Abstract
Background
Systemic antioxidants, such as oral Polypodium leucotomos extract (PLE), have been found to have photoprotective properties.
Objective
This study was designed to objectively evaluate the molecular and photobiologic effects of oral administration of PLE.
Methods
22 subjects with Fitzpatrick skin phototype I–III were enrolled. On day 1, subjects were irradiated with visible light, ultraviolet A1 (UVA1) and ultraviolet B (UVB; using 308-nm excimer laser). Evaluation was done immediately and 24 hours after irradiation. On days 3 and 4, irradiation and evaluation process was repeated after ingestion of PLE.
Results
Clinical assessments and colorimetry data showed a decrease in UVB -induced changes in 17 out of 22 subjects post-PLE administration; histology findings demonstrated such a decrease in all 22 subjects.
Limitations
Only 2 doses of PLE were given. Furthermore, subjects with skin phototypes I–III only were studied.
Conclusion
This is the first demonstration that PLE has objective measurable suppressive effects on UVB-induced erythema within 2 hours of administration. The results suggest that PLE can potentially be utilized as an adjunctive therapy to lessen the negative photobiologic effects of UVB. In addition, this is the first study to demonstrate the potential correlation between non-invasive colorimetry outcomes and UVB induced molecular damage.
Keywords: Polypodium leucotomos extract, Minimal erythema dose, Ultraviolet, Excimer Laser, Colorimetry, Cyclobutane pyrimidine dimers, Sunburn cells
INTRODUCTION
Biologic effects of exposure to solar radiation include erythema, tanning, photoaging and photocarcinogenesis. Most of these are due to the direct and indirect effects of ultraviolet (UV) radiation. However, for those with skin phototypes IV–VI, visible light has been shown to induce intense and persistent pigmentation.1–3
Sunscreen and photoprotective clothing are effective photoprotective measures. However, neither of these are sufficiently utilized. Studies show that sunscreens are applied at below half the tested concentration, and protective clothing is not worn due to the subject’s concern about heat retention.4, 5 In addition, transparent organic and inorganic sunscreens do not prevent transmission of wavelengths in the visible spectrum.6 Therefore, oral supplements with photoprotective properties may be helpful in reducing UV-induced injury when other photoprotective measures fail.
Polypodium leucotomos is a tropical fern grown in Central and South America whose extract (PLE) has photoprotective benefits through its antioxidative, chemoprotective, immunomodulatory, and anti-inflammatory effects.7 These properties are believed to be due to several of the fern’s polyphenols: caffeic, chlorogenic, ferulic, hydroxycinnamic, p-coumaric, and vanillic acids.7 PLE. As an antioxidant, PLE enhances the ability of endogenous antioxidant systems to neutralize superoxide anions, lipid peroxides and hydroxyl radicals, which are formed in the skin following exposure to UV and visible radiation.8–10 In addition, lower levels of UV-induced COX-2 expression, p53 suppressor gene mutations, cyclobutane pyrimidine dimers, epidermal proliferation, sunburn cells, and inflammatory infiltrate are seen in vitro and in animal modules following PLE administration.11, 12 Cumulatively, these findings form the scientific basis behind previously reported human clinical trials evaluating the use of PLEin the treatment of vitiligo, melasma, and polymorphous light eruption, and in the prevention of skin cancer.11, 13–19
Given the growing evidence supporting PLE’s photoprotective effect and its broadening use in management of cutaneous disorders, this study was designed to quantify its effect on minimal erythema dose (MED) and clinicaland histological changes in skin phototypes I–III subjects after exposure to visible light, UVA1 (UVA1) and B (UVB) radiation, the latter using 308-nm excimer laser as the light source.
MATERIALS AND METHODS
Study subjects
Twenty-two healthy men and women with skin phototypes I–III were enrolled. The study was approved by the institutional review board of Henry Ford Hospital (IRB #8386)in August, 2013. Informed consent was obtained from all subjects. All guidelines from the Declaration of Helsinki were followed. Subjects with history of skin cancer, photoaggravated conditions or medication were excluded. Those who were active tanners, pregnant, lactating, or known to have hypersensitivity to PLE were excluded. All subjects stated a willingness to limit participation in increased outdoor activities during the trial. A urine pregnancy test was performed on females reporting no menstruation in the prior 3 weeks. The first subject was enrolled on Dec 16, 2013.
