Abstract
DDT and pyrethroid insecticides were among the earliest neurotoxins identified to act on voltage-gated sodium channels. In the 1960s, equipped with, at the time, new voltage-clamp techniques, Professor Narahashi and associates provided the initial evidence that DDT and allethrin (the first commercial pyrethroid insecticide) caused prolonged flow of sodium currents in lobster and squid giant axons. Over the next several decades, continued efforts by Prof. Narahashi’s group as well as other laboratories led to a comprehensive understanding of the mechanism of action of DDT and pyrethroids on sodium channels. Fast forward to the 1990s, genetic, pharmacological and toxicological data all further confirmed voltage-gated sodium channels as the primary targets of DDT and pyrethroid insecticides. Modifications of the gating kinetics of sodium channels by these insecticides result in repetitive firing and/or membrane depolarization in the nervous system. This mini-review focuses on studies from Prof. Narahashi’s pioneer work and more recent mutational and computational modeling analyses which collectively elucidated the elusive pyrethroid receptor sites as well as the molecular basis of differential sensitivities of insect and mammalian sodium channels to pyrethroids.
Keywords: Sodium channels, Insecticides, DDT, Pyrethroids
1. Introduction
Voltage-gated sodium channels are essential for the generation and propagation of action potentials in almost all excitable cells. Because of their crucial role in regulating membrane excitability, sodium channels are the primary target of a broad range of naturally occurring neurotoxins, which are produced by plants and animals for defense or predation, and also therapeutic drugs and insecticides (Cestele and Catterall, 2000; Wang and Wang, 2003). These neurotoxins bind to distinct receptor sites and alter sodium channel function by blocking the pore or altering gating of sodium channels (Cestele and Catterall, 2000; Wang and Wang, 2003). For example, tetrodotoxin, isolated from the puffer fish, blocks sodium ion current by binding to a receptor site located at the extracellular opening of the ion-conducting pore. Lipid-soluble alkaloids, such as batrachotoxin (BTX) and veratridine, cause persistent activation of sodium channels at resting membrane potentials by binding to a site in the inner pore (Du et al., 2011; Tikhonov and Zhorov, 2005) where they block inactivation and shift the voltage-dependence of activation to more negative membrane potentials. Identification and characterization of distinct and pharmacologically relevant receptor sites of these neurotoxins in sodium channels played a key role in understanding the molecular bases of their diverse and unique effects on the function of sodium channels (Catterall and Swanson, 2015).
Sodium channels are the primary target of action of DDT and pyrethroid insecticides. Although the use of DDT, the first organochlorine insecticide, is largely banned, it is still one of the recommended insecticides for malaria control in Africa. Pyrethroids (Fig. 1) are synthetic analogues of pyrethrum from the flower extracts of Chrysanthemum species (Elliott, 1977) and are used extensively in the control of insect pests and vectors of numerous human diseases. Pyrethroids are grouped into two categories, Type I and Type II, based on their distinct poisoning symptoms, effects on nerve preparations, and chemical structures (Narahashi, 1986). The mode of action of DDT and pyrethroids has been extensively reviewed by T. Narahashi (Narahashi, 1986, 1988, 1992, 1996; Narahashi et al., 1992) and other investigators (Bloomquist, 1996; Bloomquist and Soderlund, 1988; Soderlund, 2010, 2012). Historically, studies on the mode of action of DDT and pyrethroids were conducted using vertebrate and invertebrate nerve preparations. Professor Narahashi and associates performed the first voltage clamp study with DDT and later with allethrin, a first commercial pyrethroid insecticide, and discovered that both DDT and allethrin cause prolonged opening of sodium channels (Narahashi and Anderson, 1967; Narahashi and Haas, 1967, 1968). More detailed voltage clamp analyses of the action of DDT and Type I and Type II pyrethroids by Narahashi’s group (Refs. in Narahashi, 2000) and also other groups, e.g., (Vijverberg and van den Bercken, 1982; Vijverberg et al., 1982), revealed that DDT and both Type I and Type II pyrethroids inhibit channel deactivation and inactivation and stabilize the open state of sodium channels causing prolonged opening of the channels. More recent studies on the effects of pyrethroids on insect and mammalian sodium channels expressed in Xenopus oocytes have confirmed and extended these earlier findings (Soderlund, 2010, 2012).
Fig. 1.
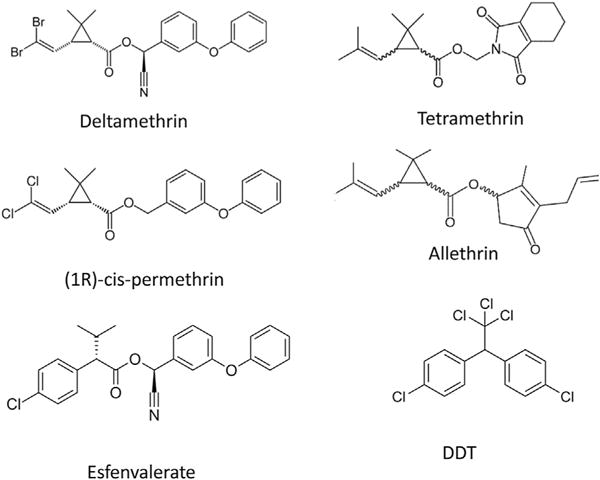
Structural formulae of representative pyrethroids and DDT.
