Introduction
Humans are constantly exposed to pathogenic microbes. The first line of cellular host defense is composed of “professional” phagocytes, cells that efficiently recognize pathogens, internalize them, and then marshal an array of antimicrobial mechanisms to destroy them. Nevertheless, successful pathogens evade or survive such attack. A particularly subversive strategy is to manipulate normal phagocyte behaviors to benefit the microbe, sometimes even turning the phagocyte from a threat to a safe haven. In this environment, the microbes can multiply while protected from immune surveillance, and in some cases, even travel to the most protected host site, the brain. This gives rise to the Trojan horse analogy: like the wooden horse that carried hidden enemies through the gates into the walled city of Troy, phagocytes carry intracellular microbes through the blood–brain barrier (BBB) into the central nervous system (CNS).
Immune cells in the brain
Traditionally, the brain has been considered an immune-privileged site because it lacks the normal robust inflammatory responses to antigenic challenges. However, it does have an active immune surveillance system [1] that involves the extravasation of leukocytes, mostly monocytes and lymphocytes, into the meninges and cerebrospinal fluid (CSF). This process follows the same general events that occur in other tissues: rolling of the leukocyte, arrest, crawling, and then transendothelial migration [2]. Because the brain microvascular endothelial cells (BMECs) of the BBB are joined by tight junctions and embedded in a proteinaceous matrix [3], transmigrating leukocytes rarely cross the BBB directly. Instead, they cross into the outer meningeal spaces, where the vasculature is devoid of tight junctions, and from this site they monitor the CSF for the presence of immune signals. Additionally, a recently discovered brain lymphatic system samples the perivascular spaces, bypassing the physical cellular barrier composed of BMECs [4, 5]. Even in healthy individuals, therefore, phagocytes are in close proximity to brain tissue, poised to act upon immune signals.
CNS phagocytes actively respond to signals generated by developmental changes, injury, disease, or infection. Such signals include interferons produced by endothelial cells in response to viral pathogens, chemotactic peptides like N-formyl-methionyl-leucyl-phenylalanine (fMLP) generated by bacterial pathogens, and inflammatory cytokines released by epithelial cells in response to fungal pathogens [6]. Microglia, phagocytes that are the only resident immune cells in the brain, also produce cytokines and chemokines to recruit other effector cells to that site. Rapid response to these signals is enabled by the normal presence of phagocytes and lymphocytes in the meninges. However, tight regulation of this response is crucial because adult neurons in the CNS generally do not regenerate; if these cells are damaged by any activities of infiltrating phagocytes, they cannot be replaced, potentially resulting in permanent damage. The BBB helps to limit immune infiltration from the blood, aiding the host to mount an immune response that is robust enough to contain infection yet limited to prevent tissue damage. For the most part, this balance is maintained, and brain infection is prevented or controlled.
Phagocytes as Trojan horses
A model for Trojan horse transit into the brain
In the absence of trauma, pathogens that cause lethal brain infections (e.g., those in Table 1) reach it from remote sites, generally traveling in the bloodstream. For microbes that use Trojan horse transit, the first step is infection of a phagocyte in the periphery (Fig 1A). Once internalized, the pathogen may actively manipulate the phagocyte to promote migration towards the brain [7]. Alternatively, it may suppress phagocyte activation (and consequent sequestration in the tissue of origin), allowing the infected cell to circulate normally throughout the body (Fig 1B). Once an infected phagocyte reaches the brain, it adheres to the luminal side of brain capillaries (with or without activation of BMECs) and crosses the BBB, either paracellularly (between BMECs) or transcellularly (through BMECs) (Fig 1C). After brain entry, the pathogen may exit its Trojan horse to infect other neural structures (Fig 1D). This model has been elucidated in the most detail for HIV and other viruses [8, 9], but studies reviewed below suggest that similar strategies are used by other microbes that are the focus of this review: bacteria, fungi, and parasites. Aspects of this model may also apply to non-CNS pathogens, such as mucosal pathogens that use phagocytes to disseminate (see “Perspectives and conclusions”).
