The characteristic structural feature of a new two-dimensional Zn coordination polymer is an infinite polymeric layer parallel to the crystallographic (132) plane.
Keywords: crystal structure, coordination polymer, zinc complex, two-dimensional layer
Abstract
The title compound, poly[bis{μ2-4,4′-bis[(1,2,4-triazol-1-yl)methyl]biphenyl-κ2 N 4:N 4′}bis(nitrato-κO)zinc(II)], [Zn(NO3)2(C18H16N6)2]n, is a two-dimensional zinc coordination polymer constructed from 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl units. It was synthesized and characterized by elemental analysis and single-crystal X-ray diffraction. The ZnII cation is located on an inversion centre and is coordinated by two O atoms from two symmetry-related nitrate groups and four N atoms from four symmetry-related 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl ligands, forming a distorted octahedral {ZnN4O2} coordination geometry. The linear 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl ligand links two ZnII cations, generating two-dimensional layers parallel to the crystallographic (132) plane. The parallel layers are connected by C—H⋯O, C—H⋯N, C—H⋯π and π–π stacking interactions, resulting in a three-dimensional supramolecular architecture.
Chemical context
Over the past few decades, the self-assembly of coordination polymers (CPs) or metal–organic frameworks (MOFs) based on metal ions or clusters and organic ligands has attracted much attention, owing to their intriguing molecular topologies and potential applications. Multidentate ligands derived from 1,2,4-triazole that contain an aromatic core have been used for this purpose, examples being 1,4-bis(1H-1,2,4-triazol-1-ylmethyl)benzene (Wang et al., 2007 ▸; Ding & Zou, 2010 ▸; Zhu et al., 2010 ▸), 1,3-bis(1H-1,2,4-triazol-1-ylmethyl)benzene (Zhang et al., 2012 ▸; Ge et al., 2008 ▸; Zhu et al., 2015 ▸), 1,2-bis(1H-1,2,4-triazol-1-ylmethyl)benzene (Yang et al., 2009 ▸; Zhao et al., 2017 ▸; Zhang et al., 2013 ▸), 1,3,5-tris(1H-1,2,4-triazol-1-ylmethyl)benzene (Li et al., 2012 ▸; Yin et al., 2009 ▸; Shi et al., 2011 ▸), 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl (Mu et al., 2011 ▸; Ren et al., 2010 ▸; Ni et al., 2010 ▸). Hydrothermal synthesis has been proved to be an effective method for the construction of these new coordination polymers. In this study, a new two-dimensional CP, viz. poly[bis{μ2-4,4′-bis[(1,2,4-triazol-1-yl)methyl]biphenyl-κ2 N 4:N 4′}bis(nitrato-κO)zinc], [Zn(NO3)2(C18H16N6)2]n, was synthesized under hydrothermal conditions by the reaction of Zn(NO3)2·6H2O and 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl at 313 K for 48 h. We report here its crystal structure and its elemental analysis.
Structural commentary
The title complex crystallizes in the triclinic space group P
 ; the asymmetric unit of the structure consists of one ZnII cation (site symmetry
; the asymmetric unit of the structure consists of one ZnII cation (site symmetry  ), one nitrate anion and one 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl ligand.
