Abstract
Aim
This study aimed to evaluate the treatment result of intensity-modulated radiation therapy (IMRT) in a large number of Japanese patients with prostate cancer.
Background
A total of 1091 patients with localized prostate cancer were recruited between March 2006 and July 2014. The patients were stratified into low- (n = 205 [18.8%]), intermediate- (n = 450 [41.2%]), high- (n = 345 [31.6%]), and very high-risk (n = 91 [8.3%]) groups according to the National Comprehensive Cancer Network classification. All patients were irradiated via IMRT at a dose of 74–78 Gy with or without androgen-deprivation therapy. The mean follow-up period was 50 months (range, 2–120 months).
Results
The biochemical failure-free rate (BFFR), the clinical failure-free rate, and the overall survival rate at the 5-year follow-up for all patients was 91.3%, 96.2%, and 99.1%, respectively. In univariate analysis, the prostate-specific antigen (PSA) levels (≤20 vs. >20 ng/ml) were significantly correlated with BFFR. A trend toward higher BFFR was noted in patients with a Gleason score (GS) of ≤7 than in patients with GS ≥8. In multivariate analysis, only PSA (≤20 vs. >20 ng/ml) was significantly correlated with BFFR. The cumulative incidence rate of gastrointestinal and genitourinary toxicity (≥grade 2) at the 5-year follow-up was 11.4% and 4.3%, respectively.
Conclusions
The findings of this study indicate that IMRT is well tolerated and is associated with both good long-term tumor control and excellent outcomes in patients with localized prostate cancer.
Keywords: Prostate cancer, Intensity-modulated radiation therapy, External beam radiation therapy
1. Background and aim
In 2012, the world wide incidence of prostate cancer was approximately 1,095,000 cases, resulting in 307,000 deaths.1 In Japan, prostate cancer is the most commonly diagnosed cancer in men, with a projected incidence of 92,600 cases (16% of 576,100 cancer cases at all primary sites) in 2016. In 2014, 11,507 patients died of prostate cancer.2
Among the optimal treatment options for prostate cancer is external beam radiotherapy3; it is less invasive than other treatment modalities and can be administered to patients with comorbidities for which surgery is contraindicated. Intensity-modulated radiation therapy (IMRT) has been widely used for external beam irradiation of localized prostate cancers. Several studies have revealed that IMRT is safer than three-dimensional conformal radiation therapy (3D-CRT), particularly in terms of gastrointestinal (GI) adverse events.4, 5, 6, 7 The safety of IMRT also allows dose escalation, leading to better tumor control.8 However, most large, single-institution studies on the efficacy of IMRT for localized prostate cancer have been performed in Western institutions. To the best of our knowledge, studies with more than 1000 patients with prostate cancer treated with IMRT are yet to be performed in Japan. Because the physique and prostate glands of Asians are smaller than those of Westerners, the outcomes and toxicity might differ between Asian and Western patients. Accordingly, this retrospective study aimed to evaluate the outcomes and late toxicity associated with IMRT among Japanese patients with prostate cancer.
2. Material and methods
2.1. Patients
Between March 2006 and July 2014, 1252 patients with prostate cancer were treated with IMRT at our institution. Patients with lymph node and distant metastases and those whose prostate-specific antigen (PSA) levels were not measured at least once after IMRT were excluded. As a result, 1091 patients were included in this analysis. Patient characteristics are shown in Table 1. All tumors were histologically confirmed as adenocarcinomas. The median patient age was 70 years (range, 38–80 years). The median PSA level at diagnosis was 8.53 ng/ml (range, 2.65–370.00 ng/ml). A total of 427 (39.1%), 331 (30.3%), 38 (3.5%), 125 (11.5%), 122 (11.2%), 39 (3.6%), and 9 (0.8%) patients had cT1c, T2a, T2b, T2c, T3a, T3b, and T4, respectively. The patients had a Gleason Score (GS) of ≤6 (n = 316 [29.0%]), 7 (n = 482 [44.2%]), 8 (n = 175 [16.0%]), 9 (n = 104 [9.5%]), and 10 (n = 14 [1.3%]). Following the National Comprehensive Cancer Network (NCCN) classification, 205 (18.8%), 450 (41.2%), 345 (31.6%), and 91 (8.3%) patients belonged to the low-, intermediate-, high-, and very high-risk groups, respectively.9 Low-risk was defined as T1c–T2a, PSA levels <10 ng/ml, and GS ≤6. Intermediate-risk was defined as T2b–T2c, GS = 7, or PSA levels of 10–20 ng/ml. High-risk was defined as T3a, ≤3 cores with GS 8–9 (primary GS ≠ 5). Very high-risk was defined as T3b–T4, primary GS = 5, or >4 cores with GS = 8–10. The mean follow-up period was 50 months (range, 2–120 months).
