Abstract
Mesofauna taxa fill key trophic positions in soil food webs, even in terrestrial–marine boundary habitats characterized by frequent natural disturbances. Salt marshes represent such boundary habitats, characterized by frequent inundations increasing from the terrestrial upper to the marine pioneer zone. Despite the high abundance of soil mesofauna in salt marshes and their important function by facilitating energy and carbon flows, the structure, trophic ecology and habitat-related diet shifts of mesofauna species in natural salt marsh habitats is virtually unknown. Therefore, we investigated the effects of natural disturbance (inundation frequency) on community structure, food web complexity and resource use of soil mesofauna using stable isotope analysis (15N, 13C) in three salt marsh zones. In this intertidal habitat, the pioneer zone is exposed to inundations twice a day, but lower and upper salt marshes are less frequently inundated based on shore height. The mesofauna comprised 86 species / taxa dominated by Collembola, Oribatida and Mesostigmata. Shifts in environmental disturbances influenced the structure of food webs, diversity and density declined strongly from the land to the sea pointing to the importance of increasing levels of inundation frequency. Accordingly, the reduced diversity and density was associated by a simplification of the food web in the pioneer zone as compared to the less inundated lower and upper salt marsh with a higher number of trophic levels. Strong variations in δ15N signatures demonstrated that mesofauna species are feeding at multiple trophic levels. Primary decomposers were low and most mesofauna species functioned as secondary decomposers or predators including second order predators or scavengers. The results document that major decomposer taxa, such as Collembola and Oribatida, are more diverse than previously assumed and predominantly dwell on autochthonous resources of the respective salt marsh zone. The results further suggest that Mesostigmata mostly adopt an intraguild predation lifestyle. The high trophic position of a large number of predators suggests that intraguild predation is of significant importance in salt marsh food webs. Presumably, intraguild predation contributes to stabilizing the salt marsh food web against disturbances.
Introduction
Salt marshes are widespread along the European coasts and cover 20% of the area of the North Atlantic Wadden Sea [1]. The exposure to frequent inundations and the clear elevational gradient with the upper salt marsh, lower salt marsh and pioneer zone make them to ideal model systems to study, how physical and biological disturbance factors interact to create pattern in natural communities [2]. Although salt marshes provide important ecosystem services such as biomass production, supply of food sources, nitrogen and carbon cycling, they are also heterogeneous habitats, and plants and animals must cope with increasing abiotic disturbance due to increasing frequency of inundations towards the pioneer zone [3–7]. Depending on shore height, the pioneer zone is exposed to inundations twice a day [8], which are less frequent in the lower salt marsh with 150–250 times a year and the upper salt marsh with 35–70 times a year [9, 10]. The consequences of the harsh and dynamic abiotic environmental conditions results in typical vegetation zonation [10–13], which corresponds to a shift from terrestrial C3 to marine C4 plants/algae along the land sea gradient [14, 15]. Similar to the plant based zonation, the organic material of the soil differs between zones, depending on decomposition rates, the amount of deposition, and its origin from either marine or terrestrial resources. C3 and C4 material differ in their photosynthetic pathway resulting in distinct stable carbon isotopic signals allowing identification of their contribution as basal resources of food webs [16–19].
Intertidal communities historically played an important role in the development of community ecology since they occur across pronounced abiotic and biotic disturbed conditions along the land-sea gradient [20], encompassing a large number of microhabitats with varying niches on small spatial scales [21]. Disturbance is known to strongly determine ecological patterns and processes, affecting diversity and dynamics, as well as the food web structure of communities [6, 20, 22, 23]. Consequently, ecological settings are complex and largely control the isotopic structure of salt marsh soil food webs [10, 14, 24–26]. However, despite the central role of terrestrial soil mesofauna in facilitating energy and carbon flows between trophic levels, their structure, trophic ecology and habitat-related diet shifts in salt marsh food webs is virtually unknown.
The salt marsh soil community is strongly size structured and previous studies investigated distribution patterns [27–29], food web structure and feeding preferences of macro-invertebrates [14, 30–32] as well as the influence of autochthonous and allochthonous resource material on community structure [5, 33–35]. Macro-invertebrates comprise different feeding types allowing to utilize diverse food resources, accordingly their diet changes along the environmental gradient [32, 36, 37]. However, previous studies mostly focused on higher trophic levels and neglected the complexity and functional role of small mesofauna species such as Collembola, Oribatida and Mesostigmata.
Collembola and Oribatida are diverse and abundant and play multiple roles in salt marsh food webs [38, 39]. They may significantly affect bacterial density and biomass [40], are important decomposers and microbivores, thereby functioning as primary and secondary decomposers as well as predators and scavengers. Small predators such as Mesostigmata are among the most effective predators in soils and sediments [41], but knowledge of their trophic ecology is based primarily on laboratory observations with only few species studied in detail [42]. Further, mesofauna taxa are major food sources for macro-invertebrates [43–45]. The few studies existing suggest that mesofauna abundance and diversity differ substantially between salt marsh zones with the diversity being low in the lower salt marsh and increasing with plant cover at higher salt marsh zones [46–48]. Increased abundance and diversity is likely to affect food web structure with the number of trophic levels increasing with habitat productivity and resource availability [49].
In order to understand the effect of disturbances on mesofauna ecology in salt marshes, we investigated the mesofauna community structure, trophic levels and resource use of abundant species along a small-scale salt marsh gradient of the German North Sea using stable isotopes. The analysis of natural variations in stable isotope ratios is a powerful tool to study the trophic structure of soil animal communities [19, 50–52], and the flux of carbon from terrestrial and marine realms into animal food webs [53, 54]. We expected (1) soil mesofauna diversity and density to decrease from the upper salt marsh to the pioneer zone correlated with more frequent flooding and associated abiotic variations, (2) the reduced density and diversity to be associated with a simplification of the mesofauna food web in the pioneer zone as compared to the less disturbed lower and upper salt marsh with a higher number of trophic levels, and (3) dominant mesofauna species such as Collembola, Oribatida and Mesostigmata feeding at multiple trophic levels functioned as secondary decomposers or predators including second order predators or scavengers.
Materials and methods
Study site
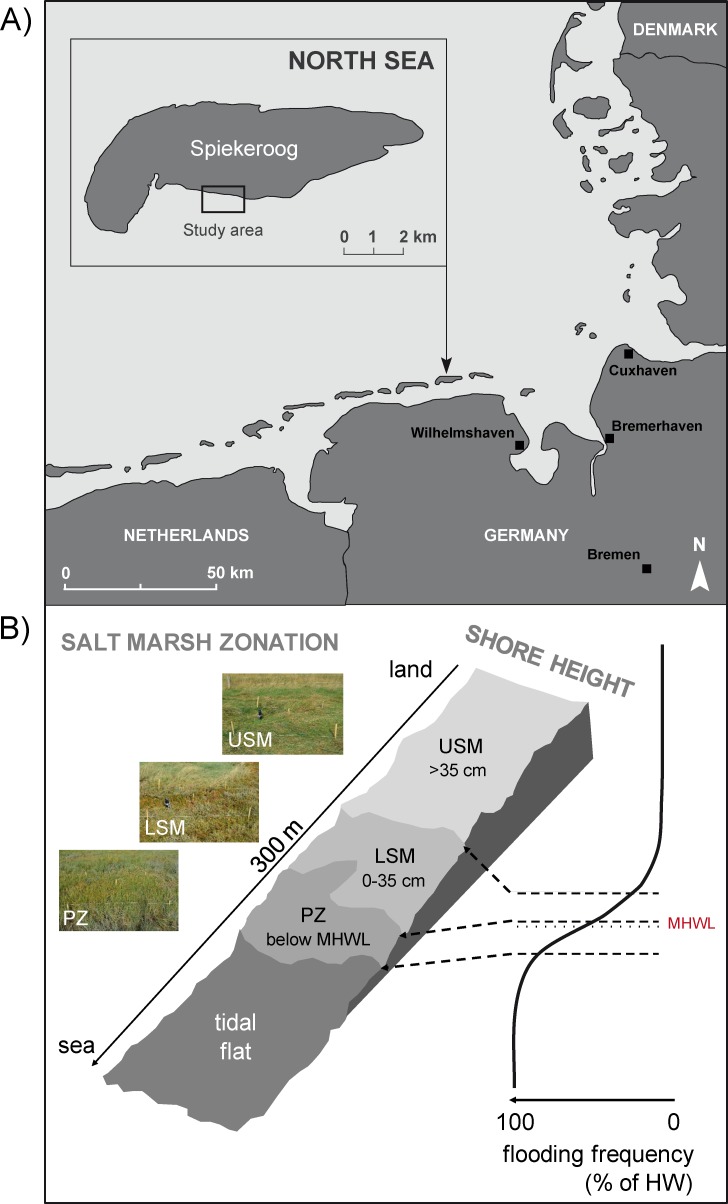
The study was performed in the salt marshes of the North Sea dune island Spiekeroog (53°45’2”- 53°47’1”N, 7°40’0”- 7°49’1”E), which belongs to the East Frisian Islands, forming part of the Wadden Sea National Park of Lower Saxony, Germany (Fig 1A). The Wadden Sea National Park of Lower Saxony gave the permission to conduct the study on this site, the current study did not involve endangered or protected species. Unlike many salt marshes along the German coastline, the salt marsh on Spiekeroog is not affected by extensive agricultural activities or urban development. Thereby, the island provides the opportunity to study the distribution and trophic structure of soil invertebrates under natural settings. Salt marshes are located in the southern part of the island, which is sheltered from northerly winds and incoming tides ranging 1 to 3 m. The boundary between terrestrial and marine habitats is characterized by halophytic plants close to the mean high water line (MHWL) and consists of three vegetation zones: upper salt marsh, lower salt marsh and pioneer zone (Fig 1B). The sampling plots were selected according to these vegetation zones depending on shore height (upper salt marsh: >35 cm above MHWL, lower salt marsh: 0–35 cm above MHWL, pioneer zone: below MHWL) and frequency of inundation (upper salt marsh: 35–70 times a year, lower salt marsh: 150–250 times a year, pioneer zone: inundations twice a day; Fig 1B). The dominant C3 plant of the upper salt marsh is Elytrigia atherica, whereas the lower salt marsh predominantly is colonized by two specialized C3 plant species, Atriplex portulacoides and Puccinellia maritima. Vegetation in the pioneer zone is dominated by the C3 plant Salicornia stricta and the C4 plant Spartina anglica (Fig A and Table D in S1 File). Furthermore, C4 macroalgae species such as Enteromorpha sp. and Ulva lactuca are widespread in the pioneer zone (Fig A and Table D in S1 File).