), one nitrate anion and one 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl ligand.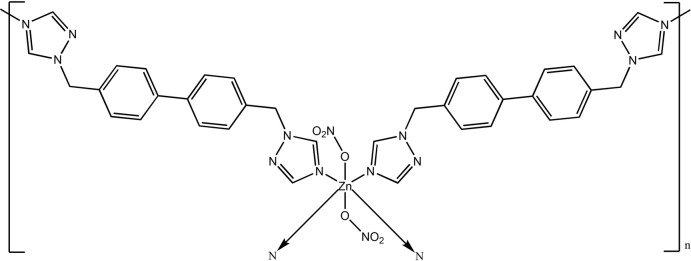
As shown in Fig. 1 ▸, each ZnII cation exhibits a slightly distorted octahedral {ZnN4O2} coordination geometry and is coordinated by four N atoms (N1, N4, N1i and N4i) from four symmetry-related organic ligands and two O atoms (O3 and O3i) from two symmetry-related nitrate groups (see Fig. 1 ▸ for symmetry code). The Zn—O [2.191 (2) Å] and Zn—N bond lengths [2.124 (3)–2.168 (2) Å] are in agreement with corresponding bond lengths found in previously reported ZnII coordination polymers. For the title coordination polymer, the ZnII cation is coordinated by four 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl ligands and two nitrate anions, and each organic ligand in turn connects two ZnII cations to generate a two-dimensional layer parallel to the crystallographic (132) plane. The organic ligand adopts a cis,cis substituent conformation. The two distinct Zn⋯Zn distances are 18.397 (3) and 18.964 (3) Å (see Fig. 2 ▸). The two benzene rings of the 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl ligand lie nearly in one plane [dihedral angle = 0.00 (2)°]. The two triazole groups of the 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl ligand are inclined to the plane of the central biphenyl groups, with dihedral angles of 80.050 (2) (C1/C2/N1/N2/N3) and 85.511 (2)° (C10/C11/N4/N5/N6). Four adjacent ZnII cations are connected by four linear organic ligands and form a 72-membered macrocyclic ring in the above-mentioned two-dimensional layer (see Fig. 2 ▸).
Figure 1.
The asymmetric unit of (I), showing the atom-numbering scheme. Displacement ellipsoids drawn at the 25% probability level. [Symmetry code: (i) −x, 2 − y, −z.]
Figure 2.
The two-dimensional layer parallel to the crystallographic (132) plane.
Supramolecular features
Neighbouring layers are linked to each other by by weak interactions (Table 1 ▸), including C—H⋯O, C—H⋯N, C—H⋯π [C11—H11⋯Cg1ii = 3.6756 (8) Å and C12—H12⋯Cg2iii = 3.5252 (7) Å; Cg1 and Cg2 are the centroids of the triazole (C1/C2/N1/N2/N3) and phenyl (C4–C9) rings, respectively; symmetry codes: (ii) 2 − x, −y, −z; (iii) 1 − x, 1 − y, −z] contacts and π–π stacking interactions [Cg1⋯Cg1ii = 3.6296 (10) Å]. These interactions, together with the covalent interactions in the infinite two-dimensional polymeric-like layer, make up a three-dimensional supramolecular structure.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3B⋯O1i | 0.97 | 2.31 | 3.2728 (7) | 170 |
| C3—H3B⋯O1A i | 0.97 | 2.33 | 3.2765 (7) | 165 |
| C10—H10⋯O2A ii | 0.93 | 2.53 | 3.0888 (6) | 115 |
| C14—H14⋯O2ii | 0.93 | 2.46 | 3.5454 (7) | 158 |
| C15—H15⋯N6iii | 0.93 | 2.58 | 3.482 (16) | 162 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Database survey
A search in the Cambridge Structural Database (Groom et al., 2016 ▸) for zinc and the 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl moiety gave eight hits. Seven of them are constructed by 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl units and different carboxylate ligands. One example is a chain structure based on Zn and 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl (PUQWAA; Ni et al., 2010 ▸).
