Abstract
Sodium channel blocker insecticides (SCBIs) are a relatively new class of insecticides that are represented by two commercially registered compounds, indoxacarb and metaflumizone. SCBIs, like pyrethroids and DDT, target voltage-gated sodium channels (VGSCs) to intoxicate insects. In contrast to pyrethroids, however, SCBIs inhibit VGSCs at a distinct receptor site that overlaps those of therapeutic inhibitors of sodium channels, such as local anesthetics, anticonvulsants and antiarrhythmics. This review will recount the development of the SCBI insecticide class from its roots as chitin synthesis inhibitors, discuss the symptoms of poisoning and evidence supporting inhibition of VGSCs as their mechanism of action, describe the current model for SCBI-induced inhibition of VGSCs, present a model for the receptor for SCBIs on VGSCs, and highlight differences between data collected from mammalian and insect experimental models.
Keywords: Voltage-gated sodium channels, sodium channel blocker insecticides, metaflumizone, indoxacarb, state-dependent channel block, insecticide receptors
1. INTRODUCTION
Voltage-gated sodium channels (VGSC) are vital components of excitatory cells and are responsible for the rising phase of action potentials. The critical roles that VGSCs play in cell excitability have made them excellent targets for a wide variety of natural toxins used in predation and self-defense by some organisms, as well as synthetic insecticides used to control agricultural, structural, or medical insect pests. Among these, several classes of insecticides, including pyrethroids and sodium channel blocker insecticides (SCBIs), target and modify or block the activity of VGSCs, thus disrupting the function of the nervous system [1, 2].
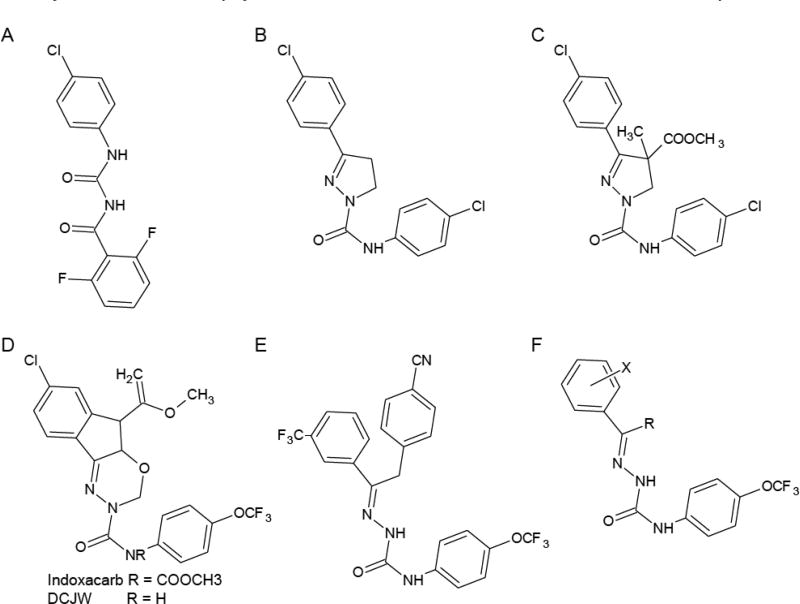
It has been over 40 years since the discovery of the first SCBIs. In the early 1970’s, researchers were attempting to identify new insecticides based on the structure of 1-benzoyl-3-phenylureas (e.g. diflubenzuron, Fig. 1A) that were effective inhibitors of chitin synthesis and insect development [3, 4]. The newly discovered insecticides, as exemplified by PH 60-41 (Fig. 1B), clearly elicited their toxic effect through a separate mechanism different from inhibition of chitin synthesis, as they caused neurotoxic symptoms such as convulsions, uncoordinated movement, cessation of feeding, and death [3, 4]. Structure-activity and optimization experiments led to the development of a series of PH compounds that exhibited the same mechanism of action with variations on efficiency [5–7]. However, this work did not lead to the development of any commercially used insecticides because this group of chemicals suffered from intolerable photoaromatization with loss of activity and soil persistence [8, 9].
Fig. (1).
Structures of diflubenzuron and the development of SCBI insecticides. A. diflubenzuron. B. PH 60-41. C. RH-3421. D. Indoxacarb and DCJW. E. Metaflumnizone. F. The proposed SCBI toxophore.
After a decade or so, the SCBI chemistry was revisited as a source for insecticidal compounds, and modifications were made to the dihydropyrazole structure, particularly at position 4 of the pyrazoline ring, to generate new compounds like RH-3421 (Fig. 1C) with high insecticidal activity [10]. Interestingly, the latter substitutions led to the introduction of a chiral center, and subsequent experiments revealed that the S enantiomer was 10 to 100 times more effective than the R enantiomer at causing toxicity in insects [11]. The RH compounds had high insecticidal activity and exhibited reduced photolability, environmental persistence, and lipophilicity as compared to their PH series predecessors [10, 12]. However, the RH compounds, despite these improvements, were also associated with high levels of mammalian toxicity. The acute oral LD50’s for RH compounds were typically greater than 1000 mg/kg, but daily administration of doses far below these levels resulted in an unexpected, delayed-onset neurotoxicity (50 mg/kg/day caused 100% mortality after 15 days) that was unacceptable for a widely used insecticide [5, 7, 10, 12]. Not surprisingly, therefore, no commercial insecticides have been produced based on the dihydropyrazole chemistry.
During a similar period, other research groups were independently working on developing arylalkylbenhydrolpiperidines (BZP) into commercially viable SCBIs. These compounds were based on the planar ring structures of nominine or cinnarizine derived from molecules isolated from bacterial cultures [13]. BZPs showed good insecticidal activity causing symptoms consistent with the SCBI class [13–15]. These compounds have a piperidine or piperidine N-oxide ring as the central moiety that lacks the chiral center present in the dihydropyrazole compounds and showed low mammalian toxicity. Despite this, however, no commercial insecticides have been produced with the BZP structure.
Expansive efforts continued to try to capitalize on the enormous insecticidal potential of the SCBI class and compounds with structures related to the dihydropyrazoles of the 1970’s and 1980’s. By altering the dihydropyrazole structure to an oxadiazine, researchers created indoxacarb (Fig. 1D), the first SCBI to achieve registration as a commercial insecticide [16]. Indoxacarb is highly insecticidal with low mammalian toxicity and environmental persistence, and due to these properties, indoxacarb has been labeled as a reduced risk insecticide [17–19]. Indoxacarb is different from its SCBI predecessors in that it is bioactivated in insects by an amidase or esterase, which removes a carbomethoxy group from the amide nitrogen to form a more active metabolite, called DCJW (Fig. 1D) [19, 20]. Interestingly, this metabolic step also occurs in mammals, though much less efficiently, and indoxacarb is more often detoxified through a separate mechanism [18, 20]. The disparate metabolic fates of indoxacarb in insects versus mammals is likely to be at least partially, if not fully, responsible for the selective toxicity of indoxacarb, as opposed to its dihydropyrazole predecessors.
Metaflumizone (Fig. 1E) is the second, and only other, member of the SCBI class to be commercialized as an insecticide [21, 22], and it features a semicarbazone structure that is essentially the opened-ring version of the dihydropyrazoles. Interestingly, metaflumizone, like the BZPs, lacks a chiral center, and therefore lacks the stereoselectivity demonstrated by RH-3421 and related dihydropyrazoles or indoxacarb. Despite this difference, however, metaflumizone does contain a shared core structure with dihydropyrazoles and indoxacarb which has been proposed by Takagi et al. [22] (Fig. 1F) to be the toxophore responsible for the insecticidal activity of the SCBI class (BZPs do not contain this central structural element yet induce similar symptoms of poisoning and so are included in the SCBI class). Metaflumizone, like indoxacarb, is highly insecticidal with low mammalian toxicity [22–24]. For a more in-depth review of the history of this class of insecticides, please see several recently published reviews [2, 16, 18, 21, 22, 25–27].