2. Distinct site of action of pyrethroids in the sodium channel
Interest in elucidating the site of action of pyrethroids in sodium channels has been a long-standing pursuit, starting with the works of Prof. Narahashi in the early 1980s. Prof. Narahashi and associates used several neurotoxins, including TTX, BTX and grayanotoxin (GTX), as tools to demonstrate that pyrethroids act on a site different from the sites of these toxins (Lund and Narahashi, 1982; Takeda and Narahashi, 1988). Furthermore, Lund and Narahashi (1982) began detailed voltage clamp analyses of pyrethroid-sodium channel interactions using internally perfused squid giant axons. They used active and inactive isomers of tetramethrin to explore the mechanism underlying pyrethroid stereospecificity. Tetramethrin has four isomers: active isomers 1R-cis and 1R-trans, and inactive isomers 1S-cis and 1S-trans (Nishimura and Narahashi, 1978). Lund and Narahashi (1982) revealed that 1R-cis- and 1R-trans- isomers of tetramethrin were effective in causing a prolonged sodium current, but 1S-cis- and 1S-trans-isomers had no effect. Furthermore, the inactive 1S-trans-tetramethrin antagonized the action of the active 1R-trans- and 1R-cis-tetramethrin in a non-competitive manner. So did the inactive 1S-cis-tetramethrin when tested against 1R-trans-tetramethrin. However, the inactive 1S-cis-tetramethrin competes with the active 1R-cis-tetramethrin for the same binding site and antagonizes the action of the active isomer. Based on their findings Lund and Narahashi (1982) proposed that there are multiple binding sites for pyrethroids in the sodium channel.
Earlier electrophysiological and pharmacological studies from other laboratories also indicate that the pyrethroid receptor site is distinct from, yet allosterically coupled with several other receptor sites, such as site 2 to which BTX and GTX bind (Refs. in Catterall, 1992; Gordon, 1997; Lazdunski et al., 1988). Specific binding of radiolabeled pyrethroids was detected in rat brain membrane preparations (Trainer et al., 1997). However, attempts to characterize specific binding of pyrethroids to insect nerve membrane preparations have failed because of the extreme hydrophobicity of pyrethroids, resulting in extremely high nonspecific binding (Dong, 1993; Pauron et al., 1989; Rossignol, 1988).
3. Molecular mechanism of knockdown resistance to DDT and pyrethroids in insect disease vectors and pests
Pyrethroids are extensively used to control agricultural pests and vectors of diseases because of their relatively low mammalian toxicity and relatively favorable environmental properties. Currently, pyrethroid-treated bed nets are some of the most powerful control measures used to limit malaria morbidity and mortality worldwide (WHO, 2007, 2009). However, intensive use of DDT first and then pyrethroids over several decades has led to the development of resistance in many insect populations.
One major mechanism of resistance is known as knockdown resistance (kdr) (Soderlund and Bloomquist, 1990). Since its initial report in the house fly (Busvine, 1951; Milani, 1954), kdr has been documented globally in almost all medically and agriculturally significant arthropod pests (Rinkevich et al., 2013; Soderlund, 2005, 2012). So far, more than 50 sodium channel mutations are associated with pyrethroid resistance in diverse arthropod pests (Dong et al., 2014); more than 20 of these mutations have been confirmed to reduce the pyrethroid sensitivity of insect sodium channels, DmNav from the fruit fly, Drosophila melanogaster; BgNav from the German cockroach, Blattella germanica; Vssc1 from the housefly, Musca domestica; or AaNav from the yellow fever mosquito, Aedes aegypti, expressed in the African clawed frog Xenopus laevis oocyte expression system (Du et al., 2013; Rinkevich et al., 2013). Identification of kdr mutations not only provides precise molecular markers for rapidly assessing the frequency of resistance alleles in field populations, but has also proven to be extremely valuable for elucidating structural features of sodium channels that are critical for the binding and action of pyrethroids (see below).
4. Molecular evidence for dual pyrethroid/DDT receptor sites in insect sodium channels
The molecular identity of pyrethroid binding site on the sodium channel was a major unresolved issue in sodium channel pharmacology for decades. Recent accumulation of data on kdr mutations and advances in homology modeling of sodium channels has made it possible to unravel the molecular identity of the elusive pyrethroid receptor sites.
The alpha subunit of eukaryotic voltage-gated sodium channels folds from a single polypeptide chain and comprises four homologous repeat domains (I–IV), each having six transmembrane helical segments (S1–S6) and a membrane re-entering P-loop (Catterall, 2012; Dong et al., 2014), see Fig. 2. Segments S1–S4 in each domain constitute a voltage-sensing module, which is connected through a linker-helix S4–S5 (L45) to the pore module. Each domain provides to the pore module an outer helix S5, a pore-lining inner helix S6, and a P-loop. The pore module encircles the central pore, while the four voltage-sensing modules are arranged around the pore module. In response to membrane depolarization, the S4 segments move outward, initiating opening of the activation gate formed by cytoplasmic parts of S6s. At the extracellular view, repeat domains I, II, III and IV are arranged clockwise (Dudley et al., 2000). In the absence of high-resolution structures of eukaryotic sodium channels, X-ray structures of homotetrameric potassium and prokaryotic sodium channels, such as KcsA (Doyle et al., 1998), Kv1.2 (Long et al., 2005) and NavAb (Payandeh et al., 2011) are used to build homology models of mammalian and insect sodium channels. Computational docking of drugs and toxins in these models, is used to integrate various experimental data, including mutational analysis of ligand binding sites, and predict atomic details of ligand-channel complexes, e.g. (Du et al., 2011; Lipkind and Fozzard, 2005; Tikhonov and Zhorov, 2005, 2007). The ligand-bound channel models, in turn, are used for further experimental studies.
Fig. 2.
