Abstract
We synthesized a new type of upper critical solution temperature (UCST) thermally responsive polymers (TRPs) with varying responsive temperatures (cloud points). We then grafted one of the TRPs with a cloud point of 42°C on the surface of mesoporous silica nanoparticles (MSN) using disulfide bonds to achieve a novel, dual responsive release system. With this system, the cargo release profiles are responsive to both temperature and reducing agents. When loaded with doxorubicin hydrochloride (DOX), the system could deliver DOX into breast cancer cells (SK-BR-3) in a controlled fashion and present high toxicity.
Graphical Abstract
A novel, dual responsive and intracellular delivery system was developed by grafting UCST-type polymers onto the surface of mesoporous silica nanoparticles through disulfide bonds.
Traditional intravenous cancer therapy is inefficient and could lead to undesirable side effects.1 Localized and controlled drug delivery systems have the potential to increase the effectiveness of cancer therapy and minimize healthy tissue exposure to toxic chemo- and radiotherapy reagents.2 Therefore controlled drug delivery systems are highly desired3 in advanced cancer therapy.4
Due to the increasing popularity of temperature-mediated (thermal) therapies, many localized in vivo temperature-regulating technologies suitable for human patient treatments in a clinical setting, including near infrared light, microwave and ultrasound, have been developed.5 Low temperature hyperthermia (39–41°C for times up to 72 h) and moderate temperature hyperthermia (42–45°C for 15–60 min repeatedly) have been utilized in clinical trials without harmful side effects.5 Because of the minimally invasive nature, such technologies are actively investigated to increase temperature locally to achieve spatially and temporally controlled drug delivery. Since cancer cells are more vulnerable to high temperature than normal cells, cancer drug delivery systems utilizing hyperthermia-triggered delivery systems are especially attractive because they can benefit from the hyperthermia-induced apoptosis mechanism.6
Therefore, thermally responsive polymers (TRPs) are increasingly utilized as therapeutic drug/biomolecule carriers in cancer treatment and other therapeutic applications in the past decade.7 TRPs exhibit reversible and temperature-dependent transformation between hydrophilic and hydrophobic phases, which allows for cargo loading and controlled release with thermal stimulation.8 The molecular chain morphology of TRPs changes with temperature in aqueous solution, which is the basis for constructing thermally controlled release systems.8 A coil to globule morphology transition occurs when a typical TRP responds to a temperature change at its solution temperature.9 This characteristic of TRPs with a lower critical solution temperature (LCST), such as poly(N-isopropylacrylamide) (PNIPAM), which has a cloud point around 32°C, have been widely utilized in constructing controlled release systems to improve the therapeutic efficiency of delivered drugs or biomolecules.10
Polymers with upper critical solution temperature (UCST) assume coil morphology at temperatures above UCST and undergo phase inversion to assume a globular morphology at temperatures below UCST.11 However, previously reported UCST polymers are mainly zwitterionic polymers (contain a positive ion at one location and a negative ion at another location in the polymer chain), such as sulfobetaine-based methacrylate polymers. They have limited applications in physiological systems due to the loss of thermal responsiveness in the presence of electrolytes.12 In the past few years, significant progress has been made in developing novel UCST polymers utilizing hydrogen bonding, which are less sensitive to ion concentrations than traditional zwitterionic polymers. Examples of such polymers include poly(N-acryloyl glycinamide) and ureido-derivatives.13
TRPs with an UCST higher than 37°C are hydrophobic under physiological condition. The hydrophobicity enhances the loading efficiency of anti-cancer drugs, which are frequently hydrophobic, and reduces burst release. When triggered with heat (reaching a temperature higher than UCST), the polymers become hydrophilic and more mobile for excretion.14 By conjugating polyethylene glycol (PEG) to poly(acrylamide-co-acrylonitrile), UCST polymeric micelles have been prepared and shown to increase loading and delivery efficiency for DOX.15 However, UCST polymers have not been broadly utilized in constructing drug delivery systems to achieve optimal drug delivery.
