Abstract
Background
High definition transcranial direct current stimulation (HD-tDCS) has been administered over single brain regions for small numbers of sessions. Safety, feasibility and tolerability of HD-tDCS over multiple brain regions, multiple daily stimulations and long periods are not established.
Objective
We studied safety, feasibility and tolerability of daily HD-tDCS over 2–4 brain regions for 20 sessions in healthy adults.
Methods
Five healthy adults underwent physical and neurological examination, electrocardiogram (EKG), electroencephalogram (EEG) and cognitive screening (ImpACT) before, during and after HD-tDCS. Four networks (left/right temporoparietal and frontal) were stimulated in sequence (20 min each) using HD-tDCS in 20 daily sessions. Sessions 1–10 included sequential stimulation of both temporoparietal networks, sessions 11–15 stimulations of 4 networks and sessions 16–20 two daily stimulation cycles of 4 networks/cycle (1.5 mA/network). Side effects, ImpACT scores and EEG power spectrum were compared before and after HD-tDCS.
Results
All subjects completed the trial. Adverse events were tingling, transient redness at the stimulation site, perception of continuing stimulation after end of session and one self-resolving headache. EEG power spectrum showed decreased delta power in frontal areas several days after HD-tDCS. While at the group level ImpACT scores did not differ before and after stimulations, we found a trend for correlation between decreased EEG delta power and individual improvements in ImpACT scores after HD-tDCS.
Conclusion
Prolonged, repeat daily stimulation of multiple brain regions using HD-tDCS is feasible and safe in healthy adults. Preliminary EEG results suggest that HD-tDCS may induce long lasting changes in excitability in the brain.
Keywords: HD-tDCS, tolerability, feasibility, safety, adverse events
Introduction
Transcranial direct current stimulation (tDCS) is a method which enables noninvasive electrical stimulation of the cortex via electrodes placed on the subject’s head (Paulus, 2011; Schlaug and Renja, 2008). Anodal stimulation facilitates and cathodal stimulation inhibits spontaneous neuronal activity in the underlying cortical area (Nitsche et al., 2008; Priori et al., 2009). tDCS does not induce neuronal firing, but modulates spontaneous neuronal network activity (Bindman et al., 1964a; Nitsche and Paulus, 2000; Purpura and McMurty, 1965). Changes in excitability are reflected in spontaneous firing rates and responsiveness to afferent synaptic inputs (Bindman et al., 1964b; Creutzfeldt et al., 1962).
tDCS also elicits a variety of after-effects; it modifies the synaptic microenvironment, modifies synaptic strength in an NMDA-receptor dependent fashion (similar to long-term potentiation), modulates intracortical and corticospinal neurons and may cause transient changes in the density of protein channels in neuronal membranes (Liebetanz et al., 2002; Nitsche et al., 2003a; Stagg et al., 2009). An interesting after-effect of tDCS is modulation of spontaneous neuronal oscillations (Ardolino et al., 2005). Remarkable is also the fact that constant electrical fields influence vessels and connective tissue, inflammation, cell migration, vascular motility and cellular structures (Merzagova et al., 2010).
tDCS has been used to modify and study cognitive functions in healthy humans and in patients with neuropsychiatric conditions. Anodal and cathodal tDCS modulate visual working memory but can also disrupt practice-dependent improvement during a verbal working memory task when the cerebellum is stimulated (Ferrucco et al., 2008). Anodal tDCS to the anterior temporal lobes influences memories, improves decision making, attention, learning, language and memory consolidation (Barham et al., 2016; Brunoni et al., 2012; Cappon et al., 2000; Fregni et al., 2015; Mattai et al., 2011; Paulus, 2011; Varga et al., 2011; Young et al., 2013). However, all of these phenomena are transient. The effects of repeated applications of tDCS and the potential of this technique to lead to lasting neurocognitive improvements remain unexplored.
High definition tDCS (HD-tDCS) results in more focal stimulation of selected brain regions (DaSilva et al., 2015; Dmochowski et al., 2011; Donnell et al., 2015; Edwards et al., 2013; Garnett et al., 2015; Kuo et al., 2013; Villamar et al., 2013a). HD-tDCS employs more than two small electrodes. One of the most frequently used HD-tDCS montages is the 4×1 ring set-up which employs a central electrode surrounded by four return electrodes arranged in a circle around the central electrode (Dmochowski et al., 2011; Edwards et al., 2013; Villamar et al., 2013b). HD-tDCS enhances motor cortex excitability (Caparelli-Daquer et al., 2012) similar to conventional tDCS, significantly improves verbal learning and working memory in healthy individuals (Nikolin et al., 2015), improves language in patients with stroke (Richardson et al., 2015), and is well tolerated (Borckhardt et al., 2012; Brunye et al., 2014; Kuo et al., 2013; Garnett et al., 2015; Richardson et al., 2015). As with tDCS, HD-tDCS studies have also been designed to stimulate one brain area for a small number of sessions (1–10).
Given the neuromodulatory and disease modifying potential of HD-tDCS and traditional tDCS, the question is posed whether stimulation of multiple cortical areas over months or years might favorably and permanently influence neurocognitive function. This might have therapeutic value for subjects with intellectual disabilities, for which no effective treatments currently exist, as well as neuropsychiatric disorders and dementia syndromes. Multiple clinical phase I and phase II trials with HD-tDCS have been performed with stimulation of a single cortical area at each session and a limited total number of stimulations (up to 10 daily sessions). It is therefore unknown whether daily stimulations of multiple cortical areas over a prolonged period of time (months or years) is feasible, well tolerated and safe.
The purpose of this trial was to study feasibility, tolerability, and safety of HD-tDCS administered daily over four different cortical regions for a total of 20 sessions to healthy adult subjects. We designed the stimulation protocol with the aim to generate electrical fields over the prefrontal cortex and the temporoparietal regions of both hemispheres. This design was chosen because our future goal is to attempt to modulate cognitive functions represented in these regions. The prefrontal cortex is known to be an area for executive control and has dense anatomical connections to posterior association cortices, limbic cortices and subcortical structures (Fuster, 2015). Within the temporoparietal cortex, language, attention, calculation, working memory, auditory processing and attention, visual/spatial orientation are represented, all of which comprise key components of human intelligence (Nieuwenhuys et al., 2008). We also collected pilot data on the effects of prolonged, multi-field HD-tDCS stimulation on EEG frequency distribution, in order to identify possible long-lasting changes in brain excitability induced by HD-tDCS.