Table 1.
Patient characteristics (N = 1091).
| Median age (range) (years) | 70 (38–80) |
| Median initial PSA (range) (ng/ml) | 8.53 (2.65–370.00) |
| <10 | 630 (57.7%) |
| ≥10, ≤20 | 281 (25.8%) |
| >20 | 180 (16.5%) |
| Clinical stage | |
| T1c | 427 (39.1%) |
| T2a | 331 (30.3%) |
| T2b | 38 (3.5%) |
| T2c | 125 (11.5%) |
| T3a | 122 (11.2%) |
| T3b | 39 (3.6%) |
| T4 | 9 (0.8%) |
| Gleason score | |
| ≤6 | 316 (29.0%) |
| 7 | 482 (44.2%) |
| 8 | 175 (16.0%) |
| 9 | 104 (9.5%) |
| 10 | 14 (1.3%) |
| NCCN classification | |
| Low risk | 205 (18.8%) |
| Intermediate risk | 450 (41.2%) |
| High risk | 345 (31.6%) |
| Very high risk | 91 (8.3%) |
| ADT use | |
| Yes | 646 (59.2%) |
| No | 445 (40.8%) |
PSA: prostate-specific antigen, NCCN: National Comprehensive Cancer Network, ADT: androgen-deprivation therapy.
2.2. Treatment planning
Patients were instructed to empty their bladder 60 min prior to the treatment. Each patient was positioned supine on a couch within the immobilization devices and underwent computed tomography (CT). Axial CT images of 2.5-mm thickness were obtained from the superior border of the sacroiliac joint to 5 cm below the ischial tuberosity.
The IMRT plans were created using the TomoTherapy Treatment Planning System (TomoTherapy Inc., Madison, WI, USA). The IMRT plans with helical tomotherapy (HT) were created using an inverse treatment planning system. The CT datasets with structures and contours in Pinnacle3 (Hitachi Medical Co., Tokyo) were transferred to the HT planning workstation. The clinical target volume (CTV) was defined as the prostate and the proximal portions of the seminal vesicles in patients with T1–T3a cancers. For patients with T3b cancers, the entire seminal vesicle was included in the CTV. The planning target volume (PTV) margin was set at 5 mm in all directions. For low- and intermediate-risk patients with biopsy-positive core rate ≤50%, the prescribed dose was 74 Gy; for intermediate-risk patients with biopsy-positive core rate >50%, it was 76 Gy; and for high- and very high-risk patients, it was 78 Gy. The daily fraction dose was 2.0 Gy, 5 times a week. The dose constraints for PTV were as follows: the dose administered to 95% of the PTV was >90% (>95% is preferable); the PTV receiving at least 90% of the prescribed dose was >96% (>98 is preferable); and the maximum dose was <110% of the prescribed dose. The rectum was delineated from 15 mm superior to 15 mm inferior to the PTV. As such, a rectal wall thickness of 3 mm and a bladder wall thickness of 3 mm were created. The rectum and bladder were used for optimization. Because higher doses were received by the rectal and bladder walls than the corresponding solid organs, the rectum and bladder walls were used to create a dose-volume histogram.10 The dose constraints for the rectum were as follows: V40 <60%, V60 <30%, V70 <20%, and V78 <1%. Meanwhile, the dose constraints for the bladder were as follows: V40 <60% and V70 <35%. Vx is defined as the percentage of the volume of the structure receiving at least one dose of “x” Gy. Megavoltage CT image-guided verification was performed daily prior to each treatment. The photon energy used was 6 MV.
Patients in the intermediate-, high- and very high-risk groups received androgen-deprivation therapy (ADT) that was commenced 3–6 months prior to radiotherapy. Adjuvant ADT was continued for patients with high- and very high-risk disease for a maximum of 2 years.