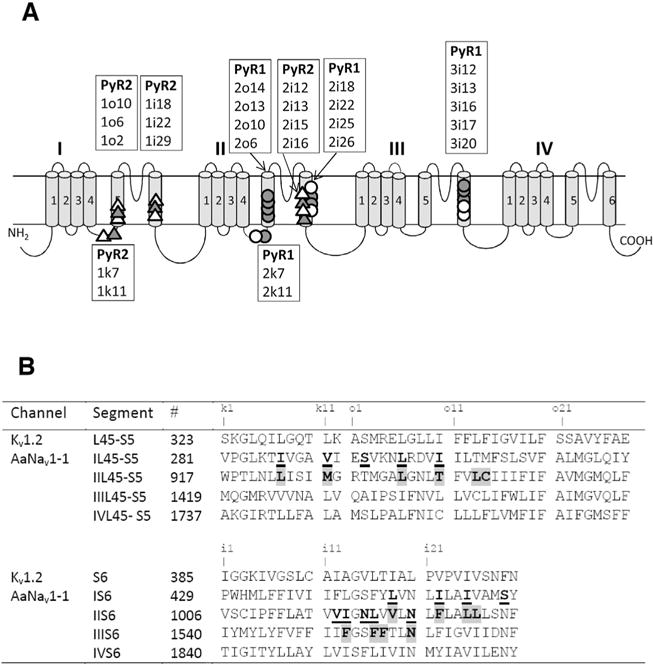
(A) Topology of sodium channel indicating residues within/around PyR1 and PyR2. The open circles and open triangles indicate, respectively, positions of mutations within/around PyR1 and PyR2, which affect action of pyrethroids. The filled circles and filled triangles indicate, respectively, positions of mutations within/around PyR1 and PyR2 that affect action of both pyrethroids and DDT, see (Du et al., 2015; Du et al., 2016) and references therein. Labels of respective positions are shown in boxes above transmembrane helices S5, S6 or below L45 linker-helices. To describe sequential positions of residues within the channel we use a residue-labeling scheme (Du et al., 2013; Zhorov and Tikhonov, 2004) where a label includes the domain number (1–4), segment type (k, the linker-helix L45; i, the inner helix S6; and o, the outer helix S5), and relative number of the residue in the segment. This provides the same labels to residues in the matching positions of the sequence alignment of sodium channels from different organisms and highlights symmetric location of residues in different channel domains. See Table 1 for more details. (B) The aligned sequences of Kv1.2 and AaNav1-1 channels. Residues predicted to contribute to PyR1 or control ligand access to PyR1 are highlighted. Residues predicted to contribute to PyR2 or control ligand access to PyR1 are underlined. Substitutions of these residues have been tested experimentally (Du et al., 2013, 2015, 2016; O’Reilly et al., 2006; Usherwood et al., 2007). Characters “k1”, “o1”, “i1”, etc. mark relative positions of residues, respectively, in the linker-helices L45, outer helices S5 and inner helices S6.
The pioneering model of the pyrethroid receptor site, PyR1, (O’Reilly et al., 2006; Usherwood et al., 2007) was elaborated for the housefly open sodium channel using as a template the X-ray structure of a voltage-gated potassium channel Kv1.2. In this model, which is based on experimental data on kdr and site-directed mutations, pyrethroids bind to the lipid-exposed interface between domains II and III and interact with pyrethroid-sensing residues in the linker-helix IIL45, the outer helix IIS5 and the inner helix IIIS6. For the first time, these studies proposed atomic-level rationale for the mechanism of action of pyrethroid insecticides, their state-dependent affinity to the sodium channel, and the role of the channel mutations that confer insecticide resistance.
Based on experimental data that become available after 2006, we elaborated a model for a second pyrethroid receptor site, PyR2, in the lipid-exposed interface between domains I and II with ligands bound to pyrethroid-sensing residues in helices IL45, IS5, IS6 and IIS6 (Du et al., 2013). We suggested that simultaneous binding of pyrethroids to both PyR1 and PyR2 is required to effectively prolong the opening of sodium channels (Du et al., 2013). This proposition is consistent with the Hill analysis, which suggests more than one pyrethroid binding site in the sodium channel (Vais et al., 2000b).
The original PyR1 and PyR2 models differ in ligand orientation and the number of transmembrane helices involved. Recently discovered kdr mutations motivated us to revise the PyR1 model. We docked two deltamethrin molecules in the PyR2 and PyR1 sites and arrived to the ternary complex model in which dibromoe-thenyl and diphenylether moieties of both ligands are oriented in the intra- and extracellular directions, respectively (Du et al., 2015). The model-driven mutagenesis identified seven new pyrethroid-sensing residues, thus supporting the dual pyrethroid-receptor sites concept. PyR1 and PyR2 receptor sites share several common features. In the proposed model, each pyrethroid receptor is formed by a linker-helix L45 and three transmembrane helices (S5 and two S6s) with helix IIS6 containing four residues that contribute to PyR1 and another four to PyR2. In homology models, which are based on the X-ray structures of homotetrameric ion channels, the folding of the L45-S5-S6 interfaces where PyR1 and PyR2 are located is rather similar. In real channels individual L45-S5-S6 interfaces may be asymmetric, as can be seen in the recent Cryo_EM structure of calcium channel Cav1.1 (Wu et al., 2015), but the general folding of the interfaces still have common features. Seven pairs of pyrethroid-sensing residues are located in symmetric positions within PyR1 and PyR2. Except for positions o10, all pyrethroid-sensing residues in PyR1 and PyR2 are hydrophobic. Particularly, at positions 2k7/3k7 and 1i25/2i25, the pyrethroid-sensing residues are leucine or isoleucine, which do not differ significantly. However, the two pyrethroid binding sites are not identical. Except for positions 1o6/2o6 and 2i20/3i20, other symmetric positions in PyR1 and PyR2 have different residues. Nevertheless, our models predict that pyrethroids bind to PyR1 and PyR2 in similar orientations, penetrating deeply into the respective domain interfaces (Fig. 3A, B) with the bulky dimethylcyclopropyl groups bound at the kink regions between L45 and S6 helices and also interacting with two S6 helices (Du et al., 2015). Binding of a ligand to two homologous receptor sites on one sodium channel is quite unique, but not unprecedented. Other examples of ion channels, which contain two similar, but not identical binding sites for agonists, are heteromeric nicotine acetylcholine receptor (Schapira et al., 2002) and glycine receptors (Dutertre et al., 2012).
