Abstract
The relation between progression of cerebral small vessel disease (SVD) and gait decline is uncertain, and diffusion tensor imaging (DTI) studies on gait decline are lacking. We therefore investigated the longitudinal associations between (micro) structural brain changes and gait decline in SVD using DTI. 275 participants were included from the Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort (RUN DMC), a prospective cohort of participants with cerebral small vessel disease aged 50–85 years. Gait (using GAITRite) and magnetic resonance imaging measures were assessed during baseline (2006–2007) and follow-up (2011 − 2012). Linear regression analysis was used to investigate the association between changes in conventional magnetic resonance and diffusion tensor imaging measures and gait decline. Tract-based spatial statistics analysis was used to investigate region-specific associations between changes in white matter integrity and gait decline. 56.2% were male, mean age was 62.9 years (SD8.2), mean follow-up duration was 5.4 years (SD0.2) and mean gait speed decline was 0.2 m/s (SD0.2). Stride length decline was associated with white matter atrophy (β = 0.16, p = 0.007), and increase in mean white matter radial diffusivity and mean diffusivity, and decrease in mean fractional anisotropy (respectively, β = − 0.14, p = 0.009; β = − 0.12, p = 0.018; β = 0.10, p = 0.049), independent of age, sex, height, follow-up duration and baseline stride length. Tract-based spatial statistics analysis showed significant associations between stride length decline and fractional anisotropy decrease and mean diffusivity increase (primarily explained by radial diffusivity increase) in multiple white matter tracts, with the strongest associations found in the corpus callosum and corona radiata, independent of traditional small vessel disease markers. White matter atrophy and loss of white matter integrity are associated with gait decline in older adults with small vessel disease after 5 years of follow-up. These findings suggest that progression of SVD might play an important role in gait decline.
Keywords: Cerebral small vessel disease (SVD), MRI, Diffusion tensor imaging (DTI), Tract-based spatial statistics (TBSS), Gait
Highlights
-
•
Relation between progression of small vessel disease and gait decline is uncertain.
-
•
We performed longitudinal MRI study in SVD patients.
-
•
Reduced WM integrity and volume was significantly related to gait decline.
-
•
Progression of SVD is important in gait decline.
1. Introduction
Gait disturbances are prevalent in older adults aged ≥ 65 years and have important consequences as they can lead to falls, functional dependence and institutionalization (Abellan van Kan et al., 2009, Cesari et al., 2005). Cerebral small vessel disease (SVD) ranks highest among vascular causes of gait decline, with evidence mainly coming from cross-sectional studies (de Laat et al., 2010, Srikanth et al., 2010). A few studies have investigated whether baseline SVD can predict gait decline over time. However, their results are inconclusive (Aribisala et al., 2013, Rosano et al., 2005, Soumare et al., 2009). In a previous study, we found no associations between baseline SVD and gait decline after 5 years in our SVD population (Van der Holst et al., 2016 Nov.). One possible hypothesis is that progression of SVD, rather than baseline SVD load, is associated with gait decline over time. The results of recent longitudinal population-based studies investigating the association between progression of SVD and gait decline are however conflicting (Callisaya et al., 2013, Moscufo et al., 2012, Silbert et al., 2008). Moreover, these studies did not include the whole SVD spectrum, nor was the white matter (WM) integrity, which can be assessed using diffusion tensor imaging (DTI), taken into account. Since a recent study showed that scalar measures of DTI were sensitive markers of SVD progression (Zeestraten et al., 2016), we hypothesized that changes in DTI measures are stronger associated with gait decline than progression of the traditional SVD markers.
Here, we investigated the longitudinal associations between changes in the traditional SVD markers (WMH, lacunes, microbleeds and brain atrophy) and changes in WM integrity (assessed by DTI), and gait decline in a population of adults with SVD, aged 50–85 years, over a period of 5 years.
2. Methods
2.1. Study population
The Radboud University Nijmegen Diffusion tensor and Magnetic resonance Cohort study (RUN DMC study) prospectively investigates the risk factors and clinical consequences of brain changes as assessed by MRI. This cohort study consists of 503 participants with SVD, aged 50–85 years at baseline (2006). The recruitment, study rationale and protocol of the RUN DMC study have been described elsewhere (van Norden et al., 2011). Inclusion criteria were age 50–85 years and SVD on neuroimaging (defined as the presence of WMH or lacunes of presumed vascular origin) (Erkinjuntti, 2002). All consecutive patients referred to our outpatient clinic who underwent diagnostic brain imaging for several reasons (e.g. stroke, TIA, cognitive complaints) were eligible for participation. Main exclusion criteria were: parkinsonism, dementia, life expectancy < 6 months, non-SVD related WM lesions and MRI contra-indications (van Norden et al., 2011).
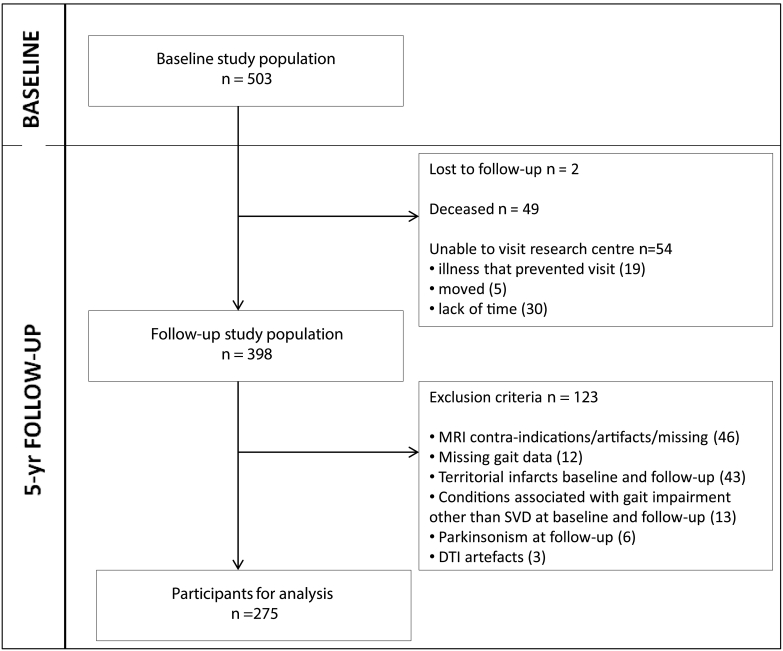
Follow-up assessment was completed in 2012. Of the 503 baseline participants, 398 participated in the follow-up assessment. For the present study, 123 participants were additionally excluded, yielding a final sample of 275. Exclusion reasons at baseline and follow-up were: missing data on MRI and gait data, territorial infarcts, parkinsonism and conditions associated with gait impairment other than SVD (see flowchart Fig. 1).
