Abstract
Background
It is unknown why functional gastrointestinal disorders (FGIDs) overlap and limited information exists on risk factors for those with overlap. Our aim was to estimate the prevalence of combinations of FGIDs including reflux (FGIDs-GER), and evaluate potential risk factors for people with multiple disorders in a representative US community.
Methods
A population-based study was conducted by mailing a valid GI symptom questionnaire to an age- and gender- stratified random sample of residents of Olmsted County, MN. Rome III definitions were used to identify people with FGIDs, and GER was defined by weekly or more frequent heartburn or acid regurgitation. The prevalence of people meeting multiple symptom complexes was estimated. Moreover, potential risk factors for people with multiple disorders were evaluated.
Key Results
A total of 3548 people provided data for each of the necessary symptom questions (mean age: 61 years ± 16, 54% female). Among these 3548 subjects, 2009 (57%) had no FGIDs-GER, 906 (26%) had a pure FGID-GER, 372 (10%) had 2 FGIDs-GER, and 261 (7%) had 3 or more FGIDs-GER. Somatization as assessed by a higher somatic symptom checklist score (OR=3.3, 95% CI [2.7,4.1]) was associated with an increased odds for those with 3 or more FGIDs-GER compared to subjects with a pure FGID-GER adjusting for age and gender.
Conclusions & Inferences
Symptom complex overlap is common rather than rare in the community. GER is an integral symptom complex associated with both upper and lower FGIDs. Somatization is a strong risk factor for multiple FGIDs.
Keywords: Functional GI disorders, gastroesophageal reflux, somatic symptoms, overlap, population-based study
Graphical abstract
Abbreviated abstract: This study showed that the co-existence of upper and lower FGIDs as well as gastroesophageal reflux (GER) symptoms is common in the community and not explained by chance, suggesting a common underlying pathophysiology. Moreover, the higher the somatization score, the more likely the overlap of FGID-GER complexes suggesting a dose-response like effect.
INTRODUCTION
Functional gastrointestinal disorders (FGIDs) are common disorders, and represent a variable combination of chronic or recurrent gastrointestinal (GI) symptoms not explained by known structural or biochemical abnormalities (1–4). Overlap of FGIDs has been widely reported in the literature (5–9). While such overlap with other GI disorders and/or extra-intestinal disorders might simply reflect a chance association due to the high prevalence of the disorders, several studies have confirmed that the overlap of FGIDs occurs more frequently than expected, indicating this is not likely a chance event (8–10). Moreover, patients who have two or more FGIDs are likely to have more frequent clinic visits and more severe clinical manifestations than those with one FGID (9–11). In addition, studies have shown that gastroesophageal reflux (GER) symptoms commonly co-exist in a considerable proportion of patients with upper or lower FGIDs (8–10, 12, 13). In a meta-analysis for the coexistence of IBS and GER, Lovell and Ford reported that the odd ratio of GER in individuals with IBS was four-fold that of individuals without IBS (12). Moreover, in a systematic review, Gerson et al. showed that dyspeptic symptoms were present in 38% of subjects with GER. It is conceivable that the overlap between FGIDs including GER is not coincidence, but rather reflects a common underlying pathophysiology. For example, somatization and psychological disorders frequently coexist with these functional disorders (9, 14). However, the intimate interrelationship between GER and FGIDs is often not considered when assessing risk factors (and is not part of the Rome criteria). Although the Rome criteria have been conceptually developed to maximise homogeneity within each functional gut disorder (supporting the conduct of clinical trials and pathways to drug approval), and while GER is not traditionally considered an FGID, physicians commonly encounter patients with multiple upper and lower GI symptoms in the clinic setting. Furthermore, the majority of studies assessing overlap of FGIDs have been focused on the association of only one or two FGIDs (most notably IBS or functional dyspepsia), and there are few data on risk factors for overlap of these conditions.
Because little is still known about the overlap of multiple FGIDs in the community, understanding the role of risk factors in overlap groups could expose differences in underlying pathophysiology, which has implications in terms of the development of new targeted therapies. Thus, we aimed to estimate the prevalence of combinations of FGIDs including gastroesophageal reflux (FGIDs-GER), and evaluate the potential risk factors including somatization for people with multiple disorders in a representative US community.
MATERIALS AND METHODS
A population-based study was conducted with subjects selected from an age- and gender-stratified community random sample sent a GI symptom survey in 2008-2009 which included validated gastrointestinal symptom questions. This study was approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center.
Subjects
The study milieu has been described in prior publications, but in brief (15–18) the Olmsted County, Minnesota, population comprises approximately 120,000 people of which 89% are white; socio-demographically, this community closely resembles the U.S. white population and results can be generally extrapolated to that population segment. Mayo Clinic is the major provider of medical care (16) and the Rochester Epidemiology Project records linkage system provides essentially an enumeration of the population allowing random samples to be identified and selected (16). These data resources have been utilized in a series of investigations into the epidemiology of functional GI disorders (9, 19–22). As approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center, we used this system to draw a series of random samples of Olmsted County residents stratified by age (5-year intervals between 20 and 94 years) and sex (equal numbers of men and women).