Synthesis and crystallization
Zn(NO3)2·6H2O (0.1 mmol), 4,4′-bis[(1H-1,2,4-triazol-1-yl)methyl]-1,1′-biphenyl (0.1 mmol) and water (6 ml) were mixed and placed in a thick Pyrex tube, which was sealed and heated to 413 K for 72 h. After cooling to room temperature, colourless block-shaped crystals (53% yield, based on Zn) suitable for X-ray analysis were obtained. Elemental analysis calculated for C36H32N14O6Zn: C 52.59, H 3.92, N 23.85%; found: C 52.23, H 3.74, N 23.49%.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. H atoms bonded to C atoms were positioned geometrically and allowed to ride on their parent atoms, with C—H = 0.93 Å with U iso(H) = 1.2U eq(C) for other H atoms. Atoms O1 and O2 of the nitrate group are disordered over two orientations, with occupancies of 0.511 (11) and 0.489 (11), and were refined through the use of SADI, RIGU and SIMU commands.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Zn(NO3)2(C18H16N6)2] |
| M r | 822.12 |
| Crystal system, space group | Triclinic, P

|
| Temperature (K) | 293 |
| a, b, c (Å) | 7.3257 (15), 9.0188 (18), 15.578 (3) |
| α, β, γ (°) | 81.70 (3), 77.64 (3), 68.90 (3) |
| V (Å3) | 935.4 (4) |
| Z | 1 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.72 |
| Crystal size (mm) | 0.24 × 0.22 × 0.20 |
| Data collection | |
| Diffractometer | Bruker APEXII Quazar |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| T min, T max | 0.84, 0.86 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 7292, 3271, 2589 |
| R int | 0.042 |
| (sin θ/λ)max (Å−1) | 0.595 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.051, 0.143, 1.05 |
| No. of reflections | 3271 |
| No. of parameters | 278 |
| No. of restraints | 85 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.34, −0.54 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989017012452/vn2130sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017012452/vn2130Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989017012452/vn2130Isup3.mol
CCDC reference: 1564369
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Crystal data
| [Zn(NO3)2(C18H16N6)2] | Z = 1 |
| Mr = 822.12 | F(000) = 424 |
| Triclinic, P1 | Dx = 1.459 Mg m−3 |
| a = 7.3257 (15) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 9.0188 (18) Å | Cell parameters from 7292 reflections |
| c = 15.578 (3) Å | θ = 1.6–25.1° |
| α = 81.70 (3)° | µ = 0.72 mm−1 |
| β = 77.64 (3)° | T = 293 K |
| γ = 68.90 (3)° | Block, colorless |
| V = 935.4 (4) Å3 | 0.24 × 0.22 × 0.2 mm |
Data collection
| Bruker APEXII Quazar diffractometer | 3271 independent reflections |
