Abstract
Inhibiting fatty acid oxidation is one approach to lowering glucose levels in diabetes. Skeletal muscle specific Carnitine Palmitoyltransferase 1b knockout mice (Cpt1bm-/-) comprise a model of impaired fat oxidation; and have decreased fat mass and enhanced glucose disposal and muscle oxidative capacity compared to controls. However, unfavorable effects occur relative to controls when Cpt1bm-/- mice are fed a 25% fat diet, including decreased activity and fat free mass and increased intramuscular lipid and serum myoglobin. In this study we explore if a low fat, high carbohydrate diet can ablate the unfavorable effects while maintaining the favorable phenotype in Cpt1bm-/- mice. Mice were fed either 10% fat (low fat) or 25% fat (chow) diet. Body composition was measured biweekly and indirect calorimetry was performed. Low fat diet abolishes the decreased activity, fat, and fat free mass seen in Cpt1bm-/- mice fed chow diet. Low fat diet also reduces serum myoglobin levels in Cpt1bm-/- mice and diminishes differences in IGF-1 seen between Cpt1bm-/- mice and control mice fed chow diet. Glucose tolerance tests reveal that glucose clearance is improved in Cpt1bm-/- mice relative to controls regardless of diet, and serum analysis shows increased levels of muscle derived FGF21. Electron microscopic analyses and measurements of mRNA transcripts show increased intramuscular lipids, FGF21, mitochondrial and oxidative capacity markers regardless of diet. The favorable metabolic phenotype of Cpt1bm-/- mice therefore remains consistent regardless of diet; and a combination of a low fat diet and pharmacological inhibition of CPT1b may offer remedies to reduce blood glucose.
Introduction
Considerable evidence supports the idea that oversupply of dietary fat exceeds the storage capacity of adipose tissue and leads to ectopic lipid accumulation resulting in “metabolic stress” in skeletal muscle, liver, pancreas and possibly other tissues, contributing to insulin resistance [1–4]. One prevailing theory is that impaired skeletal muscle fatty acid oxidation (FAO) [5–8] leads to the cytosolic accumulation of fatty acyl-CoAs, diacylglycerol (DAG) and ceramides that are directly linked to defects in insulin signaling [9–14]. Others report lipid oversupply via a high fat diet can actually increase FAO to the extent that carnitine and TCA cycle intermediates are limiting, leading to mitochondrial abnormalities and skeletal muscle insulin resistance [15, 16]. Thus, evidence exists that both lipotoxicity and mitochondrial dysfunction contribute to skeletal muscle insulin resistance. Determining if and how these are intertwined is of great interest, with the fundamentally important question being: Does inhibition of FAO in skeletal muscle contribute to insulin resistance?
Carnitine palmitoyltransferase 1 (Cpt1) catalyzes a rate limiting step of fatty acid oxidation via the shuttling of long chain fatty acids across the mitochondrial membrane. The enzyme is expressed as an isozyme subset, with Cpt1b being the predominant form expressed in skeletal muscle, heart, and brown adipose tissue [2, 4]. Cpt1a is found in brain, intestine, kidney, liver, lung, ovary, pancreas, and spleen [1], and an additional isoform has more recently been discovered, known as Cpt1c, which is predominately expressed in brain tissue [3]. Recent reports have begun to highlight the importance of the muscle isoform, Cpt1b, as a potential pharmacological target, indicating increases in insulin sensitivity upon its inhibition [17]. Furthermore, studies using the pharmacological inhibitors of Cpt1b, etomoxir and oxfenicine, show that these drugs do indeed stimulate increased glucose utilization [5, 18].
While these studies implicate Cpt1b as a potential target for further investigation and development of drugs used to treat type II diabetes mellitus, other studies have demonstrated detrimental side effects in their administration. Widespread inhibition of all Cpt1 isoforms by etomoxir can result in steatohepatitis [7]. Oxfenicine inhibits Cpt1b more selectively [12], but we have shown that ablation of Cpt1b in heart leads to cardiac hypertrophy and increased mortality [19]. These reports have raised questions as to the efficacy of inhibiting Cpt1b specifically in skeletal muscle without affecting the remainder of Cpt1b expressing tissues.
Using a skeletal muscle-specific CPT1b KO model, we showed that mitochondrial FAO inhibition results in a myriad of diabetes risk factors (reduced physical activity; increased circulating non-esterified fatty acids; and increased intramyocellular lipids, diacylglycerols, and ceramides) without inducing insulin resistance [14]. Perhaps more importantly, inhibition of mitochondrial FAO also reprograms muscle metabolism by increasing glucose utilization and increasing mitochondrial number, compensatory peroxisomal fat oxidation, and amino acid catabolism. At the whole body level this reprogramming results in resistance to obesity [14].
More recently we showed that inhibition of mitochondrial FAO induces FGF21 expression specifically in skeletal muscle and that FGF21 acts in a paracrine manner to increase glucose uptake under low insulin conditions and has no effect on adiposity in Cpt1bm-/- mice [13]. Though these changes result in the preservation of efficient usage of carbohydrates in Cpt1bm-/- mice, side effects such as the accumulation of intramyocelluar lipids (IMCL), myodegeneration, and decreased activity and energy expenditure also occur [14].