Study design
On day 1, each subject was irradiated with very pure visible light, UVA1 and UVB on the left side of the back; the spectral output of the light sources is shown in Table I. On day 2, 24 hours following irradiation, assessments including clinical photography, investigator’s global assessment (IGA)of erythema and pigmentation for each site, M ED, colorimetry, and skin biopsies were performed. MED was defined as trace erythema within the irradiated area.20 This process of irradiation and assessment was also repeated on days3 and 4, respectively. However, on day 3 subjects ingested 240 mg of PLE, obtained from Ferndale Laboratories (Ferndale, MI), 2 hours and 1 hour prior to irradiation (i.e, 480 mg total); irradiation was performed on the right side of the back.
Table I.
Spectral output of light sources and doses used
| Visible light radiation source | UVA1 radiation source | UVB radiation source | ||
|---|---|---|---|---|
| Device | Dolan Jenner Fiber Lite 180 | Hamamatsu Lightningcure 200 | Photomedex Xtrac Excimer Laser | |
| Spectral distribution % | UVB (290–320 nm) | 0.000003% | 0.00006% | 308 nm |
| UVA2 (320–340 nm) | 0.0000007% | 0.00008% | ||
| UVA1(340–400 nm) | 0.00004% | 97.9% | - | |
| Visible light (400–700 nm) | 95.8% | 0.74% | - | |
| Near infrared | 4.17% (700–1600 nm) | 1.37% (700–800 nm) | - | |
| Doses (J/cm2) | 80, 160, 320, 480 | 22, 27, 33, 39, 47 | 0.10, 0.15, 0.20, 0.25, 0.30, 0.35 | |
Abbreviations: UVA, ultraviolet A; UVA1, ultraviolet A1; UVB, ultraviolet B.
Light sources and irradiation
The visible light source was Fiber-Lite Model 180 (Dolan-Jenner Industries, Boxborough, MA) with a 150W EKE lamp with filter GG400/3mm (Schott North America, Inc., Duryea, PA) and 3-mm hot mirror (Andover Corporation, Salem, NH).
The UVA1 light source was Hamamatsu LightingCure UV Spot Light 200, 200W (Hamamatsu Photonics, Bridgewater, NJ). It emits from 240–400 nm; 2 filters were used, 3-mm WG-345 and 2-mm UG-11 (Schott North America, Inc.), which resulted in pure UVA1 (340–400 nm) radiation. The fluence-rate for both light sources was adjusted to 25 and 225 mW/cm2 for UVA1 and visible light, respectively, using an Oriel thermopile(Oriel, Stamford, CT). For irradiation, a liquid light guide with an 8-mm diameter was used for both visible light and UVA1 light source. Four visible light doses (80, 160, 320 and 480 J/cm2), and five UVA1 doses (22, 27, 33, 39, 47 J/cm2) were administered(Table I); these dose ranges were used based on the results of previous studies.3, 21 The UVB source was a 308 -nm excimer laser (Xtrac, Photomedex, Montgomeryville, PA). A spot size of 3.2 cm2 was irradiated. Standard UVB MED testing technique was used, which involves administration of6 doses (100, 150, 200, 250, 300 and 350 mJ/cm2) on the subject’s back and assessments made at 24 hours post -irradiation. The same set of doses were administered on either side (left and right) of the subject’s back. The left side of the back was irradiated on day 1, and pre-PLE assessments were made on day 2. The right side of the back was irradiated on day 3, after PLE ingestion. Post-PLE assessments were made on day 4.
Assessments
Clinical photography and IGA
Clinical photographs of the back of the subjects were taken, and IGA scores for erythema and pigmentation were assigned to each site. The same investigator assigned the IGA score pre-and post -PLE administration. The IGA scale used for this study, shown in Table S1, was developed by the study investigators at Henry Ford Hospital. An IGA score of 1 (trace erythema) was defined as the MED.
Colorimetry
It is a noninvasive objective assessment technique used to quantitatively assess erythema and pigmentation. The colorimeter consists of a spectrophotometer (Konica Minolta CM-2600d, Osaka, Japan), a xenon arc lamp and a computer. The site to be assessed was uniformly illuminated with visible light and information from the reflectance spectra was expressed as L* (lightness to darkness), a* (green to red), and b* (blue to yellow) color parameters. The “a*” value helps to objectively assess the intensity of erythema. It was normalized against the corresponding value of the subject’s adjacent normal un -irradiated skin “ao*” by calculating the ratio of a*/ao*, referred to as the relative erythema intensity. Subjects thus acted as their own control. At clinically perceptible trace erythema, an IGA of 1, the relative erythema intensity was 1.6 +0.3. Colorimetry measurements were performed for all sites during each visit.