Survey Methods
For the current study, a previously assembled age- and gender-stratified random sample (n=8006) (19–21, 23) of Olmsted County, Minnesota, residents (total=8006) were mailed validated self-report gastrointestinal symptom questionnaires (Talley Bowel Disease Questionnaire [BDQ]) in 2008 and 2009. The original BDQ (21) was designed to measure GI symptoms experienced over the previous year and to elicit a brief prior medical history with respect to somatic symptoms. The questionnaire has been modified over time. The original BDQ and modified versions have been shown to be reliable and valid measures of GI symptoms in previous studies (24, 25) and the current version of the BDQ has also been shown to be a reliable and valid measurement of bowel symptoms (26). The current BDQ incorporated 27 gastrointestinal symptoms and the somatization symptom checklist (SSC). The SSC included consists of questions about relevant symptoms and illnesses (namely headaches, backaches, insomnia, general stiffness, dizziness, and weakness), but excludes any GI items, and subjects are instructed to indicate how often each occurred (0=not a problem to 4=occurs daily) and how bothersome each was (0=not a problem to 4=extremely bothersome when occurs) during the past year, using separate 5-point scales. Total SSC scores were calculated by the average of often and bothersome of each SSC score. The SSC score has been shown to be a reliable and valid measure of somatic complaints (27, 28). In a population-based study in Olmsted County (n=461), the overall SSC score positively and significantly correlated with all SCL-90-R scales and most strongly with somatization (Pearson r=0.60, p<0.001).(27)
Priming letters were initially mailed to highlight that a GI questionnaire would be sent. Later, an explanatory letter accompanied the survey which was mailed to a total of 8006 Olmsted County residents. Reminder letters were mailed at weeks 3 to 6 after the initial mailing to non-responders. Subjects who indicated at any point that they did not wish to complete the survey were not contacted further. Otherwise, non-responders were contacted by telephone at week 9 to request their participation and verify their residence within the County. A completed questionnaire was returned by 3831 subjects, giving a response rate of 48%. A total of 50% of females responded and 45% of males, with the mean (±SD) age of respondents being 61 (±16) and non-respondents, 53 (± 18) years. Using a logistic regression model for response (no/yes), females and older subjects had slightly greater odds for response (OR [95%CI], females relative to males=1.20[1.10, 1.31], OR [95%CI] per 10 years of age=1.30 [1.26, 1.33]). We have demonstrated the absence of any major non-response bias in Olmsted County returning GI questionnaires (29). In this same population sample we have previously reported the associations of PPI use and FGIDs (30).
Definition of Symptom Categories
Subjects were classified into a priori symptom groups based on their responses to the questionnaires, which recorded their symptoms over the past three months. Subjects could have more than one disorder. Slight modifications of the Rome III criteria were used to categorize subjects.
Symptom Categories
Gastroesophageal reflux (GER)
This was defined by one or more of the following traditional reflux symptoms (31);
Heartburn (retrosternal burning pain), at least once a week 1 day/week in the last 3 months.
Acid regurgitation, at least 1 day/week in the last 3 months.
Functional dyspepsia
This was defined by symptom criteria of Rome III,(2) with one or more of following (for more than 6 months); 1) unable to finish a regular size meal more than once a week, 2) feels uncomfortably full after regular size meal more than once a week, 3) pain or burning in the upper middle abdomen (epigastrium) at least once a week (above the umbilicus).
Functional Bloating
Bloating was defined by Rome III criteria (1), recurrent feeling of bloating in abdomen or visible distension, at least 3 days/month in the last 3 months.
Irritable bowel syndrome (IBS)
IBS was defined by Rome III criteria (1), namely pain or discomfort at least 2 or 3 days per month and 2 or more of the following at least sometimes; 1) pain relieved by bowel movements (BMs), 2) change in frequency (more or fewer), 3) change in consistency (looser or harder).
Functional Constipation (FC)
FC was defined by Rome III criteria (1), all 3 criteria below had to be met:
Two or more of the following: 1) Straining during at least 25% of defecations; 2) Lumpy or hard stools in at least 25% of defecations; 3) Sensation of incomplete evacuation for at least 25% of defecations; 4) Sensation of anorectal obstruction/blockage for at least 25% of defecations; 5) Manual maneuvers to facilitate at least 25% of defecations; 6) Fewer than three defecations per week.
Loose stools rarely present without the use of laxatives
Insufficient criteria for irritable bowel syndrome
Functional diarrhea (FD)
FD was defined by Rome III criteria, (1) loose or watery stools without pain (occurring in at least 75% of stools) except if loose stools were due to laxative use. Criterion fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis.
Pure or overlap of FGID-GER complexes
Using responses from the bowel disease questionnaire, a pure FGID-GER (that met only one of the criteria for GER, dyspepsia, IBS, bloating, constipation, or diarrhea), 2 FGIDs-GER (that had any 2 way combinations of GER, dyspepsia, IBS, bloating, constipation, or diarrhea), or 3 or more FGIDs-GER (that had any 3 way combinations of GER, dyspepsia, IBS, bloating, constipation, or diarrhea) were categorized.
Statistical analysis
The associations of sociodemographic features, SSC scores, medication use, and physician visits with multiple overlap of FGIDs-GER (defined as a pure FGID-GER, 2 FGIDs-GER, or 3 or more FGIDs-GER) were evaluated using polychotomous logistic regression models. The odds ratios (OR) for overlap (and 95% confidence intervals) were estimated from the coefficients (and their standard errors) obtained in the polychotomous logistic regression models. A p-value (two-tailed) of <0.05 was considered statistically significant. All analyses utilized the SAS® statistical analysis package (SAS Institute Inc., Cary, NC).
RESULTS
Prevalence of each FGID and overlap of FGIDs-GER
Of the 3831 responders, 3548 subjects provided data for each of the necessary symptom questions for this study. The mean age of the eligible subjects was 61 years and 54% were female. Figure 1 shows the age- and sex- adjusted prevalence of each FGID-GER symptom complexes in a community.
Figure 1.
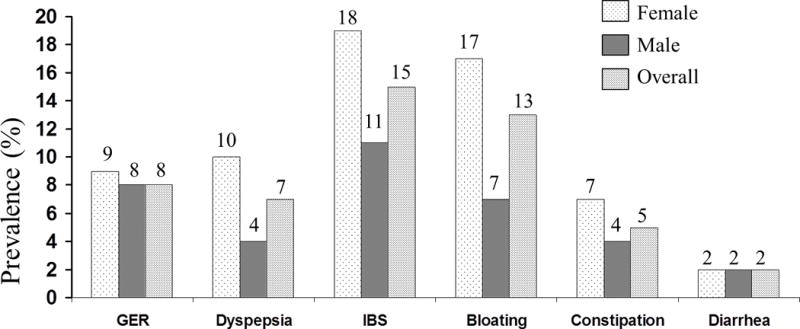
The age-and gender- adjusted prevalence of each FGID and GER.