As Cpt1b continues to develop as an intriguing target for the treatment of metabolic disorders, it is important to consider possible effects of varying dietary intake during such treatment. Known clinical variants of Cpt1b have not been functionally characterized as yet. However, maintenance strategies for individuals with Cpt1a defects involve limiting exercise, fasting, and fat intake, while also ensuring high dietary levels of carbohydrates [11]. We therefore hypothesized that Cpt1bm-/- mice might show fewer negative side effects associated with excessive fat intake when fed a diet low in fat and high in carbohydrates. In the current report we examine the effects of varying diets upon Cpt1bm-/- mice in order to determine the flexibility of their previously observed phenotypes; and to assess optimal diet conditions in systems where Cpt1b action is decreased in an attempt to maintain or even restore insulin sensitivity.
Materials and methods
Animal studies
In order to address the role of impaired skeletal muscle FAO in the development of insulin resistance, we targeted the muscle-specific isoform of carnitine palmitoyltransferase, Cpt1b. We crossed mice bearing floxed alleles of Cpt1b to Mlc1f-Cre transgenic mice for specific ablation of Cpt1b in skeletal muscle, but not cardiac tissue [9]. C57L/B6J albino ES cells were used and transferred to C57L/B6J blastocysts. Details of the Cpt1b targeting construct and breeding scheme to generate constitutive muscle-specific inactivation of Cpt1b are shown in S1A Fig of Wicks et al [14].
Animal studies were conducted at Pennington Biomedical Research Center’s AALAC-approved facility. All experiments were in compliance with the NIH Guide for the Care and Use of Laboratory Animals, and approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee (IACUC). All mice were 3–4 month old male mice on C57BL/6 background unless specified otherwise. Unless otherwise stated, mice were multi-housed, and all were exposed daily to 12 hours of light and 12 hours of dark and fed different diets as indicated (S1 Table). Mice were sacrificed by C02 asphyxiation followed by cervical dislocation, according to approved procedures of the Panel on Euthanasia of the American Veterinary Medical Association.
Animal procedures
Body composition was measured using a Bruker NMR Minispec (Bruker Corporation). Serum and plasma collections were performed by submandibular bleeding. GTT were performed following a 4-h fast by intraperitoneal injection of 20% D-glucose (40mg of glucose per mouse). Behavioral and indirect calorimetry studies were done in a 16-chamber Oxymax system (Columbus Instruments) as described previously [15, 16]. For food intake studies, a modification of previously described procedures was used [20]. Briefly, 8 week old male mice were singly housed in filter-top cages and kept at an ambient temperature of 22–23°C in a specific-pathogen free facility. Several days prior to beginning food intake measurements, bedding was removed and replaced with stainless steel wire floor inserts for recovery of food spillage. 1.5 inch diameter Polyvinylchloride nesting tubes were provided to reduce time on wire flooring.
ELISA
ELISA kits were used for measurement of IGF-1, Myoglobin, and FGF21 (Abcam, Cambridge, MA, USA; Life Diagnostics, West Chester, PA, USA; and BioVendor, Brno, Czech Republic, respectively) in serum. IGF-1 and Myoglobin were tested in the fed state, while FGF21 was assayed in overnight fasted serum.
Quantitative RT-PCR
Total RNA from red quadriceps muscle was isolated for later qRT-PCR using an RNeasy Mini Kit supplemented with DNase digestion (Qiagen). cDNA was then synthesized with an iScript cDNA synthesis kit and was used for qRT-PCR with the SYBR Green system (Bio-Rad). Analysis was conducted using the ∆∆CT procedure described previously [21]. Quantification of mouse cyclophilin B and 18s transcripts were used in all experiments as controls for normalization of gene expression. Primer details are provided in S2 Table.
Electron microscopy
Soleus muscles were fixed in 2% (wt/vol) glutaraldehyde/1% (wt/vol) formaldehyde and then postfixed in 2% (wt/vol) osmium tetroxide, en bloc stained in 0.5% uranyl acetate, and embedded in Epon-NMA. 70nm ultrathin sections were mounted on collodion-coated copper grids, stained with Reynolds lead citrate, and imaged with a JEOL 100CX transmission electron microscope at the Socolofsky Microscopy Center at Louisiana State University.
Statistical analysis
Data are expressed as mean ± s.e.m. For measurement of serum proteins and lipids, blood glucose levels, and activity Microsoft Excel software was used for analysis of variance with paired two-tailed Student’s t-tests where normality was established using GraphPad Prism software and the D'Agostino-Pearson normality test. P≤ 0.05 was considered significant. For adiposity, gene expression, and food intake studies populations were not often normally distributed or had sample sizes of N<8. For these analyses GraphPad Prism software and Mann-Whitney U Tests were performed as a measure of significant differences with P≤0.05 considered significant. JMP software from SAS was used for ANCOVA analysis.