Fig. 3.
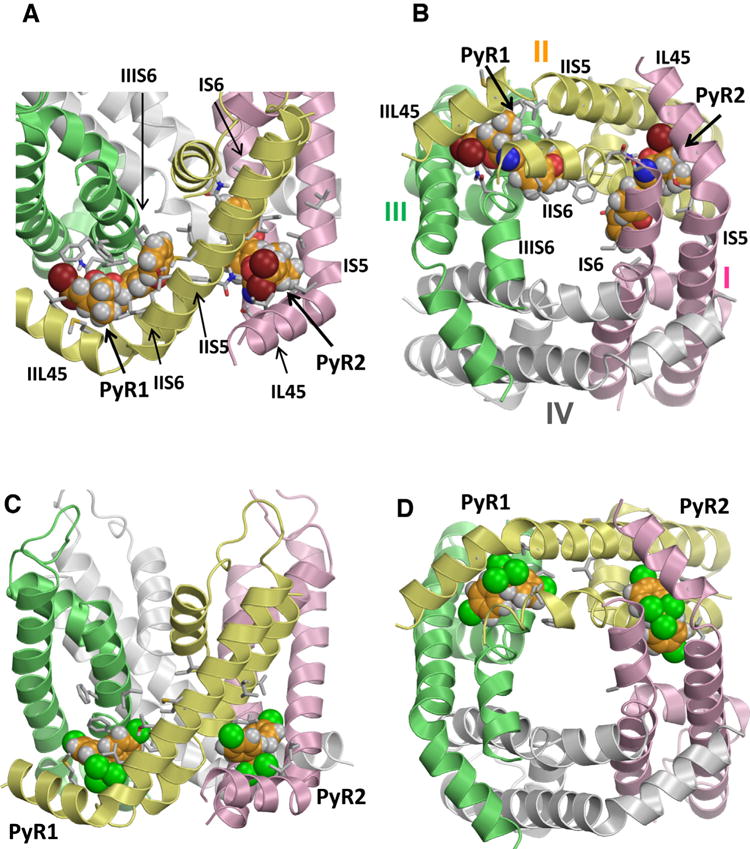
Kv1.2-based model of the open AaNav1-1 channel pore module with two DMT molecules (A,B) and two DDT molecules (C,D) docked into PyR2 and revised PyR1 sites. Helices in domains I, II, III and IV are shown by pink, yellow, green and white ribbons, respectively. Arrows point to the kink regions between helices L45 and S5 of respective receptors. Known pyrethroid-sensing residues are shown as sticks and respective helices are labeled. Carbon, oxygen, nitrogen and hydrogen atoms of the ligands are, respectively, orange, red, blue, and gray. Bromine atoms in DMT are brown and chlorine atoms in DDT are green. Note that the bulkiest moieties of the insecticides (dimethylcyclopyl group of DMT and trichloromethyl group of DDT) bind similarly between L45, S5 and S6 helices, while aromatic substituents of the bulkiest groups extend between two domains. Figures A and B are Reproduced with permission from Molecular Pharmacology (Du Y, Nomura, Y, Zhorov BS, and Dong K. Rotational symmetry of two pyrethroid receptor sites in the mosquito sodium channel. 2015; 88: 273–280). Figures C and D are originally published in the Journal of Biological Chemistry (Du et al., 2016). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The use of DDT is banned in most of the world due to its detrimental impact on the ecosystem, but indoor residual spraying of DDT is still recommended for malaria control in Africa. Mapping DDT binding sites is necessary for understanding mechanisms of resistance and modulation of sodium channels by structurally different ligands. DDT was initially proposed to bind within PyR1 with the bulky trichloromethyl group in the domain II/III interface (fenestration), between helices IIS5, IIP and IIIS6, one p-chlorophenyl ring orienting towards lipids and the other p-chlorophenyl ring extending along the IIS5 helix towards the kink between IIL45 and IIS5 (O’Reilly et al., 2006). We recently docked two DDT molecules into the Kv1.2-based mosquito sodium channel model and predicted that both ligands can bind simultaneously within PyR1 and PyR2 (Du et al., 2016). The bulky trichloromethyl group of each DDT molecule fits snugly between four helices in the bent domain interface, while two p-chlorophenyl rings extend into two wings of the interface (Fig. 3C, D). Model-driven mutagenesis and electrophysiological analysis confirmed these propositions and revealed ten previously unknown DDT-sensing residues within PyR1 and PyR2 (Du et al., 2016).
5. Molecular basis of differential sensitivities of insect and mammalian sodium channels to pyrethroids
In mammals, at least nine different sodium channel isoforms (Nav1.1 to Nav1.9) are expressed (Catterall et al., 2003; Goldin et al., 2000). Nav1.1, Nav1.2, Nav1.3 and Nav1.6 are predominately expressed in the central nervous system (CNS); Nav1.7, Nav1.8 and Nav1.9 in the peripheral nervous system (PNS); and Nav1.4 and Nav1.5 mainly in skeletal and cardiac muscles, respectively. Mammalian sodium channel isoforms also exhibit distinct electrophysiological and pharmacological properties (Dib-Hajj et al., 2002; Goldin, 2001; Yu and Catterall, 2003). Selective expression of different sodium channel genes contributes to the specialized function of sodium channels in various mammalian tissues and cell types (Yu and Catterall, 2003).