Indoxacarb and metaflumizone are currently employed to control a variety of insect pests in agricultural, structural, and medical settings [28–30]. Indoxacarb is marketed as technical grade chemical and under the trade names of Steward and Avaunt for field application in agricultural settings. In addition, indoxacarb is also sold under the trade names Advion and Arilon for control of structural pests such as ant and cockroaches or Activyl for control of ticks and fleas in cats and dogs. Metaflumizone is also marketed as an anti-flea and tick measure in cats (Promeris), but was withdrawn from use in dogs due to its association with an autoimmune disease called pemphagus foliaceus [28, 29]. In addition, metaflumizone is also used in fire ant control (Siesta) and in field crop applications as Alverde.
The widespread utility of the SCBIs and their unique chemistry and mode of action underline the importance of this class of insecticides. As such, it is imperative to understand the mechanisms and molecular biology behind the mode of action of, resistance to, and selectivity between insects and mammals so that we may not only preserve this mode of action in the face of insecticide resistance, but also improve the safety and utility of this class of insecticides. Accordingly, in this review, we will discuss the molecular mechanism of action of SCBIs, the binding site for SCBIs in VGSCs, documented cases of resistance to SCBIs, as well as mammalian intoxication and how it may differ from that observed in insects.
2. MECHANISM OF ACTION OF SCBIs
2.1. Symptoms of Toxicity
Initial experiments documented a neurological syndrome associated with SCBI intoxication in insects, which included convulsions, uncoordinated movement, cessation of feeding, and death [3, 4, 19, 31–33]. In addition to convulsions and tremors, intoxication with SCBIs also led to a distinctive pseudoparalytic state wherein insects kick their legs or convulse violently when disturbed, even though they appeared to be completely paralyzed [19, 32–34]. This unique syndrome persisted for days in intoxicated cockroaches [32], and distinguishes SCBIs from all other insecticide classes.
Much less information is available in the published literature on the symptoms associated with SCBI toxicity in mammals than in insects. Both metaflumizone and indoxacarb are considered reduced risk insecticides, with the majority of the metabolized insecticides being excreted either in urine or feces. However, in rats, acute poisoning with indoxacarb is accompanied by symptoms associated with neurotoxicity, including piloerection, hunched posture, ataxia, spasms, lethargy, tremors, abnormal gate, splayed rear legs, and salivation at 250 mg/kg [35, 36]. Interestingly, metaflumizone failed to cause any mortality in rats fed an acute does of 5000 mg/kg, though some rats temporarily showed symptoms including piloerection and dyspnea [37]. Subchronic feeding studies with either indoxacarb or metaflumizone have shown some evidence of toxicity or skin sensitization, and the “no observed adverse effect level” is around 100 mg/kg/day for both [35, 37]. Poisoning has only been reported in humans a few times, and is typically associated with methemoglobinemia [38–41], wherein a higher than normal proportion of hemoglobin in red blood cells is present as methemoglobin, and thus unable to release the oxygen that it is carrying.
2.2. Effects of SCBIs on Nerve Preparations
Further experimentation helped to elucidate the mechanisms underlying the neurological effects generated by SCBI intoxication. In insect nerve preparations, dihydropyrazoles, indoxacarb, metaflumizone, and BZPs all block spontaneous neural activity in both central and peripheral nerves [19, 32–34]. Interestingly, evoked motor activity is not affected by SCBI poisoning in these preparations, which is consistent with the ability of insects in the pseudoparalytic state to kick and convulse following mechanical stimulation. These results suggested that SCBIs inhibit the pacemaker activity of spontaneously active cells. Interestingly, these cells typically operate at membrane potentials that are considerably more positive (depolarized) than their motor neuron counterparts [32].
Subsequent experiments evaluated the response of arthropod stretch receptors to SCBI intoxication in crayfish (Procambarus clarkii), American cockroach (Periplaneta americana), Manduca sexta, Helicoverpa zea, and Spodoptera frugiperda [32–34]. In each case, SCBIs inhibited stretch receptor activity. Furthermore, in the well-studied crayfish stretch receptor [42], dihydropyrazoles elevated the threshold for action potential generation in response to depolarizing stimulation without any effect on passive membrane qualities or generator currents. In these preparations, manipulation of the amount of curl in the crayfish tail changes the membrane potential of the associated stretch receptor neurons – the more the tail curls, the greater the depolarization of the receptor. In this case, curling of the tail resulted in increased thresholds for spike generation caused by dihydropyrazoles [32]. Since the threshold for spike generation is largely based on VGSC function, these results suggested that VGSCs were inhibited by SCBI application and suggested that the effects of SCBIs on VGSCs may be state-dependent.
The mechanism of block of VGSCs was initially explored in the crayfish giant axon using the voltage clamp electrophysiological technique to manipulate the states of VGSCs by controlling the membrane potential of the cell [43]. Typically, VGSCs open in response to a depolarization of the membrane potential. A few milliseconds after opening, channels close (or fast–inactivate). Inactivation of the channel in response to a quick depolarization is called fast inactivation, and occurs on a time scale of a few milliseconds. In contrast, longer depolarizations (hundreds of milliseconds or longer) result in the development of slow inactivation, which is a slow modulatory function of VGSCs [44]. Fast and slow inactivation as a function of voltage can be accurately measured by using a two-pulse protocol, wherein an initial conditioning pulse to a depolarized potential is given, followed by a second test pulse (after a recovery period) to measure Na+ current. By varying the length of the conditioning pulse and the length of the recovery period before the test pulse, fast and slow inactivation can be measured separately. In the crayfish giant axon, the dihydropyrazole, RH-3421 had no effect on VGSC current at hyperpolarized, or very negative potentials [43]. However, depolarization of the membrane allowed the onset of a slowly developing inhibition of VGSCs by RH-3421. Furthermore, this inhibition happened over the range of membrane potentials in which slow inactivation developed and shifted the curve of the voltage dependence of slow inactivation in the hyperpolarizing (negative) direction, suggesting that SCBIs bind to VGSCs in the slow inactivated state [43]. Interestingly, repolarization of the membrane to very negative potentials relieved block by SCBIs whereas washing out with recording solution did not.
These results suggested that SCBIs are only capable of interacting with slow inactivated VGSCs, but it was unclear whether this was a result of the SCBI receptor site being in the optimal conformation for binding during slow inactivation or the slow kinetics of SCBI association with VGSCs. To test this, internal enzymatic treatment of axons with trypsin or/and N-bromoacetamide was performed to remove slow or fast inactivation, respectively [45, 46], or both. These experiments, showed that RH-3421 can associate with the fast inactivated state (in the absence of slow inactivation), as well as with the open state (in the absence of both fast and slow inactivation) if sufficient time is provided [43]. Thus, it appears that SCBIs are limited to interact with the slow inactivated state as a result of their very slow kinetics of association.
Not surprisingly, experiments on cockroach (P. americana) dorsal unpaired median neurons produced similar results with indoxacarb and DCJW as compared to the voltage clamp experiments in crayfish with dihydropyrazoles. Indoxacarb blocks the action potentials generated by current injection [47], and it blocks both type I and type II VGSCs expressed in these neurons [48], though type I VGSCs were more sensitive to inhibition by SCBIs than type II [49]. What is more, both indoxacarb and DCJW cause significant hyperpolarizing shifts in the voltage dependence of inactivation (slow inactivation or a combination of slow and fast inactivation), but DCJW was much more potent than indoxacarb in inhibiting Na+ current in these experiments [47], which is consistent with intoxication experiments in insects [19].