Histology
Skin biopsies were performed on day 2 and day 4. On day 2, two 4-mm punch biopsies were obtained from unirradiated, normal skin (control biopsy) and the site of MED/trace erythema (left side of back). On day 4, another4 -mm punch biopsy was obtained from the site of MED/trace erythema (right side of back). All 3 skin biopsies were stained for markers of inflammation (COX-2), apoptosis (sunburn cells), DNA damage (cyclobutane pyrimidine dimers), and cell proliferation (cyclin D1 and proliferating cell nuclear antigen).
Immunohistochemistry
Biopsy samples were embedded in paraffin wax and sectioned at 5 μm. After de-paraffinization and rehydration, the manufacturer’s protocol was followed for antigen retrieval (Antigen Unmasking Solution, Vector Laboratories, Burlingame, CA). Expose Mouse and Rabbit Specific HRP/DAB detection IHC kit (Abcam, Cambridge, MA) was used for immune-histochemical staining of different antigens. Additional sections running in parallel but with omission of the primary antibodies served as the negative controls.
Antibodies for proliferating cell nuclear antigen, Ki67, cyclin D1 andCox-2 were purchased from Abcam. Cyclobutane pyrimidine dimers were stained with anti-thymine dimer antibody from Kamiya Biomedical Company (Seattle, WA).
The sunburn cells were counted on the hematoxylin and eosin stained slides. The stained sections were examined with an Olympus BX51 microscope fitted with an Olympus DP71 digital camera (Olympus America, Inc., Center Valley, PA). The number of positive cells at 40x magnification were counted (cyclobutane pyrimidine dimers, Ki67, proliferating cell nuclear antigen and cyclin D1) and an average from 6 randomly selected and distinct fields was taken as the final score. The staining intensity for COX-2 were scored on a basis of 5 staining intensity levels (0 = negative, 1 = weak, 2 = moderate, 3 = strong, and 4 = very strong). An average score of intensities from 6 different fields at 40x was calculated for each biopsy. All specimens were read in a blinded manner.
Statistical analysis
Statistical analysis was performed to compare the MED values, IGA scores, relative erythema intensity, dose response slope (DR slope) and histology findings of day 2, referred to as pre-PLE, to those on day 4, referred to as post-PLE. Percent reduction in biomarkers was assessed by the following(Table II):
Table II.
Values for UV-induced biomarkers pre- and post-PLE
| Biomarker | Units | Non-UV treated skin1 | UVB irradiated skin pre-PLE1 | UVB irradiated skin post-PLE1 | % reduction following PLE | P value (pre-vs post-PLE) |
|---|---|---|---|---|---|---|
| Proliferating cell nuclear antigen | Number of positive cells/40x field | 80.4 ± 17.8 | 134.7 ± 29.8 | 89.9 ± 23.6 | 83% | <.0005 |
| Sunburn cells | Number of positive cells/40x field | 8.2 ± 3.2 | 20.1 ± 9.0 | 11.0 ± 4.6 | 76% | .00024 |
| Cyclobutane pyrimidine dimers | Number of positive cells/40x field | 10.0 ± 5.3 | 97.0 ± 30.2 | 69.5 ± 23.3 | 32% | .001 |
| Cyclin D1 | Number of positive cells/40x field | 37.1 ± 20.8 | 71.7 ± 37 | 42.2 ± 25 | 85% | .0052 |
| COX-2 | Average staining intensity | 2.0 ± 0.6 | 3.8 ± 0.4 | 2.4 ± 0.05 | 78% | <.0005 |
| Ki67 | Number of positive cells/40x field | 8.8 ± 3.0 | 14.5 ± 3.4 | 8.6 ± 2.5 | 100% | <.0005 |
PLE, Polypodium leucotomos extract; UV, ultraviolet.
Mean ± standard deviation for 22 patients
Mean = average of 6 fields/section at 40x
Comparisons were made using two-tailed paired-sample t-test. In cases where distributional normality was significantly violated, the non-parametric Wilcoxon signed-rank test was used due to the paired nature of the data. Statistical significance was set at P< .05 and all analyses were done using OriginPro software (version 9.1, OriginLab Corporation, Northampton MA).
