubMed] [Google Scholar]
- 15.WHO Collaborating Centre for Drug Statistics Methodology Guidelines for ATC classification and DDD assignment. 2013. [Internet]; [cited 2013 Mar 5]. Available from: http://www.whocc.no/filearchive/publications/1_2013guidelines.pdf.
- 16.Furu K, Wettermark B, Andersen M, et al. . The nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106:86–94. [DOI] [PubMed] [Google Scholar]
- 17.Kaikkonen P, Harsia-Alatalo J.. Medicine reimbursement system and approval of medicine prices In: Finnish Medicines Agency Fimea and Social Insurance Institution. Finnish statistics on medicines. Helsinki: Edita Prima Oy; 2012. p. 71–80. [Google Scholar]
- 18.Martikainen J, Häkkinen U, Enlund H.. Adoption of new antiglaucoma drugs in Finland: impact of changes in copayment. Clin Ther. 2007;29:2468–2476. [DOI] [PubMed] [Google Scholar]
- 19.American Geriatrics Society 2012 Beers Criteria Update Expert Panel American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilmer SN, Mager DE, Simonsick EM, et al. . A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167:781–787. [DOI] [PubMed] [Google Scholar]