| Radiation source: microfocus sealed X-ray tube, Incoatec Iµs | 2589 reflections with I > 2σ(I) |
| Mirror optics monochromator | Rint = 0.042 |
| Detector resolution: 7.9 pixels mm-1 | θmax = 25.0°, θmin = 3.0° |
| 0.5° ω and 0.5° φ scans | h = −8→8 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | k = −10→9 |
| Tmin = 0.84, Tmax = 0.86 | l = −18→18 |
| 7292 measured reflections |
Refinement
| Refinement on F2 | 85 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.051 | H-atom parameters constrained |
| wR(F2) = 0.143 | w = 1/[σ2(Fo2) + (0.079P)2 + 0.2956P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 3271 reflections | Δρmax = 0.34 e Å−3 |
| 278 parameters | Δρmin = −0.54 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Zn1 | 0.000000 | 1.000000 | 0.000000 | 0.0406 (2) | |
| O3 | 0.1631 (4) | 1.1607 (3) | 0.00189 (17) | 0.0574 (7) | |
| N1 | 0.2322 (4) | 0.8123 (3) | 0.05733 (18) | 0.0441 (7) | |
| N2 | 0.4790 (5) | 0.6937 (4) | 0.1274 (2) | 0.0482 (7) | |
| N3 | 0.4061 (6) | 0.5766 (4) | 0.1198 (2) | 0.0602 (9) | |
| N4 | 0.1701 (4) | 0.9540 (4) | −0.12813 (18) | 0.0457 (7) | |
| N5 | 0.3961 (5) | 0.8456 (4) | −0.23645 (19) | 0.0466 (7) | |
| N6 | 0.2787 (6) | 0.9865 (5) | −0.2713 (2) | 0.0729 (11) | |
| N7 | 0.1398 (5) | 1.2530 (4) | 0.0601 (2) | 0.0570 (8) | |
| C1 | 0.2590 (6) | 0.6550 (5) | 0.0771 (3) | 0.0554 (10) | |
| H1 | 0.179295 | 0.606514 | 0.061538 | 0.066* | |
| C2 | 0.3743 (6) | 0.8301 (4) | 0.0904 (2) | 0.0474 (8) | |
| H2 | 0.397217 | 0.925660 | 0.087958 | 0.057* | |
| C3 | 0.6466 (6) | 0.6585 (5) | 0.1731 (3) | 0.0601 (10) | |
| H3A | 0.687840 | 0.751186 | 0.165279 | 0.072* | |
| H3B | 0.757323 | 0.571442 | 0.146296 | 0.072* | |
| C4 | 0.5997 (6) | 0.6146 (4) | 0.2700 (3) | 0.0513 (9) | |
| C5 | 0.7455 (7) | 0.5120 (7) | 0.3132 (4) | 0.0902 (17) | |
| H5 | 0.873179 | 0.468413 | 0.281685 | 0.108* | |
| C6 | 0.7082 (8) | 0.4711 (8) | 0.4028 (4) | 0.0927 (17) | |
| H6 | 0.812884 | 0.404978 | 0.430318 | 0.111* | |
| C7 | 0.5224 (6) | 0.5251 (4) | 0.4518 (3) | 0.0532 (9) | |
| C8 | 0.3756 (8) | 0.6273 (6) | 0.4079 (3) | 0.0813 (16) | |
| H8 | 0.246945 | 0.667746 | 0.439147 | 0.098* | |
| C9 | 0.4126 (8) | 0.6717 (6) | 0.3195 (3) | 0.0823 (16) | |
| H9 | 0.309014 | 0.741721 | 0.292602 | 0.099* | |
| C10 | 0.3284 (6) | 0.8298 (5) | −0.1520 (2) | 0.0515 (9) | |
| H10 | 0.384162 | 0.743198 | −0.114162 | 0.062* | |
| C11 | 0.1465 (6) | 1.0486 (5) | −0.2035 (2) | 0.0588 (10) | |
| H11 | 0.046686 | 1.147437 | −0.206804 | 0.071* | |
| C12 | 0.5625 (7) | 0.7311 (5) | −0.2909 (3) | 0.0658 (13) | |