Fig 1.
(A) Map of study area: Spiekeroog, East Frisian Islands, Germany, North Sea. (B) Salt marsh zonation: upper salt marsh (USM, >35 cm above MHWL), lower salt marsh (LSM, 0–35 cm above MHWL) and pioneer zone (PZ, below MHWL) from the land to the sea in relation to shore height and frequency of inundations (USM: 35–70 times a year, LSM: 150–250 times a year, PZ: inundations twice a day).
Experimental design and sampling
Six sampling plots of 1.80 x 1.80 m (3.24 m2) were established in each of the three salt marsh zones (upper salt marsh, lower salt marsh and pioneer zone) in the frame of the BEFmate-project [55]. On 24th September 2014, soil samples were collected from the upper 5 cm of each sampling plot using a soil corer (Ø 5 cm). Each sampling plot consisted of four subplots 90 x 90 cm (0.81 m2), two samples were taken in two of the four subplots resulting in four pooled samples per plot per vegetation zone (72 samples overall). To avoid pseudoreplication, the mean of these four samples was used for statistical analysis resulting in a total number of 18 samples. Soil cores were placed in plastic containers and transported to the laboratory for extracting the animals. Furthermore, at each of the three vegetation zones, vascular plants, macroalgae and organic matter were sampled by hand or with a spatula for analysis of potential food resources, resulting in 21 samples with three replicates each, in total 63 samples. The samples were stored in plastic bags at -10°C until drying and further processing.
Sample processing
In the laboratory, living soil invertebrates were extracted using a high gradient heat extractor [56]. This extraction method is not suitable to extract nematodes, therefore nematodes were excluded from soil invertebrate analysis. Soil invertebrates were fixed in 70% ethanol and stored at -10°C until identification. Preservation in 70% ethanol little affects 13C signatures of animals [57], but 13C signatures vary with e.g., body size and cuticle thickness as well as life stage associated with changes in the proportion of fat reserves. To exclude these variations in δ13C signatures, we used specimens of similar body size and focused mainly on adult individuals.
For identification individuals were placed on glass-slides, analyzed to species or genus level and counted under a binocular eyepiece or microscope. Following identification and depending on the number of individuals, a minimum of two replicates for each dominant mesofauna species (Collembola, Oribatida and Mesofauna) was prepared for stable isotope measurements, in total 27 species. Soil invertebrates were dried at 40°C for 24 to 48 h and placed in a desiccator. Samples >10 µg dry mass were weighed on a fine scale (Cubis, Sartorius, Göttingen, Germany) and transferred into tin capsules. Large species (>400 µm) were fragmented and homogenized using mortar and pestle, whereas smaller mesofauna species (<400 µm) were bulked with a maximum of five specimens per sample (the final weight of the samples is given in supporting information Table B in S1 File). Food resources (vascular plants and macroalgae) were washed with tap water to remove sediment and smaller algae before drying at 40°C for 24 to 48 h. After drying, organic matter samples were passed through 250–2000 μm screen in order to remove larger organic debris, shells and stones. Resources were homogenized using a mortar and the fine powder was transferred into tin capsules. The capsules filled with soil invertebrates and food resources were closed and wrapped into pellets, placed into well plates and stored in a desiccator until stable isotope analysis.
Stable isotope analysis
To estimate the trophic position of animals in the salt marsh food web, natural variations in stable isotope ratios (15N/14N and 13C/12C) were analyzed [50, 58]. δ15N values were used to delineate the trophic position of the species and to ascribe species to trophic levels we assumed a consistent enrichment of 3.4 δ units per trophic level [50, 59]. In contrast to 15N, trophic level fractionation of 13C is low and varies between -0.5‰ [60] and 1 ‰ [61]. Therefore, δ13C signatures of consumers resemble that of their diet and can be used to evaluate the sources of carbon of consumers if the isotopic signatures of the sources are different [50], i.e. organisms feeding on C3 plants can be distinguished from those feeding on C4 plants [62].
Stable isotope ratios were analyzed using a coupled system consisting of an elemental analyzer (for mesofauna: Euro EA 3000, EuroVector S.p.A, Milan, Italy; for plants: NA 1500, 2500, Carlo Erba, Milan, Italy) and a gas mass spectrometer (Finnigan Delta V Plus, Thermo Electron, Bremen, Germany). The mean standard deviation ranged between <0.1 and 0.2 ‰ [63]. Signatures of stable isotopes were expressed using the δ notation with δX (‰) = [(Rsample—Rstandard)/Rstandard] x 1000. X represents the target isotope of δ13C or δ15N (‰), R the heavy-to-light isotope ratios (13C/12C and 15N/14N) of samples and standard, respectively. Vienna Pee Dee River Belemnite (PDB) and atmospheric nitrogen served as primary standards for δ13C and δ15N, respectively. Acetanilide was used for internal calibration. Deviation of stable isotope ratios of animals and potential resources were expressed using the Δ notation representing the differences between the soil animal stable isotope ratio and the respective ratio of salt marsh resources.
Stable isotope signatures of the organic matter varied considerably at the three salt marsh zones (δ13C values of -25.9 ± 0.76‰, -29.9 ± 0.26‰ and -13.5 ± 0.06‰ for the upper salt marsh, lower salt marsh and pioneer zone, respectively; respective δ15N values of 4.6 ± 0.50‰, 10.2 ± 0.45‰ and 12.0 ± 0.04‰). To determine the trophic positions of soil invertebrates, stable isotope signatures were normalized to the mean of the organic matter signature of the respective salt marsh zone (calibration factors for δ13C of +2.8‰, +6.8‰ and -9.6‰ for the upper salt marsh, lower salt marsh and pioneer zone, respectively; respective calibration factors for δ15N of +4.3‰, -1.3‰, -3.0‰). Although normalizing stable isotope values to that of soil organic matter may inadequately reflect the baseline of the animal community, normalization to dead organic matter has been shown to considerably improve comparison of trophic positions of soil invertebrates across different habitats [42].
Statistical analysis
Community structure of the three salt marsh zones were analyzed by canonical correspondence analysis (CCA) and non-metric multidimensional scaling (NMDS) using CANOCO 5 (Microcomputer Power, Ithaca, USA, 2012). The data set of the NMDS was subsequently used for discriminant function analysis (DFA) in STATISTICA 7.0. For the CCA and the NMDS, non-identified individuals were excluded and the data were log-transformed.
In case of significant DFA, analysis of variance (ANOVA) and Tukey's HSD (honestly significant difference) test were performed for soil mesofauna density and diversity along the salt marsh gradient. Further, to inspect differences in the number of trophic levels between salt marsh zones and the relationship between the δ13C values of basal resources from the land to the sea ANOVA was conducted using R statistical programming environment version 2.4.0 (R Development Core Team 2007). Prior to ANOVAs data were inspected for normality using Shapiro-Wilks test using STATISTICA 7.0 (StatSoft, Inc., Tulsa, OK, 2004); the test generally was not significant (p > 0.05).
To estimate the relative intake of potential food sources of consumers (Collembola and Oribatida), the Bayesian mixing model FRUITS 2.1.1 Beta (Food Reconstruction Using Isotopic Transferred Signals) was used [64]. Mean values for the stable isotopes of three food groups (C3 plants/algae, C4 plants/algae and organic material, which contained C3 and/or C4 plants/algae) were used to determine the relative contributions of basal resources to Collembola and Oribatida nutrition in the salt marsh.