Differential sensitivities of mammalian sodium channels to pyrethroids were documented in electrophysiological studies using neuronal tissue preparations. Rat dorsal root ganglion (DRG) neurons have two types of current, tetrodotoxin-sensitive (TTX-S), carried primarily by Nav1.2, 1.3, 1.6, and 1.7 sodium channels, and tetrodotoxin-resistant (TTX-R), carried mainly by Nav1.8 channels (Rush et al., 2007). Narahashi and associates showed that TTX-S channels were less sensitive to pyrethroids than TTX-R channels in the same neurons (Ginsburg and Narahashi, 1993; Song and Narahashi, 1996; Tatebayashi and Narahashi, 1994). More recent comparison of pyrethroid sensitivity among mammalian sodium channels expressed in Xenopus oocytes by Soderlund et al., confirmed and extended these interesting pharmacological observations. Rat Nav1.2, Nav1.4, and Nav1.7 channels are almost insensitive to pyrethroids, whereas rat Nav1.3; Nav1.6 and Nav1.8 sodium channels are relatively more sensitive to pyrethroids (Choi and Soderlund, 2006; Du et al., 2013; Peng et al., 2009; Smith et al., 1998; Tan and Soderlund, 2009, 2010, 2011; Vais et al., 1997; Wang et al., 2001; Warmke et al., 1997). However, insect sodium channels are much more sensitive to pyrethroids than their mammalian counterparts (Du et al., 2013; Warmke et al., 1997), which partially contributes to the selective toxicity of pyrethroids. Identification of pyrethroid-sensing residues in insect sodium channels has proven to be a valuable resource for elucidating the molecular basis of selective toxicity of pyrethroids. At the position corresponding to the M918T (M2k11T) kdr mutation in IIL45 (see Fig. 2 legend for description of residue labels), mammalian sodium channels have an isoleucine (Fig. 4). Substitution of the isoleucine in the rNav1.2 channel with a methionine increased channel sensitivity to pyrethroids (Vais et al., 2000). Pyrethroid-sensitive rat sodium channel isoforms also contain isoleucine at this position and the I2k11M mutation further increases the sensitivity of rat Nav1.8 sodium channels to cismethrin (Soderlund and Lee, 2001). More recently, substitutions of leucine in IL45 and methionine in IS5 in rNav1.2 and rNav1.4, respectively, with valine and isoleucine, which occur in insect sodium channels, also enhanced the sensitivity of rat sodium channels to pyrethroids (Du et al., 2013). Furthermore, these residues are conserved in all known mammalian sodium channels. These results indicate that isoleucine in IIL45, leucine in IL45 and methionine in IS5 contribute to the lower sensitivity of mammalian versus insect sodium channels to pyrethroids.
Fig. 4.
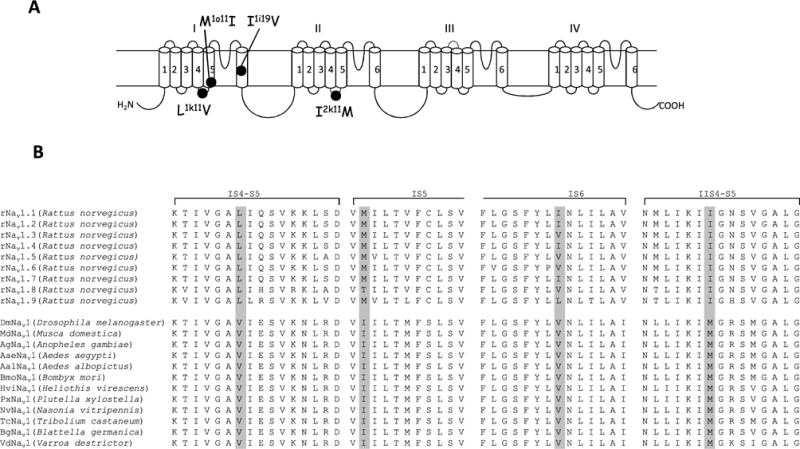
Molecular basis of different pyrethroid sensitivities among insect and mammalian sodium channels. (A) Topology of the Nav1.4 protein indicating the residues that contribute to the resistance of mammalian sodium channels to pyrethroids. (B) Sequence alignments of mammalian and insect sodium channels in the regions that are critical for the binding and action of pyrethroids.
Identification of kdr mutations in IS6 also helped uncover the molecular determinants for the differential sensitivities of mammalian sodium channel isoforms to pyrethroids (Oliveira et al., 2013). Valine to isoleucine or leucine substitutions in IS6 (V409I/L, V1i19I/L) reduced cockroach sodium channel sensitivity to pyrethroids (Oliveira et al., 2013). At the corresponding position, a valine is also present in Nav1.3, Nav1.5, Nav1.6 and Nav1.8 channels, but an isoleucine is found in Nav1.1, Nav1.2, and Nav1.4, which are more resistant to pyrethroids than Nav1.3, Nav1.5, Nav1.6 and Nav1.8 channels (Soderlund, 2012). As expected, a valine substitution of isoleucine (I433 V, Ii19V) enhanced the sensitivity of rNav1.4 channels to deltamethrin (Oliveira et al., 2013).
6. Challenges for the structure-based design of new insecticides
Mapping ligand binding sites in ion channels is based on results of mutational and electrophysiological studies. A mutation may affect binding of a ligand directly, by modifying a ligand-binding residue, or indirectly, e.g. by changing the channel conformation or population of different channel states. Discriminating direct and indirect effects of mutations is difficult. One approach is comparison of electrophysiological properties of wild-type and mutated channels. If a mutation dramatically affected gating properties, it may have affected ligand action indirectly. Another problem is that homology models of pseudo-heteromeric sodium channels, which are based on X-ray structures of homotetrameric channels, are not precise enough to predict energetics of ligand-channels interactions, which would help better understand interactions of Type I and Type II pyrethroids and other ligands with PyR1 and PyR2. However, these problems do not undermine the importance of studies that combine experimental approaches, including site-directed mutagenesis and electrophysiology, and molecular modeling. Future efforts towards discovery and development of new insecticides should involve close collaborations with synthetic chemists.