Fig. 1.
Flowchart of the study sample
Abbreviations: DTI: diffusion tensor imaging; MRI: magnetic resonance imaging; SVD: small vessel disease.
Of the 503 baseline participants, 2 participants were lost to follow-up, 49 had died and 54 refused an in-person follow-up examination, but their clinical endpoints were available; 398 participated in the follow-up assessment. For the present study, we included 275 participants, 123 participants were additionally excluded because of because of (i) MRI contra-indications, MRI artifacts or missing values at follow-up (n = 46), (ii) missing data on follow-up GAITRite (n = 12) (because they were wheelchair bound, because of home visit or because of technical problems), (iii) territorial infarcts at baseline and follow-up imaging (n = 43), because these infarcts were considered as a potential confounder, (iv) conditions associated with gait impairment other than SVD which prevented participants from walking unaided at baseline and follow-up (n = 13) (joint fusion, severe arthritis, severe polyneuropathy, leg amputation, severe vision problems, severe cardiopulmonary diseases, severe peripheral arterial disease and psychogenic gait disturbance), (v) parkinsonism during follow-up examination (n = 6), because apart from SVD other pathologies as amyloid pathology, Lewy body pathology and nigrastriatal dopaminergic loss could play a role in gait deterioration in these patients, and (vi) DTI artifacts (n = 3).
2.2. Standard protocol approvals, registration, and patient consents
All participants signed an informed consent form. The Medical Review Ethics Committee region Arnhem-Nijmegen approved the study.
2.3. Gait measurement and gait impairment
The assessment of gait was performed by using a 5.6 m electronic portable walkway (GAITRite, MAP/CIR Inc., Havertown, PA), which has an excellent test-retest reliability and validity (Bilney et al., 2003, Menz et al., 2004). Participants were instructed to walk over the walkway at their comfortable walking speed. In order to measure steady-state walking, they started two meters before the walkway and stopped two meters behind it. The following gait parameters were averaged over two walks: gait speed (m/s) and its components stride length (m) (the distance between the heel points of two consecutive footprints of the same foot) and cadence (number of steps per minute). Changes in these gait parameters were calculated as the difference between follow-up and baseline assessment.
2.4. MRI protocol
A cerebral MRI was acquired on a 1.5-Tesla scanner at baseline and follow-up (baseline: Magnetom Sonata; follow-up: Magnetom Avanto Tim (76 × 32); Siemens Medical Solutions, Erlangen, Germany). The same 8-channel head coil was used at baseline and follow-up. The protocol included: a T1-weighted 3D magnetization-prepared rapid gradient-echo (MPRAGE) imaging (baseline: repetition time (TR)/echo time (TE)/inversion time (TI) 2250 ms/3.68 ms/850 ms, flip angle = 15°, voxelsize 1.0 × 1.0 × 1.0 mm; follow-up: TR/TE/TI 2250 ms/2.95 ms/850 ms, flip angle = 15°, voxelsize 1.0 × 1.0 × 1.0 mm); a Fluid-attenuated inversion recovery (FLAIR) sequence (baseline: TR/TE/TI 9000 ms/84 ms/2200 ms, voxelsize 1.2 × 1.0 × 5.0 mm (interslice gap 1 mm); follow-up: TR/TE/TI 14240 ms/89 ms/2200 ms, voxelsize 1.2 × 1.0 × 2.5 mm (interslice gap 0.5 mm); a transversal T2 ∗ weighted gradient echo sequence (baseline and follow-up: TR/TE 800 ms/26 ms, voxelsize 1.3 × 1.0 × 5.0 mm (interslice gap 1 mm)) and a DTI sequence (baseline: TR/TE 10100 ms/93 ms, voxelsize 2.5 × 2.5 × 2.5 mm; 4 unweighted scans, 30 diffusion weighted scans with b-value = 900 s/mm2; follow-up: TR/TE 10200 ms/95 ms, voxelsize 2.5 × 2.5 × 2.5 mm; 7 unweighted scans, 61 diffusion weighted scans with b-value = 900 s/mm2).
2.5. Traditional SVD markers and brain volumetry
Traditional SVD makers (WMH, lacunes and microbleeds) were rated according to the STRIVE criteria (Wardlaw et al., 2013). Lacunes were manually rated on FLAIR/T1-weighted scans and microbleeds on T2 ∗ -weighted MRI scans by raters blinded to clinical data. The follow-up FLAIR images were resliced to match the slice thickness of baseline FLAIR images, limiting the differences in partial volume effects between baseline and follow-up scans. To determine the effects of change in slice thickness of the FLAIR sequence, we calculated WMH volumes for odd and even slices separately. Intrarater and interrater reliabilities were good (for lacunes: weighted kappa values 0.87 and 0.95, respectively, and for microbleeds: 0.85 and 0.86, respectively). WMH were segmented by using an in-house developed semi-automatic detection method on baseline and follow-up FLAIR sequences (Ghafoorian et al., 2016). All scans were visually checked by 1 rater and corrections were made when segmentation failures had occurred. Total WMH volume was calculated by summing all segmented areas multiplied by slice thickness.