The effects of SCBIs on VGSCs are not limited to insects. Treatment of neurons isolated from rat dorsal root ganglion (DRG) showed that effects on mammalian Na+ currents are similar to those observed in insects [50, 51]. Na+ current in DRG neurons was inhibited by treatment with either indoxacarb or DCJW and the voltage dependence of slow inactivation was shifted in the hyperpolarizing direction by both compounds. Also, DCJW was significantly more potent as an inhibitor of VGSCs in rat DRG neurons than indoxacarb. These results show that both mammalian and insect VGSCs are inhibited by SCBIs in a similar manner, and that differences in sensitivities to indoxacarb between insect and mammalian VGSCs are unlikely to be the source of the greater susceptibility of insects to SCBI intoxication.
2.3. Effects of SCBIs on VGSCs Expressed in Xenopus Oocytes
Heterologous expression in the unfertilized oocytes of the African clawed frog, Xenopus laevis, is an excellent model system for studying the function and pharmacology of VGSCs. The functional properties or sensitivity to agonists/antagonists of different VGSC isoforms, splice variants, or orthologs of different species can be directly compared by expressing the channels in this common model system. Accordingly, heterologous expression in Xenopus oocytes is an invaluable tool to study the molecular mechanism of block of SCBIs in both mammalian and insect VGSCs.
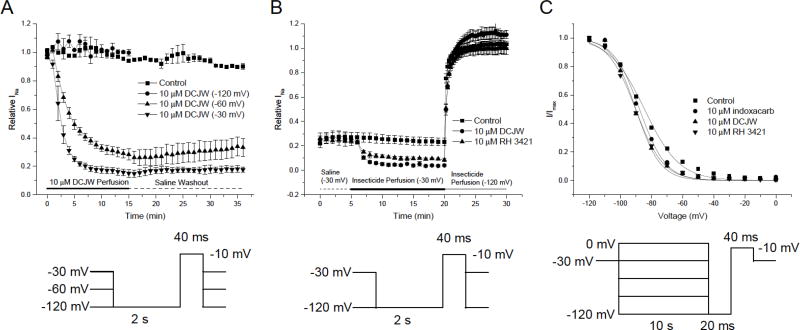
Mammals have nine VGSC isoforms, each of which has unique functional and pharmacological properties [52]. Initial experiments exploring the effects of SCBIs on mammalian VGSCs expressed in Xenopus oocytes focused on examining the effects of SCBIs on Nav1.4, the skeletal muscle sodium channel, due to the large, robust currents achieved with this VGSC and its well-documented pharmacology and electrophysiology [53–57]. As expected, DCJW caused a slowly developing, voltage-dependent inhibition of Nav1.4 current (Fig. 2A). At negative holding potentials, neither DCJW nor indoxacarb had any effect on Nav1.4 current, but as the holding potential was depolarized (−60 or −30 mV), inhibition of Nav1.4 current by DCJW increased (Fig. 2A). Indoxacarb had no effect on Nav1.4 current at any holding potential. Additionally, while DCJW and indoxacarb had no effect on the voltage dependence of fast inactivation, DCJW significantly shifted the voltage dependence of slow inactivation in the hyperpolarizing direction (Fig. 2C). Metaflumizone also produced similar results when used to treat Nav1.4 channels expressed in Xenopus oocytes, causing voltage-dependent inhibition of Na+ current and a hyperpolarizing shift in the voltage dependence of slow, but not fast, inactivation [57]. Na+ current in these experiments were only restored by repolarization of the membrane potential to very negative values (−120 mV), but not by washout with insecticide-free recording solution (Fig. 2B), suggesting that only transition of the VGSCs to a resting state relieved block of Na+ current. However, in contrast to DCJW and indoxacarb, metaflumizone caused a significant depolarizing shift in the voltage dependence of activation [57]. This result suggests that metaflumizone may interact with the resting state of Nav1.4 channels, an interaction that has not been observed for any other SCBI. The potential interaction of metaflumizone with the resting state of VGSCs adds a new dimension to the mechanism of inhibition of VGSCs by metaflumizone and possibly future SCBIs.
Fig. (2).
Voltage dependent block of rat Nav1.4 channels expressed in Xenopus oocytes. A. The slow time course of block of rat Nav1.4 channels maintained at a holding potential of -120, -60, or -30 mV by 10 μM DCJW. B. Recovery from block by DCJW or RH-3421 by repolarization of the holding potential from -30 mV (0 to 20 min) back to -120 mV (20 to 30 min). C. Voltage dependence of slow inactivation curves showing the hyperpolarizing shift induced by DCJW and RH-3421. Voltage protocols are shown below each panel. Solid lines indicate insecticide perfusion, whereas dashed lines indicate saline perfusion. Reproduced from KS Silver and DM Soderlund, State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides, Neurotoxicology 26, 397-406, 2005, with permission from Elsevier.
Subsequent expression of four isoforms of mammalian VGSCs (Nav1.2, Nav1.4, Nav1.5, or Nav1.8) in Xenopus oocytes permitted comparison of their relative sensitivity to SCBI inhibition in the presence or absence of the β1 auxiliary subunit, while also confirming similar effects of SCBIs on these isoforms [54]. Interestingly, whereas the overall effects of SCBIs on the four VGSC isoforms was similar (voltage-dependent inhibition and hyperpolarizing shifts in the voltage dependence of slow inactivation), each isoform showed a unique pattern of sensitivity to each of the tested SCBIs (indoxacarb, DCJW, or RH-3421). Nav1.4 was most sensitive to DCJW, and Nav1.2, Nav1.5, and Nav1.8 were decreasingly sensitive to DCJW and similarly sensitive to RH-3421. These results are consistent with previous findings in rat DRG neurons where tetrodotoxin-sensitive currents (Nav1.2 and Nav1.4) were more sensitive to inhibition by DCJW than tetrodotoxinresistant currents (Nav1.5 and Nav1.8) [51]. Indoxacarb, on the other hand, had no effect on Nav1.4 at any holding potential, Nav1.8 was moderately sensitive to indoxacarb, and the other two isoforms were somewhere in between. Inclusion of the β1 auxiliary subunit likewise had variable effects on SCBI activity depending on the channel and tested compound, either reducing the sensitivity of Nav1.2 to inhibition by RH-3421 or increasing the hyperpolarizing shift in the voltage dependence of slow inactivation of Nav1.4 caused by DCJW or RH-3421 [54]. These results show that whereas these VGSC isoforms are rather similar in sequence and, presumably, in structure (their different gating properties were probably not relevant since these experiments were carried out at potentials when channels are 100% inactivated), their sensitivity to SCBIs can vary widely between isoforms. The differences in sequence and structure of these isoforms may provide clues to the molecular components of the SCBI receptor site on VGSCs.
Similar experiments have also been carried out with insect VGSCs expressed in Xenopus oocytes using the VGSC from German cockroach (Blattella germanica). Unlike mammals, insects have only a single VGSC gene that undergoes extensive differential splicing or RNA editing to generate functionally distinct variations [1, 60–62]. Indoxacarb, DCJW, and metaflumizone caused voltage-dependent inhibition of BgNav1-1a channels that was only relieved by hyperpolarization of the membrane potential and induced hyperpolarizing shifts in the voltage dependence of slow inactivation [58], and similar results were obtained for metaflumizone in M. sexta larvae [33]. Interestingly, two variants of the German cockroach VGSC, BgNav1-1 and BgNav1-4, appeared to have different sensitivities to DCJW when oocytes were held at −90 mV, with DCJW inhibiting nearly 60% of the current in BgNav1-4 channels but having no effect on BgNav1-1 [59]; a result that corresponds with the differential sensitivity of type I and II VGSCs to SCBIs in cockroach DUM neurons noted above [47, 49]. However, further experiments revealed that a single amino acid change at position 1689 (the C-end of the voltage-sensing helix S4 in repeat 4) from lysine in BgNav1-1 to glutamine in BgNav1-4 shifted the voltage dependence of both fast and slow inactivation in the hyperpolarizing direction. Thus, at −90 mV, a larger percentage of BgNav1.4 channels were in inactivated states resulting in a greater percentage of inhibition. This hypothesis was proven when membrane potential was depolarized to −60 mV, whereupon BgNav1-1 and BgNav1-4, having similar levels of inactivation, were similarly sensitive to inhibition by DCJW.