| H12A | 0.509806 | 0.678916 | −0.324861 | 0.079* | |
| H12B | 0.641231 | 0.649898 | −0.252615 | 0.079* | |
| C13 | 0.6941 (6) | 0.8103 (4) | −0.3527 (3) | 0.0530 (10) | |
| C14 | 0.8175 (7) | 0.8650 (6) | −0.3228 (3) | 0.0722 (13) | |
| H14 | 0.821652 | 0.853057 | −0.262825 | 0.087* | |
| C15 | 0.9375 (7) | 0.9384 (6) | −0.3803 (3) | 0.0684 (13) | |
| H15 | 1.021917 | 0.973475 | −0.358088 | 0.082* | |
| C16 | 0.9348 (5) | 0.9608 (4) | −0.4696 (2) | 0.0431 (8) | |
| C17 | 0.8101 (7) | 0.9040 (6) | −0.4990 (3) | 0.0738 (14) | |
| H17 | 0.804965 | 0.915631 | −0.558860 | 0.089* | |
| C18 | 0.6915 (8) | 0.8296 (6) | −0.4409 (3) | 0.0750 (14) | |
| H18 | 0.608813 | 0.792084 | −0.462688 | 0.090* | |
| O1 | −0.0075 (14) | 1.3759 (11) | 0.0613 (8) | 0.0890 (19) | 0.500 (12) |
| O2 | 0.2754 (14) | 1.2075 (12) | 0.1048 (6) | 0.0792 (17) | 0.500 (12) |
| O1A | −0.0249 (12) | 1.3242 (11) | 0.1028 (7) | 0.0807 (18) | 0.500 (12) |
| O2A | 0.2775 (13) | 1.2743 (14) | 0.0828 (7) | 0.0812 (17) | 0.500 (12) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Zn1 | 0.0365 (3) | 0.0492 (4) | 0.0287 (3) | −0.0098 (2) | −0.0025 (2) | 0.0033 (2) |
| O3 | 0.0658 (17) | 0.0635 (16) | 0.0492 (15) | −0.0325 (14) | −0.0020 (13) | −0.0084 (13) |
| N1 | 0.0444 (16) | 0.0499 (17) | 0.0331 (15) | −0.0123 (13) | −0.0074 (13) | 0.0027 (13) |
| N2 | 0.0514 (18) | 0.0509 (17) | 0.0384 (16) | −0.0136 (14) | −0.0123 (14) | 0.0052 (13) |
| N3 | 0.077 (2) | 0.0481 (18) | 0.060 (2) | −0.0216 (17) | −0.0251 (19) | 0.0036 (16) |
| N4 | 0.0463 (17) | 0.0559 (18) | 0.0310 (15) | −0.0158 (14) | −0.0048 (13) | 0.0013 (13) |
| N5 | 0.0506 (17) | 0.0545 (17) | 0.0347 (16) | −0.0245 (15) | 0.0054 (14) | −0.0049 (14) |
| N6 | 0.072 (3) | 0.088 (3) | 0.042 (2) | −0.019 (2) | 0.0022 (19) | 0.0074 (19) |
| N7 | 0.0616 (17) | 0.0610 (18) | 0.0580 (19) | −0.0299 (14) | −0.0085 (15) | −0.0136 (15) |
| C1 | 0.065 (3) | 0.052 (2) | 0.052 (2) | −0.0185 (19) | −0.022 (2) | −0.0003 (18) |
| C2 | 0.052 (2) | 0.0469 (19) | 0.0385 (19) | −0.0165 (17) | −0.0040 (17) | 0.0047 (15) |
| C3 | 0.052 (2) | 0.071 (3) | 0.057 (3) | −0.020 (2) | −0.020 (2) | 0.009 (2) |
| C4 | 0.059 (2) | 0.048 (2) | 0.046 (2) | −0.0146 (17) | −0.0201 (18) | 0.0056 (17) |
| C5 | 0.044 (3) | 0.133 (5) | 0.070 (3) | −0.017 (3) | −0.018 (2) | 0.044 (3) |
| C6 | 0.057 (3) | 0.133 (5) | 0.070 (3) | −0.021 (3) | −0.028 (3) | 0.047 (3) |
| C7 | 0.071 (3) | 0.043 (2) | 0.047 (2) | −0.0121 (18) | −0.027 (2) | 0.0009 (16) |
| C8 | 0.083 (3) | 0.077 (3) | 0.041 (2) | 0.025 (2) | −0.010 (2) | −0.006 (2) |