Table 1. Deaths due to CNS infection by select obligate and facultative intracellular microbes.
| Pathogen | Growth inside phagocytes |
Pathology | Burden (yearly deaths, in thousands)1 |
|---|---|---|---|
| Streptococcus pneumoniae | Yes | Meningitis | 113 [35] |
| Neisseria meningitidis | Yes | Meningitis | 73.3 [35] |
| Mycobacterium tuberculosis | Yes | Meningitis, encephalitis, intracranial tuberculoma, brain abscesses | 55.6 [35]2 |
| Listeria monocytogenes | Yes | Meningitis, encephalitis, ventriculitis, choroiditis, brain abscesses | 0.19 (US) [36] |
| Escherichia coli K1 | Yes | Meningitis | Rare |
| Cryptococcus neoformans | Yes | Meningitis, encephalitis, cerebral cryptococcomas | 181 [18] |
| Histoplasma capsulatum | Yes | Meningitis, encephalitis | 80 [37]3 |
| Coccidioides immitis | Yes | Meningitis, brain abscesses | <0.20 (US) [38] |
| Candida albicans | Yes4 | Meningitis, encephalitis | <0.10 (US) [39]5 |
| Other fungi (Aspergillus, Mucor, Blastomyces) | Yes4 | Brain abscesses, meningitis, cerebral stroke | Rare |
| Plasmodium falciparum | No | Brain microvessel obstruction | 584 [35]6 |
| Trypanosoma cruzi | Yes | Meningitis, encephalitis | 3.0 [35]7 |
| Toxoplasma gondii | Yes | Encephalitis | 0.20 (US) [39]8 |
1 Worldwide deaths as reported in [35] unless denoted (by “US”) as United States burden only.
2 This represents 5% of all deaths due to disseminated TB.
3 This represents all extrapulmonary infections; a value for deaths due to CNS infection alone is not available.
4 These fungi change morphology when inside phagocytes, killing the host cell.
5 Most deaths due to invasive candidiasis (approximately 350,000; [37]) are not attributable to CNS infection. This value is based on the US incidence and mortality rate (24%) for CNS candidiasis and the current US population of HIV+ patients.
6 This represents 80% of all deaths due to P. falciparum.
7 This represents one-third of all Chagas disease deaths; most are due to heart failure.
8 This value is based on the US incidence and mortality rate (14%) for T. gondii encephalitis and the current US population of HIV+ patients.
Abbreviations: CNS, central nervous system; TB, tuberculosis.
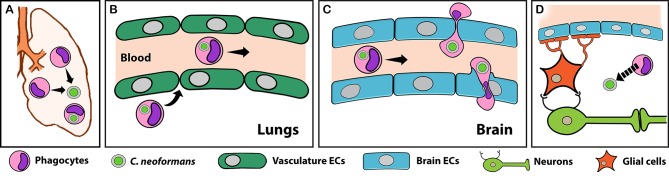
Fig 1. The role of phagocytes as Trojan horses for CNS pathogens.
Most neuroinvasive pathogens first infect organs outside the CNS, such as the lungs or the intestines. Cryptococcus neoformans infection of the lungs is shown here as an example. Once infection is established, phagocytes (pink cells) are recruited to these sites (A), where they engulf the pathogen. Some infected phagocytes leave the site of infection and enter the bloodstream, facilitated by the highly permeable vasculature (green) of peripheral organs (B). Through a process that is poorly understood, many of these home to the CNS. Once there, infected phagocytes may act as Trojan horses, traversing the BBB (blue cells) with the pathogen as a passenger (C). Although both paracellular (top) and transcellular (bottom) transmigration can occur, the latter is most likely due to the presence of tight junctions in the BBB (C). Once inside the brain, pathogens can potentially exit their Trojan horses and infect other neural structures (D). Parts of this model (A and B) also apply to phagocyte-assisted dissemination and infection outside of the CNS (see text). BBB, blood–brain barrier; CNS, central nervous system; ECs, endothelial cells. Arrows indicate movement; broken arrow indicates fungal egress.