Results
Increased fat intake leads to decreased gain of fat and fat free mass in Cpt1bm-/- mice
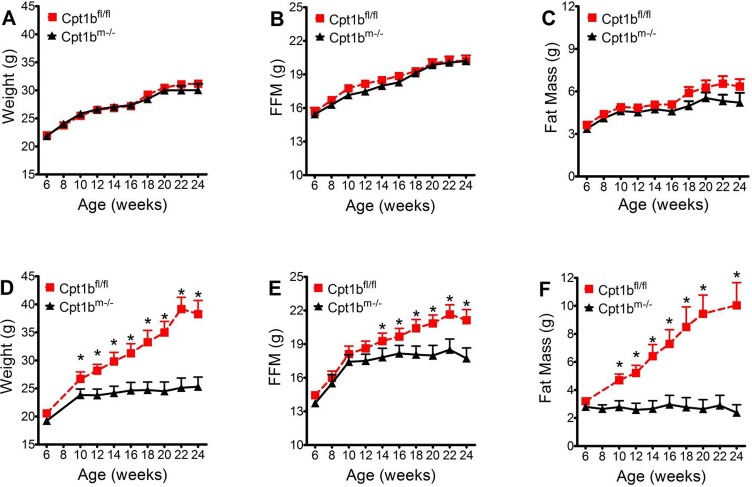
In order to determine if phenotypic differences between Cpt1bm-/- mice and control (Cpt1bfl/fl) mice could be ablated by varying nutrient composition within diets, mice were fed low fat (10% fat) or chow (25% fat) diet (S1 Table). As fat content of diet increases, body weight and fat mass also increase in control animals (Fig 1). However, an inverse relation between dietary fat and body mass appears in Cpt1bm-/- mice, where, as fat content of diet increases, body fat and fat free mass decrease (Fig 1). Because these two diets were nearly matched for energy density and protein level, these differences in fat and fat free mass gain can be attributed to the differences in caloric contributions from fats and carbohydrates between the diets.
Fig 1. Diet effects on Cpt1bm-/- adiposity.
Control mice (red dashed lines, N = 10 animals per time point on chow and N = 8 animals per time point on low fat diet) and Cpt1bm-/- mice (black lines, N = 9 animals per time point on chow and N = 12 animals per time point on low fat diet) were monitored for 24 weeks to assess gain of Weight, Fat, and FFM. Results are compared between groups fed low fat diet (A-C) and chow diet (D-F). Asterisks indicate significance with P ≤ 0.05.
Caloric intake and weight gain are restored when Cpt1bm-/- mice switch to low fat diet
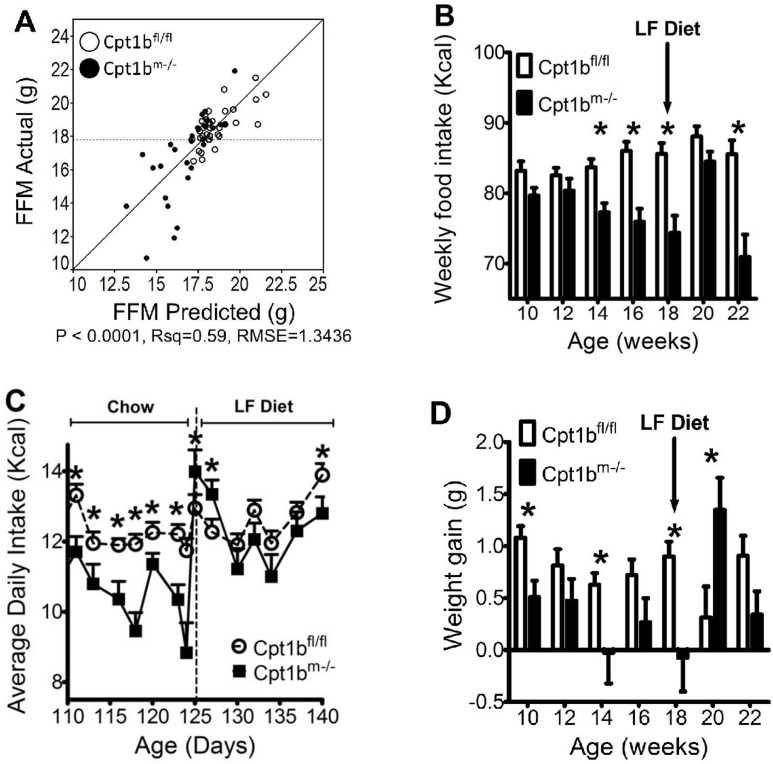
We have reported that the most substantial weight differences between control and Cpt1bm-/- animals are due to differences in fat mass, which begins to deviate several weeks before differences in food intake become significant (Fig 1 and reference [14]). ANCOVA analyses suggest that the differences in FFM vary strongly with average daily Kcal intake. When either weight or fat mass are plotted as a function of average daily Kcal intake while accounting for animal genotype as a covariable, there is a strong genotype effect with a p value of < 0.005 (S1 Fig). If FFM is used in place of either weight or fat mass gain, the genotype specific effect is almost completely diminished, exhibiting a p value of 0.968 (Fig 2A). This suggests that decreased energy consumption and decreased FFM are closely intertwined, and that fat mass is not driving food intake.
Fig 2. Increased caloric intake as a result of low fat feeding explains increased gain of FFM.
(A) ANCOVA analysis conducted by varying FFM as a function of genotype and average daily Kcal intake. White circles represent control mice, while black circles represent Cpt1bm-/- mice. (B) Average weekly Kcal intake is plotted for control (white) and Cpt1bm-/- (black) mice during this food switching study. (C) Average daily food intake was monitored for control (white circles) and Cpt1bm-/- (black squares) mice that were fed 25% fat diet for 125 days, followed by switching to low fat diet. (D) Average weekly weight gain during this food switching study is shown for control (white) and Cpt1bm-/- (black) mice. N = 15 animals per genotype for chow diet and N = 10 animals per genotype for low fat diet. Asterisks indicate significance with P ≤ 0.05.