RESULTS
All subjects exhibited a baseline erythematous response to UVB irradiation, but limited -to-no response was observed following exposure to UVA1 and visible light. Thus, the remainder of this section will focus on results related to UVB irradiation sites. A decrease in UVB -induced changes was seen post-PLE administration. This decrease was detected by noninvasive clinical assessments, colorimetry, as well as by histology.
Clinical photography and investigator’s global assessment (IGA)
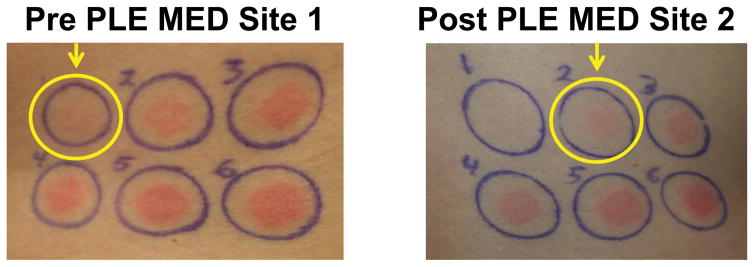
Seven of 22 subjects exhibited an increase in MED following PLE administration, as assessed by IGA. Figure 1 is a set of representative clinical photographs demonstrating an increase in MED for a subject from 100 mJ/cm2 pre -PLE to 150 mJ/cm2 post -PLE administration. The increase in the post-PLE MED values did not reach statistical significance (P> .05).
Figure 1.
(UVB response) Pre -PLE MED site 1 (left), post-PLE MED site 2 (right), is a set of representative clinical photographs demonstrating an increase in MED for a subject from 100 mJ/cm2 pre -PLE to 150 mJ/cm2 post -PLE administration. Abbreviations: MED, minimal erythema dose; PLE, Polypodium leucotomos extract.
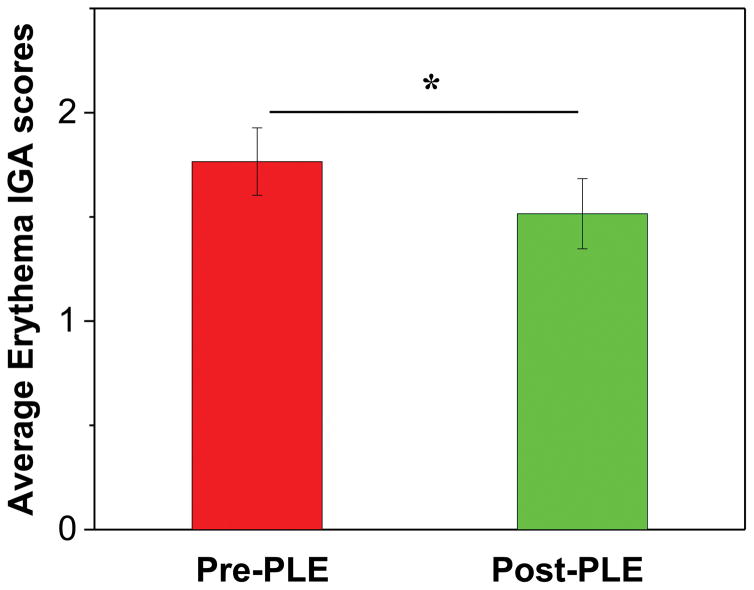
Each subject’s mean IGA score over the 6 combined doses was calculated from the left (pre-PLE) and the right (post-PLE) side. The post-PLE IGA scores were 19 % lower than the pre-PLE (P< .05) (Fig 2).
Figure 2.
(UVB response)Average erythema investigator’s global assessment scores. Mean ± standard error of the mean. Asterisk represents statistically significant change (P <.05). Abbreviations: IGA, investigator’s global assessment; PLE, Polypodium leucotomos extract.