Bacterial infections
The most common causes of bacterial meningitis are the facultative intracellular pathogens Streptococcus pneumoniae and Neisseria meningitidis. Because they survive in the blood and can independently interact with BMECs to enter the brain, these microbes do not require Trojan horse transit, although this mechanism may contribute to S. pneumoniae infection [10]. In contrast, Trojan horses play a central role in infections by another leading cause of bacterial meningitis, Listeria monocytogenes.
L. monocytogenes is a pathogen of humans and domesticated animals that invades the brain parenchyma, unlike most neuroinvasive bacteria, which are limited to the meninges (Table 1). This distinct pathology may relate to its use of Trojan horse invasion, which was first suggested by histological studies showing parasitized phagocytes in the brain tissue of infected mice [11]. Further suggestive of a Trojan horse mechanism were reports that phagocytosis of L. monocytogenes causes the release of immune signals and activation of BMECs [12], both of which would promote the recruitment of additional leukocytes to the site of infection. More direct support for this mechanism came from the observation that infected mice treated with gentamicin to kill extracellular bacteria still developed CNS infection [13]. This occurred regardless of the initial route of infection, consistent with a general model whereby phagocytes are recruited to the site of infection, engulf the pathogen, and then disseminate (Fig 1). Bolstering this observation, injection of L. monocytogenes-infected bone marrow myeloid cells caused faster and greater brain colonization than injection of free bacteria [14]. In this study, which used chimeric mice that expressed a fluorescent protein in their bone marrow cells, an increase in fluorescent signal was observed in the brain as the infection progressed, also consistent with a Trojan horse model. The bone marrow and spleen are among the first organs infected by L. monocytogenes. Interestingly, phagocytes infected in these tissues cannot kill the bacteria but do up-regulate chemokine receptors, making them ideal Trojan horses [15].
Fungal infections
Fungal infections are responsible for up to 1.6 million deaths every year [3], and the ones affecting the CNS have the highest morbidity and mortality [16]. Although several fungal pathogens cause meningitis (Table 1), the only one to frequently do so is Cryptococcus neoformans. Most people have been exposed to this environmental yeast [17]. While healthy individuals are generally asymptomatic, in immunocompromised individuals, the initial pulmonary infection can subsequently disseminate to the CNS. As a result, C. neoformans is the most common causative agent of meningitis in sub-Saharan Africa and a leading cause of death in HIV+ individuals, killing close to 200,000 people each year [18].
As with L. monocytogenes, early evidence for Trojan horse transit of C. neoformans came from histological examination of brains from infected mice [19]. This work was complemented by studies supporting the role of phagocytes in cryptococcal dissemination from the lungs to the brain. For example, depletion of alveolar macrophages reduced dissemination from the lungs [20], systemic monocyte depletion after lung infection reduced fungal burden in other organs [21], and intravenous administration of C. neoformans-associated macrophages caused higher brain burden than infection with free cryptococci. More recently, direct evidence for Trojan horse transit has come from two studies using in vitro models of brain endothelia. Both groups cultured human cerebral microvascular endothelial cell (hCMEC) monolayers on permeable membranes separating the upper (“blood”) and lower (“brain") compartments of tissue culture wells. In one study, a monocytic cell line was first incubated with C. neoformans, which was engulfed by or adhered to the phagocytes, and the samples were then stained to mark any externally adherent fungi. This mixture was added to the upper chamber, and one day later, monocytes containing unstained fungi were found in the lower chamber, suggesting that Trojan horse crossing had occurred [22]. (Interestingly, the same experiments performed with Cryptococcus gattii, a species that primarily causes lung infections, showed less barrier crossing.) In the other study, our group used a flow cytometry strategy to isolate primary human monocytes or macrophages that contained only a single internalized cryptococcal cell. We used this population to directly compare Trojan horse and free fungal transit across a similar BBB model and found that both mechanisms contribute to overall transmigration [23]. We further showed that immune signals that are normally generated during cryptococcal infection preferentially stimulate Trojan horse transit and that this mode of entry provides an alternative for fungal mutants that cannot otherwise traverse the BBB. Finally, we used live microscopy to directly visualize C. neoformans-infected phagocytes as they crossed model BBB by forming transendothelial pores in the hCMEC. Our microscopic observations also suggested that phagocytes may serve as “taxis” in addition to Trojan horses, contributing to brain infection by picking up the cryptococci (which survive poorly in blood) at distal sites and delivering them to the BBB, where the free fungi can cross independently.