To further examine the relationship between food intake and body weight, we examined the effect of switching animals fed Chow diet to low fat after 125 days of age. As observed before [14], Cpt1bm-/- mice begin decreasing food intake by twelve weeks of age and stop gaining weight, while control mice eat more with age and continue to gain weight (Fig 2B). The low fat diet switch resulted in an overall increase in average food intake for Cpt1bm-/- mice, which occurred immediately upon switching diets (Fig 2B and 2C). The increase in food intake was accompanied by a concomitant increase in body weight (Fig 2D). While Cpt1bm-/- mice gain more weight when switched from chow to low fat diet, control animals show a decrease. However, these effects on food intake and weight gain were transient, subsiding after about 4 weeks of low fat feeding (Fig 2B and 2D).
Levels of IGF1, myoglobin, NEFA, ketones, and activity change in Cpt1bm-/- mice depending upon diet
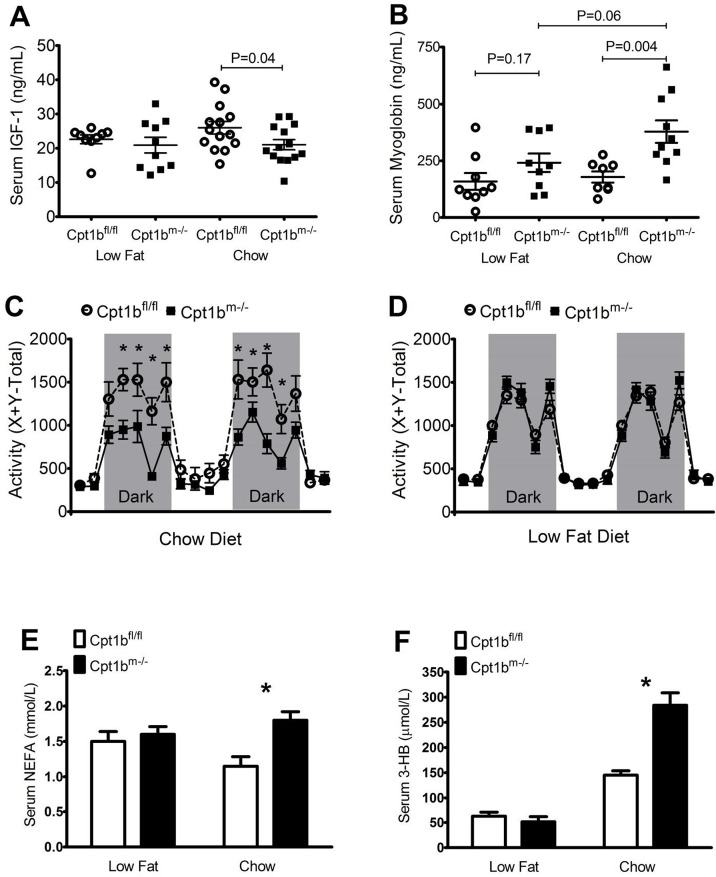
Decreased nutrient intake has been shown to decrease body size via lower production of IGF-1 [22, 23]. In line with these findings, we have shown that serum levels of IGF-1 between control and Cpt1bm-/- mice show significant differences when mice eat chow diet [13]. In the present study, serum levels of IGF-1 were also significantly lower in Cpt1bm-/- mice relative to controls when fed chow diet. However, there is no significant difference when mice are fed low fat diet (Fig 3A).
Fig 3. Low fat diet restores levels of IGF-1, myoglobin, activity, and serum lipids in Cpt1bm-/- mice.
(A) Serum levels of IGF-1 are plotted for control (white circles) and Cpt1bm-/- (black squares) mice for each of the diets tested at 4–6 months (N = 10 animals per genotype for low fat diet and N = 14 animals per genotype for chow diet). (B) Serum myoglobin levels in animals fed either low fat or chow diets (N = 9 animals per genotype for low fat diet; and N = 10 control and 8 Cpt1bm-/- for chow diet). (C-D) Activity variation is plotted for control (white circles) and Cpt1bm-/- (black squares) mice fed chow diet (C) (N = 9 animals per genotype) or low fat diet (D) (N = 12 animals per genotype). Grey sections indicate periods of darkness, while white sections indicate periods of light. (E-F) Serum levels of NEFA (E) and serum levels of 3-hydroxybutyrate (F) in control (white) and Cpt1bm-/- (black) mice fed chow or low fat diet (N = 9 for control animals and N = 10 for Cpt1bm-/- animals). Asterisks indicate significance with P ≤ 0.05.
An additional negative characteristic of Cpt1bm-/- mice fed chow diet is a slight loss of muscle function and elevated levels of serum myoglobin relative to controls [14]. Similar results were found in Cpt1bm-/- mice fed low fat diet, although the results become much less statistically significant (Fig 3B). Circulating myoglobin levels are significantly higher in Cpt1bm-/- mice fed chow diet when compared to controls. Feeding the low fat diet, however, lowers myoglobin levels in Cpt1bm-/- mice to the point where they are no longer significantly higher than controls fed the same diet (p = 0.17), but are lower than Cpt1bm-/- mice fed a chow diet with marginal significance (p = 0.06). Cpt1bm-/- mice on a low fat, high carbohydrate diet therefore show a partial correction of the muscle damage observed in Cpt1bm-/- mice eating a chow diet.