Materials and Methods
Study design and statistical considerations
The study design was an open label pilot trial. The study conformed to the ethical standards of the Helsinki Declaration (1964) and was approved by the Institutional Review Board of the University of Wisconsin Madison. All patients provided written informed consent and the study took place at the Neurophysiology laboratories of the Department of Neurology. The trial is listed under www.clinicaltrials.gov.
The primary objective was to assess feasibility, tolerability and safety of HD-tDCS, administered for a total of 20 sessions in healthy adult subjects (Moore et al., 2011). The chosen sample size was 5 subjects. This decision was based on published recommendations about the design of pilot studies, the aims of which are defined in terms of a binary outcome such as compliance with a protocol or occurrence of adverse events. For planning these types of studies, anticipated confidence interval widths can be considered for potential sample sizes. Confidence intervals based on binomial theory are able to convey the realistic level of uncertainty about such point estimates when planning these studies (Carter, 2004). For example, if n = 5 participants are observed to experience 0 serious adverse events, the 90% exact upper limit for the serious adverse event rate would be 37%, i.e., CI is [0%, 37%]. We decided that this level of precision was acceptable based on consideration of the stage of the investigation, time for study completion and costs of the study and that this sample size would provide enough confidence for the design of subsequent larger Phase I clinical trials using this protocol.
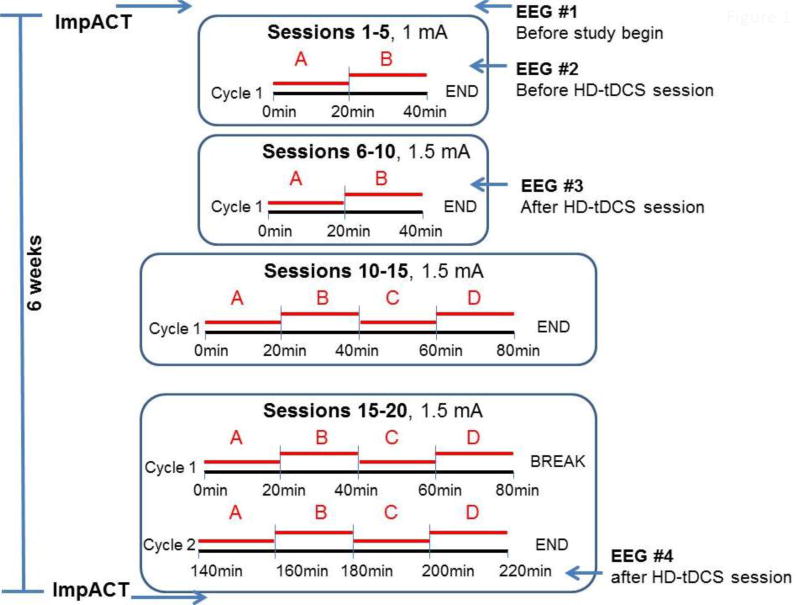
Only one session was performed in each 24 hrs period. There were 1 or 2 stimulation cycles in each session. During each cycle, 2–4 networks were stimulated sequentially for 20 min each. During sessions 1–10 participants were subjected to one cycle including stimulation of 2 networks. During sessions 11–15 they were subjected to one cycle consisting of stimulation of 4 networks. Finally, sessions 16–20 consisted of 2 cycles and during each cycle 4 networks were stimulated in sequence. A minimum of 60 min was allowed to subside between the end of cycle 1 and the beginning of cycle 2. Networks were stimulated sequentially for 20 min each.
Intensity of each network stimulation was 1mA during sessions 1–5 and 1.5 mA for the remainder of the study. The study design is shown in figure 1 and table 1.
Fig 1. Diagram of HD-tDCS stimulation protocol and timing of ancillary studies.
A: left temporoparietal network; B: right temporoparietal network; C: left frontal network; D: right frontal network.
Table 1.
HD-tDCS protocol applied in this study. A maximum of 5 sessions/week were administered. All 20 stimulations were completed within 6 weeks.
| Current Intensity |
Networks stimulated in sequence for 20 min each |
Daily cycles |
Number of stimulation sessions |
Duration of stimulation |
Interval between cycles |
|---|---|---|---|---|---|
| 1mA | 2 (R+L temporoparietal) | 1 | 5 | 40 min | N/A |
| 1.5mA | 2 (R+L temporoparietal) | 1 | 5 | 40 min | N/A |
| 1.5mA | 4 | 1 | 5 | 80 min | N/A |
| 1.5mA | 4 | 2 | 5 | 160 min | 60 min |
The study was defined to be positive if 5 healthy adults were accrued within a study period of 12 months and at least 4 of 5 participants had successful completion. Tolerability was measured as the proportion of subjects able to complete 20 sessions on the assigned treatment within 6 weeks. Procedures to monitor subject safety included vital signs measurements, physical/neurologic examinations, electrocardiograms and electroencephalograms. Clinically significant changes in the safety measures were documented as adverse events.
The secondary objective was to collect pilot data on feasibility of efficacy endpoints. We studied impact of our HD-tDCS protocol on EEG amplitudes and coherences in the lower (<8Hz) and higher (>9Hz) frequency ranges and coherences in the lower (<8Hz) and gamma (30–80 Hz) frequency ranges in healthy adults.
Research participants
Following approval by the University of Wisconsin Health Sciences Institutional Review Board (IRB) and the Federal Drug Administration (FDA, IDE G120203/S001), study subjects were recruited from the Madison Metropolitan Area. Information was placed on the University webpage, and flyers within UW Madison Campus and UW Hospital and Clinics. The subjects entered the study within a period of 12 months, they had to be ≥ 18 and ≤ 45 years of age at the time of enrollment, be able to consent, have a normal physical and neurologic examination, normal electrocardiogram (EKG) and electroencephalogram (EEG). They had to either be enrolled in college or have a bachelor’s or higher degree to ensure that they have a normal IQ. Exclusion criteria were previous or current medical history of epilepsy, neurologic, cardiac, endocrinologic, renal, chronic infectious, metabolic, psychiatric disease or cancer, abnormal EEG or EKG.