The overall age-and sex- adjusted prevalence of each syndrome in the community were as follows; 8% had GER, 7% had dyspepsia, 15% had IBS, 13% had bloating, 5% had constipation, and 2% had diarrhea. Among these 3548 subjects, 2009 (57%) had no FGIDs-GER, 906 (26%) had a pure FGID-GER, 372 (10%) had 2 FGIDs-GER, and 261 (7%) had 3 or more FGIDs-GER (Figure 2). Table 1 shows the clinical features of subgroups according to the FGIDs-GER combination status. Notably, subjects reporting combinations of FGIDs-GER were younger, more likely to be of female gender, have higher SSC scores, and report more physician visits relative to subjects without any FGID-GER. Especially, subjects with 3 or more FGIDs-GER had the highest SSC scores, the most physician visits, and the highest proportion of medication usage, including proton pump inhibitors, calcium channel blockers, antispasmodic agents, antidepressants, or narcotics.
Figure 2.
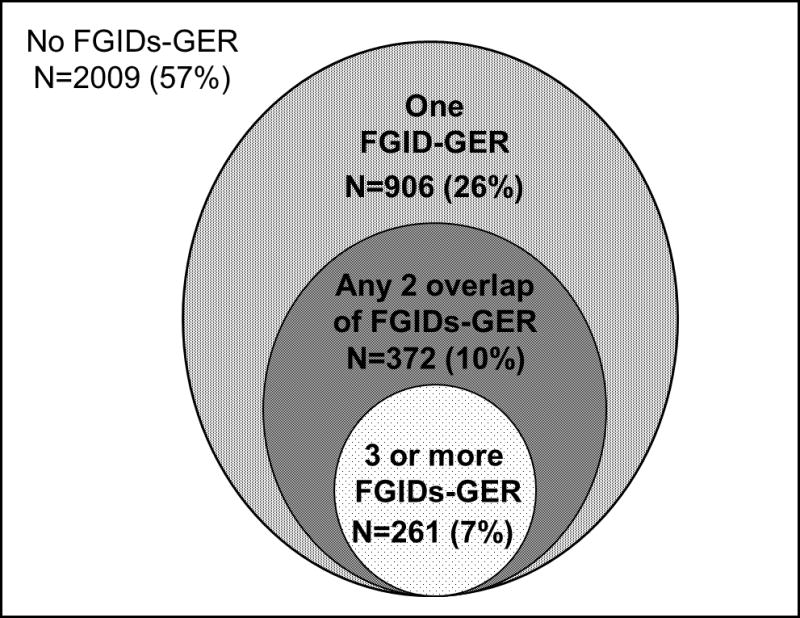
Distribution of FGID-GER complexes. FGID- functional gastrointestinal disorder. GER –gastroesophageal reflux symptoms.
Table 1.
Clinical features of subgroups according to FGID-GER combinations.
| 3 or more FGIDs-GER n = 261 |
2 FGIDs-GER n = 372 |
One FGID-GER n = 906 |
No FGIDs-GER n = 2009 |
|
|---|---|---|---|---|
| Age, mean ± SD (yr) | 58 ± 16 | 58 ± 16 | 61 ± 16 | 62 ± 15 |
| Male gender | 66 (25%) | 111 (30%) | 383 (42%) | 1064 (53%) |
| Female gender | 195 (75%) | 261 (70%) | 523 (58%) | 945 (47%) |
| Visiting a physician >5 times + | 64 (25%) | 60 (16%) | 111 (12%) | 134 (7%) |
| Medication usage | ||||
| Proton pump inhibitor use | 112 (43%) | 138 (38%) | 197 (22%) | 273 (14%) |
| Calcium channel blocker use | 18 (7%) | 23 (6%) | 52 (6%) | 113 (6%) |
| Antispasmodic use | 18 (7%) | 10 (3%) | 13 (1%) | 14 (1%) |
| Antidepressant use | 78 (30%) | 99 (27%) | 140 (16%) | 197 (10%) |
| Narcotic use | 54 (21%) | 72 (20%) | 121 (14%) | 168 (8%) |
Somatization score in combination of FGIDs-GER
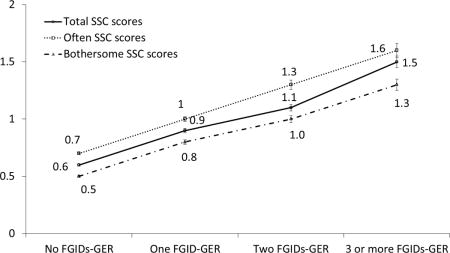
Table 2 shows the SSC score according to the number of FGID-GER complexes. In subjects with 3 or more FGIDs-GER, the mean total SSC score was 1.5 (± 0.8), that is, the highest score among the groups, and the means of the individual SSC scores were also the highest. For all individual SSC items, the more subjects having FGIDs-GER, the greater the SSC scores. Figure 3 shows the mean scores of often, bothersome, and total SSC scores according to FGIDs-GER status. The mean scores for often, bothersome, and total SSC scores increased stepwise with the number of FGIDs indicating a dose-response like relationship.
Table 2.
The mean score of individual somatic complaints from the somatic symptom checklist (SSC) in subjects with FGIDs.
| 3 or more FGIDs-GER n = 261 |
2 FGIDs-GER n = 372 |
One FGID-GER n = 906 |
No FGIDs-GER n = 2009 |
|
|---|---|---|---|---|
| SSC score, total | 1.5 ± 0.8 | 1.1 ± 0.6 | 0.9 ± 0.6 | 0.6 ± 0.5 |
| Individual somatic complaints | ||||
| Backache | 1.8 ± 1.3 | 1.5 ± 1.2 | 1.2 ± 1.1 | 0.9 ± 1.0 |
| Headache | 1.3 ± 1.2 | 1.0 ± 1.0 | 0.7 ± 0.9 | 0.5 ± 0.7 |
| Insomnia | 1.9 ± 1.3 | 1.4 ± 1.1 | 1.1 ± 1.1 | 0.8 ± 0.9 |
| General stiffness | 2.0 ± 1.4 | 1.7 ± 1.2 | 1.4 ± 1.2 | 1.0 ± 1.1 |
| Dizziness | 0.8 ± 1.1 | 0.5 ± 0.9 | 0.4 ± 0.8 | 0.2 ± 0.5 |
| Weakness | 0.9 ± 1.2 | 0.6 ± 1.0 | 0.5 ± 0.9 | 0.2 ± 0.6 |
Figure 3.