In addition to muscle damage, we have previously shown that Cpt1bm-/- mice have reduced activity relative to control mice when fed the 25% fat chow diet [14]. However, control and Cpt1bm-/- mice have similar levels of activity when fed a 10% fat diet (Fig 3C and 3D). Cpt1bm-/- mice also show increases in serum levels of nonesterified fatty acids (NEFA) compared to controls when fed chow diet [14]. This is also true of 3-hydroxybutyrate, indicating an increase in ketogenesis within Cpt1bm-/- mice relative to controls on chow diet (Fig 3E and 3F). Though we see increases in serum lipids of Cpt1bm-/- mice fed chow diet, we have also examined the liver expression levels of genes involved in ketogenesis, fatty acid synthesis and transport, lipid oxidation, glucose oxidation, and gluconeogenesis; and have found no significant increases in any of these genes with the exception of Pepck, indicating a potential increase in gluconeogenesis (data not shown). Much like the serum hormones and activity levels discussed above, the differences seen between genotypes in these lipid metabolites is diminished during low fat feeding. The changes in the levels of these biomarkers and behavioral patterns together with the abolition of weight gain differences suggest that many of the negative physiological effects upon Cpt1bm-/- mice fed chow diet are restored to control levels with low fat diet feeding.
Diet has little effect on glucose clearance and muscle physiology of Cpt1bm-/- mice
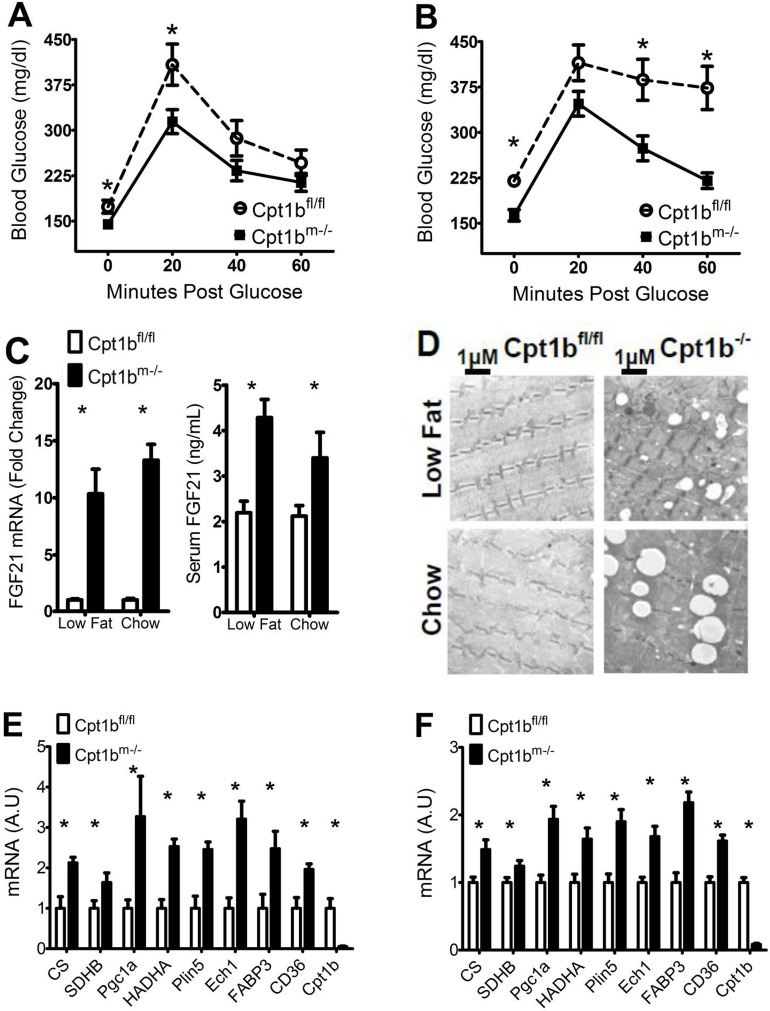
Our previous findings support that Cpt1bm-/- mice have improved glucose clearance relative to control animals when fed chow diet [14]. This finding holds consistent regardless of diet, with blood glucose levels remaining lower in Cpt1bm-/- mice at baseline and throughout the time course of glucose tolerance tests in mice fed either chow or low fat diet (Fig 4A and 4B). For control animals, increased fat content of food predicts increased blood glucose levels; and decreases in blood glucose within sixty minutes post glucose injection are minor relative to Cpt1bm-/- mice. This difference in blood glucose levels is somewhat diminished in animals fed low fat diet, although initial increases in glucose in control animals are significantly larger (Fig 4A). These data suggest that glucose uptake is somewhat enhanced in Cpt1bm-/- mice relative to controls regardless of diet.
Fig 4. Diet has little effect on glucose clearance and muscle physiology of Cpt1bm-/- mice.