Results
Community structure
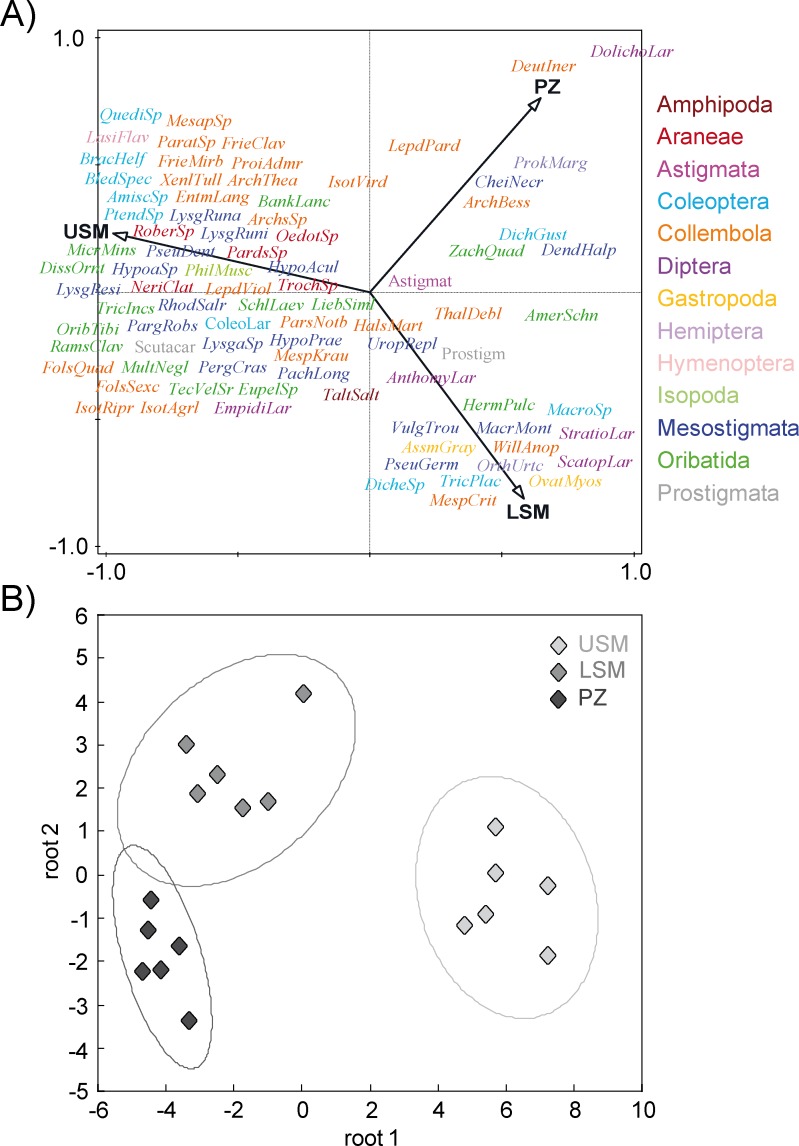
The salt marsh soil community comprised 86 taxa, of which 61 were identified to species level: 1 Amphipoda, 5 Araneae, 1 Astigmata, 9 Coleoptera, 24 Collembola, 5 Diptera, 2 Gastropoda, 2 Hemiptera, 1 Hymenoptera, 1 Isopoda, 19 Mesostigmata, 14 Oribatida and 2 Prostigmata (see Table A in S1 File for full species list). Canonical correspondence analysis (CCA) separated the community of the upper salt marsh from that of the lower salt marsh and pioneer zone, reflecting the variations in distribution patterns of soil mesofauna between the vegetation zones (Fig 2A). Discriminant function analysis (DFA) indicated that soil animal community structure of the upper salt marsh significantly differenced between and that of the lower salt marsh and the pioneer zone (Fig 2B; Tukey’s HSD: p < 0.0001).
Fig 2.
(A) Soil mesofauna community structure: canonical correspondence analysis (CCA) based on the density of 86 soil animal taxa (log-transformed) along the studied salt marsh gradient: upper salt marsh (USM), lower salt marsh (LSM) and pioneer zones (PZ). For full species names see Table A in S1 File. Eigenvalues of axis 1 = 0.6610 and axis 2 = 0.3214. (B) Discriminant function analysis (DFA) of soil invertebrate community of the three salt marsh zones: USM, LSM and PZ; ellipses represent confidence ranges at p < 0.05.
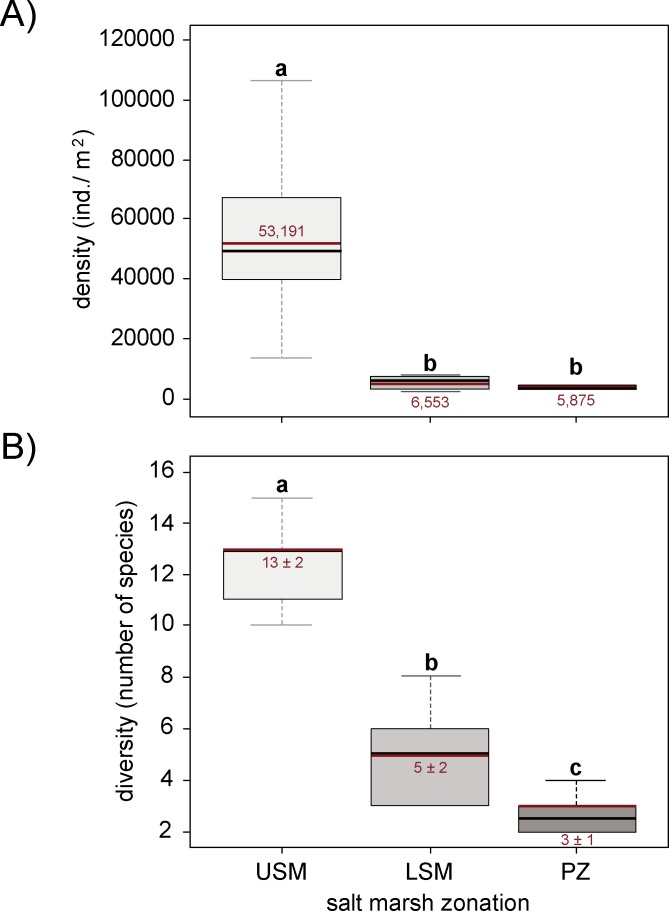
Density of the soil mesofauna significantly declined from the upper salt marsh to the lower salt marsh and pioneer zone (F2,15 = 13.26, p < 0.001; Fig 3A). Similarly, species diversity varied significantly between the three salt marsh zones (F2,15 = 61.18, p < 0.001; Fig 3B), but declined more continuously from the upper salt marsh to the lower salt marsh to the pioneer zone. The mesofauna was dominated by species of Collembola, Oribatida and Mesostigmata, which mainly occurred in the upper and lower salt marsh (see Table A in S1 File for species list). In contrast, the pioneer zone was dominated by species connected to littoral habitats with high frequency of inundation, e.g. the two Collembola species Archisotoma besselsi and Thalassaphorura debilis, the three Oribatida species Ameronothrus schneideri, Hermannia pulchella and Zachvatkinibates quadrivertex, and the Mesostigmata species Cheiroseius necorniger and Dendrolaelaps halophilus.
Fig 3.
(A) Density and (B) diversity of soil mesofauna species at the upper salt marsh (USM), lower salt marsh (LSM) and pioneer zone (PZ). Boxplots represent mean (red line) and median (black line) of density and diversity. Different letters represent significant differences between zones (Tukey’s HSD test, p < 0.05).
Trophic structure
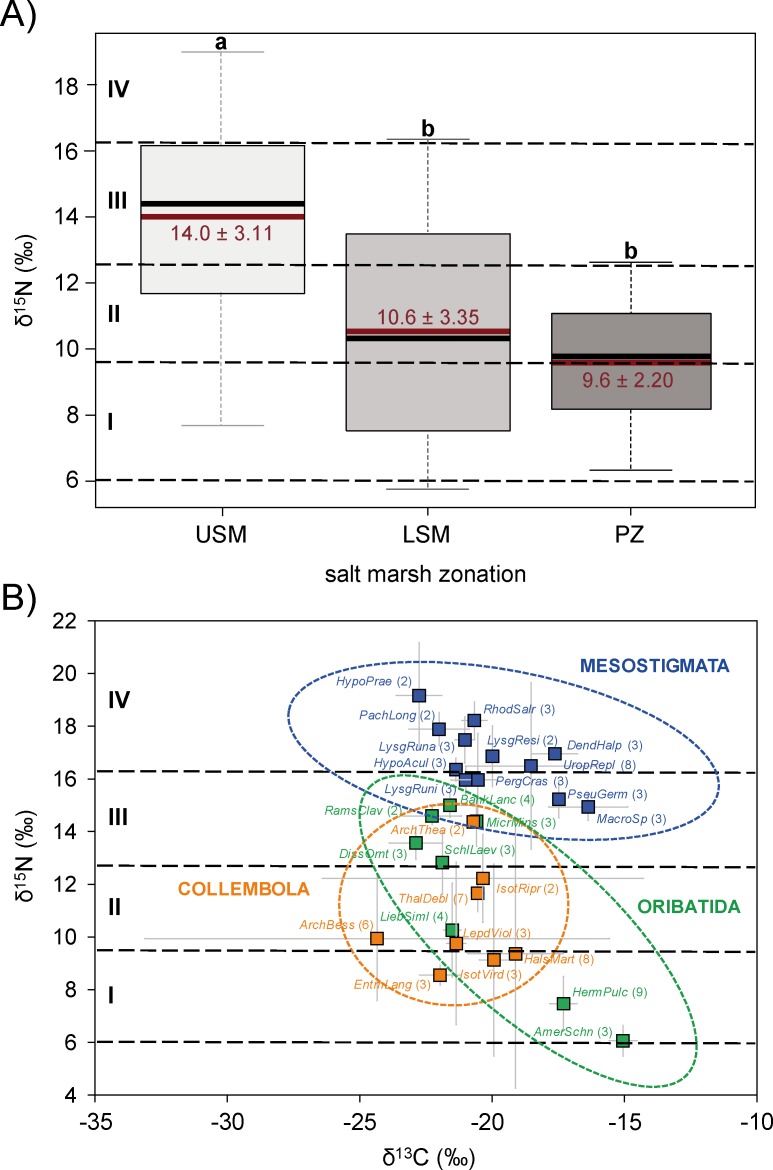
As indicated by δ15N signatures, the complete mesofauna food web consisted of four trophic levels: (I) primary decomposers (6.1–9.5‰), (II) secondary decomposers (9.5–12.9‰), (III) first order predators (12.9–16.3‰) and (IV) second order predators (16.3–19.7‰). However, the trophic structure of the mesofauna food web changed significantly along the land sea transect (F2,50 = 9.08, p < 0.000). On average, δ15N values of the upper salt marsh (14.4 ± 3.14‰) exceeded those of the lower salt marsh (11.4 ± 4.23‰) and pioneer zone (9.1 ± 3.24‰) suggesting shorter food chains in the latter two zones, with the food web in the upper salt marsh comprising four trophic levels whereas that in the pioneer zone comprising only two trophic levels (Fig 4A, Table B in S1 File).