Colorimetry
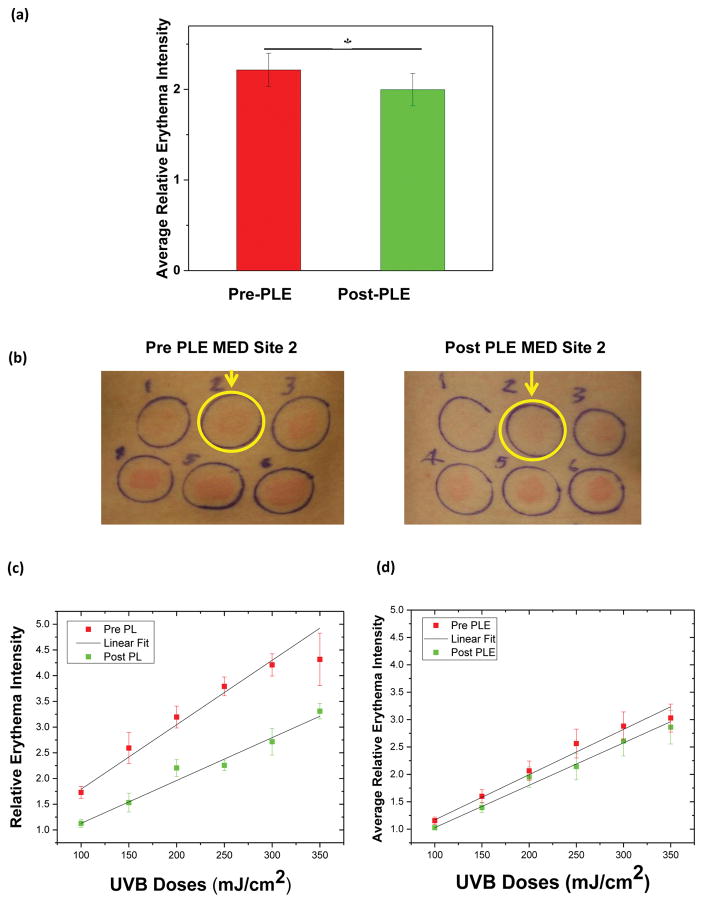
The projection of true color of L* a* and b* on the a* axis was analyzed for objectively assessing relative erythema intensity. This normalized value represented the number of folds the irradiated site was higher in erythema intensity compared to adjacent normal skin. Each subject’s mean relative erythema intensity over the 6 combined UVB doses was calculated from the left (pre-PLE) and the right (post-PLE) side. The post -PLE relative erythema intensity was 8% lower than that of the pre-PLE (P< .05) (Fig 3a). This is a substantial difference considering the fact that the quantity compared was the number of folds change from baseline for pre- and post-PLE.
Figure 3.
(UVB response) (a) Average pre -and post -PLE relative erythema intensity. Mean ± standard error of the mean. Asterisk represents statistically significant change (P <.05). (b) Pre-PLE MED site 2, 150 mJ/cm2 (left), post-PLE MED site 2, 150 mJ/cm2 (right), is a set of representative clinical photographs demonstrating the decrease in erythema intensity although no change in MED. (c)Pre and post -PLE relative erythema intensity as a function of UVB dose for 1 subject. Mean ± standard deviation. The pre PLE MED for this subject was 100 (linear range 50 – 280 mJ/cm2) and post-PLE MED was 150 (linear range 75–420 mJ/cm2). Although all data points have been shown in the graph, slopes were calculated corresponding to the linear section only. (d) Average pre and post-PLE relative erythema intensity for all subjects as a function of UVB doses. Mean ± standard error of the mean. Abbreviations: MED, minimal erythe ma dose; PLE, Polypodium leucotomos extract; UVB, ultraviolet B.
An increase in MED was detected for 7of the 22 subjects by both colorimetry and IGA scores. In another 10 subjects, the MED (assessed by subjective IGA) remained the same post-PLE; however, there was a decrease in erythema intensity for a given dose of UVB as assessed objectively by colorimetry (Fig 3b). Thus, 17 of 22 subjects demonstrated a decrease in UVB -induced changes following PLE administration. Four subjects remained same with no change in MED or erythema intensity, and one subject had a decrease in MED.
Figure 3c is a representative of pre - and post-PLE relative erythema intensity for 1 subject as a function of UVB doses. It clearly reflects a decrease in the magnitude of the relative erythema intensity post -PLE and also shows the difference in the slope with post-PLE being less steep. This slope for pre -and post -PLE as a function of UVB doses, was analyzed for each subject and is referred to as the DR slope. The DR was found to be linear in the dose range of 0.5–2.8 MED. In this domain, the average post-PLE DR slope was 18% lower than the pre-PLE (P< .05).