As suggested earlier, use of the Xenopus heterologous expression system allows direct comparison of the sensitivity of different splice variants, isoforms, or conspecific orthologs of VGSCs. We have already noted instances where different splice variants of German cockroach VGSCs [59] and mammalian VGSC isoforms [54] had different sensitivities to SCBIs, but the utility of the Xenopus expression system also allows side-by-side comparison of the different sensitivities of insect (in this case BgNav1-1) and mammalian (Nav1.4) VGSCs to indoxacarb, DCJW, or metaflumizone. Interestingly, inhibition of BgNav1-1 or Nav1.4 caused by DCJW or metaflumizone is similar with no real differences between the two at voltages that induced partial or complete inactivation [53, 57, 58]. In contrast, BgNav1-1 was much more sensitive to inhibition by indoxacarb (30%) than Nav1.4 (which was unaffected by indoxacarb), despite BgNav1-1 being tested at voltages that caused only 50% inactivation (Nav1.4 channels were 100% inactivated, which was expected to result in greater inhibition by SCBIs) [53, 58]. However, another mammalian VGSC (Nav1.8) showed greater sensitivity to indoxacarb, which was comparable to that observed in BgNav1-1 [54, 58]. These results, like those from the nerve preparations, suggest that the selective toxicity of SCBIs for insects is likely not associated with greater sensitivity of insect VGSCs to these insecticides, but is more likely associated with the different metabolic fates of SCBIs in insects versus mammals [16, 18].
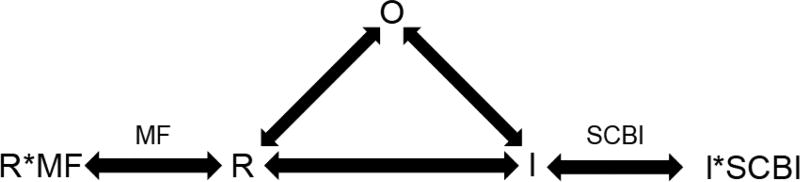
The results of all of these experiments suggest a basic scheme of how SCBIs interact with VGSCs (Fig. 3); this interaction is the same in mammalian and insect VGSCs and depends very little on the SCBI compound. Of primary importance is the interaction of SCBIs with the inactivated and specifically slow inactivated state of VGSCs. The slow kinetics of SCBI binding seem to favor interaction with the long-lived slow inactivated state and cause inhibition of Na+ current over the voltage range where slow inactivation occurs. Upon binding, SCBIs trap VGSCs in an inactivated state, reducing the number of channels available for opening upon stimulation. However, it is apparent from previous studies [43] that should the channel be able to reach an open, conducting state, Na+ current would still be inhibited by the blocking effect of an SCBI bound to the channel. Interestingly, metaflumizone has the ability to affect the voltage dependence of activation [57] as a result of interacting with the resting state. This is a new feature of the SCBI class, which could be exploited in the development of future members of this group.
Fig. (3).
Basic scheme representing the interaction of SCBIs with resting (R), Open (O), and inactivated (I) VGSCs. VGSCs can transit between any of these states, and each state may or may not have several substates (e.g. – the inactivated state involves both fast and slow substates). SCBIs preferentially bind to the inactivated states of VGSCs, and specifically the slow inactivated state due to their slow kinetics of association, and trap the channels in this state, shifting a larger portion of the VGSCs to a non-conducting state. Metaflumizone (MF) can also bind to the resting and fast inactivated states, adding a new dimension to the effects of this insecticide on VGSCs.
3. THE SCBI RECEPTOR SITE ON VGSCS
3.1. Similarities between the Actions of Local Anesthetics and SCBIs on VGSCs
Observations that SCBIs are state-dependent inhibitors of VGSCs raised the question of whether or not they share a similar or overlapping binding site with therapeutic sodium channel blockers, including local anesthetics (LAs). Several lines of biochemical, pharmacological, and physiological data suggest that this may be the case. The evidence for this has been reviewed in great depth previously [2, 26, 27] and here the issue will only be briefly covered.
Both SCBIs and LAs are voltage-dependent inhibitors of VGSCs [33, 43, 48, 51, 53–59, 63, 64], causing shifts in the voltage dependence of slow inactivation curve. LAs, however, are also able to cause both tonic (resting channel) and use-dependent (open and inactivated channel) block, probably as a result of the much faster association kinetics for LAs (though metaflumizone may bind to resting Nav1.4 channels [57]). SCBIs can also associate with both open and fast inactivated states if slow inactivation is removed [43], but in normal conditions, the kinetics of association between SCBIs and VGSCs appears to be far too slow to allow binding to the fast inactivated and open states, with the possible exception of metaflumizone in rat Nav1.4 channels expressed in oocytes [57]. SCBIs, other than metaflumizone, also appear to have no affinity for the resting state of VGSCs.
Inhibition of veratridine (VTD)-stimulated uptake of radioactively-labeled Na+ into, or binding of a radioactively-labeled batrachotoxin derivative (batrachotoxinin A 20-α-benzoate, BTX-B) to rat brain synaptosomes were historically key systems for examining inhibition of VGSC function in mammals. VTD and batrachotoxin are highly specific agonists that bind to the same site and cause VGSCs to open and allow Na+ to pass through [65]. In these assays, both dihydropyrazoles and LAs are effective inhibitors of BTX-B binding to rat brain synaptosomes [66, 67] as well as radiosodium uptake into rat brain synaptosomes when stimulated by VTD [68–70]. Additionally, RH-3421 competitively interfered with inhibition of VTD-stimulated radiosodium uptake by dibucaine (an LA), and vice versa [70].
In addition to pharmacological and biochemical evidence, electrophysiological experiments have demonstrated a competitive interaction of LAs and SCBIs. In rat Nav1.4 channels expressed in Xenopus oocytes, phenytoin, an anticonvulsant, reduced the efficacy of DCJW to inhibit Na+ current [53]. Metaflumizone also effectively reduced the ability of lidocaine to cause use-dependent block of rat Nav1.4 channels following repeated test pulses [57]. This evidence all suggests that LAs and SCBIs share overlapping receptor sites on VGSCs.
3.2. Mapping the SCBI Receptor on Mammalian VGSCs
VGSCs are large transmembrane proteins consisting of 24 transmembrane segments arranged in four repeats (I-IV) with six transmembrane segments each (S1-S6) connected by intra- and extracellular loops [52, 71]. Repeats I-IV are arranged around a central pore with the S6 segments of each repeat lining the ion conduction pathway. Upon depolarization, the S4 segments of each voltage-sensing domain, which has several positively charged residues, move, causing a conformational change in the pore domain, resulting in channel opening. Considerable effort has been undertaken to identify the molecular determinants of the LA receptor on mammalian VGSCs using the Xenopus heterologous expression system in combination with alanine scanning mutagenesis [72–79]. Most of these efforts have focused on the S6 transmembrane segments of each repeat due to the fact that LAs are channel blockers and the S6 segments line the channel pore. Data from this research have led to the development of a model for the interaction of LAs and VGSCs that shows the involvement of the transmembrane segments IS6, IIIS6 and IVS6, and highlights IVS6 as playing a much more important role than the other two [64, 80, 81]. In particular, the phenylalanine at position 1579 and the tyrosine at position 1586 (numbered according to the rat Nav1.4 sequence) have been shown to be consistently involved in the action of a wide variety of sodium channel blockers, and therefore these residues were the focus of initial attempts to identify the molecular determinants of SCBI activity in rat VGSCs [55, 57].