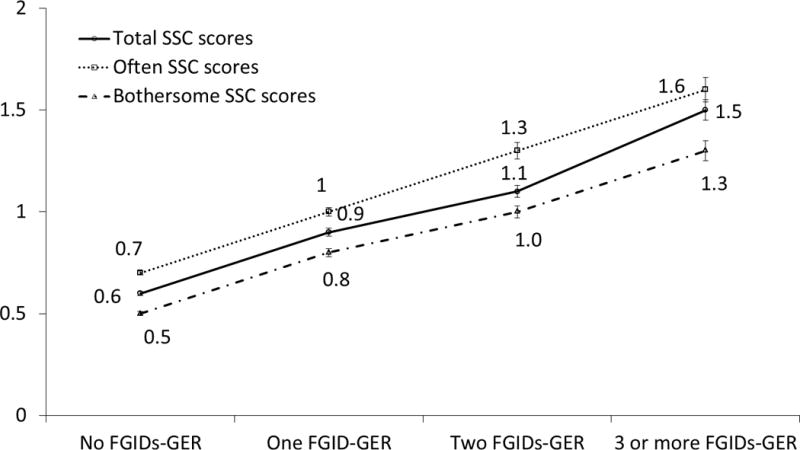
Somatic Symptom Checklist (SSC) scores in relation to the number of concurrent functional gastrointestinal disorders (FGIDs) (Mean ± S.E.). FGIDs-GER, functional gastrointestinal disorders and gastroesophageal symptom complexes.
Potential risk factors for combination of FGIDs-GER
Table 3 summarizes overall OR of potential risk factors for combinations of FGID-GER complexes compared to those with one FGID-GER or without FGIDs-GER. Younger age, female gender, and frequent physician visits were all associated with an increased odds for those with 3 or more FGIDs-GER and separately 2 FGIDs-GER compared to subjects with a pure FGID-GER. Notably, those with more FGID-GER complexes had higher odds than that those with lesser numbers of FGIDs-GER. Further, medication usage (including proton pump inhibitor (PPI), antispasmodic use, antidepressants, and narcotics), was also associated with an increased odds for those with three or more FGIDs-GER compared to a pure FGID-GER or no FGIDs-GER. The comparison between a pure FGID-GER and no FGIDs-GER is summarized in supplemental table 1. Since the GER symptom group was likely to be heterogeneous, we further assessed the potential risk factors for pure GER, a pure FGID, GER with 2 or more FGIDs, or 3 or more FGID without GER (supplemental table 1). Even though GER was separated, the overall findings were similar.
Table 3.
Multivariate comparison of factors associated with 3 or more FGIDs-GER, 2 FGIDs-GER, or one FGID-GER among residents of Olmsted County, Minnesota (Odd Ratios, 95% confidence intervals)
| Variables | 3 or more FGIDs-GER | 2 FGIDs-GER | ||
|---|---|---|---|---|
| vs. 1 FGID-GER | vs. no FGIDs-GER | vs. 1 FGID-GER | vs. no FGIDs-GER | |
| Age per 10 years | 0.86 (0.78, 0.95) | 0.83 (0.76, 0.91) | 0.87 (0.80, 0.95) | 0.85 (0.78, 0.92) |
| Female vs. Male (reference) | 2.04 (1.46, 2.85) | 3.01 (2.19, 4.13) | 1.52 (1.15, 2.00) | 2.26 (1.76, 2.92) |
| Visiting a physician > 5 times | 2.00 (1.35, 2.96) | 3.43 (2.33, 5.03) | 1.22 (0.84, 1.78) | 2.06 (1.43, 2.97) |
| PPI use | 2.47 (1.80, 3.39) | 4.15 (3.05, 5.64) | 2.11 (1.60, 2.79) | 3.56 (2.72, 4.65) |
| Calcium channel blocker use | 1.01 (0.54, 1.91) | 0.70 (0.38, 1.29) | 1.10 (0.63, 1.90) | 0.80 (0.47, 1.35) |
| Antispasmodic use | 3.68 (1.67, 8.11) | 5.59 (2.48, 12.58) | 1.34 (0.56, 3.23) | 1.86 (0.76, 4.56) |
| Antidepressant use | 1.42 (0.98, 2.04) | 1.85 (1.30, 2.62) | 1.60 (1.16, 2.20) | 2.10 (1.56, 2.83) |
| Narcotic use | 1.04 (0.69, 1.56) | 2.74 (1.94, 3.86) | 1.26 (0.89, 1.79) | 1.71 (1.22, 2.40) |
FGID, functional gastrointestinal disorders; GER, gastroesophageal reflux; PPI, proton pump inhibitors.
Relationship between the number of FGIDs-GER and SSC scores
Table 4 shows the odds ratio for total and each SSC score for 3 or more FGIDs-GER and 2 FGIDs-GER, compared to those not meeting criteria for any FGID-GER. The highest total SSC scores (OR 8.5; 95% CI 6.9–10.6) was observed in patients with three or more FGIDs-GER compared with those not meeting criteria for any FGID-GER. Notably, the odds of each individual SSC score increased stepwise according to having more FGID-GER combinations, supporting a dose-response relationship.
Table 4.