2.3. Evaluation
Biochemical failure was established according to the Phoenix criteria.11 Local failure was confirmed via biopsy. Clinical failure was diagnosed based on the imaging modalities, such as CT, magnetic resonance imaging, or bone scintigraphy. Failure-free rate and survival rate were defined as the time between the completion of IMRT and the development of any event. Biochemical failure-free rate (BFFR), clinical failure-free rate (CFFR), and overall survival (OS) rate was calculated using the Kaplan–Meier method, and group comparisons were made using the log-rank test. The Cox proportional hazard model was used for both univariate and multivariate analyses to evaluate the predictors of BFFR, CFFR, and OS. The independent variables were as follows: age, T stage (T1c–T2c vs. T3a–T4), GS (≤7 vs. ≥8), PSA (≤20 vs. >20 ng/ml) and the presence or absence of ADT use. Variables with a p-value of <0.10 in univariate analysis were subjected to multivariate analysis.
The cumulative incidence rate of grade >2 GI and genitourinary (GU) toxicities according to the Common Terminology Criteria for Adverse Events (v4.0) was calculated using the Kaplan–Meier method.12
A p value <0.05 was considered to indicate a significant difference.
3. Results
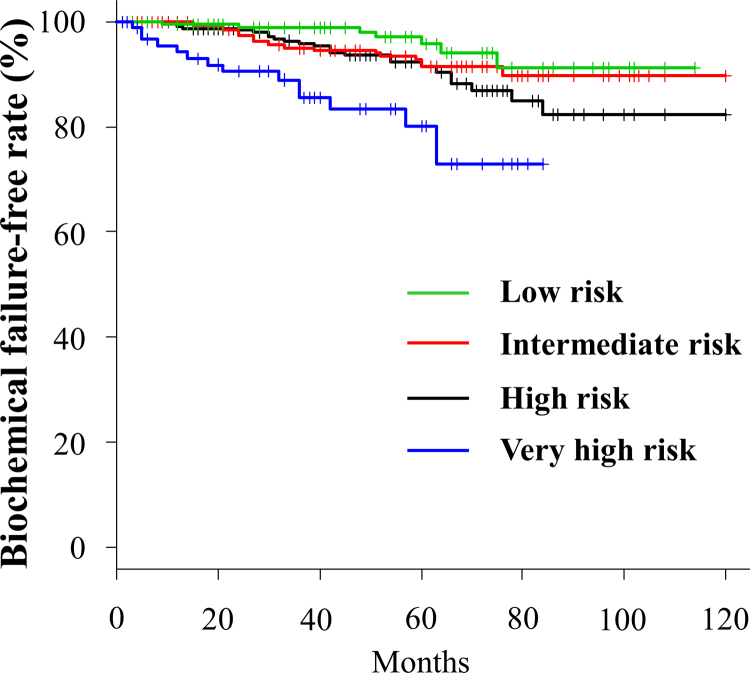
PSA failure was observed in 72 (6.6%) cases. The BFFRs at the 3- and 5-year follow-up for the low-, intermediate-, high-, and very high-risk groups were 98.9% and 95.7%, 94.9% and 91.4%, 95.9% and 91.4%, and 85.5% and 80.2%, respectively (Fig. 1). In univariate analysis, PSA (≤20 vs. >20 ng/ml) was significantly correlated with BFFR. A trend toward higher BFFR was noted more in patients with GS of ≤7 than in those with GS of ≥8. In multivariate analysis, PSA (≤20 vs. >20) was also significantly correlated with BFFR (Table 2).
Fig. 1.
Biochemical failure-free rate for each risk group.
Table 2.
Summary of univariate and multivariate analyses results for biochemical-failure free rate.
| Parameters | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age | 1.002 (0.965–1.040) | 0.9227 | ||
| PSA (<20 vs. ≥20) | 3.082 (1.912–4.967) | <0.0001 | 2.929 (1.799–4.770) | <0.0001 |
| T stage (T1c–T2c vs. T3a–T4) | 1.513 (0.867–2.638) | 0.1449 | 1.301 (0.793–2.137) | 0.2976 |
| Gleason score (≤7 vs. ≥8) | 1.569 (0.966–2.549) | 0.0689 | ||
| ADT (Yes vs. No) | 1.051 (0.656–1.684) | 0.8360 | ||
CI: confidence interval, PSA: prostate specific antigen, ADT: androgen-deprivation therapy.