Automated segmentation on T1 images of baseline and follow-up was performed using Statistical Parametric Mapping 12 unified segmentation routines (SPM12; Wellcome Department of Cognitive Neurology, University College London, United Kingdom, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), in order to obtain gray matter (GM), WM and cerebrospinal fluid (CSF) probability maps. To avoid the erroneous segmentation of WM regions with WMH as GM, the T1 images were first corrected using the binary maps of WMH by replacing the voxel intensities of WMH with the average intensity of the normal-appearing WM on the T1 images. The volumes were calculated by summing all the voxel volumes belonging to the tissue class. All images were visually checked for co-registration errors and motion and/or segmentation artifacts. Total brain volume was taken as the sum of total GM and WM volume. GM volume was composed of the volume of the neocortex, basal ganglia and thalamus.
To account for inter-scan-effects, we corrected the normalized follow-up brain volumes for the difference in intracranial volume (ICV; sum of GM, WM and CSF) between baseline and follow-up by multiplying all volumes by the factor ‘ICV baseline/ICV follow-up’. Next, all volumes were normalized to the baseline ICV to adjust for head size (Colliot et al., 2008). Note that all brain volume represent relative volume (% of the intracranial volume). We calculated brain volume change and changes in the number of lacunes and microbleeds as the difference between follow-up and baseline.
2.6. DTI analysis
Diffusion data were preprocessed and analyzed according to a previous described procedure (van Norden et al., 2011) for baseline and follow-up DTI scans. In short, after eddy current and motion artifacts corrections on the raw diffusion data, we created fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) images using DTIFit within FSL. Next, the DTI images between two time-points were first registered to a half-way space to limit the effects of partial volume, after which these images were fed into the tract-based spatial statistics (TBSS) pipeline (Smith et al., 2006). An FA skeleton was created by thinning the mean FA image based on the FA values. This skeleton was then thresholded at 0.3 to include major WM tracts. The images of MD, AD and RD were subsequently projected on this mean FA skeleton, by applying the projection vectors from each participant's FA-to-skeleton transformation. Changes in the diffusion parameters were derived by calculating the difference between DTI measures of the skeleton at follow-up and baseline.
2.7. Statistical analysis
Statistical analyses were performed using IBM (Armonk, NY) SPSS Statistics 20. To compare the baseline characteristics of participants who were included and those not, we used age and sex-adjusted ANCOVA or logistic regression analysis. For those included, gait and imaging characteristics at baseline and follow-up were compared by using a paired t-test, Wilcoxon signed rank test or McNemar test when appropriate. Multiple linear regression analysis was used to investigate the association between change in each gait variable and change in the different MRI and DTI measures. Since the baseline imaging markers were not associated with gait decline (Van der Holst et al., 2016 Nov.), these measures were not included in the subsequent analyses. Adjustments were made for age, sex, follow-up duration (time between baseline and follow-up assessment), height and baseline gait variable. WMH volume was log transformed, because of the skewed distribution. The variance inflation factor (VIF) was calculated for all regression models to test for the presence of multicollinearity. The VIF scores were low for all models (scores were below 3, where VIF-scores > 5 are considered to reflect high multicollinearity). Regression coefficients were presented as standardized beta-values.
Voxel-wise statistical analyses for changes in TBSS data and individual gait variables were performed by using permutation-based statistical interference tool for non-parametric approach as part of the FSL toolbox (randomize). The number of permutation tests was set at 5000. Significant associations were determined by using a threshold-free cluster enhancement with a p-value < 0.05, corrected for multiple comparisons. Adjustments were made for follow-up duration, age, sex, height and baseline gait variable (model A) and additionally for changes in MRI measures (including WMH volume, number of lacunes and microbleeds, WM and GM volume) (model B).
3. Results
The total study population consisted of 275 participants with a mean (SD) follow-up duration of 5.4 (0.2) years and a mean age at baseline of 62.9 (8.2) years; 56.4% was male. Characteristics of the participants included in this study and those not included are shown in Table 1. Those not included were older, had a poorer cognitive performance, slower gait speed, higher WMH volume, more lacunes, lower WM and GM volume and lower FA and higher MD values of the WM.
Table 1.
Baseline characteristics of the study population.
| Baseline characteristics | Participants included n = 275 |
Participants not included n = 228 |
p-value for difference |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), years | 62.9 (8.2) | 69.0 (8.3) | < 0.001a |
| Male sex, No. (%) | 155 (56.4) | 129 (56.6) | 0.96b |
| MMSE score, mean (SD) | 28.5 (1.5) | 27.7 (1.8) | 0.004a |
| Gait characteristics | n = 275 | n = 224c | |
| Gait speed, mean (SD), m/s | 1.4 (0.2) | 1.2 (0.3) | < 0.001a |
| Gait impairment (< 1.0 m/s), no. (%) | 12 (4.4) | 58 (25.9) | < 0.001b |
| Imaging measuresd | n = 275 | n = 227e | |
| WMH volume, median (IQR), mL | 2.2 (0.8–6.3) | 7.2 (2.6–15.9) | 0.001a |
| WM volume, mean (SD), mL | 467.6 (37.9) | 439.6 (50.2) | < 0.001a |
| GM volume, mean (SD), mL | 622.8 (49.1) | 586.8 (50.2) | < 0.001a |
| Lacunes presence, No. (%) | 44 (16.0) | 88 (38.6) | < 0.001b |
| Microbleeds presencef, No. (%) | 39 (14.3) | 44 (19.5) | 0.81b |
| WM global FA, mean (SD) | 0.33 (0.02) | 0.32 (0.02)g | 0.01a |
| WM global MD, mean (SD), × 10− 3 mm2/s | 0.88 (0.04) | 0.91 (0.05)g | < 0.001a |
Abbreviations: FA: fractional anisotropy; GM: gray matter; IQR: interquartile range; MD: mean diffusivity; MMSE: Mini Mental State Examination; SD: standard deviation; WM: white matter; WMH: white matter hyperintensity.