(A-B) GTT are shown for control (white circles) and Cpt1bm-/- (black squares) mice that were fed (A) low fat diet (N = 8 animals per genotype) or (B) chow diet (N = 15 animals per genotype). (C) Levels in control (white) and Cpt1bm-/- mice (black) of FGF21 mRNA in red quadriceps muscle (left panel), and FGF21 protein in serum; (right panel) (N = 8 animals per genotype for low fat diet and N = 10 animals per genotype for chow diet). (D) EM showing increased IMCL in soleus of Cpt1bm-/- (right) mice relative to controls (left). (E-F) (N = 4 animals per genotype for low fat diet and N = 6 animals per genotype for chow diet) Relative levels of markers of mitochondrial biogenesis and lipid usage in control (white) and Cpt1bm-/- (black) mouse red quadriceps muscle from mice fed (E) low fat diet or (F) chow diet. Asterisks indicate significance with P ≤ 0.05.
One of the adaptations within Cpt1bm-/- mice contributing to enhanced glucose disposal is the increased expression and secretion of FGF21 specifically from skeletal muscle [13]. Regardless of diet, Cpt1bm-/- mice show significantly higher expression and serum levels of FGF21 (Fig 4C). The persistence of this characteristic within Cpt1bm-/- mice relative to controls is likely a factor in their enhanced glucose disposal regardless of dietary composition.
Although Cpt1bm-/- mice have enhanced glucose disposal, they have decreased FAO, and lipid accumulation in skeletal muscle [14]. We therefore sought to test if this phenotype is consistent in both diets. We examined skeletal muscle using electron microscopy (EM) to check for the presence of increased IMCL. As with Cpt1bm-/- mice fed chow diet, those on low fat diet also show markedly increased lipid droplet formation in skeletal muscle over control mice (Fig 3D). Coordinately, the lower fat diet does not affect the upregulation of Fabp3, Cd36, and Plin5 genes within Cpt1bm-/- mice fed chow diet, which encode for lipid transport and droplet forming proteins (Fig 4E and 4F). Much like Cpt1bm-/- muscle from mice fed chow diet, muscle from Cpt1bm-/- mice fed low fat diet also display increased levels of markers of mitochondrial FAO such as Hadha and Ech1 (Fig 4E and 4F).
Mitochondrial marker numbers are also enhanced within Cpt1bm-/- muscle, and mitochondria are closely associated with the excessive lipid droplets formed therein [14]. Expression levels of PGC1α, an important regulator of mitochondrial number are upregulated relative to control mice within Cpt1bm-/- muscle in mice fed both chow and low fat diets, as are levels of mRNA counterparts of proteins involved in the citric acid cycle and oxidative phosphorylation such as Cs and SdhB (Fig 4E and 4F). Though only red muscle was used for EM and mRNA analysis (soleus and red quadriceps, respectively), our previous report shows consistency of these results in white muscle as well [14]. To support these findings further, assays of the ratios of mitochondrial genes Cytb and Cox2 to the genomic gene β-globin within white quadriceps are increased roughly 1.5 fold in Cpt1bm-/- mice relative to controls (S2 Fig). These results assert that many of the physiological changes within skeletal muscle that contribute to a lean phenotype with enhanced glucose disposal persist regardless of dietary fat or carbohydrate intake.
Discussion
Cpt1bm-/- mice gain weight normally up to 10–12 weeks of age when fed a 25% fat chow diet. Control mice continue to increase food intake with age and gain primarily fat mass. In contrast, Cpt1bm-/- mice eat less with age when fed chow diet, their fat mass stays essentially the same as it was at 10–12 weeks of age throughout life, and FFM peaks around 16 weeks of age (Fig 1). If the amount of fat in the diet is dropped to 10%, control mice do not become obese and Cpt1bm-/- mice actually gain the same amount of fat mass as control mice. These data indicate that there may be an aversion to fat in the diet by Cpt1bm-/- mice, because when switched from the 25% fat diet to the 10% fat diet Cpt1bm-/- mice have a temporary increase in food intake (Fig 2). However, it should be noted that fat mass becomes significantly different between control and Cpt1bm-/- mice well before food intake becomes significantly different [14]. Such decreases in food intake do not occur until significant differences in FFM emerge, and ANCOVA analysis suggests that FFM levels are driving food intake (Fig 2). Therefore it is possible that Cpt1bm-/- mice do not overeat on higher fat diets.
Cpt1bm-/- mice have improved glucose disposal regardless of the amount of dietary fat, indicating that pharmacological inhibition of CPT1b may be an effective means of treating metabolic disorders hallmarked by insulin resistance and obesity. In addition, the skeletal muscle from these mice resembles that from well-trained athletes [24], with increased mitochondrial biogenesis, oxidative capacity, and IMCL, yet they are hypoactive and have some muscle damage when they are fed even a moderate, 25% fat diet [14]. We show here the effects on Cpt1bm-/- mice of altering the fat and carbohydrate content in diet, and report significant ablation of inactivity and muscle damage by inhibition of skeletal muscle Cpt1b when mice eat a diet with a fat content of 10% (Table 1). Overall, the results of this study indicate that a high carbohydrate, low fat diet can ensure that Cpt1bm-/- mice retain fat, FFM and activity at similar levels relative to control mice, while also displaying a healthier metabolic phenotype with increased glucose disposal.