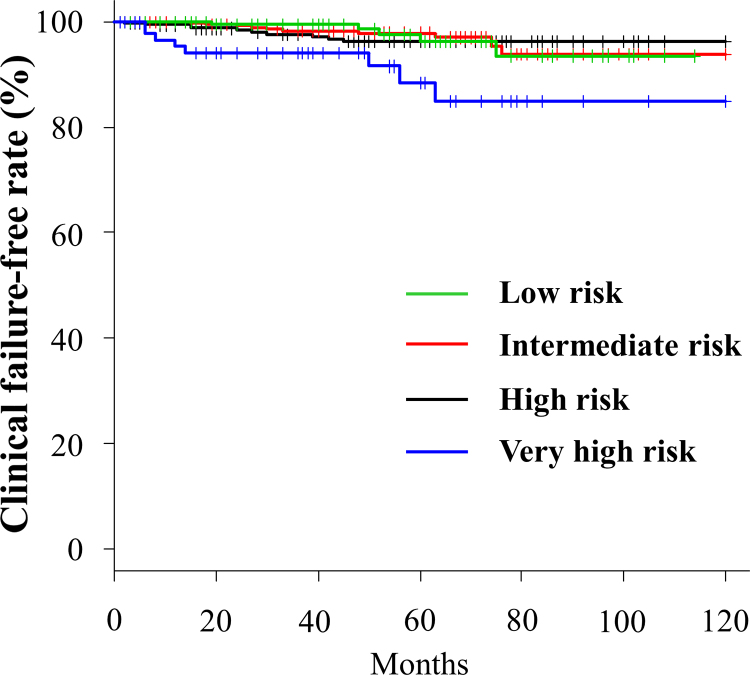
Clinical failure was observed in 33 (3.0%) cases. The sites of first failure were the prostate (local), pelvic node, para-aortic node, bone, and lungs in 8, 10, 1, 14, and 1 cases, respectively. One patient was concurrently diagnosed with pelvic node recurrence and bone metastasis. The CFFRs at the 3- and 5-year follow-up for the low-, intermediate-, high-, and very high-risk groups were 99.4% and 96.2%, 98.3% and 97.8%, 97.6% and 96.2%, and 94.1% and 88.4%, respectively (Fig. 2). In univariate analysis, PSA (≤20 vs. >20) and T stage (T1c–T2c vs. T3a–T4) were significantly correlated with CFFR. A trend toward higher CFFR was noted in patients with GS of ≤7 than in those with GS of ≥8. In multivariate analysis, no factor was significantly correlated with CFFR (Table 3).
Fig. 2.
Clinical failure-free rate for each risk group.
Table 3.
Summary of univariate and multivariate analyses results for clinical-failure free rate.
| Parameters | Univariate |
Multivariate |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age | 1.019 (0.964–1.078) | 0.5090 | ||
| PSA (<20 vs. ≥20) | 2.453 (1.188–5.066) | 0.0153 | 1.872 (0.816–4.294) | 0.1386 |
| T stage (T1c–T2c vs. T3a–T4) | 2.288 (1.088–4.810) | 0.0290 | 1.506 (0.633–3.583) | 0.3549 |
| Gleason score (≤7 vs. ≥8) | 1.920 (0.955–3.861) | 0.0672 | 1.567 (0.754–3.258) | 0.2288 |
| ADT (Yes vs. No) | 1.262 (0.621–2.566) | 0.5201 | ||
CI: confidence interval, PSA: prostate specific antigen, ADT: androgen-deprivation therapy.