Efforts aimed at modeling the pore regions (S5s, S6s, and the membrane re-entrant P-loops connecting S5 and S6) of VGSCs have been undertaken and have adopted a new nomenclature for residues in these segments to permit direct comparisons of amino acid locations in one repeat with those in other repeats as well as amino acid positions in VGSCs of different organisms [82, 83]. Amino acid residues are therefore named by the repeat in which they are located (1-4), the segment (i, the inner helix, o, the outer helix), and the relative number of the residue in that segment. Accordingly, the F1579 residue would be labeled F4i15 and Y1586 as Y4i22 in this nomenclature, which will be used throughout the rest of this review (though, when necessary, the actual residue numbers will also be given).
Introduction of alanine at F4i15 or Y4i22 had both expected and surprising effects on SCBI inhibition of Nav1.4 channels. As expected, mutation of F4i15 to an alanine significantly reduced the sensitivity of Nav1.4 channels to state-dependent block by DCJW, RH-3421, and metaflumizone [55, 57]. These results were consistent with published reports showing reductions in LA activity following mutation of the same residue to alanine [72, 80], and confirms that LAs and SCBIs share an overlapping binding site in mammalian VGSCs. In contrast, mutation Y4i22A unexpectedly increased the sensitivity of Nav1.4 channels to state-dependent inhibition by indoxacarb (wildtype Nav1.4 was insensitive to indoxacarb, but its Y4i22A mutant was inhibited), DCJW, RH-3421, and metaflumizone [55, 57], suggesting that the receptor sites for LAs and SCBIs overlap at the level of F4i15, but not at the level of Y4i22.
Subsequent experimentation also identified another residue that may be important for the activity of metaflumizone on VGSCs. A valine at position 787 (V2i18) is critical for determining the voltage dependence of slow inactivation, and mutation V2i18K shifts the voltage dependence of slow inactivation in the hyperpolarizing direction, whereas mutations V2i18C or V2i18A shift it in the depolarizing direction, resulting in incomplete slow inactivation [56, 84]. Previous publications suggest that hyperpolarizing shifts in the voltage dependence of slow inactivation enhance sensitivity to SCBIs as was observed in cockroach sodium channels at a different residue [59], whereas depolarizing shifts should decrease channel sensitivity. The results from these experiments, however, showed varying effects of these three mutations on the ability of indoxacarb or DCJW to cause inhibition of Na+ current with no correlation to their effects on the voltage dependence of slow inactivation, indicating that V2i18 does not interact with indoxacarb or DCJW [56]. In contrast, all three mutations reduced the sensitivity of Nav1.4 channels to inhibition by metaflumizone, implying that V2i18 is involved in the binding of metaflumizone to VGSCs. The results of these mutagenesis experiments allow us to conclude that F4i15, but not Y4i22, is a critical residue for the activity of SCBIs in VGSCs, and as such the receptor for SCBIs partially overlaps with that of LAs. However, since helix IIS6 (where V2i18 is located) is important for metaflumizone, but not LA activity, and Y4i22 is critical for LA, but not SCBI activity, the receptors for SCBIs and LAs have molecular determinants that are unique to each class of chemicals.
3.3. Mapping the SCBI Receptor Site in Insect VGSCs
Experiments to map both the SCBI and LA receptors have been undertaken using the cockroach (B. ger-germanica) sodium channel (BgNav1-1) in order to both identify the molecular determinants of receptors for these classes of chemicals and to understand how these receptors might differ between insect and mammalian VGSCs. As in mammalian VGSCs, these studies focused on the importance of F4i15 and Y4i22 (F1817 and Y1824 in BgNav1-1) residues in IVS6 in determining the sensitivity of the BgNav1-1 channels to inhibition by LAs or SCBIs. Channels bearing alanine mutations at these positions were significantly less sensitive to use- and frequency-dependent block by lidocaine, though tonic block and stabilization of slow inactivated channels was unaffected, suggesting that each of these residues is necessary for lidocaine activity [63]. In contrast, the F4i15A and Y4122A mutations had no effect or slightly increased sensitivity to inhibition by DCJW or metaflumizone [58]. The activity of SCBIs on the F4i15A mutant is in direct contrast to the results obtained in mammalian VGSCs. Thus, it seems that the receptor sites for SCBIs in insect and mammalian VGSCs may be different.
3.4. Target Site-mediated Resistance as a Clue to the SCBI Receptor
Observations of target site-mediated resistance in insect populations treated with SCBIs can also reveal amino acids that contribute to the SCBI receptor site. Until recently there had been many reports of resistance to SCBIs, but the mechanisms were typically associated with detoxification, such as upregulation of P450 monooxygenases [85–87]. Importantly, the first report of VGSC mutations causing resistance to SCBIs was recently published. Populations of the diamondback moth (Plutella xylostella) from Baiyun, Guangdong Province in China have VGSCs bearing one of two point mutations in IVS6 that were associated with 750-fold and 70-fold resistance to indoxacarb and metaflumizone, respectively, in these insects [88]. One of these sites, F4i15 (F1845 in the diamondback moth) has been shown to be highly important for SCBI activity in mammalian VGSCs, but unimportant in cockroach VGSCs [55, 57, 58]. The mutation detected in these moths, however, was from phenylalanine to a tyrosine (F4i15Y), and insertion of this mutation into BgNav1-1 channels expressed in oocytes significantly reduced the potency of indoxacarb, DCJW, metaflumizone, and lidocaine [89]. The second mutation, V4i18I (V1582 in BgNav1-1, or V1848 in P. xylostella), also reduced sensitivity of BgNav1-1 channels to inhibition by indoxacarb, DCJW, metaflumizone, and lidocaine [89]. In contrast to V4i18I, mutation V4i18A increased sensitivity to metaflumizone and lidocaine and did not affect the activity of indoxacarb or DCJW. This result is similar to previous experiments using alanine mutagenesis to identify residues involved in the SCBI receptor in BgNav1-1 and Nav1.4. Mutations F4i15A in BgNav1-1 or Y4i22A in Nav1.4, like V4i18A in BgNav1-1, increased sensitivity to SCBIs, suggesting that these residues may not be involved in binding of SCBIs [55, 57, 58]. These conclusions, however, must now be reconsidered since natural mutation at these residues to isoleucine or tyrosine confers resistance to SCBIs in field populations of P. xylostella [88, 89].
3.5. The SCBI Receptor
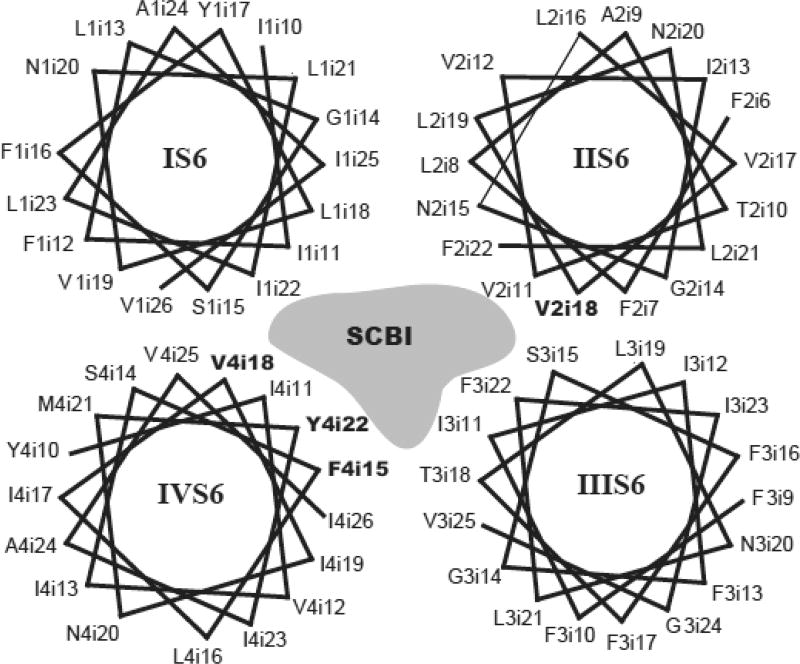
Data collected from all of these experiments, suggest that the receptor for SCBIs partially overlaps that of LAs, and the model for LA binding to VGSCs may assist in further exploring the molecular interactions of SCBIs with VGSCs. However, specific SCBIs clearly interact with residues that are not involved in the binding of other SCBI compounds or LAs (e.g. V2i18A affects channel sensitivity to metaflumizone, but not indoxacarb or DCJW [56]). As such, we propose that the SCBI receptor includes residues in IVS6, which are critical to the action of LAs. These residues are F4i15 and V4i18, and possibly Y4i22, though further experiments are necessary with substitutions other than alanine. In addition, V2i18, a residue in IIS6 which faces the pore, but does not contribute to LA binding, also contributes to the metaflumizone receptor (Fig. 4). Other residues may participate in binding SCBIs to VGSCs, but additional experiments combined with computational modeling (as has been done for pyrethroids [82]) are necessary for a more complete understanding of the molecular bases of interactions between SCBIs and VGSCs [90].