| C9 | 0.091 (4) | 0.075 (3) | 0.045 (2) | 0.021 (3) | −0.027 (2) | 0.002 (2) |
| C10 | 0.052 (2) | 0.055 (2) | 0.0356 (19) | −0.0112 (17) | 0.0015 (17) | 0.0035 (16) |
| C11 | 0.049 (2) | 0.068 (2) | 0.036 (2) | −0.0013 (19) | 0.0002 (17) | 0.0047 (18) |
| C12 | 0.079 (3) | 0.055 (2) | 0.057 (3) | −0.033 (2) | 0.028 (2) | −0.017 (2) |
| C13 | 0.058 (2) | 0.047 (2) | 0.045 (2) | −0.0194 (18) | 0.0153 (18) | −0.0088 (17) |
| C14 | 0.087 (3) | 0.100 (3) | 0.034 (2) | −0.048 (3) | 0.002 (2) | 0.000 (2) |
| C15 | 0.075 (3) | 0.108 (4) | 0.039 (2) | −0.054 (3) | −0.004 (2) | −0.002 (2) |
| C16 | 0.0386 (18) | 0.0427 (18) | 0.0388 (18) | −0.0073 (14) | 0.0024 (15) | −0.0055 (15) |
| C17 | 0.087 (3) | 0.114 (4) | 0.038 (2) | −0.060 (3) | −0.007 (2) | 0.003 (2) |
| C18 | 0.085 (3) | 0.111 (4) | 0.051 (3) | −0.066 (3) | −0.002 (2) | −0.005 (2) |
| O1 | 0.091 (3) | 0.077 (3) | 0.080 (4) | −0.006 (3) | −0.008 (3) | −0.016 (3) |
| O2 | 0.094 (3) | 0.080 (4) | 0.075 (3) | −0.035 (3) | −0.032 (3) | −0.003 (3) |
| O1A | 0.080 (3) | 0.075 (3) | 0.074 (4) | −0.020 (3) | 0.009 (3) | −0.016 (3) |
| O2A | 0.085 (3) | 0.088 (4) | 0.087 (4) | −0.041 (3) | −0.030 (3) | −0.008 (3) |
Geometric parameters (Å, º)
| Zn1—O3 | 2.191 (2) | C4—C5 | 1.367 (6) |
| Zn1—O3i | 2.191 (2) | C4—C9 | 1.376 (6) |
| Zn1—N1i | 2.167 (3) | C5—H5 | 0.9300 |
| Zn1—N1 | 2.167 (3) | C5—C6 | 1.385 (7) |
| Zn1—N4i | 2.124 (3) | C6—H6 | 0.9300 |
| Zn1—N4 | 2.124 (3) | C6—C7 | 1.363 (7) |
| O3—N7 | 1.263 (4) | C7—C7ii | 1.505 (8) |
| N1—C1 | 1.359 (5) | C7—C8 | 1.376 (6) |
| N1—C2 | 1.322 (5) | C8—H8 | 0.9300 |
| N2—N3 | 1.372 (4) | C8—C9 | 1.375 (7) |
| N2—C2 | 1.318 (5) | C9—H9 | 0.9300 |
| N2—C3 | 1.465 (5) | C10—H10 | 0.9300 |
| N3—C1 | 1.314 (5) | C11—H11 | 0.9300 |
| N4—C10 | 1.318 (5) | C12—H12A | 0.9700 |
| N4—C11 | 1.353 (5) | C12—H12B | 0.9700 |
| N5—N6 | 1.365 (5) | C12—C13 | 1.506 (5) |
| N5—C10 | 1.310 (5) | C13—C14 | 1.359 (6) |
| N5—C12 | 1.475 (5) | C13—C18 | 1.364 (6) |
| N6—C11 | 1.307 (5) | C14—H14 | 0.9300 |
| N7—O1 | 1.237 (8) | C14—C15 | 1.387 (6) |
| N7—O2 | 1.249 (8) | C15—H15 | 0.9300 |
| N7—O1A | 1.237 (7) | C15—C16 | 1.381 (5) |
| N7—O2A | 1.221 (8) | C16—C16iii | 1.487 (7) |
| C1—H1 | 0.9300 | C16—C17 | 1.375 (5) |
| C2—H2 | 0.9300 | C17—H17 | 0.9300 |
| C3—H3A | 0.9700 | C17—C18 | 1.390 (6) |
| C3—H3B | 0.9700 | C18—H18 | 0.9300 |
| C3—C4 | 1.501 (6) | ||
| O3—Zn1—O3i | 180.0 | C5—C4—C3 | 120.3 (4) |
| N1—Zn1—O3i | 92.22 (11) | C5—C4—C9 | 116.5 (4) |
| N1i—Zn1—O3 | 92.22 (11) | C9—C4—C3 | 123.2 (4) |
| N1i—Zn1—O3i | 87.78 (11) | C4—C5—H5 | 119.1 |