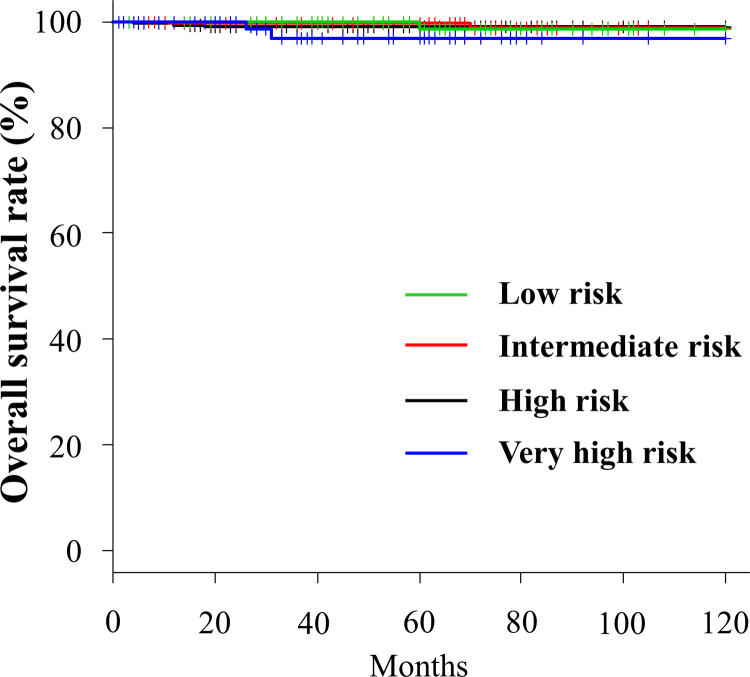
Eight (0.7%) patients died during follow-up. Among them, only 2 died due to prostate cancer, while 3 died due to another cancer and the other 3 due to pneumonia. The OS rates at the 3- and 5-year follow-up for the low-, intermediate-, high-, and very high-risk groups were 100% and 98.7%, 99.8% and 99.8%, 99.0% and 99.0%, and 96.9% and 96.9%, respectively (Fig. 3).
Fig. 3.
Overall survival rate for each risk group.
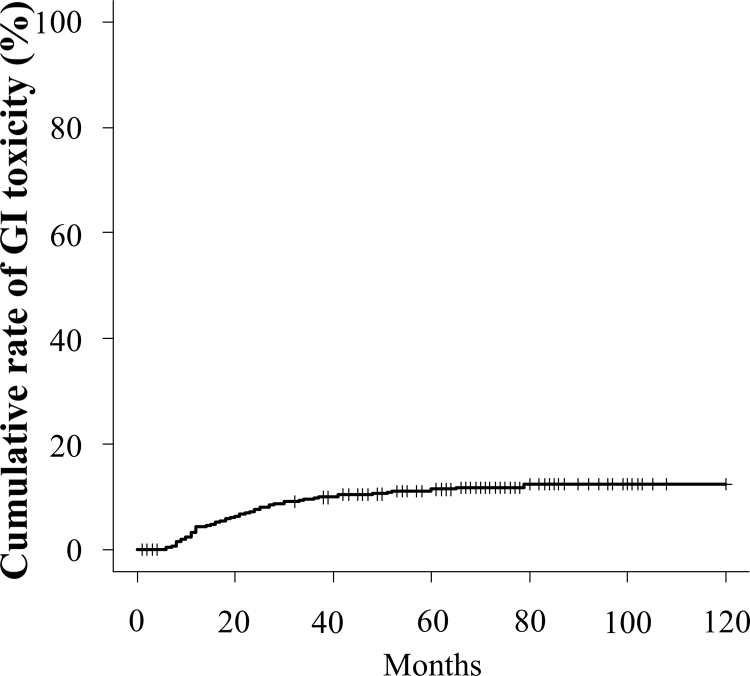
Grade ≥2 GI toxicity was observed in 102 (9.3%) cases. Among them, 25 (2.3%) cases were grade 3. Meanwhile, no cases of grade 4 GI toxicity were noted. The cumulative incidence rate of GI toxicity (≥grade 2) at the 3- and 5-year follow-up was 9.8% and 11.4%, respectively (Fig. 4).
Fig. 4.
Cumulative incidence rate of grade >2 gastrointestinal adverse events.