Age and sex adjusted using ANCOVA.
Age and sex adjusted using logistic regression.
4 participants were excluded because of missing values on baseline gait.
Brain volumes are represented normalized to the total intracranial volume.
1 participant was excluded because of imaging artifacts.
2 participants in both groups were excluded because of missing values of baseline microbleeds.
3 participants were excluded because of baseline DTI artifacts.
Table 2 shows the gait and imaging characteristics of our study population at baseline and follow-up. Mean gait speed decline was 0.2 m/s (SD0.2) over 5 years (p < 0.001, one sample t-test), mainly due to reduction in stride length (mean decline of 0.2 m (SD0.1), p < 0.001). We found a non-significant decrease of cadence (mean decline 0.9 steps/min (SD7.5), p = 0.06).
Table 2.
Comparison of gait and imaging measures at baseline and follow-up (n = 275).
| Characteristic | Baseline | Follow-up | p-value |
|---|---|---|---|
| Gait characteristics | |||
| Gait speed, mean (SD), m/s | 1.38 (0.21) | 1.19 (0.21) | < 0.001a |
| Stride length, mean (SD), m | 1.46 (0.18) | 1.26 (0.18) | < 0.001a |
| Cadence, mean (SD), steps/min | 113.9 (9.4) | 113.0 (9.3) | 0.06a |
| Imaging measures | |||
| WMH volume, median (IQR), mL | 2.2 (0.8–6.3) | 2.8 (1.2–8.0) | < 0.001b |
| WM volume, mean (SD), mL | 467.6 (37.9) | 457.0 (42.8) | < 0.001a |
| GM volume, mean (SD), mL | 622.8 (49.1) | 612.0 (50.2) | < 0.001a |
| Lacunes presence, no. (%) | 44 (16.0) | 61 (22.2) | < 0.001c |
| Microbleeds presenced, no. (%) | 39 (14.3) | 56 (20.4) | < 0.001c |
| FA of skeleton, mean (SD) | 0.49 (0.03) | 0.47 (0.03) | < 0.001a |
| MD of skeleton, mean (SD), × 10− 3 mm2/s | 0.80 (0.04) | 0.82 (0.05) | < 0.001a |
Abbreviations: AD: axial diffusivity; FA: fractional anisotropy; GM: gray matter; IQR: interquartile range; MD: mean diffusivity; RD: radial diffusivity; SD: standard deviation; WM: white matter; WMH: white matter hyperintensity.
Paired t-test.
Wilcoxon signed rank test.
McNemar test.
Respectively 2 and 1 participant(s) had missing values of microbleeds at baseline and follow-up.
There was a significant increase of WMH volume, presence of lacunes and microbleeds and MD, RD and AD values of WM tracts between baseline and follow-up. A significant decrease was seen for WM and GM volume and FA value of the WM tracts during follow-up (Table 2).
3.1. Progression of traditional SVD markers and gait decline
A decline in WM volume was associated with a decline in stride length (β = 0.16, p = 0.007), after adjustment for age, sex, follow-up duration, height, and baseline stride length (Table 3). No significant associations were found for changes in stride length and the other SVD markers, or for changes in gait speed or cadence and changes in all traditional SVD markers.
Table 3.
Association between changes in imaging measures and changes in gait.
| Change in gait parameters | |||
|---|---|---|---|
| Change in imaging measures | Δ gait speed (m/s) | Δ stride length (m) | Δ cadence (steps/min) |
| ΔWMH volumea, mL | − 0.03 | − 0.05 | 0.02 |
| ΔWM volume, mL | 0.10 | 0.16** | 0.04 |
| ΔGM volume, mL | 0.08 | 0.10 | 0.06 |
| ΔLacunes, no. | 0.04 | 0.01 | 0.06 |
| ΔMicrobleeds, no. | − 0.09 | − 0.10 | − 0.07 |
| ΔFA of skeleton | 0.06 | 0.10* | 0.01 |
| ΔMD of skeleton, × 10− 4 mm2/s | − 0.06 | − 0.12* | 0.05 |
| ΔRD of skeleton, × 10− 4 mm2/s | − 0.07 | − 0.14** | 0.02 |
| ΔAD of skeleton, × 10− 4 mm2/s | 0.01 | − 0.03 | 0.07 |
Abbreviations: AD: axial diffusivity; FA: fractional anisotropy; GM: gray matter; MD: mean diffusivity; RD: radial diffusivity; WM: white matter; WMH: white matter hyperintensity.
Data are standardized beta-values.
Adjustments were made for age, sex, follow-up duration, height and baseline gait parameter (baseline gait speed, stride length or cadence respectively).
*p < 0.05, **p < 0.01.
Bold values indicate significance at p < 0.05, false discovery rate-corrected.
Δ indicates difference between follow-up and baseline assessment.
Log transformed.
3.2. Changes in DTI parameters and gait decline
Only significant associations were found for changes in DTI measures and changes in stride length, and not for changes in gait speed or cadence. An increase of RD value and MD value of WM tracts was associated with a decline in stride length (respectively, β = − 0.14, p = 0.009; β = − 0.12, p = 0.018), and to a lesser extent between a decrease of FA and stride length decline (β = 0.10, p = 0.049) (Table 3).