| N1—Zn1—O3 | 87.78 (11) | C4—C5—C6 | 121.8 (5) |
| N1i—Zn1—N1 | 180.0 | C6—C5—H5 | 119.1 |
| N4—Zn1—O3i | 94.65 (10) | C5—C6—H6 | 119.1 |
| N4i—Zn1—O3 | 94.65 (10) | C7—C6—C5 | 121.9 (5) |
| N4—Zn1—O3 | 85.35 (10) | C7—C6—H6 | 119.1 |
| N4i—Zn1—O3i | 85.35 (10) | C6—C7—C7ii | 122.4 (5) |
| N4i—Zn1—N1i | 90.36 (12) | C6—C7—C8 | 116.2 (4) |
| N4i—Zn1—N1 | 89.64 (12) | C8—C7—C7ii | 121.4 (5) |
| N4—Zn1—N1 | 90.36 (12) | C7—C8—H8 | 118.9 |
| N4—Zn1—N1i | 89.64 (12) | C9—C8—C7 | 122.2 (4) |
| N4i—Zn1—N4 | 180.0 | C9—C8—H8 | 118.9 |
| N7—O3—Zn1 | 128.5 (2) | C4—C9—H9 | 119.3 |
| C1—N1—Zn1 | 130.5 (3) | C8—C9—C4 | 121.4 (4) |
| C2—N1—Zn1 | 126.5 (2) | C8—C9—H9 | 119.3 |
| C2—N1—C1 | 102.7 (3) | N4—C10—H10 | 124.8 |
| N3—N2—C3 | 120.7 (3) | N5—C10—N4 | 110.5 (4) |
| C2—N2—N3 | 109.9 (3) | N5—C10—H10 | 124.8 |
| C2—N2—C3 | 129.4 (3) | N4—C11—H11 | 123.6 |
| C1—N3—N2 | 102.0 (3) | N6—C11—N4 | 112.9 (4) |
| C10—N4—Zn1 | 128.1 (3) | N6—C11—H11 | 123.6 |
| C10—N4—C11 | 103.9 (3) | N5—C12—H12A | 109.2 |
| C11—N4—Zn1 | 128.0 (3) | N5—C12—H12B | 109.2 |
| N6—N5—C12 | 122.6 (3) | N5—C12—C13 | 112.2 (3) |
| C10—N5—N6 | 109.0 (3) | H12A—C12—H12B | 107.9 |
| C10—N5—C12 | 128.3 (4) | C13—C12—H12A | 109.2 |
| C11—N6—N5 | 103.8 (3) | C13—C12—H12B | 109.2 |
| O1—N7—O3 | 115.4 (6) | C14—C13—C12 | 121.5 (4) |
| O1—N7—O2 | 130.6 (7) | C14—C13—C18 | 118.1 (4) |
| O2—N7—O3 | 114.0 (5) | C18—C13—C12 | 120.4 (4) |
| O1A—N7—O3 | 122.8 (5) | C13—C14—H14 | 119.5 |
| O2A—N7—O3 | 123.5 (6) | C13—C14—C15 | 121.0 (4) |
| O2A—N7—O1A | 113.6 (7) | C15—C14—H14 | 119.5 |
| N1—C1—H1 | 122.6 | C14—C15—H15 | 119.2 |
| N3—C1—N1 | 114.8 (3) | C16—C15—C14 | 121.7 (4) |
| N3—C1—H1 | 122.6 | C16—C15—H15 | 119.2 |
| N1—C2—H2 | 124.7 | C15—C16—C16iii | 120.9 (4) |
| N2—C2—N1 | 110.7 (3) | C17—C16—C15 | 116.7 (3) |
| N2—C2—H2 | 124.7 | C17—C16—C16iii | 122.4 (4) |
| N2—C3—H3A | 108.9 | C16—C17—H17 | 119.4 |
| N2—C3—H3B | 108.9 | C16—C17—C18 | 121.2 (4) |
| N2—C3—C4 | 113.5 (3) | C18—C17—H17 | 119.4 |
| H3A—C3—H3B | 107.7 | C13—C18—C17 | 121.4 (4) |
| C4—C3—H3A | 108.9 | C13—C18—H18 | 119.3 |
| C4—C3—H3B | 108.9 | C17—C18—H18 | 119.3 |
Symmetry codes: (i) −x, −y+2, −z; (ii) −x+1, −y+1, −z+1; (iii) −x+2, −y+2, −z−1.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3B···O1iv | 0.97 | 2.31 | 3.2728 (7) | 170 |
| C3—H3B···O1Aiv | 0.97 | 2.33 | 3.2765 (7) | 165 |
| C10—H10···O2Av | 0.93 | 2.53 | 3.0888 (6) | 115 |
| C14—H14···O2v | 0.93 | 2.46 | 3.5454 (7) | 158 |
| C15—H15···N6vi | 0.93 | 2.58 | 3.482 (16) | 162 |
Symmetry codes: (iv) x−1, y+1, z; (v) −x+1, −y, −z; (vi) x−1, y, z.