Histology
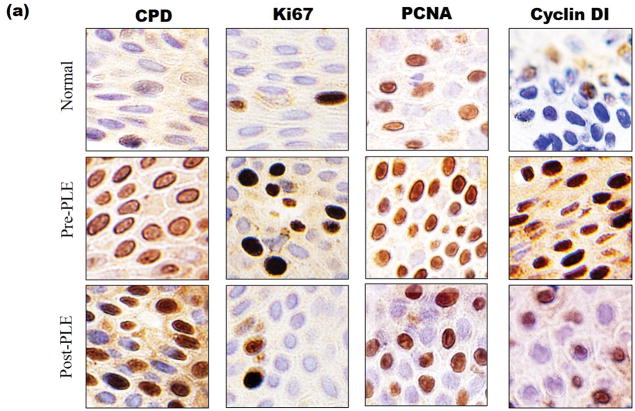
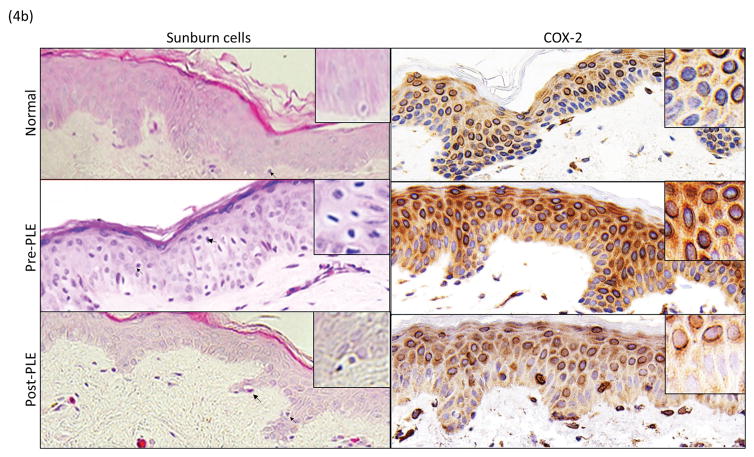
Studies were conducted to evaluate the effect of PLE on biomarkers associated with UV damage. These included parameters associated with DNA damage and apoptosis (sunburn cells and cyclobutane pyrimidine dimers), inflammation (COX-2) and proliferation (cyclin D1, Ki67 and proliferating cell nuclear antigen). There was a significant reduction in the deleterious effects of UV radiation on all of these biomarkers (P <.05) (Table II and Figs 4a and b). For, cyclobutane pyrimidine dimers, the improvement was 32%. For the other biomarkers, the improvement ranged from 76%–100%.
Figure 4.
(UVB response)(a) Changes in biomarkers of UV radiation exposure before and after PLE. Representative photomicrographs (20x) of stained nuclei for CPD, Ki67, PCNA and Cyclin D1. (b)Changes in biomarkers of UV radiation exposure before and after PLE. Representative photomicrographs (20x) of sunburn cells and cytoplasmic staining for COX-2. Abbreviations: CPD, cyclobutane pyrimidine dimers; MED, minimum erythema dose; PCNA, proliferating cell nuclear antigen; PLE, Polypodium leucotomos extract; UV, ultraviolet.
DISCUSSION
This is the first demonstration that PLE has objective measurable suppressive effects on UVB -induced erythema within 2 hours of administration, which provides further insight on the photoprotective effects of PLE. PLE did exhibit significant chemoprotective and anti-inflammatory properties against UVB-induced damage as indicated by a decrease in the IGA scores, relative erythema intensity as well as associated biomarkers following PLE administration.
Lighter skin individuals exhibit a steep DR slope, implying that a small increase in dose will result in a considerable increase in erythema. On the other hand, darker skin individuals have been shown to have a relatively flatter/less steep DR slope indicating the need of larger dose increments to induce differences in erythema.22 The less steep post -PLE DR slope in our study (Fig 3c) indicates that PLE had induced tolerance to UVB radiation, shifting the subject’s response towards that of a higher/darker skin phototype. Thus, an individual’s photobiologic responses (clinical and molecular) to UV radiation across a spectrum of dosages, which we term one’s photocapacity, improves following PLE ingestion.
Molecular damage has been reported to have a linear association after subjects were exposed to 0.5–3 MEDs of solar -simulating radiation;23 our study found similar linear association for relative erythema intensity and UVB doses(Fig 3d). This demonstrates a potential correlation between the colorimetry outcome of relative erythema intensity and molecular damage, suggesting the possibility of using colorimetry to serve as a noninvasive surrogate for quantification of cyclobutane pyrimidine dimers formation.