Fig. (4).
Helical wheel scheme showing the S6 transmembrane segments from each repeat with residues that are implicated in SCBI activity highlighted in bold (F4i15, V4i18, Y4i22, V2i18). A hypothetical SCBI molecule is pictured in the channel pore.
CONCLUSION
SCBIs are potent, state-dependent inhibitors of VGSCs, which have a receptor site that is unique to this class of insecticides, yet overlaps with that of therapeutic VGSC inhibitors such as LAs. The novel chemistry and target site of SCBIs make this class invaluable for controlling insect pests, particularly those that have become resistant to other classes of insecticides. Target site-mediated resistance has been documented in a few populations of P. xylostella in China [88, 89], and it is only a matter of time until this happens again in other insect species. Therefore, further studies are necessary to understand the mechanisms through which SCBIs inhibit VGSCs and the molecular determinants that mediate these interactions. These advancements will direct efforts to monitor for resistance by identifying residues that could be involved in SCBI binding, allowing better management of resistance when it develops. In any case, SCBIs will continue to be an important tool for managing insect pests for the foreseeable future.
Acknowledgments
This work was supported by the National Institutes of Health Grant M057440 from NIGMS (to K.D. and B.S.Z.).
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
References
- 1.Dong K, Du Y, Rinkevich FD, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17. doi: 10.1016/j.ibmb.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver K, Du Y, Nomura Y, Oliviera EE, Salgado VL, Zhorov BS, Dong K. In: Target Receptors in the Control of Insect Pests: Part II. Cohen E, editor. Vol. 46. Academic Press: Advances in Insect Physiology; UK: 2014. pp. 389–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulder R, Gijswijt MJ. The laboratory evaluation of two promising new insecticides which interfere with cuticle deposition. Pestic Sci. 1973;4:737–745. [Google Scholar]
- 4.Mulder R, Wellinga K, van Daalen JJ. A new class of insecticides. Naturwissenschaften. 1975;62:531–532. doi: 10.1007/BF00609070. [DOI] [PubMed] [Google Scholar]
- 5.Grosscurt AC, van Hes R, Wellinga K. 1- Phenylcarbamoyl-2-pyrazolines, a new class of insecticides 3. synthesis and insecticidal properties of 3,4-diphenyl-1- phenylcarbamoyl-2-pyrazolines. J Agric Food Chem. 1979;27:406–409. [Google Scholar]
- 6.van Hes R, Wellinga K, Grosscurt AC. 1- Phenylcarbamoyl-2-pyrazolines: a new class of insecticides 2. synthesis and insecticidal properties of 3,5-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J Agric Food Chem. 1978;26:915–918. [Google Scholar]
- 7.Wellinga K, Grosscurt AC, Van Hes R. 1-Phenylcarbamoyl-2-pyrazolines: a new class of insecticides 1. synthesis and insecticidal properties of 3- phenylcarbamoyl-2-pyrazolines. J Agric Food Chem. 1977;25:987–992. [Google Scholar]
- 8.Fuehr F, Mittelstaedt W, Wieneke J. Bilanzversuche mit 14C-markiertem 1-(4-chlorophenylcarbamoyl)-3-(4- chlorophenyl)-4-phenyl-2-pyrazolin (PH 60-42) nach boden- und spritzapplikation in freilandlysimetern. Chemosphere. 1980;9:469–482. [Google Scholar]
- 9.Scheele B. Untersuchung des photoabbaus von PH 60-42 auf maisblaettern, glasplatten und bodenduennschichtplatten. Chemosphere. 1980;9:483–494. [Google Scholar]
- 10.Jacobson RM. In: Recent Advances in the Chemistry of Insect Control II. Crombie L, editor. Royal Society of Chemistry; Cambridge: 1990. pp. 206–212. [Google Scholar]
- 11.Hasan R, Nishimura K, Okada M, Akamatsu M, Inoue M, Ueno T. Stereochemical basis for the insecticidal activity of carbamoylated and acylated pyrazolines. Pestic Sci. 1996;46:105–112. [Google Scholar]
- 12.Meier GA, Silverman IR, Ray PS, Cullen TG, Ali SF, Marek CA, Webster CA. In: American Chemical Society Symposium Series 504. Baker DR, Fenyes JG, Steffens JJ, editors. ACS Books; Washington D.C.: 1992. pp. 313–326. [Google Scholar]
- 13.Lyga J, Lyga I, Silverman R, Ali SF, Cullen TG, Cohen DH, Simmons KA. In: Synthesis and Chemistry of Agrochemicals VI. Baker DR, Fenyes JG, Selby TP, Stevenson TM, editors. American Chemical Society; Washington D.C.: 2002. pp. 199–210. [Google Scholar]
- 14.Leong D, Bloomquist JR, Bempong J, Dybas JA, Kinne L, Lyga J, Marek FL, Nicholson RA. Insecticidal arylalkylbenzhydrolpiperidines: novel inhibitors of voltage-sensitive sodium and calcium channels in mammalian brain. Pest Manag Sci. 2001;57:889–895. doi: 10.1002/ps.352. [DOI] [PubMed] [Google Scholar]
- 15.Wierenga JM, Warkentin DL, Staetz CA, Pitts DL, Dybas JA. Insecticidal activity of Narylalkylbenzhydrolpiperidines. Pest Manag Sci. 2002;58:1266–1272. doi: 10.1002/ps.598. [DOI] [PubMed] [Google Scholar]
- 16.McCann SF, Cordova D, Andaloro JT, Lahm GP. In: Modern Crop Protection Compounds. Kraemer W, Schirmer U, editors. Vol. 3. Wiley; Weinheim: 2007. pp. 1031–1047. [Google Scholar]
- 17.Harder HH, Riley SL, McCann SF, Irving SN. Brighton Crop Protection Conference - Pests and Diseases. 1996. pp. 449–454. [Google Scholar]
- 18.Wing KD, Andaloro JT, McCann SF, Salgado VL. In: Insect Control: Biological and Synthetic Agents. Gilbert LI, Gill SS, editors. Elsevier; New York: 2010. pp. 35–57. [Google Scholar]
- 19.Wing KD, Schnee ME, Sacher M, Connair M. A novel oxadiazine insecticide is bioactivated in lepidopteran larvae. Arch Insect Biochem Physiol. 1998;37:91–103. [Google Scholar]
- 20.Wing KD, Sacher M, Kagaya Y, Tsurubuchi Y, Mulderig L, Connair M, Schnee M. Bioactivation and mode of action of the oxadiazine indoxacarb in insects. Crop Protect. 2000;19(8-10):537–545. [Google Scholar]
- 21.Salgado VL. In: Insect Control. Gilbert LI, Gill SS, editors. Elsevier; New York: 2010. pp. 58–59. [Google Scholar]
- 22.Takagi K, Hamaguchi H, Nishimatsu T, Konno T. Discovery of metaflumizone, a novel semicarbazone insecticide. Vet Parasitol. 2007;150(3):177–181. doi: 10.1016/j.vetpar.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 23.BASF. Metaflumizone worldwide technical brochure. BASF Agricultural Products. 2007 [Google Scholar]
- 24.Hempel K, Hess FG, Boegi C, Fabian E, Hellwig J, Fegert I. Toxicological properties of metaflumizone. Vet Parasitol. 2007;150(3):190–195. doi: 10.1016/j.vetpar.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Silver K, Soderlund DM. Action of pyrazoline-type insecticides at neuronal target sites. Pestic Biochem Physiol. 2005;81:136–143. [Google Scholar]