Odds ratio of somatization score and each items for subjects with 3 or more FGIDs-GER, 2 FGIDs-GER, vs. one FGID-GER or controls
| Variables | 3 or more FGIDs-GER | 2 FGIDs-GER | ||
|---|---|---|---|---|
| vs. 1 FGID-GER | vs. no FGIDs-GER | vs. 1 FGID-GER | vs. no FGIDs-GER | |
| SSC score, total | 3.32 (2.69, 4.09) | 8.52 (6.86, 10.59) | 1.79 (1.49, 2.16) | 4.60 (3.80, 5.57) |
| Often Score | 2.98 (2.46, 3.61) | 6.57 (5.40, 7.98) | 1.65 (1.40, 1.95) | 3.64 (3.07, 4.30) |
| Bothersome Score | 2.99 (2.44, 3.68) | 7.47 (6.03, 9.25) | 1.78 (1.47, 2.15) | 4.43 (3.65, 5.38) |
| Individual somatic complaints | ||||
| Backache | 1.58 (1.40, 1.78) | 2.11 (1.88, 2.37) | 1.29 (1.16, 1.43) | 1.72 (1.56, 1.90) |
| Headache | 1.74 (1.51, 2.01) | 2.44 (2.12, 2.81) | 1.28 (1.12, 1.47) | 1.79 (1.58, 2.04) |
| Insomnia | 1.70 (1.50, 1.93) | 2.30 (2.04, 2.59) | 1.21 (1.08, 1.35) | 1.64 (1.47, 1.81) |
| General stiffness | 1.59 (1.41, 1.79) | 2.18 (1.94, 2.45) | 1.31 (1.18, 1.46) | 1.80 (1.64, 1.99) |
| Dizziness | 1.69 (1.46, 1.95) | 2.67 (2.30, 3.10) | 1.19 (1.02, 1.37) | 1.88 (1.62, 2.18) |
| Weakness | 1.63 (1.43, 1.85) | 2.63 (2.30, 3.01) | 1.26 (1.12, 1.43) | 2.04 (1.79, 2.32) |
OR’s adjusted for age per 10 years, and female gender; FGID, functional gastrointestinal disorders; GER, gastroesophageal reflux; SSC, somatic symptom checklist score
DISCUSSION
In this study, we found significant proportions of people in the community experienced a combination of FGIDs-GER symptoms; the overall proportion reporting two combination of FGIDs-GER was estimated to be 10% and the proportion of 3 or more FGIDs-GER was 7% in this representative US community. We observed that younger age, female gender, higher somatic symptom score, more physician visits and more medication usage were significantly associated with increased numbers of FGID-GER complexes relative to subjects free of these FGID-GER symptom complexes. Of interest, people who have more FGID-GER complexes were significantly more likely to also report features traditionally attributed to somatization. Overall, those with a high somatic symptom score were 8.5 fold more likely to have three or more FGIDs and GER, compared to those without any FGID-GER, and a dose-response like relationship was observed. The strong and consistent associations, and dose-response, would suggest a strong relationship between FGID-GER complexes and somatization.
While it has been established patients with FGIDs seeking health care commonly present with other bowel symptoms and/or extraintestinal complaints, studies have generally been limited by failing to consider a broad range of FGIDs and GER symptoms together (5, 10, 12). The majority of studies evaluating the overlap of FGIDs have focused on only one or two FGID combinations, most notably IBS and dyspepsia. A striking observation in the present study is the strong link between FGIDs and reflux symptoms that is not explained by chance and suggests common pathophysiological pathways may exist. GER symptoms are conceptualised to arise in many cases from increased transient lower esophageal sphincter relaxations that promote proximal esophageal acid reflux. Functional dyspepsia at least in a subset is linked to failure of fundic relaxation (fundic disaccommodation), and recently it has been observed in patients with GER that gastric disaccommodation may induce increased TLESRs after meals suggesting a possible mechanism linking GER and functional dyspepsia (32). On the other hand, increased bacterial fermentation in the colon secondary to dysbiosis has been speculated to be important in symptom generation in IBS (hence the benefit of a low FODMAP diet), and colonic fermentation of indigestible carbohydrates has been demonstrated to increase TLESRs in one study again suggesting a possible pathophysiological link (33). If GER is a fundamental pathophysiological feature of selected FGIDs which will require more experimental work to confirm, then exclusion of those with reflux symptoms from clinical trials may be in error. In functional dyspepsia, exclusion of those with coexistent reflux symptoms was observed to result in no benefit over placebo with the prokinetic itopride, compared with including this overlap group where positive findings were observed (34, 35). It is important to note that subjects with GER symptoms in the current study likely represent a mixed population including some with functional heartburn or oesophageal hypersensitivity and others with pathological acid reflux.
The Rome criteria for FGIDs are largely mutually exclusive in terms of defining each FGID that for the purposes of research aims to promote homogeneity for clinical trial enrolment (1–3). However, in the real world, physicians commonly encounter patients with multiple unexplained gut and extraintestinal symptoms. Several studies have demonstrated that IBS or dyspepsia, as representative FGIDs, commonly coexist with other distinct disorders, including fibromyalgia, chronic fatigue syndrome, temporomandibular joint disorder, chronic pelvic pain and interstitial cystitis more often than expected by chance (14, 36–39). We found that more than half of subjects who had FGIDs-GER symptoms complained of 2 or more different syndromes in a representative community sample. In FGIDs, there are data implicating the gut may drive extraintestinal symptoms via the response to intestinal inflammation (gut-brain disorders). In functional dyspepsia for example, excess duodenal eosinophilia has been observed and independently confirmed (40, 41). Further, in H. pylori negative functional dyspepsia circulating homing T cells and elevated cytokine levels including TNF alpha have been reported, and correlated with delayed gastric emptying, providing further evidence of intestinal inflammation driving upper gut dysfunction (42). In IBS, similarly elevated circulating TNF-alpha levels have been observed that correlated to anxiety, implicating gut inflammation in driving psychological distress in IBS (a gut-brain syndrome) (43). While common gut mechanisms may account for some cases with both upper and lower FGIDs such as subtle intestinal inflammation that is perhaps more extensive in those with more complaints (44), an alternative explanation is underlying somatization, possibly a brain-gut disorder and manifestation of visceral hypersensitivity that accounts for a substantial proportion of cases (45–47). Longitudinal epidemiological data suggest in FGIDs that about half may begin with psychological distress first then gut symptoms develop (implicating a brain-gut syndrome) while the other half may commence with gut symptoms and then psychological distress manifests (a gut-brain syndrome) (48).