Our results, and those of previous studies, showed that PLE is a promising adjunctive method of oral photoprotection to traditional methods of photoprotection, which includes seeking shade, wearing photoprotective clothing, wide brimmed hats and sunglasses, and using sunscreen on exposed areas. While traditional photoprotection limits photon exposure, oral antioxidants and photoprotectants, such as PLE, green tea extract, and silymarin, reduce free-radical induced cellular damage and thereby may improve the biologic photocapacity of individuals.24 There are limitations to our study. Only 2 doses of PLE were given 2 hours before exposure to UVB radiation. Furthermore, to facilitate evaluation of erythema, only subjects with skin phototypes I–III were studied. The UVA1 and visible light doses administered could have been on the lower side for the skin phototypes included in the study to induce erythema and pigmentation responses. This most likely is the major reason that our investigation did not show an effect of PLE on UVA1 or visible light.
Future objective clinical investigations on subjects with skin phototypes IV–VI are needed to determine the role of PLE in mitigating the undesired effects of visible light and UVA irradiation. Additionally, studies assessing the optimal dosage and duration of administration are needed to maximize the potential systemic photoprotective effects of PLE.
Supplementary Material
Acknowledgments
Funding source: This study was sponsored by Ferndale Healthcare, Inc.
ABBREVIATIONS
- CPD
cyclobutane pyrimidine dimers
- DR
dose response
- IGA
investigator’s global assessment
- MED
minimum erythema dose
- PCNA
proliferating cell nuclear antigen
- PLE
Polypodium leucotomos extract
- UV
ultraviolet
- UVA1
ultraviolet A1
- UVB
ultraviolet B
Footnotes
Prior Presentations: Oral presentations at the 3rd Annual Meeting of the Skin of Color Society (SOCS); San Francisco, CA; March 2015; and at the 23rd Annual Meeting of the Photomedicine Society; Denver, CO; March 2014. Poster presentation at the 2015 Fall Clinical Dermatology Conference; Las Vegas, NV; October 2015 and the 23rd World Congress of Dermatology; Vancouver, BC, Canada; June 2015.
Clinical Trials Registration: Not Applicable
Conflict of Interest: P.I. is a sub-investigator for Ferndale Laboratories, Estee Lauder and Clinuvel. I.K. is a sub-investigator for Ferndale Laboratories, Estee Lauder, Johnson and Johnson and Allergan. J.L.G. is a sub-investigator for Ferndale Laboratories. C.A.E. is an investigator and consultant for Ferndale Laboratories. H.W.L. has served as a consultant for Pierre Fabre, and has received research grant support from Estee Lauder, Ferndale Laboratories and Allergan. I.H.H is an investigator for Ferndale Laboratories, Estee Lauder, Allergan, Johnson and Johnson and Clinuvel.
References
- 1.Duteil L, Cardot-Leccia N, Queille-Roussel C, Maubert Y, Harmelin Y, Boukari F, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822–6. doi: 10.1111/pcmr.12273. [DOI] [PubMed] [Google Scholar]
- 2.Hexsel CL, Mahmoud BH, Mitchell D, Rivard J, Owen M, Strickland FM, et al. A clinical trial and molecular study of photoadaptation in vitiligo. Br J Dermatol. 2009;160:534–9. doi: 10.1111/j.1365-2133.2008.08943.x. [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud BH, Ruvolo E, Hexsel CL, Liu Y, Owen MR, Kollias N, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. The Journal of investigative dermatology. 2010;130:2092–7. doi: 10.1038/jid.2010.95. [DOI] [PubMed] [Google Scholar]
- 4.Boggild AK, From L. Barriers to sun safety in a Canadian outpatient population. J Cutan Med Surg. 2003;7:292–9. doi: 10.1007/s10227-003-0126-9. [DOI] [PubMed] [Google Scholar]
- 5.Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Arch Dermatol. 2002;138:1319–25. doi: 10.1001/archderm.138.10.1319. [DOI] [PubMed] [Google Scholar]
- 6.Kaye ET, Levin JA, Blank IH, Arndt KA, Anderson RR. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351–5. [PubMed] [Google Scholar]
- 7.Choudhry SZ, Bhatia N, Ceilley R, Hougeir F, Lieberman R, Hamzavi I, et al. Role of oral Polypodium leucotomos extract in dermatologic diseases: a review of the literature. J Drugs Dermatol. 2014;13:148–53. [PubMed] [Google Scholar]