Parasitic infections
Parasitic infections cause high burdens of disease in low- and middle-income countries, with almost 800,000 deaths in 2015 (Table 1). Several parasites cause devastating CNS pathology, either while remaining in the vasculature—like the parasite that causes malaria—or by crossing the BBB. Here we focus our discussion on a parasite that is estimated to infect one-third of the world, Toxoplasma gondii [24].
T. gondii is acquired orally and colonizes the gastrointestinal tract. In healthy humans, a robust immune response halts the rapid parasite proliferation that would cause severe acute disease in an immunocompromised host. However, even immunocompetent individuals do not completely clear the infection and remain chronically infected with quiescent parasite cysts, mainly in tissues of the CNS and skeletal muscle. Support for Trojan horse transport of T. gondii derives from studies similar to those mentioned above for other pathogens, mainly adoptive transfer studies showing that parasitized monocytes or dendritic cells cause brain infection faster than free parasites [25]. Consistent with these observations, the injection of intracellular parasites together with antibodies against CD11b, which blocks phagocyte migration, reduced brain burden 2-fold. Furthermore, enhanced transendothelial migration of infected leukocytes has been observed in some, although not all, in vitro studies [26, 27]; another study using a robust BBB model consisting of brain endothelia and astrocytes reported the preferential transmigration of infected monocytes [28]. Lastly, T. gondii Trojan horse transit has been visualized in vitro, although these studies used an activated non-brain endothelial cell line (human umbilical vein endothelial cells [HUVECs])[29].
As with the other pathogens discussed here, free T. gondii likely also cross the BBB, although the relative frequency of the two processes is not known. Notably, infection causes endothelial cells to become activated, with up-regulation of adhesion molecules and down-regulation of junctional complexes [28]; both of these processes could stimulate phagocyte transmigration and thus promote Trojan horse transit. Intravital microscopy has also shown that BMECs serve as a replicative niche for T. gondii and that intracellular replication is required for egress (through host cell lysis) into the CNS [30]. Interestingly, the same experiments did not show Trojan horse movement, although they did reveal infected phagocytes trapped on the vascular side of brain vessels; these may act as taxis (as with C. neoformans), serving as a source of free parasites to infect BMECs or cross the BBB. This idea has recently been supported by the observation that adhesion of infected leukocytes to endothelial cells in vivo triggers parasite egress [31].
Perspectives and conclusions
Only a few pathogens cause significant pathology in the brain, yet they collectively lead to over 1 million deaths every year (Table 1). Understanding how these microbes cross the BBB has implications not only for the development of new treatments for these diseases but also for our understanding of the basic immunobiology of the CNS. Here we have presented a general model for Trojan horse infection of the brain and discussed three pathogens that exploit this mechanism. While most of the experimental support for this process comes from in vitro studies, new technologies like real-time in vivo imaging are beginning to offer exciting insights into this and related processes. Beyond the CNS, bloodstream phagocytes play other critical roles in infection, such as assisting in the dissemination of Salmonella from the gut [32], providing a protected replicative niche for Leishmania parasites [33], and harboring latent Mycobacterium reservoirs [34]. Clearly, understanding the complex interactions between phagocytes and pathogens is of the utmost importance if we wish to elucidate important steps in pathogenesis that can be targeted for efficient control of these deadly infections.
Acknowledgments
We thank Lisa Drewry and members of the Doering lab for stimulating discussions and comments on the manuscript.