Fig 4.
(A) δ15N signatures of all soil mesofauna species in the upper salt marsh (USM), lower salt marsh (LSM) and pioneer zone (PZ). Boxplots represent mean (red line) and median (black line) of δ15N signatures; different letters represent significant differences between zones (Tukey’s HSD; p < 0.05). (B) δ13C and δ15N stable isotope values (means with standard deviation) of dominant mesofauna species: Collembola (orange squares), Mesostigmata (blue squares) and Oribatida (green squares). Black dashed horizontal lines represent estimated trophic level boundaries with each trophic level spanning 3.4‰ δ15N: I = primary decomposers, II = secondary decomposers, III = first order predators, and IV = second order predators. Number of replicates are included in parentheses; see Table C in S1 File for full species names.
δ15N signatures of mesofauna species spanned from 6.1 ± 0.72‰ in A. schneideri (Oribatida) to 19.2 ± 2.01‰ in Hypoaspis praesternalis (Mesostigmata) reflecting the four trophic levels the upper salt marsh (Fig 4B, Table C in S1 File). In general, the number of primary decomposers, i.e., animals that feed mainly on organic matter, was low. Only the Oribatida species A. schneideri had 15N signatures close to that of organic matter. Collembola species were positioned in trophic levels I to III with δ15N values ranging between 8.6 ± 0.38‰ and 14.4 ± 0.07‰ (see Table C in S1 File). Most of the Collembola species were ascribed to primary or secondary decomposers with the exception of Archisotoma theae, which was positioned in trophic level III indicating first order predator lifestyle (Fig 4B). Similarly, Oribatida spanned from trophic level I to III with δ15N values ranging between 6.1 ± 0.72 and 15.0 ± 0.46‰ (see Table C in S1 File). Contrasting Collembola, however, most Oribatida species (Banksinoma lanceolata, Dissorhina ornata, Microppia minus, Ramusella clavipectinata and Scheloribates laevigatus) were ascribed to trophic level III, i.e. first order predators (Fig 4B). As expected, Mesostigmata species had the highest δ15N values ranging from 14.9 ± 0.50 to 19.2 ± 2.01‰ (see Table C in S1 File). Accordingly, the Mesostigmata community was composed of first and second order predators, i.e. trophic level III and IV (Fig 4B). High δ15N signatures of Mesostigmata species indicate that beside secondary decomposers, first order predators such as certain Oribatida and Collembola species as well as other Mesostigmata species significantly contribute to their diet.
Linkage of basal resources to consumers
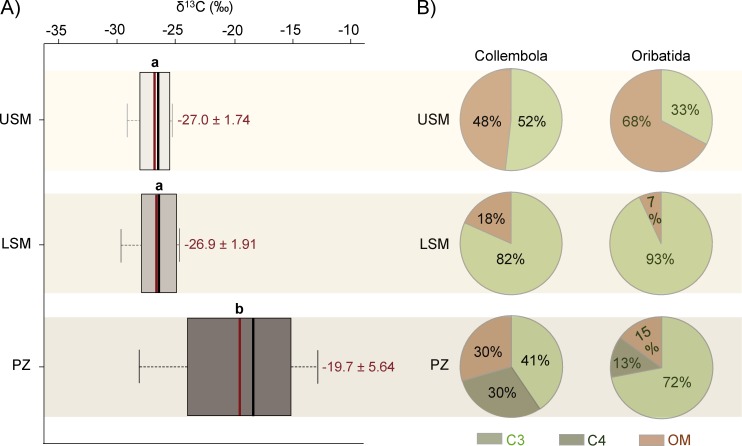
The composition of basal food resources changed significantly along the land-sea vegetation zonation (F1,18 = 7.25, p = 0.0049). δ13C values of basal food resources of the pioneer zone differed significantly from those of the upper and lower salt marsh (Fig 5A). δ13C values of basal food resources increased from -27.0 ± 1.7‰ and -26.9 ± 1.9‰ in the upper and lower salt marsh, respectively, to -19.7 ± 5.6‰ in the pioneer zone (Table D in S1 File).
Fig 5.
(A) δ13C signatures of basal food resources in the three salt marsh zones: upper salt marsh (USM), lower salt marsh (LSM) and pioneer zones (PZ). Boxplots represent mean (red line) and median (black line) of δ13C signatures; different letters represent significant differences between vegetation zones (Tukey’s HSD, p < 0.05). (B) Pie charts represent mean percentages of potential food resources [C3 plants/algae, C4 plants/algae and organic material (OM) containing C3 and/or C4 plants/algae] of Collembola and Oribatida species along the salt marsh gradient.
Mixing models suggested that Collembola and Oribatida species prefer different resources within the salt marsh zones. The upper and lower salt marsh was dominated by C3 plants and soil organic matter (containing C3 plant carbon), whereas the pioneer zone was characterized by C3 and C4 plants/algae and soil organic matter containing C3 and C4 plants/algae carbon (Fig A and Table D in S1 File). In the upper salt marsh, Collembola predominantly rely on C3 plants with 52% of their body carbon originating from this source, whereas Oribatida rely on soil organic matter with 68% of their body carbon originating from this source (Fig 5B, Table E in S1 File). In contrast, resource use of Collembola and Oribatida in the lower salt marsh strongly shifted towards C3 plants with 82% and 93% of their body carbon originating from this source and only 18% of Collembola and 7% of Oribatida body carbon originating from soil organic matter (Fig 5B, Table E in S1 File). In the pioneer zone, Collembola predominantly rely on C3 plants with a contribution of 41% and in addition use C4 plants/algae as well as organic material to a similar extent, i.e. 30% each. In comparison, Oribatida species predominantly rely on C3 plants with 72% of their body carbon originating from this source and only 13% and 15% originating from C4 plants/algae and organic material, respectively.
As indicated by the Collembola species T. debilis, which inhabited all three salt marsh zones (see Table A in S1 File), resource use of species changed along the salt marsh gradient. Based on mixing models this species predominantly incorporated C3 plant carbon in the upper and lower salt marsh (74% and 90%, respectively) whereas in the pioneer zone it predominantly fed on marine C4 plants/algae (37%; Table E in S1 File).
Discussion
Disturbance and community structure
Density and diversity of soil mesofauna decreased significantly with increasing levels of inundation frequency from the land towards the sea. This indicates that the observed community shift correlated with changes in flooding from the upper salt marsh to the pioneer zone [22, 23, 65]. An increasing level of inundation frequency to the sea induces increasing stress for mesofauna species due to variable but generally high salinity (salinity of 5–20, 20–26 and 26–32 for the upper salt marsh, lower salt marsh and pioneer zone, respectively; D. Meier, pers. comm.).
The upper salt marsh is less disturbed by inundations as those are restricted to occasional storm events, therefore species with low salt tolerance can thrive in this habitat. Accordingly, the soil fauna was dominated by species typical for terrestrial meadows i.e., Entomobrya lanuginosa, species of Folsomia, Isotoma and Lepidocyrtus (Collembola), Liebstadia similis, Multioppia neglecta, R. clavipectinata, S. laevigatus and Tectocepheus velatus sarekensis (Oribatida), Rhodacarus salaries and Uropoda repleta (Mesostigmata). Abiotic disturbances become more frequent in the lower salt marsh and in particular in the pioneer zone. Therefore, the lower salt marsh was inhabited by salt and desiccation tolerant species, such as Halisotoma maritima and Mesaphorura krausbaueri (Collembola), H. pulchella (Oribatida), and Pseudoparasitus germanicus (Mesostigmata). The pioneer zone is the most extreme habitat of the salt marsh with highest frequency of inundations and abiotic variations. Therefore, specialist species preferentially colonize this salty marine habitat, such as A. besselsi and T. debilis (Collembola), A. schneideri, H. pulchella and Z. quadrivertex (Oribatida), and C. necorniger and D. halophilus (Mesostigmata). Colonization of the lower salt marsh and pioneer zone is associated with physiological and behavioral adaptations enabling the species to cope with frequent inundations and variations in salinity.