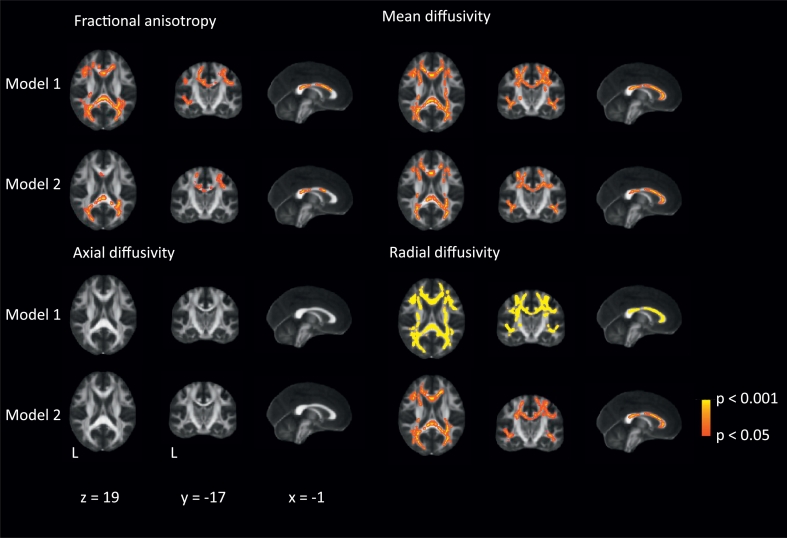
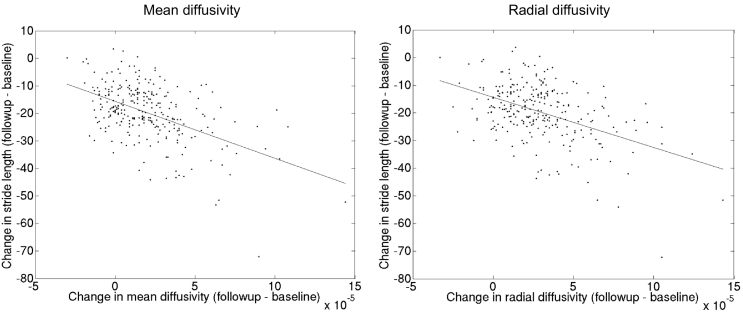
Our longitudinal TBSS analysis showed significant associations between an increase of MD (primarily explained by an increase of RD) and to a lesser extent decrease of FA and decline in stride length (Fig. 2 and Fig. 3), independent of traditional SVD markers. These associations were primarily found in the whole corpus callosum, and bilateral superior and posterior corona radiata (p < 0.005). We did not find any evidence of laterality and there was also involvement of corticospinal tracts within the corona radiata. In contrast, no significant associations were found between change in AD and decline in stride length or between changes in DTI measures and decline in gait speed or cadence after additionally controlling for the traditional SVD markers (data not shown).
Fig. 2.
Association between decrease in stride length and changes in diffusion tensor imaging measures after 5 years of follow-up.
Voxel-wise analysis of the association between changes in stride length (in centimeters) and changes in different diffusion tensor imaging measures, thresholded at P < 0.05 and corrected for multiple comparisons. Adjustments were made for age, sex, follow-up duration, height and baseline stride length (model 1) and additionally for changes in cerebral small vessel disease characteristics (white matter hyperintensities, number of lacunes, microbleeds and gray matter and white matter volume) (model 2). These images are superimposed onto the spatially normalized Montreal Neurological Institute (MNI) stereotactic space fractional anisotropy map. The x, y, and z coordinates represent the MNI coordinates of each slide.
Fig. 3.
The scatterplots shows the relation (linear regression) between changes in mean diffusivity and radial diffusivity values of the significant white matter tracts found in tract-based spatial statistic (TBSS) analysis and changes in stride length (in centimeters), respectively.
4. Discussion
In this longitudinal study, we found that WM atrophy and loss of WM integrity (indicated by an increase of MD and RD, and to a lesser extent decrease of FA) were associated with gait decline by affecting stride length in older adults with SVD after 5 years of follow-up. Changes in DTI measures associated with stride length decline were primarily found in corpus callosum, and posterior and superior corona radiata, and were independent of traditional SVD markers. In contrast, progression of the other SVD markers, including increase of WMH volume, number of lacunes and microbleeds and GM atrophy, were not associated with gait decline.
Main strengths of this longitudinal and single-center study include the quantitative assessment of gait parameters on the same GAITRite at baseline and follow-up, the follow-up duration of 5.4 years, in which participants showed a significant gait decline and progression of SVD, and the exclusion of participants with conditions associated with gait impairment other than SVD which allowed us to further elucidate the role of SVD in gait decline.
Several methodological issues need to be addressed. First, the dropout rate (45% of the study population) may have resulted in attrition bias. However, as those who were not included had a higher load of SVD and a slower gait at baseline it may be that the found associations have been underestimated. Second, due to the observational design of our study, causal inference cannot be reliably made. The possibility of reverse causality, indicating that gait deterioration leads to a sedentary lifestyle and thereby to progression of SVD and reduction of microstructural integrity, cannot be excluded. Third, despite our careful clinical investigation to exclude participants with other factors related to decline in gait from this substudy, including polyneuropathy, vision problems, rheumatic disease, degenerative joint disease, cardiopulmonary diseases and peripheral arterial disease, we cannot rule out the possibility of residual confounding by unmeasured variables that are related to both change in the microstructural integrity of the white matter and gait decline. Fourth, the effect of scanner upgrade (baseline: Sonata and follow-up: Avanto MRI scanner, using the same Siemens head coil) is unknown. It has previously been found that the volumetric measures remained reliable, even after scanner upgrade and that the variance of the volume differences relative to test-retest reproducibility did not significantly change, however it may introduce a bias in the mean volume differences (Jovicich et al., 2009). Therefore, our results on macrostructural brain changes have to be interpreted with caution. Also, the change in slice thickness of the FLAIR images at follow-up could have an impact on the segmentation results of the WMH. Caution has been taken to limit the effect of the change in slice thickness by reslicing to match slice thickness of baseline FLAIR images. Additionally, we calculated WMH volumes for odd and even slices separately and found no significant differences in the effects of change in slice thickness of the FLAIR images. The DTI protocol did not differ between baseline and follow-up, except from the number of diffusion weighted scans (30 versus 61 diffusion-encoding gradient directions). The effect of number of gradient directions is however limited, as we have applied the diffusion tensor model (with 6 degree of freedom). With regard to the scanner upgrade, a previous study showed that the DTI parameters did not differ between the scanners (Huang et al., 2012). Therefore, we consider the DTI results as robust.