Table 1. Changes in Cpt1bm-/- relative to controls fed chow and low fat diets.
| Cpt1bm-/- vs Control | ||
|---|---|---|
| Chow Diet | Low Fat Diet | |
| Muscle Metabolism mRNA | ||
| Fatty Acid Oxidation Genes | ↑ | ↑ |
| Fatty Acid Transport Genes | ↑ | ↑ |
| Pyruvate Oxidation Genes | ↑ | ↑ |
| Ox Phos Genes | ↑ | ↑ |
| Whole Body Parameters | ||
| Glucose Disposal | ↑ | ↑ |
| Body Weight | ↓ | No Change |
| Fat Mass | ↓ | No Change |
| Fat Free Mass | ↓ | No Change |
| Food Intake | ↓ | No Change |
| Activity | ↓ | No Change |
| Serum Proteins and Lipids | ||
| FGF21 | ↑ | ↑ |
| IGF-1 | ↓ | No Change |
| Myoglobin | ↑ | No Change |
| Nonesterified Fatty Acids | ↑ | No Change |
| 3-Hydroxybutyrate | ↑ | No Change |
Supporting information
(DOCX)
(DOCX)
ANCOVA analysis of weight (A) and fat mass (B) as a function of covariables genotype and average daily Kcal intake. White circles indicate controls and black circles indicate Cpt1bm-/- mice (N = 10–15 animals per genotype per diet group).
(DOCX)
(N = 6 animals per group).
(DOCX)
Acknowledgments
The authors would like to thank Tamra Mendoza and Estrellita Bermudez of the Pennington Biomedical Research Center Gene Nutrient Interactions Laboratory for sample collection and data acquisition in this work.
Support for this work was provided by NIDDK Fellowship T32DK-6458413 to J.D.W., and grant R01DK-089641 to R. L. M. This work used the PBRC facilities of the Transgenic, Comparative Biology, and Genomics Cores supported in part by COBRE (National Institute of General Medical Sciences Grant 8P20GM-103528) and the Nutrition Obesity Research Centers (National Institute of Diabetes and Digestive and Kidney Diseases Grant 2P30-DK-072476-11A1).
J.D.W., B. V., S.E.W., and J.Z. performed research. J.D.W., B. V., S.E.W., J.Z., R.C.N and R.L.M designed research and analyzed data. J.D.W and R.L.M prepared figures and wrote the manuscript. The authors declare that there is no conflict of interest associated with this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Support for this work was provided by NIDDK Fellowship T32DK-6458413 to J.D.W., and grant R01DK-089641 to R.L.M. This work used the Pennington Biomedical Research Center facilities of the Transgenic, Comparative Biology, and Genomics Cores supported in part by COBRE (National Institute of General Medical Sciences Grant 8P20GM-103528) and the Nutrition Obesity Research Centers (National Institute of Diabetes and Digestive and Kidney Diseases Grant 2P30-DK-072476-11A1).
References
- 1.Brown NF, Hill JK, Esser V, Kirkland JL, Corkey BE, Foster DW, et al. Mouse white adipocytes and 3T3-L1 cells display an anomalous pattern of carnitine palmitoyltransferase (CPT) I isoform expression during differentiation. Inter-tissue and inter-species expression of CPT I and CPT II enzymes. The Biochemical journal. 1997;327 (Pt 1):225–31. Epub 1997/11/14. ; PubMed Central PMCID: PMC1218784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esser V, Brown NF, Cowan AT, Foster DW, McGarry JD. Expression of a cDNA isolated from rat brown adipose tissue and heart identifies the product as the muscle isoform of carnitine palmitoyltransferase I (M-CPT I). M-CPT I is the predominant CPT I isoform expressed in both white (epididymal) and brown adipocytes. The Journal of biological chemistry. 1996;271(12):6972–7. Epub 1996/03/22. . [DOI] [PubMed] [Google Scholar]
- 3.Price N, van der Leij F, Jackson V, Corstorphine C, Thomson R, Sorensen A, et al. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics. 2002;80(4):433–42. Epub 2002/10/12. . [DOI] [PubMed] [Google Scholar]
- 4.Warfel JD, Vandanmagsar B, Dubuisson OS, Hodgeson SM, Elks CM, Ravussin E, et al. Examination of carnitine palmitoyltransferase 1 abundance in white adipose tissue: implications in obesity research. American journal of physiology Regulatory, integrative and comparative physiology. 2017;312(5):R816–R20. Epub 2017/03/24. doi: 10.1152/ajpregu.00520.2016 ; PubMed Central PMCID: PMC5451572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keung W, Ussher JR, Jaswal JS, Raubenheimer M, Lam VH, Wagg CS, et al. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes. 2013;62(3):711–20. Epub 2012/11/10. doi: 10.2337/db12-0259 [pii]. ; PubMed Central PMCID: PMC3581198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim T, He L, Johnson MS, Li Y, Zeng L, Ding Y, et al. Carnitine Palmitoyltransferase 1b Deficiency Protects Mice from Diet-Induced Insulin Resistance. J Diabetes Metab. 2014;5(4):361 Epub 2014/10/14. doi: 10.4172/2155-6156.1000361 ; PubMed Central PMCID: PMC4190034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21(1):89–104. Epub 2001/04/12. . [DOI] [PubMed] [Google Scholar]