Certain Collembola species are osmoconform even at high salinity, whereas others are drifting on the seawater surface allowing aerial respiration [66–68], whereas non-haloric Oribatida species developed plastron structures allowing them to respire under water [69]. Halobiont Oribatida, such as A. schneideri, H. pulchella and Z. quadrivertex, are well adapted to saline conditions and are able to withstand frequent inundation and to survive in submersion [38, 46, 70, 71]. In contrast, nothing is known about the mechanisms which enable Mesostigmata, such as the C. necorniger and D. halophilus, to inhabit frequently inundated salt marsh zones. However, widespread occurrence in the lower salt marsh and the pioneer zone suggests that they are well adapted to harsh environmental conditions and frequent inundation.
Declining diversity of mesofauna in salt marsh zones closer to the sea is in line with the “Intermediate Disturbance Hypothesis” stating that species diversity is maximized when ecological disturbance is neither too rare nor too frequent [72]. Terrestrial species not well adapted to abiotic disturbances are likely to be excluded from the semi-marine lower salt marsh zones resulting in lower species density and diversity. As a consequence, specialized taxa are most affected by disturbances, but able to tolerate the abiotic fluctuations and survive under extreme environmental conditions close to the sea due to physiological and behavioral adaptations [73, 74].
Disturbance and food web structure
Salt marsh soils provide a wide range of food resources for soil fauna communities. The δ15N signatures suggest that mesofauna species of salt marshes occupy very different trophic niches. The food web spans over four trophic levels including primary and secondary decomposers, as well as first and second order predators, suggesting that intra-guild predation is widespread and plays an important role. The broad range of δ13C signatures of salt marsh mesofauna species supports the hypothesis, that the utilization of diverse food resources along the salt marsh gradient fundamentally structures the entire food web.
Supporting the predominant role of disturbance in structuring the soil mesofauna food web, the number of trophic levels was higher in the less disturbed upper salt marsh zone and decreased to the pioneer zone. Notably, the structure of the food web at the higher salt marsh zones closely resembled that of forest ecosystems characterized by more opportunistic links and long food chains, which typically span over four trophic levels [75, 76]. Previous studies in forest ecosystems also showed Oribatida to span three to four trophic levels including primary and secondary decomposers as well as predators and/or scavengers [77]. Similar results have been obtained for Collembola with again most species functioning as secondary rather than primary decomposers [78, 79]. This suggests that similar to forest ecosystems, most decomposer taxa of salt marsh habitats do not feed on dead organic matter but occupy higher trophic levels including secondary decomposers (feeding predominantly on microorganisms), predators and/or scavengers. Presumably, similar to forests and arable systems [80, 81], rhizosphere microorganisms relying on root derived resources play an important role as food resource for decomposer species in higher salt marsh zones. In contrast, in the pioneer zone bacteria relying on marine detritus as well as algae play a major role as food resource for Collembola and Oribatida. Supporting this view the generalist Collembola species T. debilis predominantly fed on marine C4 plants/algae in the pioneer zone but predominantly on C3 plant carbon in the upper salt marsh. Similarly, other studies showed that the Collembola species Archisotoma pulchella prefers to feed on diatoms [82] and the Oribatida species Ameronothrus lineatus predominantly feeds on green algae [83, 84]. Previous studies suggested that marine resources provide most energy and resources for coastal communities and their importance extends considerably into the terrestrial realm [5]. In contrast to these findings, the use of marine-based food sources by soil invertebrates was limited to the pioneer zone, whereas invertebrates at higher salt marsh zones predominantly relied on terrestrial resources of these habitats. This points to the importance of marine resources for soil food webs of frequently inundated salt marsh habitats, whereas higher salt marsh zones, colonized by terrestrial plants, provide most of the resources for the mesofauna decomposer system. On the other hand, the results indicate that soil mesofauna species do not migrate between the salt marsh zones thereby integrating resources of different habitats.
Further, the results suggest that soil mesofauna food webs of salt marshes predominantly dwell on autochthonous resources based in large on the plants (including algae) colonizing the respective salt marsh zones. In the upper and lower salt marsh root derived resources are likely to play an important role in fueling the decomposer system, thereby resembling truly terrestrial systems such as forests but also arable systems [80, 81]. Consequently, the shift from a typical terrestrial plant system in the higher salt marsh zones to a more marine plant system in the pioneer zone is associated with fundamental changes in the decomposer system, which in turn affects the food web structure with a decreasing number of trophic levels to the sea (pioneer zone).
As indicated by high δ15N values a number of Collembola and Oribatida species function as predators and/or scavengers. Predator Collembola and Oribatida species likely are feeding at least in part on nematodes, which are highly abundant in salt marshes, similar to terrestrial soil food webs [85–87]. Notably, the results suggest predation to be more widespread in Oribatida than in Collembola. Conform to our expectations and previous studies of forest soil food webs [42], however, Mesostigmata formed the main predators in the studied salt marsh mesofauna food webs. Their high δ15N values and variable hunting strategies [88] suggest complex predator-prey relationships including intraguild predation. Further, Mesostigmata themselves likely function as prey of marine predators such as nematodes, copepods and polychaetes and this potentially contributed to the lower number of trophic levels of the mesofauna food web in the pioneer zone. By contrast, reduced predation by marine species may have contributed to the higher abundance and diversity of mesofauna, and the longer food chains in the lower and upper salt marsh [43]. However, in these habitats mesofauna likely forms important prey of macrofauna predators such as Araneae. Therefore, the relative contribution of predation as structuring force of mesofauna communities in the different salt marsh zones remains uncertain and needs further investigation.
A large number of mesofauna species (60 taxa) only occurred in one of the three vegetation zones, suggesting that habitat and / or trophic specialists are widespread. Trophic specialists occupy a limited range of niches but more effectively exploit these resources than trophic generalists [27, 89]. In contrast, 22 taxa colonized two salt marsh zones underlining that salt marsh communities are well structured with most species only being able to colonize a narrow habitat range and use the respective resources of that habitat. Only two Collembola species (A. besselsi and T. debilis) typically colonizing disturbed habitats, occurred in each of the three salt marsh zones and therefore truly represent generalists. Food generalists opportunistically use resources which are available [90] allowing them to colonize a wide range of habitats [6, 91]. However, even though habitat specialists predominated, these species presumably also used a wider range of resources rather than only single prey taxa as reflected by the dominance of secondary decomposers as well as second order predators indicating that the use of resources of different trophic levels [92] is widespread among salt marsh mesofauna species. Presumably, feeding on multiple trophic levels is favored in the heterogeneous salt marsh habitats and this likely contributes to food web stability [93].
Body mass is a major structuring factor of food webs [94], especially predator-prey interactions strongly depend on body mass ratios, therefore we expected large species to predominante in higher trophic levels. Contrasting this expectation and other food webs [95], the trophic position of mesofauna species was not significantly related to body mass (Fig B and Table B in S1 File). Again, however, this resembles terrestrial Mesostigmata communities of forests in Central Europe [42].
Conclusions
The study highlighted the complex trophic structure of mesofauna communities and their central position within salt marsh habitats. Strong changes in community structure and food web complexity along the studied salt marsh gradient suggest that the occurrence of the majority of species in salt marshes is related to inundation frequency. Parallel to the strong turnover of species, mesofauna food webs markedly changed along the salt marsh gradient from four trophic levels in the upper salt marsh to only two in the pioneer zone. Similar to terrestrial soil food webs, the number of primary decomposers was low and most species functioned as secondary decomposers or predators including second order predators or scavengers. In particular the high number of second order predator taxa suggests high incidence of intraguild predation, again resembling terrestrial soil food webs. Notably, soil animal communities in each of the three salt marsh zones predominantly relied on autochthonous resources, with marine resources being restricted mainly to the pioneer zone, and indicating that terrestrial food webs are intimately linked to rhizosphere resources. Overall, the high number of species of soil microarthropods including Collembola, Oribatida and Mesostigmata suggests that, similar to consolidated terrestrial systems, these taxa fill key trophic positions of soil food webs even at the adverse environmental conditions of marine–terrestrial boundary habitats.
Supporting information
Figure A. Mean δ13C values ± standard deviation (SD) of basal food resources (vascular plants, algae and soil organic matter) of the three salt marsh zones: upper salt marsh (USM), lower salt marsh (LSM) and pioneer zones (PZ). Light grey squares mark C3 plants/algae, black squares mark C4 plants/algae. Figure B. Relationship between body mass and trophic level as indicated by δ15N signatures of Mesostigmata (blue), Collembola (orange) and Oribatida species (green) for the three salt marsh zones: upper salt marsh (USM, dots), lower salt marsh (LSM, 296 triangles) and pioneer zone (PZ, diamonds). Dry weight of species is given in Table B in S1 File. Table A. List of soil invertebrate taxa and abbreviations. Mean density (ind./m2) and standard deviation (SD) of mesofauna species in the three salt marsh zones: upper salt marsh, lower salt marsh and pioneer zone. Table B. Mean stable isotope ratios of δ15N values (‰) and standard deviation (SD) of soil invertebrate taxa of the three salt marsh zones: upper salt marsh, lower salt marsh and pioneer zone. Table including number of replicates (n) and dry weight of individuals. Table C. Trophic structure–mean stable isotope ratios of δ13C and δ15N values (‰) and standard deviation (SD) of dominant species of Collembola, Mesostigmata and Oribatida, including taxa abbreviations and number of replicates (n). Stable isotope signatures were normalized to the mean of the organic matter signature of the respective salt marsh zone. Table D. Mean δ13C (‰) and standard deviation (SD) of basal food resources of the three salt marsh zones: upper salt marsh, lower salt marsh and pioneer zone, table including sample size (n) and type of CO2-fixation. Table E. Mixing models–mean percentage of potential food sources [C3 plants/algae, C4 plants/algae and organic material (OM) containing C3 and/or C4 plants/algae] calculated by mean values and standard deviation (SD) of stable isotope signatures (δ13C and δ15N) of Collembola and Oribatdia consumer species in the upper salt marsh, lower salt marsh and pioneer zone.