We found that higher somatization scores are associated with the multiple overlap of FGIDs-GER, compared to a pure FGID-GER or no FGIDs-GER. Further, this association was more evident in those with the greatest overlap. Increased visceral perception or visceral hypersensitivity has been suggested as a unifying pathophysiological mechanism that underlies several FGIDs. Independent studies have clearly demonstrated that patients with IBS or dyspepsia have increased sensitivity to visceral distension (49–51). Further, Corsetti et al. (51) showed that first perception of gastric distention was significantly lower in patients with both FD and IBS, compared to patients with FD only. Other studies have shown that patients with IBS can have somatic hypersensitivity (52, 53). Calderella et al. (52) showed that pain thresholds at the subcutis and muscle level were significantly lower in IBS patients than in normal subjects. Thus, increased sensitivity to perception may be one of the possible common mechanisms in those with overlapping FGID-GER complexes and result in a high somatization score. Further, altered brain activation in response to visceral or somatic pain stimuli have been demonstrated in IBS, FD, and other extraintestinal somatization disorders including fibromyalgia, compared to healthy controls (46, 54–56). Consistent with these observations are the findings that among those with gut and extraintestinal manifestations, brain areas related to sensory and affective processing regions are commonly activated by noxious stimuli (46, 54, 55). Our population-based data support the concept that a brain-gut disorder (as identified by somatization) characterises a substantial proportion of those with FGIDs and is likely often integral to the disease experience. Recently, Pinto-Sanchez et al. (57) demonstrated that the prevalence of anxiety and depression increased in a stepwise manner with the number of co-existing FGIDs and/or frequency and/or severity of gastrointestinal symptoms. Other studies have shown an association between somatization and true psychiatric disorders including anxiety and depression (58, 59). The increased prevalence of somatization in multiple combinations in FGIDs may induce more health care seeking behaviour, compared to pure or no FGIDs, but also may suggest an underlying role for circulating pro-inflammatory factors such a homing small intestinal T cells or cytokines in some cases from an underlying subtle intestinal inflammation leading to FGID symptoms (60).
The present study showed that demographic characteristics of FGIDs including younger age, and being female were more likely to be associated with overlap or having more than one FGID, compared to subjects with only one FGID or controls. Gender differences in some FGIDs, most notably in IBS, are well established (57, 61, 62). Other unexplained disorders including fibromyalgia and chronic fatigue syndrome are also more prevalent in women than in men. Notably, most of FGIDs, especially IBS, and other extraintestinal somatization disorders are more prevalent in the reproductive years, between puberty and menopause, a finding yet to be adequately explained (4).
Even though we applied modified criteria from Rome III criteria for FGIDs, it was reassuring that we observed the overall age- and gender adjusted prevalence of GER was 8%, dyspepsia 7%, IBS 15%, bloating 13%, constipation 5%, and diarrhea 2% in a representative community sample, all prevalence rates very similar to prior studies in Olmsted County (20, 27, 63) and elsewhere (4, 64). This suggests selection bias is unlikely to account for our findings. Further responders were similar to non-responders in this study. In other research from Olmsted County, we have shown response bias is extremely low risk in responders to the BDQ (29). The strengths of the current study include the investigation of a random community sample. We avoided just studying health care seeking subjects which should have minimized or avoided selection bias. Symptom based criteria of FGIDs are well accepted, and a carefully validated questionnaire was applied. Limitations of our work include the lack of detail about psychological and psychiatric conditions including depression or anxiety status. Also, we cannot conclude that there is a definite causal association between symptom-based GI disorders and somatization disorders because of the inherent difficulties in a cross-sectional study design. A possible additional limitation was a lack of clinical detail regarding structural disease, although in most cases we would expect investigations to be negative or normal as others have confirmed (65). Despite these limitations, this study presents important characteristics of the overlap of multiple FGIDS group in a community. While our data may not be generalized to the whole U.S. population because the racial composition of this community is predominantly white, and while the prevalence of GI symptom complexes may vary by ethnic group, at a minimum our data are generalizable to Caucasians across the United States.
In summary, this study provides strong evidence of the co-existence of multiple FGIDs with GER in a community. Our results also demonstrate that overlap of FGIDs is common rather than rare in the community and is not explained by chance. The findings support common pathophysiological pathways in the FGIDs and GER. Higher somatization scores, that is more severe and frequent somatic symptoms, are a risk factor for multiple FGID-GER complexes. Further studies including large, prospective, and longitudinal approaches comprehensively assessing gut and extraintestinal symptoms are warranted. We conclude that symptom complex overlap is common in the community and multiple somatic symptoms are a major risk factor for overlap of FGIDs and GER.
Supplementary Material
Key Points.
This study evaluated the epidemiology of co-existence of upper and lower FGIDs in the general population, to identify potential risk factors as the underlying pathophysiology that explains co-existent FGIDs is poorly understood.
We found that the co-existence of upper and lower FGIDs as well as gastroesophageal reflux (GER) symptoms is common in the community and not explained by chance, suggesting a common underlying pathophysiology.
Younger age, female gender, and higher somatization score are important risk factors for overlapping FGID-GER complexes.
The higher the somatization score, the more likely the overlap of FGID-GER complexes suggesting a dose-response like effect.
Acknowledgments
The authors wish to thank Lori R. Anderson for her assistance in the preparation of the manuscript.
Funding
This study was made possible in part using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01- AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Abbreviations
- BDQ
Bowel Disease Questionnaire
- GERD
gastroesophageal reflux disease
- PPI
proton pump inhibitors
- REP
Rochester Epidemiology Project
- SSC
Somatic Symptom Checklist
Footnotes
Disclosures
Dr. Talley and Mayo Clinic have licensed the Talley Bowel Disease Questionnaire.