- 26.Silver K, Song W, Nomura Y, Salgado VL, Dong K. Mechanism of action of sodium channel blocker insecticides (SCBIs) on insect sodium channels. Pestic Biochem Physiol. 2010;97:87–92. doi: 10.1016/j.pestbp.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Stein RT, Silver KS, Soderlund DM. Indoxacarb, metaflumizone, and other sodium channel inhibitor insecticides: mechanism and site of action on mammalian voltage-gated sodium channels. Pestic Biochem Physiol. 2013;106:101–112. doi: 10.1016/j.pestbp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rugg D, Hair JA. Dose determination of a novel formulation of metaflumizone plus amitraz for the treatment and control of fleas (Ctenocephalides felis) and ticks (Rhipicephalus sanguineus) on dogs. Vet Parasitol. 2007;150:203–208. doi: 10.1016/j.vetpar.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Rust MK, Rugg D, Rock D. Metaflumizone - A new ectoparasiticide for dogs and cats - Preface. Vet Parasitol. 2007;150(3):175–176. [Google Scholar]
- 30.Agency, U.S., Environmental Protection Agency. Pesticide Fact Sheet: Indoxacarb. 2000 [Google Scholar]
- 31.Salgado VL. Mechanism of action of 1-phenylcarbamoyl-2-pyrazoline insecticides. Pestic Sci. 1988;24:243–344. [Google Scholar]
- 32.Salgado VL. Mode of action of insecticidal dihydropyrazoles: selective block of impulse generation in sensory nerves. Pestic Sci. 1990;28:389–411. [Google Scholar]
- 33.Salgado VL, Hayashi JH. Metaflumizone is a novel sodium channel blocker insecticide. Vet Parasitol. 2007;150(3):182–189. doi: 10.1016/j.vetpar.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 34.Bloomquist JR, Payne GT, Kinne L, Lyga J, Leong D, Nicholson RA. Toxicity and mode of action of benzhydrolpiperidines and related compounds in insects. Pesticid Biochem Physiol. 2002;73:18–26. [Google Scholar]
- 35.California Environmental Protection Agency. Department of Pesticide Regulation. Indoxacarb. 2015 http://www.cdpr.ca.gov/docs/risk/toxsums/pdfs/5331.pdf.
- 36.Wismer T, Means C. Toxicology of newer insecticides in small animals. Vet Clin N Amer Small. 2012;42(2):335. doi: 10.1016/j.cvsm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 37.California Environmental Protection Agency. Department of Pesticide Regulation. Metaflumizone. 2015 http://www.cdpr.ca.gov/docs/risk/toxsums/pdfs/5935.pdf.
- 38.Park JS, Kim H, Lee SW, Min JH. Successful treatment of methemoglobinemia and acute renal failure after indoxacarb poisoning. Clin Toxicol. 2011;49(8):744–746. doi: 10.3109/15563650.2011.602080. [DOI] [PubMed] [Google Scholar]
- 39.Prasanna L, Rao SM, Singh V, Kujur R, Gowrishankar Indoxacarb poisoning: an unusual presentation as methemoglobinemia. Indian J Crit Care Med. 2008;12(4):198–200. doi: 10.4103/0972-5229.45082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y-J, Lin Y-L, Huang H-Y, Hsu B-G. Methemoglobinemia induced by indoxacarb intoxication. Clin Toxicol. 2010;48(7):766–767. doi: 10.3109/15563650.2010.503657. [DOI] [PubMed] [Google Scholar]
- 41.Oh JS, Choi KH. Methemoglobinemia associated with metaflumizone poisoning. Clin Toxicol (Phila) 2014;52(4):288–290. doi: 10.3109/15563650.2014.900180. [DOI] [PubMed] [Google Scholar]
- 42.Wiersma CAG, Furshpan E, Florey E. Physiological and pharmacological observations on muscle organs of the crayfish, Cambarus clarkii Girard. J Exp Biol. 1953;30:136–151. [Google Scholar]
- 43.Salgado VL. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol Pharmacol. 1992;41:120–126. [PubMed] [Google Scholar]
- 44.Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol. 2003;13(3):284–290. doi: 10.1016/s0959-4388(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 45.Oxford GS, Wu CH, Narahashi T. Removal of sodium channel inactivation in squid giant axons by N-bromoacetamide. J Gen Physiol. 1978;71:227–247. doi: 10.1085/jgp.71.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starkus JG, Shrager P. Modification of slow sodium inactivation in nerve after internal perfusion with trypsin. A J Physiol: Cell Physiol. 1978;255:C238–C244. doi: 10.1152/ajpcell.1978.235.5.C238. [DOI] [PubMed] [Google Scholar]
- 47.Lapied B, Grolleau F, Sattelle DB. Indoxacarb, an oxadiazine insecticide, blocks insect neuronal sodium channels. Br J Pharmacol. 2001;132(2):587–595. doi: 10.1038/sj.bjp.0703853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao X, Ikeda T, Salgado VL, Yeh JZ, Narahashi T. Block of two subtypes of sodium channels in cockroach neurons by indoxacarb insecticides. Neurotoxicology. 2005;26(3):455–465. doi: 10.1016/j.neuro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Lavialle-Defaix C, Moignot B, Legros C, Lapied B. How does calcium-dependent intracellular regulation of voltage-dependent sodium current increase the sensitivity to the oxadiazine insecticide indoxacarb metabolite decarbomethoxylated JW062 (DCJW) in insect pacemaker neurons? J Pharmacol Exp Ther. 2010;333(1):264–272. doi: 10.1124/jpet.109.163519. [DOI] [PubMed] [Google Scholar]
- 50.Tsurubuchi Y, Kono Y. Modulation of sodium channels by the oxadiazine insecticide indoxacarb and its N-decarbomethoxylated metabolite in rat dorsal root ganglion neurons. Pest Manag Sci. 2003;59(9):999–1006. doi: 10.1002/ps.652. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X, Ikeda T, Yeh JZ, Narahashi T. Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology. 2003;24(1):83–96. doi: 10.1016/s0161-813x(02)00112-2. [DOI] [PubMed] [Google Scholar]
- 52.Goldin AL. Resurgence of sodium channel research. Ann Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 53.Silver K, Soderlund DM. State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides. Neurotoxicology. 2005;26:397–406. doi: 10.1016/j.neuro.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Silver K, Soderlund DM. Differential sensitivity of rat voltage-sensitive sodium channel isoforms to pyrazoline-type insecticides. Toxicol App Pharmacol. 2006;214:209–217. doi: 10.1016/j.taap.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 55.Silver K, Soderlund DM. Point mutations at the local anesthetic receptor site modulate the state-dependent block of rat Nav1.4 sodium channels by pyrazoline-type insecticides. Neurotoxicology. 2007;28:655–663. doi: 10.1016/j.neuro.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 56.von Stein RT, Soderlund DM. Compound-specific effects of mutations at Val787 in DII-S6 of Nav1.4 sodium channels on the action of sodium channel inhibitor insecticides. Neurotoxicology. 2012;33(5):1381–1389. doi: 10.1016/j.neuro.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Stein RT, Soderlund DM. Role of the local anesthetic receptor in the state-dependent inhibition of voltage-gated sodium channels by the insecticide metaflumizone. Mol Pharmacol. 2012;81(3):366–374. doi: 10.1124/mol.111.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silver K, Nomura Y, Salgado VL, Dong K. Role of the sixth transmembrane segment of domain IV of the cockroach sodium channel in the action of sodium channel-blocker insecticides. Neurotoxicology. 2009;30(4):613–621. doi: 10.1016/j.neuro.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song W, Liu Z, Dong K. Molecular basis of differential sensitivity of insect sodium channels to DCJW, a bioactive metabolite of the oxadiazine insecticide indoxacarb. Neurotoxicology. 2006;27(2):237–244. doi: 10.1016/j.neuro.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong K. Insect sodium channels and insecticide resistance. Invert Neurosci. 2007;7(1):17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong K. In: Insect Pharmacology, Channels, Receptors, Toxins and Enzymes. Gilbert LI, Gill SS, editors. Academic Press; New York: 2010. pp. 25–27. [Google Scholar]