We found that a decline in gait speed is primarily caused by a decline in stride length and not in cadence in our population. This is in line with our previous finding that stride length is a more sensitive marker for gait abnormalities in SVD compared to cadence and gait speed (de Laat et al., 2010).
Furthermore, we showed that WM atrophy and changes in DTI measures were associated with gait decline in older adults with SVD. The association between WM atrophy and gait decline has been reported earlier by (Callisaya et al. (2013). In contrast to this study, we found no significant associations between gait decline and progression of WMH volume, or progression of other traditional SVD markers. These findings suggest that diffusional measures have the potential to serve as a better disease marker of SVD burden than the standard MRI measures, which could be used in future studies. There are several reasons for the limitations of the traditional markers based on conventional MRI, which need to be taken into account when planning future studies (De Guio et al., 2016). First, quantification of lesions, such as white matter hyperintensities and lacunes, can be labor-intensive and subjected to bias due to segmentation error. Second, the volume of these lesions rely on dichotomization of the tissue (into normal and abnormal), disregarding the continuous degree of severity. In contrast, DTI can capture the gradual changes both within the lesions and in the normal-appearing white matter. Third, the associations between these lesions and clinical symptoms are generally weak or inconsistent (Holtmannspotter et al., 2005, Nitkunan et al., 2008). Fourth, the diffusional measures correlate more stronger with clinical symptoms, including gait (de Laat et al., 2011), cross-sectionally and longitudinally (this study). Several longitudinal studies have shown that diffusional measures had the smallest sample size estimates compared to other SVD markers (Baykara et al., 2016, Benjamin et al., 2016). Fourth, the limited contribution of each of these SVD markers to gait decline might also be explained by the possible regional-specific associations of these SVD markers on gait decline or a threshold-effect rather than a dose-dependent relation, as some evidence has been found for WMH (Soumare et al., 2009). Also, the changes of the traditional MRI markers of SVD are greater and are easier to detect in more severe patients (van Leijsen et al., 2017). Since we included a rather healthy study population covering the whole spectrum of SVD and excluded severe patients with among other parkinsonism, the variation of MRI markers over time might less resulting in no significant associations with gait decline. Furthermore, it might be that WM microstructural integrity is a moderator in the association between progression of traditional SVD markers and gait decline, as one cross-sectional study showed that in participants with a greater WM microstructural integrity WMH were less strongly associated with gait in comparison to those with a low WM microstructural integrity (Rosario et al., 2016).
Regional analysis of DTI determined several WM tracts involved in gait decline commonly affected by SVD pathology (Srikanth et al., 2010). Consistent with, and extending our previous cross-sectional study (de Laat et al., 2011), we demonstrated that the strongest associations between changes of DTI measures and decline in stride length were found in corpus callosum and corona radiata. The corpus callosum is an important WM tract in motor control, as this region contains commissural fibers connecting multiple cortical areas involved in gait planning, initiation and execution, including frontal, parietal and occipital cortices (Chao et al., 2009). Fibers from these regions converse into the corona radiata that as such contains projection fibers that are involved in motor pathways and thus plays a pivotal role in motor function (Jang, 2009). Also, the involvement of corticospinal tracts within the corona radiata, which is a critical pathway involved in gait function, could contribute to the decline in stride length observed in this study. Our results suggest that progression of disruption of these WM tracts is associated with gait decline in SVD.
We found the strongest association with increase in MD, and especially RD values of the WM tracts and gait decline. This is consistent with data from a recent study showing that MD is a more sensitive marker for SVD progression in comparison to FA in a SVD population over a period of 3 years (Zeestraten et al., 2016). An increase of MD, primarily explained by an increase in RD, is thought to represent demyelination in homogeneous parallel WM regions (Song et al., 2003), which might be related to volume reduction of WM. This result might provide some support for the role of (ischaemic) demyelination in SVD related gait decline above axonal damage/degeneration (reflected by an increase in AD), which has also been described in neuropathological studies of SVD (Englund, 2002). However, as DTI measures are dependent on eigenvalue sorting, it may be difficult to obtain reliable measures in complex WM architecture (e.g. areas with crossing fibers) or pathology (e.g. SVD) (Wheeler-Kingshott and Cercignani, 2009) and therefore our results should be interpreted with caution. Our results might suggest that changes in MD of the WM, especially of the corpus callosum and corona radiata, could serve as an early marker of gait decline in an SVD population.
SVD is a dynamic and highly variable disease process with progression and in some regression of SVD (van Leijsen et al., 2017, Wardlaw et al., 2017). In a recent study reduction in blood pressure was associated with reduction in WMH volume and MD over 1 year period after minor stroke (Wardlaw et al., 2017). This suggests that better blood pressure control could attenuate or even reverse the WMH growth and MD increase of the white matter, and consequently could lead to better gait function over time. The results of randomized controlled trials of blood pressure are however so far mixed. Nevertheless better vascular risk factors management (including hypertension) remains important in reducing the potential risk of brain pathology and gait decline.