Specific Author Contributions
Rok Seon Choung, Alan R. Zinsmeister. Richard Locke III, and Nicholas J. Talley participated in the design, analysis, and writing of the manuscript. Alan R. Zinsmeister and Cathy D. Schleck provided the statistical analysis.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Choung RS, Locke GR., 3rd Epidemiology of IBS. Gastroenterol Clin North Am. 2011;40:1–10. doi: 10.1016/j.gtc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy TM, Jones RH, Hungin AP, O’Flanagan H, Kelly P. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut. 1998;43:770–774. doi: 10.1136/gut.43.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung HK, Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Diarrhea-predominant irritable bowel syndrome is associated with diverticular disease: a population-based study. Am J Gastroenterol. 2010;105:652–661. doi: 10.1038/ajg.2009.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen S, Jensen TH, Henriksen SL, et al. Overlap of symptoms of gastroesophageal reflux disease, dyspepsia and irritable bowel syndrome in the general population. Scandinavian journal of gastroenterology. 2015;50:162–169. doi: 10.3109/00365521.2014.983157. [DOI] [PubMed] [Google Scholar]
- 8.Jung HK, Halder S, McNally M, et al. Overlap of gastro-oesophageal reflux disease and irritable bowel syndrome: prevalence and risk factors in the general population. Aliment Pharmacol Ther. 2007;26:453–461. doi: 10.1111/j.1365-2036.2007.03366.x. [DOI] [PubMed] [Google Scholar]
- 9.Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Overlap of dyspepsia and gastroesophageal reflux in the general population: one disease or distinct entities? Neurogastroenterol Motil. 2012;24:229–234, e106. doi: 10.1111/j.1365-2982.2011.01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaji M, Fujiwara Y, Shiba M, et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 2010;25:1151–1156. doi: 10.1111/j.1440-1746.2010.06249.x. [DOI] [PubMed] [Google Scholar]
- 11.Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Characteristics of functional bowel disorder patients: a cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther. 2014;39:312–321. doi: 10.1111/apt.12573. [DOI] [PubMed] [Google Scholar]
- 12.Lovell RM, Ford AC. Prevalence of gastro-esophageal reflux-type symptoms in individuals with irritable bowel syndrome in the community: a meta-analysis. Am J Gastroenterol. 2012;107:1793–1801. doi: 10.1038/ajg.2012.336. quiz 1802. [DOI] [PubMed] [Google Scholar]
- 13.Gerson LB, Kahrilas PJ, Fass R. Insights into gastroesophageal reflux disease-associated dyspeptic symptoms. Clin Gastroenterol Hepatol. 2011;9:824–833. doi: 10.1016/j.cgh.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Kim SE, Chang L. Overlap between functional GI disorders and other functional syndromes: what are the underlying mechanisms? Neurogastroenterol Motil. 2012;24:895–913. doi: 10.1111/j.1365-2982.2012.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurland LT, Molgaard CA. The patient record in epidemiology. Sci Am. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Int J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 20.Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ., 3rd Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–934. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 21.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 22.Choung RS, Locke GR, 3rd, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Alternating bowel pattern: what do people mean? Aliment Pharmacol Ther. 2006;23:1749–1755. doi: 10.1111/j.1365-2036.2006.02953.x. [DOI] [PubMed] [Google Scholar]
- 23.Choung RS, Locke GR, 3rd, Rey E, et al. Factors associated with persistent and nonpersistent chronic constipation, over 20 years. Clin Gastroenterol Hepatol. 2012;10:494–500. doi: 10.1016/j.cgh.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talley NJ, Phillips SF, Melton J, 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Annals of internal medicine. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 25.Halder SL, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Melton LJ, 3rd, Talley NJ. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology. 2007;133:799–807. doi: 10.1053/j.gastro.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Rey E, Locke GR, 3rd, Jung HK, et al. Measurement of abdominal symptoms by validated questionnaire: a 3-month recall timeframe as recommended by Rome III is not superior to a 1-year recall timeframe. Aliment Pharmacol Ther. 2010;31:1237–1247. doi: 10.1111/j.1365-2036.2010.04288.x. [DOI] [PubMed] [Google Scholar]
- 27.Choung RS, Locke GR, 3rd, Zinsmeister AR, Schleck CD, Talley NJ. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol. 2009;104:1772–1779. doi: 10.1038/ajg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attanasio V, Andrasik F, Blanchard EB, Arena JG. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. Journal of behavioral medicine. 1984;7:247–257. doi: 10.1007/BF00845390. [DOI] [PubMed] [Google Scholar]
- 29.Choung RS, Locke GR, Schleck CD, et al. A low response rate does not necessarily indicate non-response bias in gastroenterology survey research: A population-based study. Journal of Public Health. 2013;21:87–95. [Google Scholar]
- 30.Choung RS, Locke GR, Schleck CD, Zinsmeister AR, Talley NJ. Associations between medication use and functional gastrointestinal disorders: a population-based study. Neurogastroenterol Motil. 2013;25:413–419, e298. doi: 10.1111/nmo.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 32.Pauwels A, Altan E, Tack J. The gastric accommodation response to meal intake determines the occurrence of transient lower esophageal sphincter relaxations and reflux events in patients with gastro-esophageal reflux disease. Neurogastroenterol Motil. 2014;26:581–588. doi: 10.1111/nmo.12305. [DOI] [PubMed] [Google Scholar]
- 33.Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003;124:894–902. doi: 10.1053/gast.2003.50159. [DOI] [PubMed] [Google Scholar]
- 34.Talley NJ, Tack J, Ptak T, Gupta R, Giguere M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740–746. doi: 10.1136/gut.2007.132449. [DOI] [PubMed] [Google Scholar]