Electrode montage and design
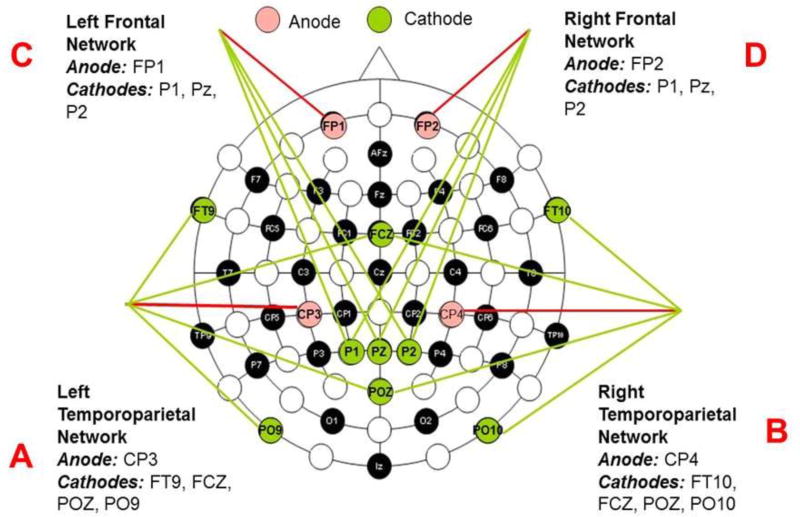
When conducting HD-tDCS, specially designed insets, electrodes, stimulation protocols, and conductive gels are used. Appropriate instrumentation, electrode design, and protocols are considered important for HD-tDCS safety and comfort. All materials were purchased from Soterix Medical (New York, USA). A flexible plastic EEG cap was placed on each participant’s head and held in place with a chin strap. The HD-tDCS anode casings were placed as outlined in figure 2. The electrode casings were secured in the EEG cap and were injected with 3 mL of Signa Gel (Parker Laboratories, NJ), with more applied if needed. The electrodes were then placed into the gel solution inside the casings and held in place with the casing caps. Impedance values were examined for each of the 5 electrodes and were all verified to be <2 quality units. The HD-tDCS device was attached to a 1×1 low-intensity DC stimulator (Soterix Medical, New York, USA) and the device was ramped to 1 or 1.5 mA and maintained at this current for 20 minutes.
Fig 2. Diagram of stimulation principle and outline of the HD-tDCS stimulation session design. There were four networks applied as noted on the diagram.
Networks were stimulated sequentially in the sequence left temporoparietal (20 min), right temporoparietal (20 min), left frontal (20 min), right frontal (20 min). Total duration of one full HD-tDCS cycle was 80 min. Only 1 daily cycle was applied in sessions 1–15, and 2 daily cycles in sessions 16–20.
For each stimulation the target was identified by a standard 10/10 EEG coordinate, and was found on the head using a tape measure. The center active electrode (anode) was placed over this target site within the EEG cap, while the remaining electrodes (cathodes) were positioned to surround the target electrode as shown in figure 2. The result was focal neuromodulation under the area surrounding the active anodal electrode which was also the target.
With the selected electrode placement we aimed to generate electrical fields over the prefrontal cortex and the temporoparietal regions of both hemispheres. We used an approximate 4×1 set-up with the anode placed over the centroparietal cortex and a set-up consisting of an anodal electrode over the frontopolar region and 3 cathodal electrodes over the parietal cortex to stimulate 4 networks, left and right temporoparietal and left and right frontal (Fig 2). Networks were stimulated in sequence and each network was stimulated for 20 min at a time.
All procedures were conducted at the University of Wisconsin Hospitals and Clinics. The electrodes were placed every day and the stimulation sessions were monitored by trained study personnel who remained on site. Subjects were evaluated in person in the UW Hospitals and Clinics by a health professional at screening and at weeks 4 and 8. A study member was on call and available to the study participants 24/7.
Neuropsychological battery to assess cognitive abilities
Participants underwent Immediate Post Concussion Assessment and Cognitive Testing (ImpACT) (Lovell et al., 2006) at the screening visit and at conclusion of the study. ImpACT is a brief (~20 minute) computerized cognitive screening battery that assesses verbal memory, visual memory, reaction time, and impulsivity. ImpACT is designed for short test-retest intervals and is routinely used to assess cognition in the context of traumatic brain injury. This test is not designed to evaluate specific networks, it is merely used to screen for a global encephalopathy as it occurs following traumatic brain injury. Our purpose in using this test was to screen for an encephalopathy triggered by our extended multiple field HD-tDCS protocol.
EEG acquisition, data preprocessing and statistical analysis
Electroencephalograms (EEGs) were standard 20 minute clinical EEGs acquired without sedation and sampled at 1024 Hz. Electrodes were placed in the standard 10–20 arrangement and saved in referential montage with linked ears as the reference. Up to 32 channels of reformatted EEG were displayed in several bipolar and referential montages. EEGs were performed in the Neurophysiology laboratories at the University of Wisconsin Hospital and Clinics.
EEG analysis used EEGlab software (https://sccn.ucsd.edu/eeglab/) and in-house Matlab code. First, EEG data were filtered between 1 and 40 Hz. Artifact rejection was performed by visual inspection of the EEG to exclude bad channels as well as epochs with eye movement and muscle activity contamination (Landness et al., 2011).. EEG data were then average referenced and delta power was computed at each channel computing the root-mean-square of the signal in the 1–4 Hz band (Murphy et al., 2011).