Funding Statement
This work was funded by Science and technology research projects of jilin province department of education (JJKH20170186KJ) grant .
References
- Bruker (2016). APEX3. Bruker AXS Inc., Madison, Wisconsin, USA.
- Ding, B. & Zou, H.-A. (2010). Acta Cryst. E66, m932. [DOI] [PMC free article] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Ge, H., Liu, K., Yang, Y., Li, B. & Zhang, Y. (2008). Inorg. Chem. Commun. 11, 260–264.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst. 48, 3–10. [DOI] [PMC free article] [PubMed]
- Li, Q.-X., Shi, X.-J. & Chen, L.-C. (2012). Acta Cryst. E68, m1299. [DOI] [PMC free article] [PubMed]
- Mu, Y., Song, Y., Wang, C., Hou, H. & Fan, Y. (2011). Inorg. Chim. Acta, 365, 167–176.
- Ni, T., Shao, M., Zhu, S., Zhao, Y., Xing, F. & Li, M. (2010). Cryst. Growth Des. 10, 943–951.
- Ren, C., Liu, P., Wang, Y.-Y., Huang, W.-H. & Shi, Q.-Z. (2010). Eur. J. Inorg. Chem. pp. 5545–5555.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shi, X., Li, Q., Zhang, Y., Chang, X. & Hou, H. (2011). J. Coord. Chem. 64, 3918–3927.
- Wang, W.-B., Wang, L.-Y., Li, B.-L. & Zhang, Y. (2007). Acta Cryst. E63, m2416–m2417.
- Yang, Y., Feng, Y.-F., Liang, N., Li, B.-L. & Zhang, Y. (2009). J. Coord. Chem. 62, 3819–3827.
- Yin, X.-J., Zhou, X.-H., Gu, Z.-G., Zuo, J.-L. & You, X.-Z. (2009). Inorg. Chem. Commun. 12, 548–551.
- Zhang, Z., Ma, J.-F., Liu, Y.-Y., Kan, W.-Q. & Yang, J. (2013). CrystEngComm, 15, 2009–2018.
- Zhang, H.-K., Wang, X., Wang, S. & Wang, X.-D. (2012). Acta Cryst. E68, m856. [DOI] [PMC free article] [PubMed]
- Zhao, S., Zheng, T.-R., Zhang, Y.-Q., Lv, X.-X., Li, B.-L. & Zhang, Y. (2017). Polyhedron, 121, 61–69.
- Zhu, X., Guo, Y. & Zou, Y.-L. (2010). Acta Cryst. E66, m85. [DOI] [PMC free article] [PubMed]
- Zhu, X., Yang, Y., Jiang, N., Li, B., Zhou, D., Fu, H. & Wang, N. (2015). Z. Anorg. Allg. Chem. 641, 699–703.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989017012452/vn2130sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989017012452/vn2130Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989017012452/vn2130Isup3.mol
CCDC reference: 1564369
Additional supporting information: crystallographic information; 3D view; checkCIF report