In conclusion, our data suggest that WM atrophy and loss of WM integrity, especially of the corpus callosum and corona radiata, are associated with gait decline over a period of 5 years in older adults with SVD. These findings favor a role for WM pathology progression in gait decline in patients with SVD and should therefore be considered as one of the possible causes of gait decline. Future studies should investigate the reproducibility of our results and the potential of DTI as surrogate and early marker of gait impairment in SVD, for example in clinical trials. Meanwhile, clinical practitioners should focus on prevention strategies directed against progression of WM pathology in order to maintain ambulatory function in an aging society.
Disclosure
This study was supported by the “Dutch Brain Foundation” and by the “Netherlands Organization for Scientific Research”. Dr. Tuladhar is a junior staff member of the Dutch Heart Foundation (grant number 2016 T044). Dr. van Dijk received a personal fellowship from the Dutch Brain Foundation (H04–12;F2009(1)-16). Prof. de Leeuw received a personal fellowship from the Dutch Brain Foundation (H04–12;F2009(1)-16) and a VIDI innovational grant from the Netherlands Organization for Scientific Research (grant number 016.126.351).
References
- Abellan van Kan G., Rolland Y., Andrieu S., Bauer J., Beauchet O., Bonnefoy M., Cesari M., Donini L.M., Gillette Guyonnet S., Inzitari M., Nourhashemi F., Onder G., Ritz P., Salva A., Visser M., Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Aribisala B.S., Gow A.J., Bastin M.E., del Carmen Valdes Hernandez M., Murray C., Royle N.A., Munoz Maniega S., Starr J.M., Deary I.J., Wardlaw J.M. Associations between level and change in physical function and brain volumes. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baykara E., Gesierich B., Adam R., Tuladhar A.M., Biesbroek J.M., Koek H.L., Ropele S., Jouvent E., Alzheimer's Disease Neuroimaging I., Chabriat H., Ertl-Wagner B., Ewers M., Schmidt R., de Leeuw F.E., Biessels G.J., Dichgans M., Duering M. A novel imaging marker for small vessel disease based on Skeletonization of white matter tracts and diffusion histograms. Ann. Neurol. 2016;80:581–592. doi: 10.1002/ana.24758. [DOI] [PubMed] [Google Scholar]
- Benjamin P., Zeestraten E., Lambert C., Ster I.C., Williams O.A., Lawrence A.J., Patel B., MacKinnon A.D., Barrick T.R., Markus H.S. Progression of MRI markers in cerebral small vessel disease: sample size considerations for clinical trials. J. Cereb. Blood Flow Metab. 2016;36:228–240. doi: 10.1038/jcbfm.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilney B., Morris M., Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17:68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- Callisaya M.L., Beare R., Phan T.G., Blizzard L., Thrift A.G., Chen J., Srikanth V.K. Brain structural change and gait decline: a longitudinal population-based study. J. Am. Geriatr. Soc. 2013;61:1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- Cesari M., Kritchevsky S.B., Penninx B.W., Nicklas B.J., Simonsick E.M., Newman A.B., Tylavsky F.A., Brach J.S., Satterfield S., Bauer D.C., Visser M., Rubin S.M., Harris T.B., Pahor M. Prognostic value of usual gait speed in well-functioning older people--results from the health, aging and body composition study. J. Am. Geriatr. Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Chao Y.P., Cho K.H., Yeh C.H., Chou K.H., Chen J.H., Lin C.P. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum. Brain Mapp. 2009;30:3172–3187. doi: 10.1002/hbm.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colliot O., Chetelat G., Chupin M., Desgranges B., Magnin B., Benali H., Dubois B., Garnero L., Eustache F., Lehericy S. Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology. 2008;248:194–201. doi: 10.1148/radiol.2481070876. [DOI] [PubMed] [Google Scholar]
- De Guio F., Jouvent E., Biessels G.J., Black S.E., Brayne C., Chen C., Cordonnier C., De Leeuw F.E., Dichgans M., Doubal F., Duering M., Dufouil C., Duzel E., Fazekas F., Hachinski V., Ikram M.A., Linn J., Matthews P.M., Mazoyer B., Mok V., Norrving B., O'Brien J.T., Pantoni L., Ropele S., Sachdev P., Schmidt R., Seshadri S., Smith E.E., Sposato L.A., Stephan B., Swartz R.H., Tzourio C., van Buchem M., van der Lugt A., van Oostenbrugge R., Vernooij M.W., Viswanathan A., Werring D., Wollenweber F., Wardlaw J.M., Chabriat H. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J. Cereb. Blood Flow Metab. 2016;36:1319–1337. doi: 10.1177/0271678X16647396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat K.F., van Norden A.G., Gons R.A., van Oudheusden L.J., van Uden I.W., Bloem B.R., Zwiers M.P., de Leeuw F.E. Gait in elderly with cerebral small vessel disease. Stroke. 2010;41:1652–1658. doi: 10.1161/STROKEAHA.110.583229. [DOI] [PubMed] [Google Scholar]
- de Laat K.F., Tuladhar A.M., van Norden A.G., Norris D.G., Zwiers M.P., de Leeuw F.E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- Englund E. Neuropathology of white matter lesions in vascular cognitive impairment. Cerebrovasc. Dis. 2002;13(Suppl. 2):11–15. doi: 10.1159/000049144. [DOI] [PubMed] [Google Scholar]
- Erkinjuntti T. Subcortical vascular dementia. Cerebrovasc. Dis. 2002;2:58–60. doi: 10.1159/000049152. [DOI] [PubMed] [Google Scholar]
- Ghafoorian M., Karssemeijer N., van Uden I.W., de Leeuw F.E., Heskes T., Marchiori E., Platel B. Automated detection of white matter hyperintensities of all sizes in cerebral small vessel disease. Med. Phys. 2016;43:6246. doi: 10.1118/1.4966029. [DOI] [PubMed] [Google Scholar]