We conducted statistical comparisons between the 1st and 4th EEGs. The 1st EEG session was performed before study begin in all subjects. The 4th EEG session was performed several days after the last HD-tDCS session in 3 subjects and after an HD-tDCS session during week 4 in 2 subjects. Statistical analysis was performed using both statistical parametric mapping (SPM, http://www.fil.ion.ucl.ac.uk/spm) and statistical non-parametric mapping (SNPM, http://warwick.ac.uk/snpm). Topographical values of changes in delta power between 1st and 4th EEG sessions (before and after HD-tDCS intervention) were converted to two dimension images using a 32×32 grid (Kilner and Friston, 2010). Random effects analyses modeled the group effect for a delta power change between sessions 1 and 4, with the change in ImpACT scores before and after study entered as a covariate. Results were thresholded at p<0.05 corrected for multiple comparisons using family-wise error at the voxel- or cluster-level (with uncorrected parametric p=0.05 as cluster forming threshold) (Boly et al., 2017). We restricted the parametric correction for multiple comparisons for correlation between delta power and ImpACT score changes to regions showing a significant change in delta power change between session 1 and 4 at uncorrected p<0.05 (small volume correction) (Worsley et al., 1996). In order to further ensure that outliers did not drive our results, confirmatory non-parametric statistics were performed for all results (Boly et al., 2017).
Results
From September 2015 until March 2016 we recruited 6 adults who consented of whom five were eligible to participate in this trial. Mean age of participants was 23.2 years (SD 4.2, min 19.0 years, max 30 years). There were 2 female and three male participants, 4 white and 1 African American, all subjects were non-hispanic. Three subjects were attending college, one had completed undergraduate education and one had a master degree.
Mean duration of participation in the study for the five participants was 91.6 days (SD 9.7 days, min 77 and max 101 days). This included the time from signing the consent until a final phone contact 30 days after the last stimulation session.
The participants completed the 20 HD-tDCS sessions within 34.4 days (SD 5.177 days, min 30 and max 41 days).
Adverse events
There were no serious adverse events during this trial. All adverse events reported are listed in table 2, they were mild and resolved without intervention.
Table 2.
Adverse event description
| Description | Subjects | Severity | Times experienced |
Action taken |
Attribution | Expected |
|---|---|---|---|---|---|---|
| Tingling sensation | 5/5 | mild | At each session | None | Definite | Yes |
| Poor sleep | 1/5 | mild | once | None | Possible | No |
| Perception of continuing stimulation | 1/5 | mild | once | None | Probable | Yes |
| Headache | 1/5 | mild | once | None | Possible | No |
| Skin irritation | 2/5 | mild | Three sessions | None | Probable | Yes |
All subjects experienced a tingling sensation at the site of stimulation which included the anodal and cathodal electrodes. This feeling was described as mild discomfort with intensity of 2–3/10 at the beginning of stimulation and 1–2/10 after 2–3 min. Skin irritation under the anodal electrode was reported in 2 subjects during 3 sessions. One subject developed a headache during stimulation which resolved when the stimulation ended. One subject reported having the perception of continuing stimulation lasting 3 hrs after end of one session. Poor sleep for 2 nights was reported by one subject but was thought to not be related to the stimulation.
Tolerability and feasibility
All enrolled participants completed the trial within the required period of six weeks. Unpleasantness and pain induced by the respective montages and stimulation conditions were rated as mild.
Neurocognitive performance
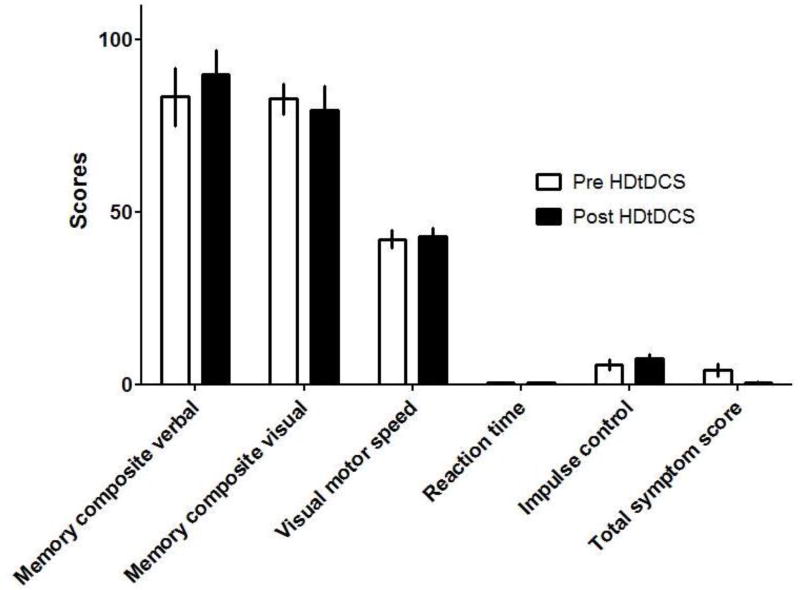
We performed the computerized immediate post concussion assessment and cognitive testing (ImPACT) which is the most widely used concussion assessment tool. The purpose of this test was not to perform a detailed assessment of neurocognitive abilities of the enrolled subjects but to screen for possible adverse effects of repeat multifield HD-tDCS stimulation on aspects of cognition which include verbal and visual memory, visual motor speed, reaction time and impulse control. We did not detect significant score differences within these 5 areas of cognition between the two assessments (fig. 3).
Fig 3.
ImpACT scores before and after 20 sessions of HD-tDCS, applied as outlined in figure 1 and table 1. Columns depict mean scores ± SD in n=5 participants. No statistically significant differences were detected when before and after treatment scores were compared using Student’s t-test.
EEG analysis
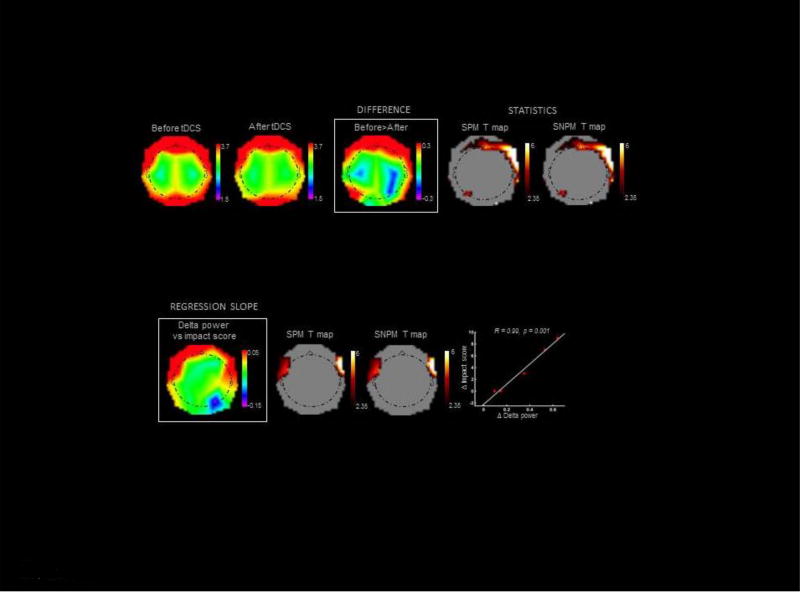
EEG power shifts from low to high oscillatory frequencies are known to reflect increases in neuronal excitability (Steriade et al., 2001). We thus hypothesized that decreases in EEG low frequencies could track long-lasting changes in excitability induced by HD-tDCS. At the group level, our subjects indeed showed decreased delta power over a set of frontal scalp regions several days after HD-tDCS ended, while no regions showed a significant delta increase (Fig. 4, top panel & Table 3). Moreover, Fig 4 (bottom panel) and Table 4 show that the decrease in delta power displayed a trend for a correlation with individual improvements in ImpACT scores after HD-tDCS.