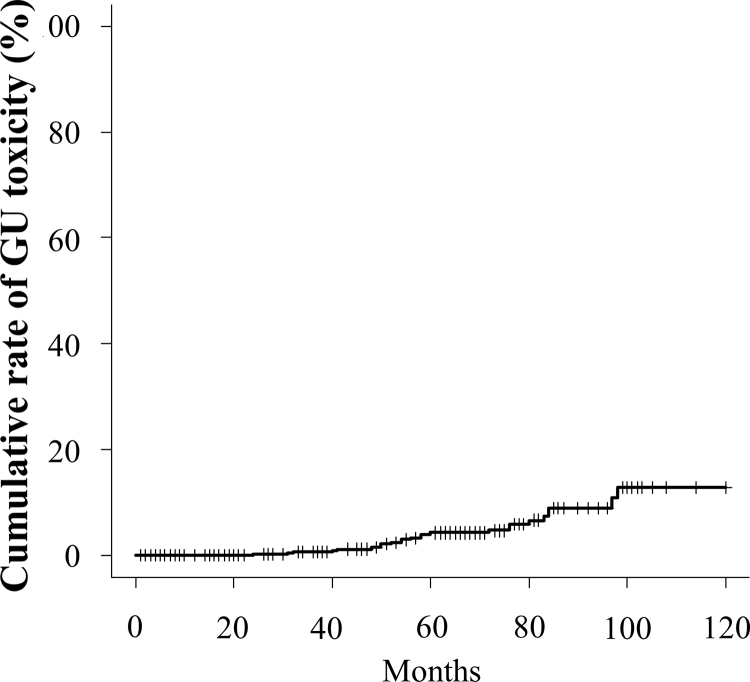
Grade ≥2 GU toxicity was observed in 32 (2.9%) cases. Among them, 3 (0.3%) cases were grade 3. No cases of grade 4 GU toxicity were noted. The cumulative incidence rate of GU toxicity (≥grade 2) at the 3- and 5-year follow-up was 0.7% and 4.3%, respectively (Fig. 5).
Fig. 5.
Cumulative incidence rate of grade >2 genitourinary adverse events.
4. Discussion
IMRT for prostate cancer is reported to have better dose coverage of CTV and local control rate than 3D-CRT.13, 14 IMRT can reduce the dose to the organs at risk, particularly the rectum, thus decreasing the rate of adverse events. The maintenance of quality of life after treatment was also better among patients who underwent IMRT than among those who underwent 3D-CRT.15, 16 Recently, IMRT has been widely used in several facilities.17
Using a large sample size from a single institution, Spratt and Zelefsky et al. treated 1002 patients with prostate cancer with IMRT and reported good results.18 Kupelian et al. treated 770 patients with prostate cancer using IMRT and also reported good results.19 However, all the large population studies were performed in Western countries. To the best of our knowledge, studies on IMRT for prostate cancer using a large sample size in Japan are yet to be conducted. Japanese studies only involved approximately 300 patients with prostate cancer treated with IMRT.20, 21, 22 Our study is the first study to evaluate the results of IMRT in more than 1000 patients with prostate cancer at a single institution in Japan.
In this study, the BFFR, CFFR, and OS at the 5-year follow-up for all patients was 91.3%, 96.2%, and 99.1%, respectively. The cumulative incidence rate of GI and GU toxicity (≥grade 2) at the 5-year follow-up was 11.4% and 4.3%, respectively. The treatment results were good and the incidence of adverse events was also within the acceptance range. In univariate analysis, the PSA (≤20 vs. >20 ng/ml) was significantly correlated with BFFR. A trend toward higher BFFR was noted more in patients with GS of ≤7 than in those with GS of ≥8. In multivariate analysis, only PSA (≤20 vs. >20 ng/ml) was significantly correlated with BFFR. Because BFFR was defined by PSA elevation, PSA was the primary factor that significantly influenced BFFR. As for CFFR, PSA (≤20 vs. >20 ng/ml) and T stage (T1c–T2c vs. T3a–T4) were significantly correlated in univariate analysis. A trend toward higher CFFR was noted more in patients with GS of ≤7 than in patients with GS of ≥8. In multivariate analysis, no factor was significantly correlated with CFFR. This result may have been influenced by the low occurrence of clinical failure.