Table 1.
Effects of mutations within the two pyrethroid receptor sites on the action of DMT and PMT. Symbols ↓, ↑, and ≈indicate decrease, increase, or insignificant change of the ligand potency, respectively.
| PyR1
|
PyR2
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mutant | Ref.a | DMT | PMT | DDT | Mutant | Ref.a | DMT | PMT | DDT |
| L22k7F/I | 1 | ↓/↓ | ≈/↑ | ≈ | I1k7A | 3 | ↓ | ↓ | |
| M2k11T | 1 | ↓ | ↓ | ↑ | V1k11A | 3,6 | ↓ | ↓ | ↓ |
| S11o2A | 5 | ↓ | ↓ | ||||||
| L2o6I | 1 | ↓ | ↓ | ↓ | L1o6I/A | 3,5,6 | ↑/≈ | ≈/↑ | ↑/ |
| T22o10I | 1 | ↓ | ↓ | ↓ | I1o10C | 3,6 | ↓ | ↓ | ≈ |
| L22o13F | 1 | ↓ | ≈ | ↓ | T1o13W/A | 3,6 | ≈/ | ≈/ | /≈ |
| C22o14A | 1 | ↓ | ↑ | ↓ | |||||
| V2i18G | 3 | ↓ | ↓ | L1i18G | 3 | ↓ | ↓ | ||
| F2i22Sb | 5,6 | ↓ | ↓ | ↓ | I11i22A | 5,6 | ↓ | ↓ | ↑ |
| L22i25A | 5,6 | ↓ | ↓ | ↓ | I11i25A | 5,6 | ≈ | ≈ | ≈ |
| L22i26A | 5 | ↓ | ↓ | ||||||
| S1i29A | 5 | ↓ | ↓ | ||||||
| I3i12A | 2,6 | ↑ | ↑ | ↓ | V22i12A/L | 5,6 | ≈/↑ | ↑/↑ | ≈/ |
| F33i13C | 3,6 | ↓ | ↓ | ↓ | I22i13M | 3,6 | ≈ | ↓ | ↓ |
| S33i15A | 2 | ≈ | ≈ | N2i15S | 5 | ↓ | ↓ | ||
| F3i16A | 2,6 | ↓ | ↓ | ↓ | L22i16F/S | 3,6 | ↓/↓ | ↓/↓ | ↓/ |
| F33i17I | 4 | ↓ | ↓ | ||||||
| N3i20A | 2 | ↓ | ↓ | N2i20A | 5 | ≈ | ≈ | ||
References: 1, (Usherwood et al., 2007); 2 (Du et al., 2009); 3 (Du et al., 2013) and references therein; 4 (Tan et al., 2005); 5, (Du et al., 2015); 6, (Du et al., 2016).
F2i22 contributes to both PyR1 and PyR2.
Acknowledgments
The research on the mechanism of action and resistance of sodium channel-targeting insecticides is supported by a grant from NIH (GM 57440) to KD and BSZ. We thank past and current research associates for their contributions to the research in the Dong lab. We dedicate this review to the late Professor T. Narahashi for his seminal contributions to the field of neuropharmacology and neurotoxicology, his pioneering work on the mode of action of pyrethroids, and his encouragement and support of Dong’s research program over the past two decades.
Abbreviations
- BTX
batrachotoxin
- DDT1
1,1-trichloro-2,2-bis(p-chlorophenyl) ethane
- DMT
deltamethrin
- GTX
grayanotoxin
- PMT
permethrin
- TTX
tetrodotoxin
- WHO
World Health Organization
References
- Bloomquist JR, Soderlund DM. Pyrethroid insecticides and DDT modify alkaloid-dependent sodium channel activation and its enhancement by sea anemone toxin. Mol Pharmacol. 1988;33(5):543–550. [PubMed] [Google Scholar]
- Bloomquist JR. Ion channels as targets for insecticides. Ann Rev Entomol. 1996;41(1):163–190. doi: 10.1146/annurev.en.41.010196.001115. [DOI] [PubMed] [Google Scholar]
- Busvine JR. Mechanism of resistance to insecticide in houseflies. Nature. 1951;168(4266):193–195. doi: 10.1038/168193a0. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Swanson TM. Structural basis for pharmacology of voltage-gated sodium and calcium channels. Mol Pharmacol. 2015;88(1):141–150. doi: 10.1124/mol.114.097659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XXXIX Compendium of voltage-gated ion channels: sodium channels. Pharmacol Rev. 2003;55(4):575–578. doi: 10.1124/pr.55.4.7. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72(Suppl. 4):S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590(11):2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82(9–10):883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- Choi JS, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Na v 1.8 sodium channels expressed in Xenopus oocytes. Toxicol Appl Pharmacol. 2006;211(3):233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S, Black JA, Cummins TR, Waxman SG. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 2002;25(5):253–259. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol. 2014;50:1–17. doi: 10.1016/j.ibmb.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K. Voltage-gated Sodium Channels as Insecticide Targets. 1993 doi: 10.1016/B978-0-12-417010-0.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280(5360):69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Du Y, Lee JE, Nomura Y, Zhang T, Zhorov BS, Dong K. Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem J. 2009;419(2):377–385. doi: 10.1042/BJ20082082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Garden D, Khambay B, Zhorov BS, Dong K. Batrachotoxin, pyrethroids, and BTG 502 share overlapping binding sites on insect sodium channels. Mol Pharmacol. 2011;80(3):426–433. doi: 10.1124/mol.111.072504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, Zhorov BS, Dong K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci U S A. 2013;110(29):11785–11790. doi: 10.1073/pnas.1305118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Zhorov BS, Dong K. Rotational symmetry of two pyrethroid receptor sites in the mosquito sodium channel. Mol Pharmacol. 2015;88(2):273–280. doi: 10.1124/mol.115.098707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Zhorov BS, Dong K. Evidence for dual binding sites for DDT in insect sodium channels. J Biol Chem. 2016 doi: 10.1074/jbc.M115.678672. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley SC, Jr, Chang N, Hall J, Lipkind G, Fozzard HA, French RJ. muconotoxin GIIIA interactions with the voltage-gated Na(+) channel predict a clockwise arrangement of the domains. J Gen Physiol. 2000;116(5):679–690. doi: 10.1085/jgp.116.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre S, Drwal M, Laube B, Betz H. Probing the pharmacological properties of distinct subunit interfaces within heteromeric glycine receptors reveals a functional betabeta agonist-binding site. J Neurochem. 2012;122(1):38–47. doi: 10.1111/j.1471-4159.2012.07755.x. [DOI] [PubMed] [Google Scholar]