Funding Statement
Work cited in this review from the Doering lab was supported by the National Institute of Allergy and Infectious Diseases (https://www.niaid.nih.gov) grants AI114549, AI102882, and AI078795 to TLD; NIH T32 AI007172 to FHS; and a Burroughs Wellcome Fund (https://www.bwfund.org) Postdoctoral Enrichment Award to FHS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123–31. Epub 2017/01/17. doi: 10.1038/ni.3666 [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15(11):692–704. Epub 2015/10/17. doi: 10.1038/nri3908 [DOI] [PubMed] [Google Scholar]
- 3.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412 Epub 2015/01/07. doi: 10.1101/cshperspect.a020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212(7):991–9. Epub 2015/06/17. doi: 10.1084/jem.20142290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41. Epub 2015/06/02. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera A, Siracusa MC, Yap GS, Gause WC. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17(4):356–63. Epub 2016/03/24. doi: 10.1038/ni.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caradonna K, Pereiraperrin M. Preferential brain homing following intranasal administration of Trypanosoma cruzi. Infect Immun. 2009;77(4):1349–56. Epub 2009/01/27. doi: 10.1128/IAI.01434-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74(5):650–6. Epub 2003/09/10. doi: 10.1189/jlb.0503207 [DOI] [PubMed] [Google Scholar]
- 9.Klein RS, Hunter CA. Protective and Pathological Immunity during Central Nervous System Infections. Immunity. 2017;46(6):891–909. Epub 2017/06/22. doi: 10.1016/j.immuni.2017.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutta R, Roy S. Chronic morphine and HIV-1 Tat promote differential central nervous system trafficking of CD3+ and Ly6C+ immune cells in a murine Streptococcus pneumoniae infection model. J Neuroinflammation. 2015;12:120 Epub 2015/06/20. doi: 10.1186/s12974-015-0341-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prats N, Briones V, Blanco MM, Altimira J, Ramos JA, Dominguez L, et al. Choroiditis and meningitis in experimental murine infection with Listeria monocytogenes. Eur J Clin Microbiol Infect Dis. 1992;11(8):744–7. Epub 1992/08/01. [DOI] [PubMed] [Google Scholar]
- 12.Drevets DA, Bronze MS. Listeria monocytogenes: epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol Med Microbiol. 2008;53(2):151–65. Epub 2008/05/09. doi: 10.1111/j.1574-695X.2008.00404.x [DOI] [PubMed] [Google Scholar]
- 13.Drevets DA, Jelinek TA, Freitag NE. Listeria monocytogenes-infected phagocytes can initiate central nervous system infection in mice. Infect Immun. 2001;69(3):1344–50. Epub 2001/02/17. doi: 10.1128/IAI.69.3.1344-1350.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Join-Lambert OF, Ezine S, Le Monnier A, Jaubert F, Okabe M, Berche P, et al. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell Microbiol. 2005;7(2):167–80. Epub 2005/01/22. doi: 10.1111/j.1462-5822.2004.00444.x [DOI] [PubMed] [Google Scholar]
- 15.Drevets DA, Schawang JE, Mandava VK, Dillon MJ, Leenen PJ. Severe Listeria monocytogenes infection induces development of monocytes with distinct phenotypic and functional features. J Immunol. 2010;185(4):2432–41. Epub 2010/07/16. doi: 10.4049/jimmunol.1000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13 Epub 2012/12/21. doi: 10.1126/scitranslmed.3004404 [DOI] [PubMed] [Google Scholar]
- 17.Srikanta D, Santiago-Tirado FH, Doering TL. Cryptococcus neoformans: historical curiosity to modern pathogen. Yeast. 2014;31(2):47–60. Epub 2014/01/01. doi: 10.1002/yea.2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017. Epub 2017/05/10. doi: 10.1016/S1473-3099(17)30243-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chretien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;186(4):522–30. Epub 2002/08/27. doi: 10.1086/341564 [DOI] [PubMed] [Google Scholar]