(DOC)
Acknowledgments
This study was performed within the research project BEFmate (Biodiversity—Ecosystem Functioning across marine and terrestrial ecosystems). We are grateful to the team of the research center Wittbülten on the East Frisian Island Spiekeroog for supporting our field work, Susanne Böning-Klein and Reinhard Langel (Centre for Stable Isotope Research and Analysis, University of Göttingen) for supporting preparation and performing measurements of stable isotope samples. Furthermore, we thank Nicole Scheunemann for supporting identification of Collembola species and Melanie Maraun for advice applying the Bayesian mixing model FRUITS. Suggestions and advice of three anonymous reviewers improved our paper and are gratefully acknowledged.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Volkswagen Foundation within the funding initiative of the Ministry for Science and Culture of Lower Saxony, Germany under project number ZN2930. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schuerch M, Dolch T, Reise K, Vafeidis AT. Unravelling interactions between salt marsh evolution and sedimentary processes in the Wadden Sea (southeastern North Sea). Progress in Physical Geography. 2014; 1–2. [Google Scholar]
- 2.Petersen J, Kers B, Stock M. TMAP-Typology of Coastal Vegetation in the Wadden Sea area. Wadden Sea Ecosystem. 2014; 32. [Google Scholar]
- 3.Witte IJ, Zijlstra JJ. The meiofauna of a tidal flat in the western part of the Wadden Sea and its role in the benthic ecosystem. Marine Ecology Progress Series. 1984; 14:129–138. [Google Scholar]
- 4.Craft CB, Broome SW, Seneca ED, Showers WJ. Estimating sources of soil organic matter in natural and transplanted estuarine marshes using stable isotopes of carbon and nitrogen. Estuarine Coastal Shelf Science. 1988; 26:633–641. [Google Scholar]
- 5.Polis GA, Hurd SD. Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. The American Naturalist. 1996; 147(3):396–423. doi: 10.1086/285858 [Google Scholar]
- 6.Polis GA, Strong DR. Food web complexity and community dynamics. American Naturalist. 1996; 147(5):813–846. doi: 10.1086/285880 [Google Scholar]
- 7.Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR. The value of estuarine and coastal ecosystem services. Ecological Monographs. 2011; 81(2):169–193. doi: 10.1890/10-1510.1 [Google Scholar]
- 8.van der Lee EM, Umlauf L. Intertidal wave mixing in the Baltic Sea: near-inertial waves in the absence of tides. Journal of Geophysical Research. 2011; 116:C10016 doi: 10.1029/2011JC007072 [Google Scholar]
- 9.Sánchez JM, Izco J, Medrano M. Relationships between vegetation zonation and altitude in a salt-marsh system in northwest Spain. Journal of Vegetation Science. 1996; 7(5):695–702. doi: 10.2307/3236381 [Google Scholar]
- 10.Bockelmann AC, Bakker JP, Neuhaus R, Lage J. The relation between vegetation zonation, elevation and inundation frequency in a Wadden Sea salt marsh. Aquatic botany. 2002; 73(3):211–221. doi: 10.1016/S0304-3770(02)00022-0 [Google Scholar]
- 11.Hild A, Niesel V, Günther CP. Study area: The backbarrier tidal flats of Spiekeroog In: Dittmann S, editor. The Wadden Sea Ecosystem: stability properties and mechanisms. Springer, Berlin Heidelberg, NewYork; 1999. pp. 299. [Google Scholar]
- 12.Silvestri S, Defina A, Marani M. Tidal regime, salinity and salt marsh plant zonation. Estuarine, coastal and shelf science. 2005; 62(1–2):119–130. doi: 10.1016/j.ecss.2004.08.010 [Google Scholar]
- 13.Röper T, Massmann G, Meyer H, Sültenfuss J. Groundwater ages, recharge conditions and hydrochemical evolution of a barrier island freshwater lens (Spiekeroog, Northern Germany). Journal of Hydrology. 2012; 454–455:173–186. doi: 10.1016/j.jhydrol.2012.06.011 [Google Scholar]
- 14.Kwak TJ, Zedler JB. Food web analysis of southern California coastal wetlands using multiple stable isotopes. Oecologia. 1997; 110 (2):262–277. doi: 10.1007/s004420050159 [DOI] [PubMed] [Google Scholar]
- 15.Tanner BR, Uhle ME, Kelley JT, Mora CI. C3/C4 variations in salt-marsh sediments: An application of compound specific isotopic analysis of lipid biomarkers to late Holocene paleoenvironmental research. Organic Geochemistry. 2007; 38(3):474–484. doi: 10.1016/j.orggeochem.2006.06.009 [Google Scholar]
- 16.Farquhar GD, Ehleringer JR, Hubick KT. Carbon isotope discrimination and photosynthesis. Annual review of plant physiology and plant molecular biology. 1989; 40:503–537. [Google Scholar]
- 17.Arp WJ, Drake BG, Pockman WT, Curtis PS, Whigham DF. Interactions between C3 and C4 salt marsh plant species during four years of exposure to elevated atmospheric CO2. Vegetatio. 1993; 104(1):133–143. doi: 10.1007/BF00048149 [Google Scholar]
- 18.Peterson BJ. Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecologica. 1999; 20(4):479–487. doi: 10.1016/S1146-609X(99)00120-4 [Google Scholar]
- 19.Tiunov AV. Stable Isotopes of carbon and nitrogen in soil ecological studies. The Biological Bulletin. 2007; 34(4):395–407. doi: 10.1134/S1062359007040127 [PubMed] [Google Scholar]
- 20.Bertness MD, Leonard GH. The role of positive interactions in communities: lessons from intertidal habitats. Ecology. 1997; 78(7):1976–1989. doi: 10.1890/0012-9658(1997)078[1976:TROPII]2.0.CO;2 [Google Scholar]
- 21.Vöge S, Reiss H, Kröncke I. Macrofauna succession in an infilling salt marsh clay pit. Senckenbergiana maritima. 2008; 38(2):93–106. doi: 10.1007/BF03055284 [Google Scholar]
- 22.Ellingsen KE, Gray JS. Spatial patterns of benthic diversity: is there a latitudinal gradient along the Norwegian continental shelf? Journal of Animal Ecology. 2002; 71(3):373–389. doi: 10.1046/j.1365-2656.2002.00606.x [Google Scholar]
- 23.Zajac RN, Lewis RS, Poppe LJ, Twichell DC, Vozarik J, DiGiacomo-Cohen ML. Responses of infaunal populations to benthoscape structure and the potential importance of transition zones. Limnology and Oceanography. 2003; 48(2):829–842. doi: 10.4319/lo.2003.48.2.0829 [Google Scholar]
- 24.Eleuterius LN, Eleuterius CK. Tide levels and salt marsh zonation. Bulletin of Marine Science. 1979; 29(3):394–400(7). [Google Scholar]
- 25.Olff HD, De Leeuw J, Bakker J, Platerink R, Van Wijnen H. Vegetation succession and herbivory in a salt marsh: changes induced by sea level rise and silt deposition along an elevational gradient. Journal of Ecology. 1997; 85(6):799–814. doi: 10.2307/2960603 [Google Scholar]
- 26.Lange G, Haynert K, Dinter T, Scheu S, Kröncke I. Adaptation of benthic invertebrates to food sources along marine-terrestrial boundaries as indicated by carbon and nitrogen stable isotopes. Journal of Sea Research. 2018; 131:12–21. doi: 10.1016/j.seares.2017.10.002 [Google Scholar]
- 27.Kneib R. Patterns of invertebrate distribution and abundance in the intertidal salt marsh: causes and questions. Estuaries. 1984; 7(4):392–412. doi: 10.2307/1351621 [Google Scholar]
- 28.Evin LA, Talley TS. Influences of vegetation and abiotic environmental factors on salt marsh invertebrates. Concepts and controversies in tidal marsh ecology. 2002; 661–707. doi: 10.1007/0-306-47534-0_30 [Google Scholar]
- 29.Salgado JP, Cabral H, Costa M. Spatial and temporal distribution patterns of the macrozoobenthos assemblage in the salt marshes of Tejo estuary (Portugal). Hydrobiologia. 2007; 587(1):225–239. doi: 10.1007/s10750-007-0685-7 [Google Scholar]
- 30.Créach V, Schricke MT, Bertru G, Mariotti A. Stable isotopes and gut analyses to determine feeding relation-ships in saltmarsh macroconsumers. Estuary Coastal Shelf Science. 1997; 44(5):599–611. doi: 10.1006/ecss.1996.0147 [Google Scholar]
- 31.Kreeger DA, Newell IE. Trophic complexity between producers and invertebrates consumers in salt marshes In: Weinstein M, Kreeger DA, editors. Concepts and controversies in tidal marsh ecology. Kluwer Academic Publishers, Dordrecht; 2000. pp. 187–220. [Google Scholar]
- 32.Dubois S, Blanchet H, Garcia A, Massé M, Galois R, Grémare A, Charlier K, Guillou G, Richard P, Savoye N. Trophic resource use by macrozoobenthic primary consumers within a semi-enclosed coastal ecosystem: stable isotope and fatty acid assessment. Journal of Sea Research. 2014; 88:87–99. doi: 10.1016/j.seares.2014.01.004 [Google Scholar]