- Elliott M. Synthetic Pyrethroids. American Chemical Society; Washington D.C: 1977. [Google Scholar]
- Ginsburg KS, Narahashi T. Differential sensitivity of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels to the insecticide allethrin in rat dorsal root ganglion neurons. Brain Res. 1993;627(2):239–248. doi: 10.1016/0006-8993(93)90326-i. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28(2):365. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Gordon D. A new approach to insect-pest control–combination of neurotoxins interacting with voltage sensitive sodium channels to increase selectivity and specificity. Invert Neurosci. 1997;3(2–3):103–116. doi: 10.1007/BF02480365. [DOI] [PubMed] [Google Scholar]
- Lazdunski M, Lombet A, Mourre C. Specific Binding Sites for Pyrethroids on the Voltage-Dependent Sodium Channel. Elsevier Science; 1988. [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol. 2005;68(6):1611–1622. doi: 10.1124/mol.105.014803. [DOI] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309(5736):897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Lund AE, Narahashi T. Dose-dependent interaction of the pyrethroid isomers with sodium channels of squid axon membranes. Neurotoxicology. 1982;3(1):11–24. [PubMed] [Google Scholar]
- Milani R. Comportamento mendeliano della resistenza alla azione abbatante del DDT: correlazione tran abbatimento e mortalia in Musca domestica L. Riv Parasitol. 1954;15:513–542. [PubMed] [Google Scholar]
- Narahashi T, Anderson NC. Mechanism of excitation block by the insecticide allethrin applied externally and internally to squid giant axons. Toxicol Appl Pharmacol. 1967;10(3):529–547. doi: 10.1016/0041-008x(67)90092-0. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Haas HG. DDT: interaction with nerve membrane conductance changes. Science. 1967;157(3795):1438–1440. doi: 10.1126/science.157.3795.1438. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Haas HG. Interaction of DDT with the components of lobster nerve membrane conductance. J Gen Physiol. 1968;51(2):177–198. doi: 10.1085/jgp.51.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T, Frey JM, Ginsburg KS, Roy ML. Sodium and GABA-activated channels as the targets of pyrethroids and cyclodienes. Toxicol Lett. 1992;64–65:429–436. doi: 10.1016/0378-4274(92)90216-7. Sec No. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Toxins that modulate the sodium channel gating mechanism. Ann N Y Acad Sci. 1986;479:133–151. doi: 10.1111/j.1749-6632.1986.tb15566.x. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Molecular and Cellular Approaches to Neurotoxicology: Past, Present and Future. Elsevier; New York: 1988. [Google Scholar]
- Narahashi T. Nerve membrane Na+ channels as targets of insecticides. Trends Pharmacol Sci. 1992;13(6):236–241. doi: 10.1016/0165-6147(92)90075-h. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Neuronal ion channels as the target sites of insecticides. Pharmacol Toxicol. 1996;78:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: past, present, and future. J Pharmacol Exp Ther. 2000;294(1):1–26. [PubMed] [Google Scholar]
- Nishimura K, Narahashi T. Structure-activity relationships of pyrethroids based on direct action on nerve. Pest Biochem Physiol. 1978;8(1):53–64. [Google Scholar]
- O’Reilly AO, Khambay BP, Williamson MS, Field LM, Wallace BA, Davies TG. Modelling insecticide-binding sites in the voltage-gated sodium channel. Biochem J. 2006;396(2):255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira EE, Du Y, Nomura Y, Dong K. A residue in the transmembrane segment 6 of domain I in insect and mammalian sodium channels regulate differential sensitivities to pyrethroid insecticides. Neurotoxicology. 2013;38C:42–50. doi: 10.1016/j.neuro.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauron D, Barhanin J, Amichot M, Pralavorio M, Berge JB, Lazdunski M. Pyrethroid receptor in the insect sodium channel: alteration of its properties in pyrethroid-resistant flies. Biochemistry. 1989;28(4):1673–1677. [Google Scholar]
- Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475(7356):353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F, Mellor IR, Williamson MS, Davies TG, Field LM, Usherwood PN. Single channel study of deltamethrin interactions with wild-type and mutated rat NaV1.2 sodium channels expressed in Xenopus oocytes. Neurotoxicology. 2009;30(3):358–367. doi: 10.1016/j.neuro.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Rinkevich FD, Du Y, Dong K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol. 2013;106(3):93–100. doi: 10.1016/j.pestbp.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DP. Reduction in number of nerve membrane sodium channels in pyrethroid resistant house flies. Pestic Biochem Physiol. 1988;32(2):146–152. [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579(Pt 1):1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira M, Abagyan R, Totrov M. Structural model of nicotinic acetylcholine receptor isotypes bound to acetylcholine and nicotine. BMC Struct Biol. 2002;2:1. doi: 10.1186/1472-6807-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Ingles PJ, Soderlund DM. Actions of the pyrethroid insecticides cismethrin and cypermethrin on house fly Vssc1 sodium channels expressed in Xenopus oocytes. Arch Insect Biochem Physiol. 1998;38(3):126–136. doi: 10.1002/(SICI)1520-6327(1998)38:3<126::AID-ARCH3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Bloomquist JR. Molecular Mechanisms of Insecticide Resistance. Chapman and Hall; New York: 1990. [Google Scholar]