- Holtmannspotter M., Peters N., Opherk C., Martin D., Herzog J., Bruckmann H., Samann P., Gschwendtner A., Dichgans M. Diffusion magnetic resonance histograms as a surrogate marker and predictor of disease progression in CADASIL: a two-year follow-up study. Stroke. 2005;36:2559–2565. doi: 10.1161/01.STR.0000189696.70989.a4. [DOI] [PubMed] [Google Scholar]
- Huang L., Wang X., Baliki M.N., Wang L., Apkarian A.V., Parrish T.B. Reproducibility of structural, resting-state BOLD and DTI data between identical scanners. PLoS One. 2012;7 doi: 10.1371/journal.pone.0047684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S.H. A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. NeuroRehabilitation. 2009;24:279–283. doi: 10.3233/NRE-2009-0479. [DOI] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Han X., Salat D., van der Kouwe A., Quinn B., Pacheco J., Albert M., Killiany R., Blacker D., Maguire P., Rosas D., Makris N., Gollub R., Dale A., Dickerson B.C., Fischl B. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. NeuroImage. 2009;46:177–192. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz H.B., Latt M.D., Tiedemann A., Mun San Kwan M., Lord S.R. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20:20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Moscufo N., Wolfson L., Meier D., Liguori M., Hildenbrand P.G., Wakefield D., Schmidt J.A., Pearlson G.D., Guttmann C.R. Mobility decline in the elderly relates to lesion accrual in the splenium of the corpus callosum. Age. 2012;34:405–414. doi: 10.1007/s11357-011-9242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitkunan A., Barrick T.R., Charlton R.A., Clark C.A., Markus H.S. Multimodal MRI in cerebral small vessel disease: its relationship with cognition and sensitivity to change over time. Stroke. 2008;39:1999–2005. doi: 10.1161/STROKEAHA.107.507475. [DOI] [PubMed] [Google Scholar]
- Rosano C., Kuller L.H., Chung H., Arnold A.M., Longstreth W.T., Jr., Newman A.B. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J. Am. Geriatr. Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]
- Rosario B.L., Rosso A.L., Aizenstein H.J., Harris T., Newman A.B., Satterfield S., Studenski S.A., Yaffe K., Rosano C. Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:968–973. doi: 10.1093/gerona/glv224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert L.C., Nelson C., Howieson D.B., Moore M.M., Kaye J.A. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71:108–113. doi: 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Soumare A., Elbaz A., Zhu Y., Maillard P., Crivello F., Tavernier B., Dufouil C., Mazoyer B., Tzourio C. White matter lesions volume and motor performances in the elderly. Ann. Neurol. 2009;65:706–715. doi: 10.1002/ana.21674. [DOI] [PubMed] [Google Scholar]
- Srikanth V., Phan T.G., Chen J., Beare R., Stapleton J.M., Reutens D.C. The location of white matter lesions and gait--a voxel-based study. Ann. Neurol. 2010;67:265–269. doi: 10.1002/ana.21826. [DOI] [PubMed] [Google Scholar]
- Van der Holst H.M., Van Uden I.W., De Laat K.F., Van Leijsen E.M., Van Norden A.G., Norris D.G., Van Dijk E.J., Tuladhar A.M., de Leeuw F.E. Baseline cerebral small vessel disease is not associated with gait decline after 5 years. Mov Disord. Clin. Pract. 2016;4:374–382. doi: 10.1002/mdc3.12435. https://doi.org/10.1002/mdc3.12435 (Nov.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leijsen E.M.C., van Uden I.W.M., Ghafoorian M., Bergkamp M.I., Lohner V., Kooijmans E.C.M., van der Holst H.M., Tuladhar A.M., Norris D.G., van Dijk E.J., Rutten-Jacobs L.C.A., Platel B., Klijn C.J.M., de Leeuw F.E. Nonlinear temporal dynamics of cerebral small vessel disease: the RUN DMC study. Neurology. 2017;89:1569–1577. doi: 10.1212/WNL.0000000000004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norden A.G., de Laat K.F., Gons R.A., van Uden I.W., van Dijk E.J., van Oudheusden L.J., Esselink R.A., Bloem B.R., van Engelen B.G., Zwarts M.J., Tendolkar I., Olde-Rikkert M.G., van der Vlugt M.J., Zwiers M.P., Norris D.G., de Leeuw F.E. Causes and consequences of cerebral small vessel disease. The RUN DMC study: a prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:29. doi: 10.1186/1471-2377-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R., Lindley R.I., O'Brien J.T., Barkhof F., Benavente O.R., Black S.E., Brayne C., Breteler M., Chabriat H., Decarli C., de Leeuw F.E., Doubal F., Duering M., Fox N.C., Greenberg S., Hachinski V., Kilimann I., Mok V., Oostenbrugge R., Pantoni L., Speck O., Stephan B.C., Teipel S., Viswanathan A., Werring D., Chen C., Smith C., van Buchem M., Norrving B., Gorelick P.B., Dichgans M., nEuroimaging, S.T.f.R.V.c.o Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J.M., Chappell F.M., Valdes Hernandez M.D.C., Makin S.D.J., Staals J., Shuler K., Thrippleton M.J., Armitage P.A., Munoz-Maniega S., Heye A.K., Sakka E., Dennis M.S. White matter hyperintensity reduction and outcomes after minor stroke. Neurology. 2017;89:1003–1010. doi: 10.1212/WNL.0000000000004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Kingshott C.A., Cercignani M. About “axial” and “radial” diffusivities. Magn. Reson. Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Zeestraten E.A., Benjamin P., Lambert C., Lawrence A.J., Williams O.A., Morris R.G., Barrick T.R., Markus H.S. Application of diffusion tensor imaging parameters to detect change in longitudinal studies in cerebral small vessel disease. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147836. [DOI] [PMC free article] [PubMed] [Google Scholar]