- 33.Riera P, Stal LJ, Nieuwenhuize J, Richard P, Blanchard G, Gentil F. Determination of food source for benthic invertebrates in a salt marsh (Aiguillon Bay, France) by carbon and nitrogen stable isotopes: importance of locally produced sources. Marine Ecology Progress Series. 1999; 187:301–307. [Google Scholar]
- 34.Colombini I, Chelazzi L, Gibson R, Atkinson R. Influence of marine allochthonous input on sandy beach communities. Oceanography and Marine Biology: An Annual Review. 2003; 41:115–159. [Google Scholar]
- 35.Doi H. Spatial patterns of autochthonous and allochthonous resources in aquatic food webs. Population Ecology. 2009; 51(1):57–64. doi: 10.1007/s10144-008-0127-z [Google Scholar]
- 36.Scaps P. A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor. Hydrobiologia. 2002; 470(1–3):203–218. doi: 10.1023/A:1015681605656 [Google Scholar]
- 37.Galván K, Fleeger JW, Fry B. Stable isotope addition reveals dietary importance of phytoplankton and microphytobenthos to saltmarsh infauna. Marine Ecology Progress Series. 2008; 359:37–49. doi: 10.3354/meps07321 [Google Scholar]
- 38.Polderman PJG. The Oribatida (Acari) of saline areas in the western part of the Dutch Wadden Sea. Netherlands Institute for Sea Research. 1974; 8(1):49–72. doi: 10.1016/0077-7579(74)90026-X [Google Scholar]
- 39.Sterzynska M, Ehrnsberger R. The distribution and diversity of Collembola in saltmarsh habitats of the German North Sea—a preliminary study. Pedobiologia. 2000; 44(3–4):402–412. doi: 10.1078/S0031-4056(04)70058-X [Google Scholar]
- 40.Perlmutter DG, Meyer JL. The impact of a stream-dwelling harpacticoid copepod upon detritally associated bacteria. Ecology. 1991; 72(6):2170–2180. doi: 10.2307/1941568 [Google Scholar]
- 41.Schneider FD, Scheu S, Brose U. Body mass constraints on feeding rates determine the consequences of predator loss. Ecology Letters. 2012; 15(5):436–443. doi: 10.1111/j.1461-0248.2012.01750.x [DOI] [PubMed] [Google Scholar]
- 42.Klarner B, Maraun M, Scheu S. Trophic diversity and niche partitioning in a species rich predator guild–Natural variations in stable isotope ratios (13C/12C, 15N/14N) of mesostigmatid mites (Acari, Mesostigmata) from Central European beech forests. Soil Biology and Biochemistry. 2013; 57:327–333. doi: 10.1016/j.soilbio.2012.08.013 [Google Scholar]
- 43.Bell SS. Meiofauna-macrofauna interactions in a high salt marsh habitat. Ecological Monographs. 1980; 50:487–505. doi: 10.2307/1942654 [Google Scholar]
- 44.Schmid-Araya JM, Schmid PE. Trophic relationship: integrating meiofauna into a realistic benthic food web. Freshwater Biology. 2000; 44(1):149–163. doi: 10.1046/j.1365-2427.2000.00594.x [Google Scholar]
- 45.Schmid-Araya JM, Hildrew AG, Robertson A, Schmid PE, Winterbottom J. The importance of meiofauna in food webs: evidence from an acid stream. Ecology. 2002; 83(5):1271–1285. doi: 10.1890/0012-9658(2002)083[1271:TIOMIF]2.0.CO;2 [Google Scholar]
- 46.Luxton M. The ecology of salt marsh Acarina. Journal of Animal Ecology. 1967; 36(2):257–277. [Google Scholar]
- 47.Luxton M. Local distribution and habitat of mites (Acari) on the sea coast of New Zealand. Journal of the Royal Society. 1990; 20(4):419–426. doi: 10.1080/03036758.1990.10426720 [Google Scholar]
- 48.Romanuk TN, Levings CD. Associations between arthropods and the supralittoral ecotone: dependence of aquatic and terrestrial taxa on riparian vegetation. Environmental Entomology. 2003; 32(6):1343–1353. doi: 10.1603/0046-225X-32.6.1343 [Google Scholar]
- 49.Persson L, Diehl S, Johansson L, Andersson G, Hamrin SF. Trophic interactions in temperate lake ecosystems–a test of food-chain theory. American Naturalist. 1992; 140(1):59–84. doi: 10.1086/285403 [Google Scholar]
- 50.Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002; 83(3):703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2 [Google Scholar]
- 51.Carlier A, Riera P, Amouroux JM, Bodiou JY, Desmalades M, Grémare A. Spatial heterogeneity in the food web of a heavily modified Mediterranean coastal lagoon: stable isotope evidence. Journal of Aquatic Biology. 2009; 5(2):167–179. doi: 10.3354/ab00147 [Google Scholar]
- 52.Pollierer MM, Langel R, Scheu S, Maraun M. Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotope ratios (15N/14N and 13C/12C). Soil Biology and Biochemistry. 2009; 41(6):1221–1226. doi: 10.1016/j.soilbio.2009.03.002 [Google Scholar]
- 53.Connolly RM, Gorman D, Guest MA. Movement of carbon among estuarine habitats and its assimilation by invertebrates. Oecologia. 2005; 144(4):684–691. doi: 10.1007/s00442-005-0167-4 [DOI] [PubMed] [Google Scholar]
- 54.Törnroos A, Nordström MC, Bonsdorff E. Coastal habitats as surrogates for taxonomic, functional and trophic structures of benthic faunal communities. PLoS ONE. 2013; 8(10):e78910 doi: 10.1371/journal.pone.0078910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balke T, Lõhmus K, Hillebrand H, Zielinski O, Haynert K, Meier D, Hodapp D, Minden V, Kleyer M. Artificial salt marsh islands: a model system for novel metacommunity experiments. Estuarine, Coastal and Shelf Science. 2017; 198:288–298. doi: 10.1016/j.ecss.2017.09.021 [Google Scholar]
- 56.Macfadyen A. Improved funnel-type extractors for soil arthropods. Journal of Animal Ecology. 1961; 30(1):171–184. doi: 10.2307/2120 [Google Scholar]
- 57.Krab EJ, van Logtestijn RSP, Cornelissen JHC, Berg MP. Reservations about preservations: storage methods affect δ13C signatures differently even in closely related soil fauna. Methods in Ecology and Evolution. 2012; 3:138–144. doi: 10.1111/j.2041-210X.2011.00126.x [Google Scholar]
- 58.Martinez del Rio C, Wolf N, Carleton SA, Gannes LZ. Isotopic ecology ten years after a call for more laboratory experiments. Biological Reviews. 2009; 84(1):91–111. doi: 10.1111/j.1469-185X.2008.00064.x [DOI] [PubMed] [Google Scholar]
- 59.Minagawa M, Wada E. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta. 1984; 48(5):1135–1140. doi: 10.1016/0016-7037(84)90204-7 [Google Scholar]
- 60.Spence KO, Rosenheim JA. Isotopic enrichment in herbivorous insects: a comparative field-based study of variation. Oecologia. 2005; 146(1):89–97. doi: 10.1007/s00442-005-0170-9 [DOI] [PubMed] [Google Scholar]
- 61.DeNiro MJ, Epstein S. Influence of diet on distribution of carbon isotopes in animals. Geochimica Cosmochimica Acta. 1978; 42(5):495–506. doi: 10.1016/0016-7037(78)90199-0 [Google Scholar]
- 62.Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics. 1987; 18:293–320. doi: 10.1146/annurev.es.18.110187.001453 [Google Scholar]
- 63.Reineking A, Langel R, Schikowski J. 15N, 13C-on-line measurements with an elemental analyzer (Carlo-Erba, NA 1500), a modified trapping box and a gas isotope mass spectrometer (Finnigan, MAT-251). Isotopenpraxis Isotopes in Environmental and Health Studies. 1993; 29(1–2):169–174. doi: 10.1080/10256019308046151 [Google Scholar]
- 64.Fernandes R, Millard AR, Brabec M, Nadeau MJ., Grootes P. Food reconstruction using isotopic transferred signals (FRUITS): A bayesian model for diet reconstruction. PLoS ONE. 2014; 9(2): e87436 doi: 10.1371/journal.pone.0087436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hewitt JE, Thrush SF, Halliday J, Duffy C. The importance of small-scale habitat structure for maintaining beta diversity. Ecology. 2005; 86(6):1619–1626. doi: 10.1890/04-1099 [Google Scholar]
- 66.Weigmann G. Zur Ökologie der Collembolen und Oribatiden im Grenzbereich Land-Meer. Zeitschrift für wissenschaftliche Zoologie. 1973; 186:295–391. [Google Scholar]
- 67.Witteveen J, Joosse EN. The effects of inundation on marine littoral Collembola. Holarctic Ecology. 1988; 11(1):1–7. doi: 10.1111/j.1600-0587.1988.tb00775.x [Google Scholar]
- 68.Witteveen J, Verhoef HA, Letschert JPW. Osmotic and ionic regulation in marine littoral Collembola. Journal of Insect Physiology. 1987; 33(1):59–66. doi: 10.1016/0022-1910(87)90105-3 [Google Scholar]