Fig 4. EEG power topographies.
Upper panel: Changes in EEG delta power after HD-tDCS treatment. From left to right: average referenced EEG delta power topographies before (EEG #1) and after (EEG #4) HD-tDCS, differences between the two, and SPM and SNPM T maps. T maps are thresholded for display at uncorrected p<0.05. A rainbow color scale is used for SWA, and a hot color scale for T values. Lower panel: Correlation between changes in EEG delta power and improvements in ImpACT scores after HD-tDCS. From left to right: slope of multiple regression between changes in EEG delta power and improvements in ImpACT scores; SPM and SNPM T maps; and distribution plot for the statistical peaks of correlation analysis. T maps are thresholded for display at uncorrected p<0.05. A rainbow color scale is used for regression slope values, and a hot color scale for T values.
Table 3.
Peaks of significance for differences in delta power before versus after tDCS
| Statistics | Side | Scalp coordinates (x, y) |
T value | P value corrected |
P value uncorrected |
|---|---|---|---|---|---|
| SPM | Right | 60 18 | 16.25 | 0.040c | <0.001 |
| SNPM | Right | 60 18 | 21.55 | 0.031c | 0.031 |
Coordinates are expressed as distance from a midline reference point for scalp analyses.
cluster-level corrected.
Table 4.
Peaks of significance for correlation between change in delta power and change in impact score
| Correlation | Side | Scalp coordinates (x, y) |
R Pearson |
P value corrected |
P value uncorrected |
R Spearman | P value Spearman |
|---|---|---|---|---|---|---|---|
| SPM | Right | 60 18 | 0.99 | 0.089svc | 0.001 | 0.97 | 0.017 |
| SNPM | Left | 60 18 | 0.100v | 0.017 |
Coordinates are expressed as distance from a midline scalp reference point.
whole-brain voxel-level corrected,
small volume corrected
All obtained electrocardiograms were normal.
Discussion
Here we present evidence that extended multiple-field HD-tDCS is feasible, well tolerated and safe in healthy adults when it is applied daily over four brain regions for a total of 20 sessions.
All enrolled subjects completed the 20 stimulation sessions within the required period of 6 weeks. Moreover, our preliminary EEG results suggest that this HD-tDCS procedure may induce long lasting changes in excitability in the human brain. EEG power spectrum showed decreased delta power in frontal areas several days after HD-tDCS sessions ended. Furthermore, we found a trend for correlation between decreased EEG delta power and individual improvements in ImpACT scores after HD-tDCS.
The maximal dose used in our study consisted of two daily cycles of stimulation for 5 sessions. Adverse events were mild and did not differ from those reported in previous studies where only one brain area was stimulated daily for up to 10 sessions.
Our results are in line with previous studies that employed the standard 4 × 1 HD-tDCS set-up (Kuo et al., 2013; Nikolin et al., 2015; Borckardt et al., 2012; Villamar et al., 2013a, b). Only mild and self-resolved adverse effects were reported after the end of each stimulation session.
TDCS dosage is defined by current dosage (measured in amperes), duration of stimulation and electrode montage (size and position of all electrodes). Current density (current dose divided by electrode size) is also an important parameter in considering dosage; especially for defining safety (Nitsche et al., 2007; Miranda et al., 2009). In our trial, the total dose multiplied with duration of stimulation applied within 6 weeks was 2,300 mA × min. In comparison, a traditional tDCS trial with ten 20 min long stimulation sessions at 2 mA over one brain region delivers a total dose of 400 mA × min which is 17.4% of the total dose we administered. The current density in our study was with 13.3 A/m2 (1.5mA / 1.13 cm2) also higher than in traditional tDCS studies which use large sponge electrodes.
The design of the stimulation parameters and length of individual sessions we chose was based on previously published literature. We selected a total current intensity of 1.5 mA/session which is slightly below that used in children with childhood onset schizophrenia. In a study by Mattai et and colleagues (2011), 20 min long tDCS sessions were administered daily over a period of 2 weeks and were found to be safe and well tolerated. Intensity of stimulation in that study was 2 mA but only one area was stimulated.
Our decision to administer daily stimulation sessions and stimulate each network for 20 min is based on findings of other investigators about the duration and quality of after effects of tDCS. Given findings by Paulus (2011) that anodal tDCS applied for more than 26 min will initially cause excitation but later on will cause cortical inhibition, we chose to limit stimulation of each network to 20 min because we wanted to achieve depolarization over the stimulated cortical areas and not inhibition. On the other hand, we did not want to use shorter periods of stimulation because our goal was to elicit longer lasting after-effects. It is known from previous work that short applications (i.e., seconds to few minutes) of anodal/cathodal tDCS result in excitability shifts during stimulation but no after-effects. In contrast, ten minutes or more of stimulation can elicit prolonged after-effects, which can be sustained for over an hour (Ardolino et al., 2005; Nitsche et al., 2000; 2008). The exact duration of effects depends on the targeted cortical area and on the type of variables assessed. For clinical purposes, longer-lasting effects are crucial.