- 8.Timmers S, Nabben M, Bosma M, van Bree B, Lenaers E, van Beurden D, et al. Augmenting muscle diacylglycerol and triacylglycerol content by blocking fatty acid oxidation does not impede insulin sensitivity. Proc Natl Acad Sci U S A. 2012;109(29):11711–6. Epub 2012/07/04. doi: 10.1073/pnas.1206868109 ; PubMed Central PMCID: PMC3406830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bothe GW, Haspel JA, Smith CL, Wiener HH, Burden SJ. Selective expression of Cre recombinase in skeletal muscle fibers. Genesis. 2000;26(2):165–6. Epub 2000/03/21. doi: 10.1002/(SICI)1526-968X(200002)26:2<165::AID-GENE22>3.0.CO;2-F [pii]. . [PubMed] [Google Scholar]
- 10.Haynie KR, Vandanmagsar B, Wicks SE, Zhang J, Mynatt RL. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes Metab. 2014;16(8):757–60. Epub 2013/12/18. doi: 10.1111/dom.12248 ; PubMed Central PMCID: PMC4057362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saudubray JM, Martin D, de Lonlay P, Touati G, Poggi-Travert F, Bonnet D, et al. Recognition and management of fatty acid oxidation defects: a series of 107 patients. Journal of inherited metabolic disease. 1999;22(4):488–502. Epub 1999/07/17. . [DOI] [PubMed] [Google Scholar]
- 12.Stephens TW, Higgins AJ, Cook GA, Harris RA. Two mechanisms produce tissue-specific inhibition of fatty acid oxidation by oxfenicine. Biochem J. 1985;227(2):651–60. ; PubMed Central PMCID: PMCPMC1144885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandanmagsar B, Warfel JD, Wicks SE, Ghosh S, Salbaum JM, Burk D, et al. Impaired Mitochondrial Fat Oxidation Induces FGF21 in Muscle. Cell reports. 2016. Epub 2016/05/18. doi: 10.1016/j.celrep.2016.04.057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicks SE, Vandanmagsar B, Haynie KR, Fuller SE, Warfel JD, Stephens JM, et al. Impaired mitochondrial fat oxidation induces adaptive remodeling of muscle metabolism. Proc Natl Acad Sci U S A. 2015;112(25):E3300–9. Epub 2015/06/10. doi: 10.1073/pnas.1418560112 [pii]. ; PubMed Central PMCID: PMC4485116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145(1):243–52. doi: 10.1210/en.2003-0452 . [DOI] [PubMed] [Google Scholar]
- 16.Wicks SE, Londot H, Zhang B, Dowden J, Klopf-Eiermann J, Fisher-Perkins JM, et al. Effect of intrastriatal mesenchymal stromal cell injection on progression of a murine model of Krabbe disease. Behav Brain Res. 2011;225(2):415–25. Epub 2011/08/16. doi: 10.1016/j.bbr.2011.07.051 ; PubMed Central PMCID: PMC3179783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T, Moore JF, Sharer JD, Yang K, Wood PA, Yang Q. Carnitine Palmitoyltransferase 1b Deficient Mice Develop Severe Insulin Resistance After Prolonged High Fat Diet Feeding. J Diabetes Metab. 2014;5 Epub 2015/01/13. doi: 10.4172/2155-6156.1000401 ; PubMed Central PMCID: PMC4286342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timmers S, Nabben M, Bosma M, van Bree B, Lenaers E, van Beurden D, et al. Augmenting muscle diacylglycerol and triacylglycerol content by blocking fatty acid oxidation does not impede insulin sensitivity. Proc Natl Acad Sci U S A. 2012;109(29):11711–6. Epub 2012/07/04. doi: 10.1073/pnas.1206868109 ; PubMed Central PMCID: PMC3406830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haynie KR, Vandanmagsar B, Wicks SE, Zhang J, Mynatt RL. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes Metab. 2014;16(8):757–60. Epub 2013/12/18. doi: 10.1111/dom.12248 ; PubMed Central PMCID: PMC4057362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruger C, Kumar KG, Mynatt RL, Volaufova J, Richards BK. Brain transcriptional responses to high-fat diet in Acads-deficient mice reveal energy sensing pathways. PLoS One. 2012;7(8):e41709 Epub 2012/09/01. doi: 10.1371/journal.pone.0041709 ; PubMed Central PMCID: PMC3425564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–88. Epub 2011/01/11. doi: 10.1038/nm.2279 ; PubMed Central PMCID: PMC3076025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straus DS, Takemoto CD. Specific decrease in liver insulin-like growth factor-I and brain insulin-like growth factor-II gene expression in energy-restricted rats. The Journal of nutrition. 1991;121(8):1279–86. Epub 1991/08/01. . [DOI] [PubMed] [Google Scholar]
- 23.Straus DS. Nutritional regulation of hormones and growth factors that control mammalian growth. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1994;8(1):6–12. Epub 1994/01/01. . [DOI] [PubMed] [Google Scholar]
- 24.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–61. doi: 10.1210/jcem.86.12.8075 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
ANCOVA analysis of weight (A) and fat mass (B) as a function of covariables genotype and average daily Kcal intake. White circles indicate controls and black circles indicate Cpt1bm-/- mice (N = 10–15 animals per genotype per diet group).
(DOCX)
(N = 6 animals per group).
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.