- 62.Soderlund DM. In: Comprehensive Molecular Insect Science. Gilbert LI, Iatrou K, Gill SS, editors. Vol. 5. Elsevier; Amsterdam: 2005. pp. 1–24. [Google Scholar]
- 63.Song W, Silver K, Du Y, Liu Z, Dong K. Analysis of the action of lidocaine on insect sodium channels. Insect Biochem. Mol. Biol. 2011;41:36–41. doi: 10.1016/j.ibmb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mike A, Lukacs P. The enigmatic drug binding site for sodium channel inhibitors. Curr Mol Pharmacol. 2010;3:129–144. doi: 10.2174/1874467211003030129. [DOI] [PubMed] [Google Scholar]
- 65.Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15(2):151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 66.Crevling CR, McNeal ET, Daly JW, Brown GB. Batrachotoxin-induced depolarization and [3H]batrachotoxinin-A 20-a-benzoate binding in a vesicular preparation from Guinea pig cerebral cortex. Mol Pharmacol. 1983;23:350–358. [PubMed] [Google Scholar]
- 67.Deecher DC, Payne GT, Soderlund DM. Inhibition of [3H]batrachotoxinin A 20-α-benzoate binding to mouse brain sodium channels by the dihydropyrazole insecticide RH 3421. Pestic Biochem Physiol. 1991;41:265–273. [Google Scholar]
- 68.Catterall WA. Common modes of drug action of Na+ channels: local anesthetics, antiarrhythmics, and anticonvulsants. Trends Pharmacol Sci. 1987;8:57–65. [Google Scholar]
- 69.Deecher DC, Soderlund DM. RH 3421, an insecticidal dihydropyrazole inhibits sodium channel-dependent sodium uptake into mouse brain preparations. Pestic Biochem Physiol. 1991;39:130–137. [Google Scholar]
- 70.Payne GT, Deecher DC, Soderlund DM. Structure-activity relationships for the action of dihydropyrazole insecticides on mouse brain sodium channels. Pestic Biochem Physiol. 1998;60:177–185. [Google Scholar]
- 71.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26(1):13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 72.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 73.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated sodium channels. Proc Nat Acad Sci USA. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang GK, Quan C, Wang SY. A common local anethetic receptor for benzocaine and etidocaine in voltage-gated m1 Na+ channels. Eur J Physiol. 1998;435:293–302. doi: 10.1007/s004240050515. [DOI] [PubMed] [Google Scholar]
- 75.Yarov-Yarovoy V, Brown J, Sharp EM, Clare JJ, Scheuer T, Catterall WA. Molecular determinants of voltage-dependent gating and binding of pore-blocking drugs in transmembrane segment IIIS6 of the Na+ channel. J Biol Chem. 2001;276:20–27. doi: 10.1074/jbc.M006992200. [DOI] [PubMed] [Google Scholar]
- 76.Yarov-Yarovoy V, McPhee JC, Idsvoog D, Pate C, Scheuer T, Catterall WA. Role of amino acid residues in transmembrane segments IS6 and IIS6 of the Na+ channel α subunit in voltage-dependent gating and drug block. J Biol Chem. 2002;277:35393–35401. doi: 10.1074/jbc.M206126200. [DOI] [PubMed] [Google Scholar]
- 77.Liu G, Yarov-Yarovoy V, Nobbs M, Clare JJ, Scheuer T, Catterall WA. Differential interactions of lamotrigine and related drugs with transmembrane segment IVS6 of voltage-gated sodium channels. Neuropharmacology. 2003;44:413–422. doi: 10.1016/s0028-3908(02)00400-8. [DOI] [PubMed] [Google Scholar]
- 78.Nau C, Wang SY, Strichartz GR, Wang GK. Point mutations at N434 in D1-S6 of mu1 Na(+) channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol Pharmacol. 1999;56(2):404–413. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- 79.Wang GK, Russell C, Wang SY. State-dependent block of voltage-gated Na+ channels by amitriptyline via the local anesthetic receptor and its implication for neuropathic pain. Pain. 2004;110(1–2):166–174. doi: 10.1016/j.pain.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 80.Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68:1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- 81.Tikhonov DB, Zhorov BS. Sodium channels: ionic model of slow inactivation and state-dependent drug binding. Biophys J. 2007;93:1557–1570. doi: 10.1529/biophysj.106.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, Zhorov BS, Dong K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Nat Acad Sci USA. 2013;110(29):11785–11790. doi: 10.1073/pnas.1305118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhorov BS, Tikhonov DB. Potassium, sodium, calcium and glutamate-gated channels: pore architecture and ligand action. J Neurochem. 2004;88(4):782–799. doi: 10.1111/j.1471-4159.2004.02261.x. [DOI] [PubMed] [Google Scholar]
- 84.O'Reilly JP, Wang SY, Wang GK. Residue-specific effects on slow inactivation at V787 in D2-S6 of Nav1.4 sodium channels. Biophys J. 2001;81:2100–2111. doi: 10.1016/S0006-3495(01)75858-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmad M, Hollingworth RM. Synergism of insecticides provides evidence of metabolic mechanisms of resistance in the obliquebanded leafroller Choristoneura rosaceana (Lepidoptera: Tortricidae) Pest Manag Sci. 2004;60(5):465–473. doi: 10.1002/ps.829. [DOI] [PubMed] [Google Scholar]
- 86.Khakame SK, Wang X, Wu Y. Baseline toxicity of metaflumizone and lack of cross resistance between indoxacarb and metaflumizone in diamondback moth (Lepidoptera: Plutellidae) J Econ Entomol. 2013;106(3):1423–1429. doi: 10.1603/ec12494. [DOI] [PubMed] [Google Scholar]
- 87.Sayyed AH, Wright DJ. Genetics and evidence for an esterase-associated mechanism of resistance to indoxacarb in a field population of diamondback moth (Lepidoptera : Plutellidae) Pest Manag Sci. 2006;62(11):1045–1051. doi: 10.1002/ps.1270. [DOI] [PubMed] [Google Scholar]
- 88.Wang XL, Su W, Zhang JH, Yang YH, Dong K, Wu YD. Two novel sodium channel mutations associated with resistance to indoxacarb and metaflumizone in the diamondback moth, Plutella xylostella. Insect Sci. 2016;23:50–58. doi: 10.1111/1744-7917.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang D, Du Y, Nomura Y, Wang X, Wu Y, Zhorov BS, Dong K. Mutations in the transmembrane helix S6 of domain IV confer cockroach sodium channel resistance to sodium channel blocker insecticides and local anesthetics. Insect Biochem Mol Biol. 2015;66:88–95. doi: 10.1016/j.ibmb.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang YQ, Du YZ, Jiang DX, Behnke C, Nomura Y, Zhorov BS, Dong K. The receptor site and mechanism of action of sodium channel blocker insecticides. J. Biol. Chem. 2016;291(38):20113–20124. doi: 10.1074/jbc.M116.742056. [DOI] [PMC free article] [PubMed] [Google Scholar]