Single-dose tDCS interventions have relatively short-lived after-effects. However, multiple stimulation sessions can induce a significant manipulation in synaptic efficacy (Froc et al., 2000; Monte-Silva et al., 2011). In fact, repeated sessions of tDCS may have cumulative effects associated with greater magnitude and duration of behavioral effects. For example, cathodal tDCS applied over 5 consecutive days is associated with cumulative motor function improvement lasting up to 2 weeks after the end of stimulation. This is an effect which is not observed when sessions are applied weekly (as opposed to daily) (Boggio et al., 2007). Alonzo and colleagues (Alonzo et al., 2012) also reported that daily stimulation achieves superior effects on synaptic plasticity than second daily stimulation. Thus, we aimed to perform 5 daily stimulation sessions without interruptions during each week.
It is currently unknown whether and how maximization and stabilization of the electrophysiologic effects of tDCS can be achieved. The optimal repetition rate and duration to promote tDCS-induced plasticity remains to be determined. In animal experiments, repetition of tDCS during the after-effects of a first stimulation session enhances efficacy (Bindman et al., 1964). On the other hand, repeated plasticity induction may result in antagonistic effects (Siebner et al., 2004).
Monte-Silva and colleagues (Monte-Silva et al., 2011) compared the effects induced by single sessions of cathodal tDCS over the motor cortex to the effects of repetitive stimulation during or after the after-effects of the first stimulation. The results showed that increasing cathodal tDCS duration (1 mA, with no inter-stimulation interval) resulted in longer lasting after-effects, typically over 1 hour (tDCS duration from 9 to 18 min prolonged the after-effects from 60 to 90 min). Interestingly, when the second stimulation was performed during the after-effects of the first, a prolongation and enhancement of tDCS-induced effects for up to 120 min after stimulation was observed. In contrast, when the second session was performed 3 or 24 hours after the first, tDCS effects on cortical excitability were mixed. Thus, there is stimulation timing–dependent plasticity regulation in the human motor cortex. On the days we administered 2 cycles of HD-tDCS, we chose to allow an interval of 2 hrs between stimulation of each network to allow for after-effects to subside. Our finding of decreased delta power in the frontal EEG several days after the end of the HD-tDCS procedure indeed suggests that our experimental procedure was successful to induce long-lasting changes in excitability in human brains.
Although tDCS and HD-tDCS differ in many aspects from other non-invasive neuromodulatory therapies in that they do not induce neuronal action potential and use weak electric currents, there still are safety concerns. If the materials contact the skin, skin irritation may occur; tissue heating may induce skin burning (Palm et al., 2008) – although mild redness is more likely related to local, vasodilatation skin changes rather than skin damage (Fregni et al., 2007). Preclinical work suggests no significant temperature increases for typical tDCS protocols (Datta et al., 2009; Minhas et al., 2010; Nitsche and Paulus, 2000). TDCS has been tested in thousands of subjects worldwide with no evidence of toxic effects to date. A large retrospective study (Poreisz et al., 2007) reviewed adverse effects in 77 healthy subjects and 25 patients who underwent a total of 567 1mA stimulation sessions. Results show that the most common effects were mild tingling sensations (75%), light itching sensation (30%), moderate fatigue (35%), and headache (11.8%); and most of these effects did not differ from those of placebo stimulation. In another study, 164 sessions of stimulation were analyzed. Other studies (Boggio et al., 2007; Ferrucci et al., 2009; Fregni et al., 2006; Loo et al., 2009; Nitsche et al., 2003a, b; Rigonatti et al., 2008; Tadini et al., 2011) also reported only mild and transient side effects. The most severe adverse event ever reported is skin lesions on the site of electrode placement (Palm et al., 2008).
In our trial we excluded subjects with any type of disease and screened before and during the study for epileptiform discharges in the EEG. The appearance of epileptiform discharges would lead to termination of participation of that particular subject. In published literature emphasis is placed on excluding subjects with epilepsy from tDCS trials. However, epileptic seizures have not been observed in a pilot trial with patients with active epilepsy who received tDCS (Fregni et al., 2006) and epileptic seizures have not been a complication of any tDCS or HD-tDCS trial ever conducted. Thus, the risk of tDCS and HD-tDCS inducing a clinical seizure, subclinical seizures or inducing other EEG abnormalities remains a theoretical one.
Although our ultimate intention with prolonged and multifield HD-tDCS is to improve cognitive abilities, the possibility that we might actually compromise cognition with this intensive protocol was a concern and we therefore performed the ImpACT battery before and after completion of the 20 HD-tDCS sessions to screen for a decline in cognitive function. As fig. 3 shows, there is no such indication, as the individual scores in the ImpACT test before and after stimulation did not differ. On the contrary, our preliminary results – with a trend for a correlation between decreased EEG delta power and improvements in ImpACT scores after HD-tDCS - suggest that HD-tDCS may rather have positive cognitive effects.
We believe that demonstrating safety, feasibility and tolerability of this intensive protocol will allow exploration of neuromodulatory potential of prolonged and multifocal HD-tDCS or tDCS. Demonstrating safety of the protocol is essential to justify application of the method for prolonged periods of time to vulnerable populations (e.g. intellectual disabilities, neuropsychiatric disorders with cognitive consequences).
It should be acknowledged that the baseline cortical excitability may be different in children and in people using pharmacotherapy or presenting with neuropsychiatric disorders. Some single-patient studies report that tDCS can induce mania/hypomania in patients with major depression (Arul-Anandam et al., 2010; Baccaro et al., 2010; Brunoni et al., 2011). This issue is likely to interfere with the chosen dosage and may also influence the type and severity of adverse events. Thus, despite successful completion of this trial, great caution should be exercised as we move towards exploring the therapeutic potential of intensive HD-tDCS protocols in vulnerable patient populations.
Acknowledgments
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The project was also supported by the Chunky Monkey Run and other gifts to the Department of Neurology, University of Wisconsin Madison.