- 35.Holtmann G, Talley NJ, Liebregts T, Adam B, Parow C. A placebo-controlled trial of itopride in functional dyspepsia. N Engl J Med. 2006;354:832–840. doi: 10.1056/NEJMoa052639. [DOI] [PubMed] [Google Scholar]
- 36.Bourke JH, Langford RM, White PD. The common link between functional somatic syndromes may be central sensitisation. Journal of psychosomatic research. 2015;78:228–236. doi: 10.1016/j.jpsychores.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Annals of internal medicine. 2001;134:868–881. doi: 10.7326/0003-4819-134-9_part_2-200105011-00011. [DOI] [PubMed] [Google Scholar]
- 38.Whitehead WE, Palsson OS, Levy RR, Feld AD, Turner M, Von Korff M. Comorbidity in irritable bowel syndrome. Am J Gastroenterol. 2007;102:2767–2776. doi: 10.1111/j.1572-0241.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 39.Choung RS, Herrick LM, Locke GR, 3rd, Zinsmeister AR, Talley NJ. Irritable bowel syndrome and chronic pelvic pain: a population-based study. J Clin Gastroenterol. 2010;44:696–701. doi: 10.1097/MCG.0b013e3181d7a368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keely S, Walker MM, Marks E, Talley NJ. Immune dysregulation in the functional gastrointestinal disorders. European journal of clinical investigation. 2015 doi: 10.1111/eci.12548. [DOI] [PubMed] [Google Scholar]
- 41.Walker MM, Aggarwal KR, Shim LS, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an Australian cohort. J Gastroenterol Hepatol. 2014;29:474–479. doi: 10.1111/jgh.12419. [DOI] [PubMed] [Google Scholar]
- 42.Liebregts T, Adam B, Bredack C, et al. Small bowel homing T cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am J Gastroenterol. 2011;106:1089–1098. doi: 10.1038/ajg.2010.512. [DOI] [PubMed] [Google Scholar]
- 43.Liebregts T, Adam B, Bredack C, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 44.Spiller R. Postinfectious functional dyspepsia and postinfectious irritable bowel syndrome: different symptoms but similar risk factors. Gastroenterology. 2010;138:1660–1663. doi: 10.1053/j.gastro.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 45.Van Oudenhove L, Vandenberghe J, Vos R, Fischler B, Demyttenaere K, Tack J. Abuse history, depression, and somatization are associated with gastric sensitivity and gastric emptying in functional dyspepsia. Psychosom Med. 2011;73:648–655. doi: 10.1097/PSY.0b013e31822f32bf. [DOI] [PubMed] [Google Scholar]
- 46.Van Oudenhove L, Vandenberghe J, Dupont P, et al. Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H(2)(15)O-PET study. Am J Gastroenterol. 2010;105:913–924. doi: 10.1038/ajg.2010.39. [DOI] [PubMed] [Google Scholar]
- 47.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. 2012;61:1284–1290. doi: 10.1136/gutjnl-2011-300474. [DOI] [PubMed] [Google Scholar]
- 49.Farre R, Vanheel H, Vanuytsel T, et al. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology. 2013;145:566–573. doi: 10.1053/j.gastro.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 50.Dorn SD, Palsson OS, Thiwan SI, et al. Increased colonic pain sensitivity in irritable bowel syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. Gut. 2007;56:1202–1209. doi: 10.1136/gut.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corsetti M, Caenepeel P, Fischler B, Janssens J, Tack J. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 2004;99:1152–1159. doi: 10.1111/j.1572-0241.2004.30040.x. [DOI] [PubMed] [Google Scholar]
- 52.Caldarella MP, Giamberardino MA, Sacco F, et al. Sensitivity disturbances in patients with irritable bowel syndrome and fibromyalgia. Am J Gastroenterol. 2006;101:2782–2789. doi: 10.1111/j.1572-0241.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 53.Moshiree B, Price DD, Robinson ME, Gaible R, Verne GN. Thermal and visceral hypersensitivity in irritable bowel syndrome patients with and without fibromyalgia. The Clinical journal of pain. 2007;23:323–330. doi: 10.1097/AJP.0b013e318032e496. [DOI] [PubMed] [Google Scholar]
- 54.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–472 e463. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang L, Berman S, Mayer EA, et al. Brain responses to visceral and somatic stimuli in patients with irritable bowel syndrome with and without fibromyalgia. Am J Gastroenterol. 2003;98:1354–1361. doi: 10.1111/j.1572-0241.2003.07478.x. [DOI] [PubMed] [Google Scholar]
- 56.Gerstner G, Ichesco E, Quintero A, Schmidt-Wilcke T. Changes in regional gray and white matter volume in patients with myofascial-type temporomandibular disorders: a voxel-based morphometry study. Journal of orofacial pain. 2011;25:99–106. [PubMed] [Google Scholar]
- 57.Pinto-Sanchez MI, Ford AC, Avila CA, et al. Anxiety and Depression Increase in a Stepwise Manner in Parallel With Multiple FGIDs and Symptom Severity and Frequency. Am J Gastroenterol. 2015;110:1038–1048. doi: 10.1038/ajg.2015.128. [DOI] [PubMed] [Google Scholar]
- 58.Gerrits MM, Vogelzangs N, van Oppen P, van Marwijk HW, van der Horst H, Penninx BW. Impact of pain on the course of depressive and anxiety disorders. Pain. 2012;153:429–436. doi: 10.1016/j.pain.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Haug TT, Mykletun A, Dahl AA. The association between anxiety, depression, and somatic symptoms in a large population: the HUNT-II study. Psychosom Med. 2004;66:845–851. doi: 10.1097/01.psy.0000145823.85658.0c. [DOI] [PubMed] [Google Scholar]
- 60.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology. 1997;112:2120–2137. doi: 10.1053/gast.1997.v112.agast972120. [DOI] [PubMed] [Google Scholar]
- 62.Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, Heitkemper MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci. 2009;54:1542–1549. doi: 10.1007/s10620-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Locke GR, 3rd, Zinsmeister AR, Fett SL, Melton LJ, 3rd, Talley NJ. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. doi: 10.1111/j.1365-2982.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 64.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 65.Chey WD, Nojkov B, Rubenstein JH, Dobhan RR, Greenson JK, Cash BD. The yield of colonoscopy in patients with non-constipated irritable bowel syndrome: results from a prospective, controlled US trial. Am J Gastroenterol. 2010;105:859–865. doi: 10.1038/ajg.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