- Soderlund DM, Lee SH. Point mutations in homology domain II modify the sensitivity of rat Nav1.8 sodium channels to the pyrethroid insecticide cismethrin. Neurotoxicology. 2001;22(6):755–765. doi: 10.1016/s0161-813x(01)00065-1. [DOI] [PubMed] [Google Scholar]
- Soderlund DM. Sodium channels. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Elsevier; Amsterdam: 2005. pp. 1–24. [Google Scholar]
- Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pestic Biochem Physiol. 2010;97(2):78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM. Molecular mechanisms of pyrethroid insecticide neurotoxicity: recent advances. Arch Toxicol. 2012;86:165–181. doi: 10.1007/s00204-011-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Narahashi T. Differential effects of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant single sodium channels. Brain Res. 1996;712(2):258–264. doi: 10.1016/0006-8993(95)01449-7. [DOI] [PubMed] [Google Scholar]
- Takeda K, Narahashi T. Chemical modification of sodium channel inactivation: separate sites for the action of grayanotoxin and tetramethrin. Brain Res. 1988;448(2):308–312. doi: 10.1016/0006-8993(88)91268-1. [DOI] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30(1):81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Nav1.6 sodium channels. Toxicol Appl Pharmacol. 2010;247(3):229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Soderlund DM. Actions of tefluthrin on rat Nav1.7 voltage-gated sodium channels expressed in Xenopus oocytes. Pestic Biochem Physiol. 2011;101(1):21–26. doi: 10.1016/j.pestbp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Liu Z, Wang R, Huang ZY, Chen AC, Gurevitz M, Dong K. Identification of amino acid residues in the insect sodium channel critical for pyrethroid binding. Mol Pharmacol. 2005;67(2):513–522. doi: 10.1124/mol.104.006205. [DOI] [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther. 1994;270(2):595–603. [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Sodium channel activators: model of binding inside the pore and a possible mechanism of action. FEBS Lett. 2005;579(20):4207–4212. doi: 10.1016/j.febslet.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Sodium channels: ionic model of slow inactivation and state-dependent drug binding. Biophys J. 2007;93(5):1557–1570. doi: 10.1529/biophysj.106.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainer VL, McPhee JC, Boutelet-Bochan H, Baker C, Scheuer T, Babin D, Demoute JP, Guedin D, Catterall WA. High affinity binding of pyrethroids to the alpha subunit of brain sodium channels. Mol Pharmacol. 1997;51(4):651–657. doi: 10.1124/mol.51.4.651. [DOI] [PubMed] [Google Scholar]
- Usherwood PNR, Davies TGE, Mellor IR, O’Reilly AO, Peng F, Vais H, Khambay BPS, Field LM, Williamson MS. Mutations in DIIS5 and the DIIS4-S5 linker of Drosophila melanogaster sodium channel define binding domains for pyrethroids and DDT. FEBS Lett. 2007;581(28):5485–5492. doi: 10.1016/j.febslet.2007.10.057. [DOI] [PubMed] [Google Scholar]
- Vais H, Williamson MS, Hick CA, Eldursi N, Devonshire AL, Usherwood PN. Functional analysis of a rat sodium channel carrying a mutation for insect knock-down resistance (kdr) to pyrethroids. FEBS Lett. 1997;413(2):327–332. doi: 10.1016/s0014-5793(97)00931-9. [DOI] [PubMed] [Google Scholar]
- Vais H, Atkinson S, Eldursi N, Devonshire AL, Williamson MS, Usherwood PN. A single amino acid change makes a rat neuronal sodium channel highly sensitive to pyrethroid insecticides. FEBS Lett. 2000;470(2):135–138. doi: 10.1016/s0014-5793(00)01305-3. [DOI] [PubMed] [Google Scholar]
- Vijverberg HP, van den Bercken J. Annotation. Action of pyrethroid insecticides on the vertebrate nervous system. Neuropathol Appl Neurobiol. 1982;8(6):421–440. doi: 10.1111/j.1365-2990.1982.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Vijverberg HP, van der Zalm JM, van der Bercken J. Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves. Nature. 1982;295(5850):601–603. doi: 10.1038/295601a0. [DOI] [PubMed] [Google Scholar]
- WHO. Insecticide-treated mosquito nets: a WHO position statement 2007 [Google Scholar]
- WHO. WHO recommended long-lasting insecticidal mosquito nets 2009 [Google Scholar]
- Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15(2):151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Wang SY, Barile M, Wang GK. A phenylalanine residue at segment D3-S6 in Na+ v1.4 voltage-gated Na channels is critical for pyrethroid action. Mol Pharmacol. 2001;60(3):620–628. [PubMed] [Google Scholar]
- Warmke JW, Reenan RAG, Wang PY, Qian S, Arena JP, Wang JX, Wunderler D, Liu K, Kaczorowski GJ, VanderPloeg LHT, Ganetzky B, Cohen CJ. Functional expression of Drosophila para sodium channels – Modulation by the membrane protein TipE and toxin pharmacology. J Gen Physiol. 1997;110(2):119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Yan Z, Li Z, Yan C, Lu S, Dong M, Yan N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 2015;350:6267. doi: 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4(3):207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhorov BS, Tikhonov DB. Potassium, sodium, calcium and glutamate-gated channels: pore architecture and ligand action. J Neurochem. 2004;88(4):782–799. doi: 10.1111/j.1471-4159.2004.02261.x. [DOI] [PubMed] [Google Scholar]