References
- Alonzo A, Brassil J, Taylor JL, Martin D, Loo CK. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimulation. 2012;5:208–213. doi: 10.1016/j.brs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. Journal of Physiology. 2005;568:653–663. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arul-Anandam AP, Loo C, Mitchell P. Induction of hypomanic episode with transcranial direct current stimulation. Journal of ECT. 2010;26:68–69. doi: 10.1097/YCT.0b013e3181a744bf. [DOI] [PubMed] [Google Scholar]
- Baccaro A, Brunoni AR, Bensenor IM, Fregni F. Hypomanic episode in unipolar depression during transcranial direct current stimulation. Acta Neuropsychiatrica. 2010;22:316–318. [Google Scholar]
- Barham MP, Enticott PG, Conduit R, Lum JA. Transcranial electrical stimulation during sleep enhances declarative (but not procedural) memory consolidation: Evidence from a meta-analysis. Neuroscience and Biobehavior Reviews. 2016;63:65–77. doi: 10.1016/j.neubiorev.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. Relation between the Size and Form of Potentials Evoked by Sensory Stimulation and the Background Electrical Activity in the Cerebral Cortex of the Rat. Journal of Physiology. 1964a;171:1–25. doi: 10.1113/jphysiol.1964.sp007358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) During Current Flow and (2) in the Production of Long-Lasting after-Effects. Journal of Physiology. 1964b;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restorative Neurology and Neuroscience. 2007;25:123–129. [PubMed] [Google Scholar]
- Boly M, Jones B, Findlay G, Plumley E, Mensen A, Hermann B, Tononi J, Maganti R. Altered sleep homeostasis correlates with cognitive impairment in patients with focal epilepsy. Brain. 2017;140:1026–1040. doi: 10.1093/brain/awx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borckardt JJ, Bikson M, Frohman H, Reeves ST, Datta A, Bansal V, Madan A, Barth K, George MS. A pilot study of the tolerability and effects of high-definition transcranial direct current stimulation (HD-tDCS) on pain perception. Journal of Pain. 2012;13:112–120. doi: 10.1016/j.jpain.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Brunoni AR. Clinical Research with Transcranial Direct Current Stimulation (tDCS): Challenges and Future Directions. Brain Stimulation. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Zanao T, Oliveira J, Bensenor IM, Fregni F. Manic psychosis following transcranial direct current stimulation and sertraline. Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:E4–5. doi: 10.1176/jnp.23.3.jnpe4. [DOI] [PubMed] [Google Scholar]
- Brunye TT, Cantelon J, Holmes A, Taylor HA, Mahoney CR. Mitigating cutaneous sensation differences during tDCS: comparing sham versus low intensity control conditions. Brain Stimulation. 2014;7:832–835. doi: 10.1016/j.brs.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Caparelli-Daquer EM, Zimmermann TJ, Mooshagian E, Parra LC, Rice JK, Datta A, Bikson M, Wassermann EM. A pilot study on effects of 4×1 high-definition tDCS on motor cortex excitability. Conference proceedings, Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Annual Conference. 2012;2012:735–738. doi: 10.1109/EMBC.2012.6346036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappon D, Jahanshahi M, Bisiacchi P. Value and efficacy of transcranial direct current stimulation in the cognitive rehabilitation: a critical review since 2000. Frontiers in Neuroscience. 2016;10:157. doi: 10.3389/fnins.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RE. Application of stochastic processes to participant recruitment in clinical trials. Control Clinical Trials. 2004;25:429–436. doi: 10.1016/j.cct.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical d-c currents on cortical neuronal activity. Experimental Neurology. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- DaSilva AF, Truong DQ, DosSantos MF, Toback RL, Datta A, Bikson M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Frontiers in Neuroanatomy. 2015;9:89. doi: 10.3389/fnana.2015.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimulation. 2009;2:201–207. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. Journal of Neural Engineering. 2011;8:046011. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- Donnell A, Nascimento T, Lawrence M, Gupta V, Zieba T, Truong DQ, Bikson M, Datta A, Bellile E, DaSilva AF. High-definition and non-invasive brain modulation of pain and motor dysfunction in chronic TMD. Brain Stimulation. 2015;8:1085–1092. doi: 10.1016/j.brs.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–275. doi: 10.1016/j.neuroimage.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Salvoro B, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation in severe, drug-resistant major depression. Journal of Affective Disorders. 2009;118:215–219. doi: 10.1016/j.jad.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S, Priori A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. Journal of Cognitive Neurosciences. 2008;20:1687–1697. doi: 10.1162/jocn.2008.20112. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche MA, Rigonatti SP, Pascual-Leone A. Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depression and Anxiety. 2006;23:482–484. doi: 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- Fregni F, Nitsche MA, Loo CK, Brunoni AR, Marangolo P, Leite J, Carvalho S, Bolognini N, Caumo W, Paik NJ, Simis M, Ueda K, Ekhitan H, Luu P, Tucker DM, Tyler WJ, Brunelin J, Datta A, Juan CH, Venkatasubramanian G, Boggio PS, Bikson M. Regulatory Considerations for the Clinical and Research Use of Transcranial Direct Current Stimulation (tDCS): review and recommendations from an expert panel. Clinical Research and Regulatory Affairs. 2015;32:22–35. doi: 10.3109/10601333.2015.980944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology perspectives on the therapeutic potential of rTMS and tDCS. National Clinical Practice in Neurology. 2007;3:383–393. doi: 10.1038/ncpneuro0530. [DOI] [PubMed] [Google Scholar]
- Froc DJ, Chapman CA, Trepel C, Racine RJ. Long-term depression and depotentiation in the sensorimotor cortex of the freely moving rat. Journal of Neuroscience. 2000;20:438–445. doi: 10.1523/JNEUROSCI.20-01-00438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 5. New York, NY: Academic Press; 2015. [Google Scholar]
- Garnett EO, den Ouden D-B. Validating a sham condition for use in high definition transcranial direct current stimulation. Brain Stimulation. 2015;8:551–554. doi: 10.1016/j.brs.2015.01.399. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Friston K. Topological inference for EEG and MEG. The Annals of Applied Statistics. 2010;4:1272–1290. [Google Scholar]
- Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M-F, Nitsche MA. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimulation. 2013;6:644–648. doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Landsness E, Bruno MA, Noirhomme Q, Riedner B, Gosseries O, Schnakers C, Massimini M, Laureys S, Tononim G, Boly M. Electrophysiological correlates of behavioural changes in vigilance in vegetative state and minimally conscious state. Brain. 2011;134:2222–2232. doi: 10.1093/brain/awr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Loo C, Martin D, Pigot M, Arul-Anandam P, Mitchell P, Sachdev P. Transcranial Direct Current Stimulation Priming of Therapeutic Repetitive Transcranial Magnetic Stimulation: A Pilot Study. Journal of ECT. 2009;25:256–260. doi: 10.1097/YCT.0b013e3181a2f87e. [DOI] [PubMed] [Google Scholar]
- Lovell MR, Collins MW, Podell K, Powell J, Maroon J. ImPACT: Immediate post-concussion assessment and cognitive testing. Pittsburgh, PA: NeuroHealth Systems, LLC; 2006. [Google Scholar]
- Mattai A, Miller R, Weisinger B, Greenstein D, Bakalar J, Tossell J, Wassermann EM, Rapoport J, Goglay N. Tolerability of transcranial direct current stimulation in childhood-onset schizophrenia. Brain Stimulation. 2011;4:275–280. doi: 10.1016/j.brs.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzagora AC, Foffani G, Panyavin I, Mordillo-Mateos L, Aguilar J, Onaral B, Oliviero A. Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage. 2010;49:2304–2310. doi: 10.1016/j.neuroimage.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, Bikson M. Electrodes for high-definition transcutaneous DC stimulation for applications in drug delivery and electrotherapy, including tDCS. Journal of Neuroscience Methods. 2010;190:188–197. doi: 10.1016/j.jneumeth.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PC, Faria P, Hallett M. What does the ratio of injected current to electrode area tell us about current density in the brain during tDCS? Clinical Neurophysiology. 2009;120:1183–1887. doi: 10.1016/j.clinph.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte-Silva KK, Kuo MF, Liebetanz D, Paulus W, Nitsche MA. Shaping the optimal repetition interval for cathodal transcranial direct current stimulation (tDCS) Journal of Neurophysiology. 2011;103:1735–1740. doi: 10.1152/jn.00924.2009. [DOI] [PubMed] [Google Scholar]
- Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clinical Translational Science. 2011;4:332–337. doi: 10.1111/j.1752-8062.2011.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Bruno MA, Riedner BA, Boveroux P, Noirhomme Q, Landsness EC, Brichant JF, Phillips C, Massimini M, Laureys S, Tononi G, Boly M. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283–291A. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewenhuys R, Voogd J, vam Heuizen C. The Human Central Nervous System. 3. Springer; Berlin, Heidelberg, New York: 2008. Telencephalon: Neocortex; pp. 491–679. [Google Scholar]
- Nikolin S, Loo CK, Bai S, Dokos S, Martin DM. Focalised stimulation using high definition transcranial direct current stimulation (HD-tDCS) to investigate declarative verbal learning and memory functioning. Neuroimage. 2015;117:11–19. doi: 10.1016/j.neuroimage.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, Boggio PS, Fregni F, Pascual-Leone A. Transcranial direct current stimulation: State of the art 2008. Brain Stimulation. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. Journal of Neurophysiology. 2007;97:3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. Journal of Physiology. 2003a;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clinical Neurophysiology. 2003b;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm U, Keeser D, Schiller C, Fintescu Z, Reisinger E, Nitsche M, Reisinger E, Padberg F. Skin lesions after treatment with transcranial direct current stimulation (tDCS) Brain Stimulation. 2008;1:386–387. doi: 10.1016/j.brs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychological Rehabilitation. 2011;1:1–16. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Research Bullettin. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimulation. 2009;2:241–245. doi: 10.1016/j.brs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular Activities and Evoked Potential Changes during Polarization of Motor Cortex. Journal of Neurophysiology. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Richardson J, Datta A, Dmochowski J, Parra LC, Fridriksson J. Feasibility of using high-definition transcranial direct current stimulation (HD-tDCS) to enhance treatment outcomes in persons with aphasia. Neurorehabilitation. 2015;36:115–126. doi: 10.3233/NRE-141199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigonatti SP, Boggio PS, Myczkowski ML, Otta E, Fiquer JT, Ribeiro RB, Nitsche MA, Pascuall-Leone A, Fregni F. Transcranial direct stimulation and fluoxetine for the treatment of depression. European Psychiatry. 2008;23:74–76. doi: 10.1016/j.eurpsy.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Renja V. Transcranial direct current stimulation: a noninvasive tool to facilitate stroke recovery. Expert Reviews in Medical Devices. 2008;5:759–768. doi: 10.1586/17434440.5.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. Journal of Neuroscience. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. Natural waking and sleep states: a view from inside neocortical neurons. Journal of Neurophysiology. 2001;85:19691985. doi: 10.1152/jn.2001.85.5.1969. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. Journal of Neuroscience. 2009;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadini L, El-Nazer R, Brunoni AR, Williams J, Carvas M, Boggio PS, Priori A, Pascual-Leone A, Fregni F. Cognitive, mood and EEG effects of noninvasive cortical stimulation with weak electrical currents. Journal of ECT. 2011;27:134–140. doi: 10.1097/YCT.0b013e3181e631a8. [DOI] [PubMed] [Google Scholar]
- Varga ET, Terney D, Atkins MD, Nikanorova M, Jeppesen DS, Uldallm P, Hjalgrim H, Beniczky S. Transcranial direct current stimulation in refractory continuous spikes and waves during slow sleep: A controlled study. Epilepsy Research. 2011;97:142–145. doi: 10.1016/j.eplepsyres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Villamar MF, Volz MS, Bikson M, Datta A, DaSilva AF, Fregni F. Technique and considerations in the use of 4×1 ring high-definition transcranial direct current stimulation (HD-tDCS) Journal of Visualized Experiments. 2013a;77:e50309. doi: 10.3791/50309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, Fregni F. et al. Focal modulation of the primary motor cortex in fibromyalgia using 4 × 1-ring high-definition transcranial direct current stimulation (HD-tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. Journal of Pain. 2013b;14:371–383. doi: 10.1016/j.jpain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Young SJ, Bertucco M, Sheehan-Stross R, Sanger TD. Cathodal Transcranial Direct Current Stimulation in Children With Dystonia A Pilot Open-Label Trial. Journal of Child Neurology. 2013;28:1238–1244. doi: 10.1177/0883073812460092. [DOI] [PubMed] [Google Scholar]