- 8.Gomes AJ, Lunardi CN, Gonzalez S, Tedesco AC. The antioxidant action of Polypodium leucotomos extract and kojic acid: reactions with reactive oxygen species. Braz J Med Biol Res. 2001;34:1487–94. doi: 10.1590/s0100-879x2001001100018. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez S, Pathak MA. Inhibition of ultraviolet-induced formation of reactive oxygen species, lipid peroxidation, erythema and skin photosensitization by polypodium leucotomos. Photodermatol Photoimmunol Photomed. 1996;12:45–56. doi: 10.1111/j.1600-0781.1996.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 10.Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901–7. doi: 10.1038/jid.2011.476. [DOI] [PubMed] [Google Scholar]
- 11.Middelkamp-Hup MA, Pathak MA, Parrado C, Goukassian D, Rius-Diaz F, Mihm MC, et al. Oral Polypodium leucotomos extract decreases ultraviolet-induced damage of human skin. J Am Acad Dermatol. 2004;51:910–8. doi: 10.1016/j.jaad.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Zattra E, Coleman C, Arad S, Helms E, Levine D, Bord E, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol. 2009;175:1952–61. doi: 10.2353/ajpath.2009.090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilera P, Carrera C, Puig-Butille JA, Badenas C, Lecha M, Gonzalez S, et al. Benefits of oral Polypodium leucotomos extract in MM high-risk patients. J Eur Acad Dermatol Venereol. 2013;27:1095–100. doi: 10.1111/j.1468-3083.2012.04659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caccialanza M, Recalcati S, Piccinno R. Oral polypodium leucotomos extract photoprotective activity in 57 patients with idiopathic photodermatoses. G Ital Dermatol Venereol. 2011;146:85–7. [PubMed] [Google Scholar]
- 15.De Las Heras ME, Ledo E, González S, Rocamora A, Ledo A. Polypodium leucotomos extract as adjuvant to PUVA therapy in the treatment of plaque psoriasis. Med Cutan Ibero Lat Am. 1997;25:103–7. [Google Scholar]
- 16.Middelkamp-Hup MA, Bos JD, Rius-Diaz F, Gonzalez S, Westerhof W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2007;21:942–50. doi: 10.1111/j.1468-3083.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Bosca A, Zapater P, Betlloch I, Albero F, Martinez A, Diaz-Alperi J, et al. Polypodium leucotomos extract in atopic dermatitis: a randomized, double-blind, placebo-controlled, multicenter trial. Actas Dermosifiliogr. 2012;103:599–607. doi: 10.1016/j.ad.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Tanew A, Radakovic S, Gonzalez S, Venturini M, Calzavara-Pinton P. Oral administration of a hydrophilic extract of Polypodium leucotomos for the prevention of polymorphic light eruption. J Am Acad Dermatol. 2012;66:58–62. doi: 10.1016/j.jaad.2010.09.773. [DOI] [PubMed] [Google Scholar]
- 19.Villa A, Viera MH, Amini S, Huo R, Perez O, Ruiz P, et al. Decrease of ultraviolet A light-induced “common deletion” in healthy volunteers after oral Polypodium leucotomos extract supplement in a randomized clinical trial. J Am Acad Dermatol. 2010;62:511–3. doi: 10.1016/j.jaad.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Otman SG, Edwards C, Gambles B, Anstey AV. Validation of a semiautomated method of minimal erythema dose testing for narrowband ultraviolet B phototherapy. Br J Dermatol. 2006;155:416–21. doi: 10.1111/j.1365-2133.2006.07273.x. [DOI] [PubMed] [Google Scholar]
- 21.Moyal D, Chardon A, Kollias N. Determination of UVA protection factors using the persistent pigment darkening (PPD) as the end point. Potodermatol Photoimmunol Photomed. 2000;16:245–9. doi: 10.1034/j.1600-0781.2000.160602.x. [DOI] [PubMed] [Google Scholar]
- 22.Westerhof W, Estevez-Uscanga O, Meens J, Kammeyer A, Durocq M, Cario I. The relation between constitutional skin color and photosensitivity estimated from UV-induced erythema and pigmentation dose-response curves. J Invest Dermatol. 1990;94:812–6. doi: 10.1111/1523-1747.ep12874671. [DOI] [PubMed] [Google Scholar]
- 23.Young AR, Potten CS, Nikaido O, Parsons PG, Boenders J, Ramsden JM, et al. Human melanocytes and keratinocytes exposed to UVB or UVA in vivo show comparable levels of thymine dimers. J Invest Dermatol. 1998;111:936–40. doi: 10.1046/j.1523-1747.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol. 2012;67:1013–24. doi: 10.1016/j.jaad.2012.02.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.