- 20.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75(10):4792–8. Epub 2007/08/01. doi: 10.1128/IAI.00587-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77(1):120–7. Epub 2008/10/22. doi: 10.1128/IAI.01065-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorrell TC, Juillard PG, Djordjevic JT, Kaufman-Francis K, Dietmann A, Milonig A, et al. Cryptococcal transmigration across a model brain blood-barrier: evidence of the Trojan horse mechanism and differences between Cryptococcus neoformans var. grubii strain H99 and Cryptococcus gattii strain R265. Microbes Infect. 2016;18(1):57–67. Epub 2015/09/16. doi: 10.1016/j.micinf.2015.08.017 [DOI] [PubMed] [Google Scholar]
- 23.Santiago-Tirado FH, Onken MD, Cooper JA, Klein RS, Doering TL. Trojan Horse Transit Contributes to Blood-Brain Barrier Crossing of a Eukaryotic Pathogen. MBio. 2017;8(1). Epub 2017/02/02. doi: 10.1128/mBio.02183-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wohlfert EA, Blader IJ, Wilson EH. Brains and Brawn: Toxoplasma Infections of the Central Nervous System and Skeletal Muscle. Trends Parasitol. 2017;33(7):519–31. Epub 2017/05/10. doi: 10.1016/j.pt.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107(1):309–16. Epub 2005/07/30. doi: 10.1182/blood-2005-02-0666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8(10):1611–23. Epub 2006/09/21. doi: 10.1111/j.1462-5822.2006.00735.x [DOI] [PubMed] [Google Scholar]
- 27.Lambert H, Dellacasa-Lindberg I, Barragan A. Migratory responses of leukocytes infected with Toxoplasma gondii. Microbes Infect. 2011;13(1):96–102. Epub 2010/10/19. doi: 10.1016/j.micinf.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 28.Lachenmaier SM, Deli MA, Meissner M, Liesenfeld O. Intracellular transport of Toxoplasma gondii through the blood-brain barrier. J Neuroimmunol. 2011;232(1–2):119–30. Epub 2010/11/26. doi: 10.1016/j.jneuroim.2010.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno N, Harker KS, Clarke EV, McWhorter FY, Liu WF, Tenner AJ, et al. Real-time imaging of Toxoplasma-infected human monocytes under fluidic shear stress reveals rapid translocation of intracellular parasites across endothelial barriers. Cell Microbiol. 2014;16(4):580–95. Epub 2013/11/20. doi: 10.1111/cmi.12239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, et al. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol. 2016;1:16001 Epub 2016/08/31. doi: 10.1038/nmicrobiol.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba M, Batanova T, Kitoh K, Takashima Y. Adhesion of Toxoplasma gondii tachyzoite-infected vehicle leukocytes to capillary endothelial cells triggers timely parasite egression. Sci Rep. 2017;7(1):5675 Epub 2017/07/20. doi: 10.1038/s41598-017-05956-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worley MJ, Nieman GS, Geddes K, Heffron F. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc Natl Acad Sci U S A. 2006;103(47):17915–20. Epub 2006/11/11. doi: 10.1073/pnas.0604054103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritter U, Frischknecht F, van Zandbergen G. Are neutrophils important host cells for Leishmania parasites? Trends Parasitol. 2009;25(11):505–10. Epub 2009/09/19. doi: 10.1016/j.pt.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 34.Nguyen L, Pieters J. The Trojan horse: survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol. 2005;15(5):269–76. Epub 2005/05/04. doi: 10.1016/j.tcb.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 35.Global Burden of Disease Study 2015. The Lancet. 2016; Available from: http://ghdx.healthdata.org/gbd-results-tool. Accessed on 15 August 2017.
- 36.Centers for Disease C, Prevention. Vital signs: Listeria illnesses, deaths, and outbreaks—United States, 2009–2011. MMWR Morb Mortal Wkly Rep. 2013;62(22):448–52. Epub 2013/06/07. [PMC free article] [PubMed] [Google Scholar]
- 37.Fungal Disease Frequency. GAFFI. 2017; Available from: http://www.gaffi.org/why/fungal-disease-frequency/. Accessed on 15 August 2017.
- 38.Valley Fever (Coccidioidomycosis). Centers for Disease Control and Prevention. 2017; Available from: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/. Accessed on 15 August 2017.
- 39.Buchacz K, Lau B, Jing Y, Bosch R, Abraham AG, Gill MJ, et al. Incidence of AIDS-Defining Opportunistic Infections in a Multicohort Analysis of HIV-infected Persons in the United States and Canada, 2000–2010. J Infect Dis. 2016;214(6):862–72. Epub 2016/08/26. doi: 10.1093/infdis/jiw085 [DOI] [PMC free article] [PubMed] [Google Scholar]