- 69.Pfingstl T, Krisper G. Plastron respiration in marine intertidal oribatid mites (Acari, Fortuyniidae and Selenoribatidae). Zoomorphology. 2014; 133(4):359–378. doi: 10.1007/s00435-014-0228-5 [Google Scholar]
- 70.Pfingstl T. Resistance to fresh and salt water in intertidal mites (Acari: Oribatida): implications for ecology and hydrochorous dispersal. Experimental and Applied Acarology. 2013; 61(1):87–96. doi: 10.1007/s10493-013-9681-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulte G. Vertikalwanderungen küstenbewohnender Milben (Acari, Oribatei). Netherlands Journal of Sea Research. 1973; 7:68–80. doi: 10.1016/0077-7579(73)90033-1 [Google Scholar]
- 72.Connell JH. Tropical rain forests and coral reefs as open non-equilibrium systems In: Anderson RM, Turner BD, Taylor LR, editors. Population Dynamics. Blackwell, Oxford; 1979. pp. 141–163. [Google Scholar]
- 73.Marx MT, Wild AK, Knollmann U, Kamp G, Wegener G, Eisenbeis G. Responses and adaptations of collembolan communities (Hexapoda: Collembola) to flooding and hypoxic conditions. Pesquisa Agropecuária Brasileira. 2009; 44(8):1002–1010. doi: 10.1590/S0100-204X2009000800032 [Google Scholar]
- 74.Weigmann G. Oribatid mites (Acari:Oribatida) from the coastal region of Portugal. Zachvatkinibates and Punctoribates (Mycobatidae). Soil Organisms. 2009; 81:85–105. [Google Scholar]
- 75.Scheu S, Falca M. The soil food web of two beech forests (Fagus sylvatica) of contrasting humus types: stable isotope analysis of a macro- and amesofauna-dominated system. Oecologia. 2000; 123(2):285–296. doi: 10.1007/s004420051015 [DOI] [PubMed] [Google Scholar]
- 76.Digel C, Curtsdotter A, Riede J, Klarner B, Brose U. Unravelling the complex structure of forest soil food webs: Higher omnivory and more trophic levels. Oikos. 2014; 123(10):1157–1172. doi: 10.1111/oik.00865 [Google Scholar]
- 77.Schneider K, Migge S, Norton RA, Scheu S, Langel R, Reineking A, Maraun M. Trophic niche differentiation in soil microarthropods (Oribatida, Acari): evidence from stable isotope ratios (15N/14N). Soil Biology and Biochemistry. 2004; 36(11):1769–1774. doi: 10.1016/j.soilbio.2004.04.033 [Google Scholar]
- 78.Chahartaghi M, Langel R, Scheu S, Ruess L. Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biology and Biochemistry. 2005; 37(9):1718–1725. doi: 10.1016/j.soilbio.2005.02.006 [Google Scholar]
- 79.Illig J, Langel R, Norton RA, Scheu S, Maraun M. Where are the decomposers? Uncovering the soil food web of a tropical montane rain forest in southern Ecuador using stable isotopes (15N). Journal of Tropical Ecology. 2005; 21(5):589–593. doi: 10.1017/S0266467405002646 [Google Scholar]
- 80.Pollierer MM, Langel R, Körner C, Maraun M, Scheu S. The underestimated importance of belowground carbon input for forest soil animal food webs. Ecology Letters. 2007; 10(8):729–736. doi: 10.1111/j.1461-0248.2007.01064.x [DOI] [PubMed] [Google Scholar]
- 81.Scheunemann N, Maraun M, Scheu S, Butenschoen O. The role of shoot residues vs. crop species for soil arthropod diversity and abundance of arable systems. Soil Biology and Biochemistry. 2015; 81:81–88. doi: 10.1016/j.soilbio.2014.11.006 [Google Scholar]
- 82.Mertens J, Beladjal L, Janssens F, Matthys P. Pitfall trapping in flooding habitats: a new technique reveals Archisotoma pulchella (Collembola: Isotomidae) as new to the Belgian fauna. Belgian Journal of Zoology. 2007; 137(2):177–181. [Google Scholar]
- 83.Schulte G. Zur Nahrungsbiologie der terrestrischen und marinen Milbenfamilie Ameronothridae. Journal of Soil Ecology. 1976; 16:332–352. [Google Scholar]
- 84.Pfingstl T. Habitat use, feeding and reproductive traits of rocky-shore intertidal mites from Bermuda (Oribatida: Fortuyniidae and Selenoribatidae). Acarologia. 2013; 53(4):369–382. [Google Scholar]
- 85.Muraoka M, Ishibashi N. Nematode-feeding mites and their feeding behaviour. Applied Entomology and Zoology. 1976; 11(1):1–7. doi: 10.1303/aez.11.1 [Google Scholar]
- 86.Rockett CL. Nematode predation by oribatid mites (Acari:Oribatida). International Journal of Acarology. 1980; 6(3):219–224. doi: 10.1080/01647958008683222 [Google Scholar]
- 87.Heidemann K, Hennies A, Schakowske J, Blumenberg L, Ruess L, Scheu S, Maraun M. Free-living nematodes as prey for higher trophic levels of forest soil food webs. Oikos. 2014; 123(10):1199–1211. doi: 10.1111/j.1600-0706.2013.00872 [Google Scholar]
- 88.Koehler HH. Predatory mites (Gamasina, Mesostigmata). Agriculture Ecosystems and Environment. 1999; 74(1–3):395–410. doi: 10.1016/S0167-8809(99)00045-6 [Google Scholar]
- 89.Clavel J, Julliard R, Devictor V. Worldwide decline of specialist species: toward a global functional homogenization? Frontiers in Ecology and the Environment. 2011; 9(4):222–228. doi: 10.1890/080216 [Google Scholar]
- 90.Coll M, Guershon M. Omnivory in terrestrial arthropods: mixing plant and prey diets. Annual Review of Entomology. 2002; 47:267–297. doi: 10.1146/annurev.ento.47.091201.145209 [DOI] [PubMed] [Google Scholar]
- 91.Ho CK, Pennings SC. Consequences of omnivory for trophic interactions on salt marsh shrub. Ecology. 2008; 89(6):1714–1722. doi: 10.1890/07-1069.1 [DOI] [PubMed] [Google Scholar]
- 92.Williams RJ, Martinez ND. Limits to trophic levels and omnivory in complex food webs: theory and data. American Naturalist. 2004; 163(3):458–468. doi: 10.1086/381964 [DOI] [PubMed] [Google Scholar]
- 93.Kratina P, LeCraw RM, Ingram T, Anholt BR. Stability and persistence of food web omnivory: Is there a general pattern? Ecosphere. 2012; 3(6):1–18. doi: 10.1890/ES12-00121.1 [Google Scholar]
- 94.Brose U. Body-mass constraints on foraging behaviour determine population and food-web dynamics. Functional Ecology. 2010; 24:28–34. [Google Scholar]
- 95.Riede JO, Brose U, Ebenman B, Jacob U, Thompson R, Townsend CR, Jonsson T. Stepping in Elton’s footprints: a general scaling model for body masses and trophic levels across ecosystems. Ecology Letters. 2011; 14(2):169–178. doi: 10.1111/j.1461-0248.2010.01568.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A. Mean δ13C values ± standard deviation (SD) of basal food resources (vascular plants, algae and soil organic matter) of the three salt marsh zones: upper salt marsh (USM), lower salt marsh (LSM) and pioneer zones (PZ). Light grey squares mark C3 plants/algae, black squares mark C4 plants/algae. Figure B. Relationship between body mass and trophic level as indicated by δ15N signatures of Mesostigmata (blue), Collembola (orange) and Oribatida species (green) for the three salt marsh zones: upper salt marsh (USM, dots), lower salt marsh (LSM, 296 triangles) and pioneer zone (PZ, diamonds). Dry weight of species is given in Table B in S1 File. Table A. List of soil invertebrate taxa and abbreviations. Mean density (ind./m2) and standard deviation (SD) of mesofauna species in the three salt marsh zones: upper salt marsh, lower salt marsh and pioneer zone. Table B. Mean stable isotope ratios of δ15N values (‰) and standard deviation (SD) of soil invertebrate taxa of the three salt marsh zones: upper salt marsh, lower salt marsh and pioneer zone. Table including number of replicates (n) and dry weight of individuals. Table C. Trophic structure–mean stable isotope ratios of δ13C and δ15N values (‰) and standard deviation (SD) of dominant species of Collembola, Mesostigmata and Oribatida, including taxa abbreviations and number of replicates (n). Stable isotope signatures were normalized to the mean of the organic matter signature of the respective salt marsh zone. Table D. Mean δ13C (‰) and standard deviation (SD) of basal food resources of the three salt marsh zones: upper salt marsh, lower salt marsh and pioneer zone, table including sample size (n) and type of CO2-fixation. Table E. Mixing models–mean percentage of potential food sources [C3 plants/algae, C4 plants/algae and organic material (OM) containing C3 and/or C4 plants/algae] calculated by mean values and standard deviation (SD) of stable isotope signatures (δ13C and δ15N) of Collembola and Oribatdia consumer species in the upper salt marsh, lower salt marsh and pioneer zone.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.