In conclusion, the findings of this study indicate that IMRT is well tolerated and is associated with both good long-term tumor control and excellent outcomes in patients with localized prostate cancer.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.World Health Organization . 2012. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx [accessed 08.08.17] [Google Scholar]
- 2.National Cancer Center, Japan . 2015. Cancer statistics in Japan 2015. http://ganjoho.jp/en/professional/statistics/table_download.html [accessed 08.08.17] [Google Scholar]
- 3.Boladeras A., Martinez E., Ferrer F. Localized prostate cancer treated with external beam radiation therapy: long term outcomes at a European comprehensive cancer centre. Rep Pract Oncol Radiother. 2016;21:181–187. doi: 10.1016/j.rpor.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahlon O., Zelefsky M.J., Shippy A. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:33D–37D. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki H., Nakamura S., Nishimura T. Transitioning from conventional radiotherapy to intensity-modulated radiotherapy for localized prostate cancer: changing focus from rectal bleeding to detailed quality of life analysis. J Radiat Res. 2014;55:1033–1047. doi: 10.1093/jrr/rru061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dolezel M., Odrazka K., Zouhar M. Comparing morbidity and cancer control after 3D-conformal (70/74 Gy) and intensity modulated radiotherapy (78/82 Gy) for prostate cancer. Strahlenther Onkol. 2015;191:338–346. doi: 10.1007/s00066-014-0806-y. [DOI] [PubMed] [Google Scholar]
- 7.Someya M., Hori M., Tateoka K. Results and DVH analysis of late rectal bleeding in patients treated with 3D-CRT or IMRT for localized prostate cancer. J Radiat Res. 2015;56:122–127. doi: 10.1093/jrr/rru080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alicikus Z.A., Yamada Y., Zhang Z. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–1437. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network NCCN Guidelines for treatment of cancer by site. Prostate Cancer. 2017 https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf [accessed 08.08.17] [Google Scholar]
- 10.Gómez L., Andrés C., Ruiz A. Dosimetric impact in the dose-volume histograms of rectal and vesical wall contouring in prostate cancer IMRT treatments. Rep Pract Oncol Radiother. 2017;22:223–230. doi: 10.1016/j.rpor.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roach M., Hanks G., Thames H. Definin biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix consensus conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Health, National Cancer Institute . 2017. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf#search=%27CTCAE%27 [accessed 08.08.17] [Google Scholar]
- 13.Zelefsky M.J., Fuks Z., Hunt M. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 14.Vora S.A., Wong W.W., Schild S.E. Analysis of biochemical control and prognostic factors in patients treated with either low-dose three-dimensional conformal radiation therapy or high-dose intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68:1053–1058. doi: 10.1016/j.ijrobp.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Lips I., Dehnad H., Kruger A.B. Health-related quality of life in patients with locally advanced prostate cancer after76 Gy intensity-modulated radiotherapy vs. 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys. 2007;69:656–661. doi: 10.1016/j.ijrobp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Namiki S., Ishidoya S., Ito A. Five-year follow-up of health-related quality of life after intensity-modulated radiation therapy for prostate cancer. Jpn J Clin Oncol. 2009;39:732–738. doi: 10.1093/jjco/hyp086. [DOI] [PubMed] [Google Scholar]
- 17.Tomita N., Kodaira T., Teshima T. Japanese structure survey of high-precision radiotherapy in 2012 based on institutional questionnaire about the patterns of care. Jpn J Clin Oncol. 2014;44:579–586. doi: 10.1093/jjco/hyu041. [DOI] [PubMed] [Google Scholar]
- 18.Spratt D.E., Pei X., Yamada J. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2013;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kupelian P.A., Willoughby T.R., Reddy C.A. Hypofractionated intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Cleveland Clinic experience. Int J Radiat Oncol Biol Phys. 2007;68:1424–1430. doi: 10.1016/j.ijrobp.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Taguchi S., Fukuhara H., Shiraishi K. Radical prostatectomy versus external beam radiotherapy for cT1-4N0M0 prostate cancer: comparison of patient outcomes including mortality. PLOS ONE. 2015;10:e0141123. doi: 10.1371/journal.pone.0141123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutani S., Ohashi T., Sakayori M. Comparison of genitourinary and gastrointestinal toxicity among four radiotherapy modalities for prostate cancer: conventional radiotherapy, intensity-modulated radiotherapy, and permanent iodine-125 implantation with or without external beam radiotherapy. Radiother Oncol. 2015;117:270–276. doi: 10.1016/j.radonc.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M., Hatano K., Fukawasa S. Therapeutic outcomes of neoadjuvant and concurrent androgen-deprivation therapy and intensity-modulated radiation therapy with gold marker implantation for intermediate-risk and high-risk prostate cancer. Int J Urol. 2015;22:477–482. doi: 10.1111/iju.12707. [DOI] [PubMed] [Google Scholar]