Multi-stimuli responsive release systems may have more advantages over single-stimulus responsive system, such as higher efficiency, possibly lower required dose, and decreased drug resistance.16 The microenvironment of cancer tissue could provide endogenous stimuli, such as over-expressed proteins and reductive glutathione (GSH), 17 to trigger responsive release and enhance targeting. Among various molecules that respond to such endogenous stimuli, cystamine contains a disulfide bond and can serve as a chemically reducible linker.18
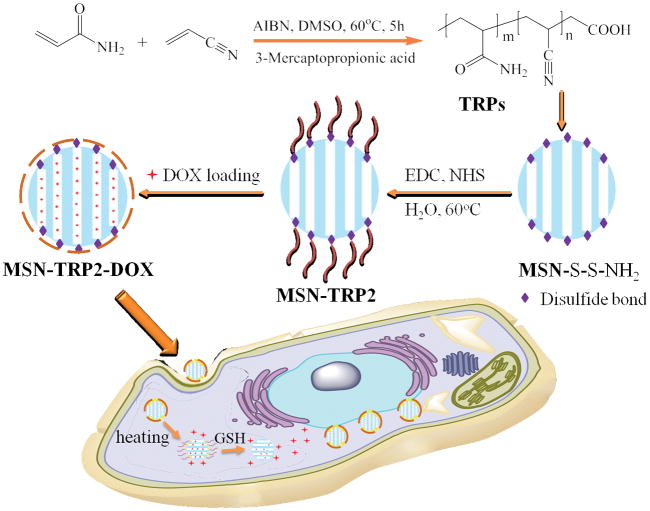
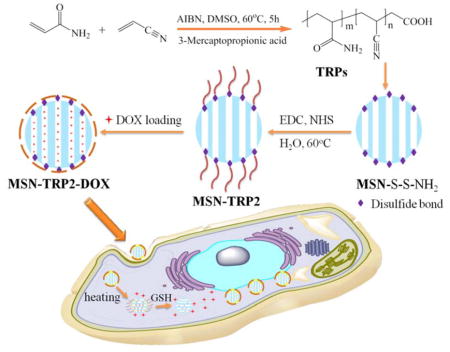
Herein, we synthesized a series of UCST polymers, TRP1, TRP2, and TRP3, by copolymerizing varying ratios of acrylamide and acrylonitronile (Scheme 1), confirmed by Fourier transform infrared spectroscopy (FTIR) and 1H-NMR spectra (Figure S1), and obtained varying cloud points of 32°C, 42°C and 50°C, respectively. Instead of using TRPs themselves as delivery vehicles, we grafted TRP2 (with a cloud point of 42°C) onto mesoporous silica nanoparticles (MSN) with disulfide-containing cystamine, aiming to develop a dually responsive intracellular delivery system with temperature-responsive and cancer cell responsive “gates” to more effectively regulate intracellular cargo release profiles (Scheme 1).
Scheme 1.
Schematic illustration of preparation and intracellular uptake of the new UCST TRP-modified MSN delivery system (MSN-TRP).
The molecular weights and compositions of the synthesized TRPs are determined using gel permeation chromatography (GPC) and FTIR (Table 1). The ratios between the two segments in the copolymers were semi-quantitatively determined by integrating ratios of infrared vibration bands of the carbonyl and nitrile groups (Figure S2). The cloud points were determined by measuring the turbidity changes of the polymer solutions with temperature changes, which were defined here as the temperatures corresponding to 50% of the transmittance at 670 nm (with a polymer concentration of 1 wt %). The cloud point positively correlates with the acrylonitrile content with high sensitivity (Table 1, Figure 1a). Furthermore, the TRP2 showed remarkable reversibility in response to temperature changes, without shift of the cloud point after three cycles of cooling and heating (Figure 1b), demonstrating its robust thermal responsive property.
Table 1.
Controlled increase of the cloud points (Tc) by adjusting the amounts of acrylonitrile (AN).
| Polymer | Mn | Mw | Mw/Mn | AN in feed, wt% | AN in polymer, wt% | Tc/°C |
|---|---|---|---|---|---|---|
| TRP1 | 13717 | 24465 | 1.80 | 14.3 | 7.5±1.3 | 32 |
| TRP2 | 18136 | 26433 | 1.46 | 15.6 | 10.1±1.1 | 42 |
| TRP3 | 10402 | 18180 | 1.75 | 16.6 | 12.8±0.9 | 50 |
Figure 1.
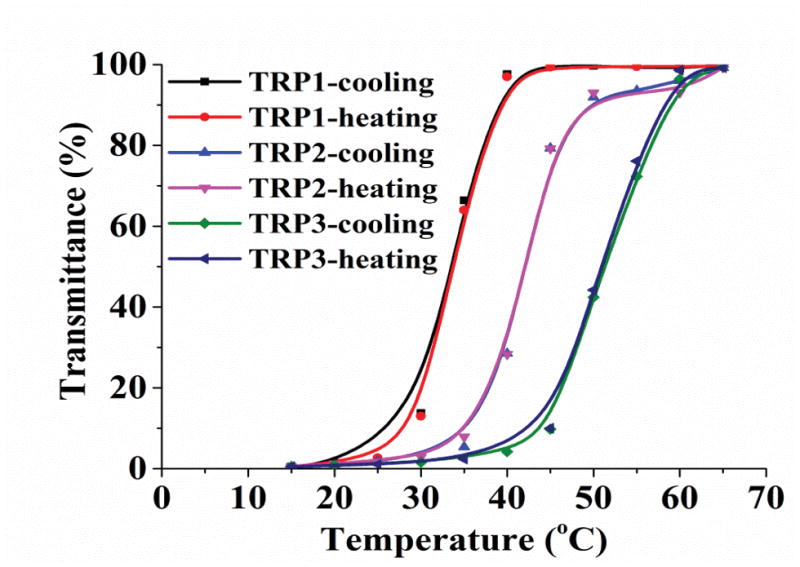
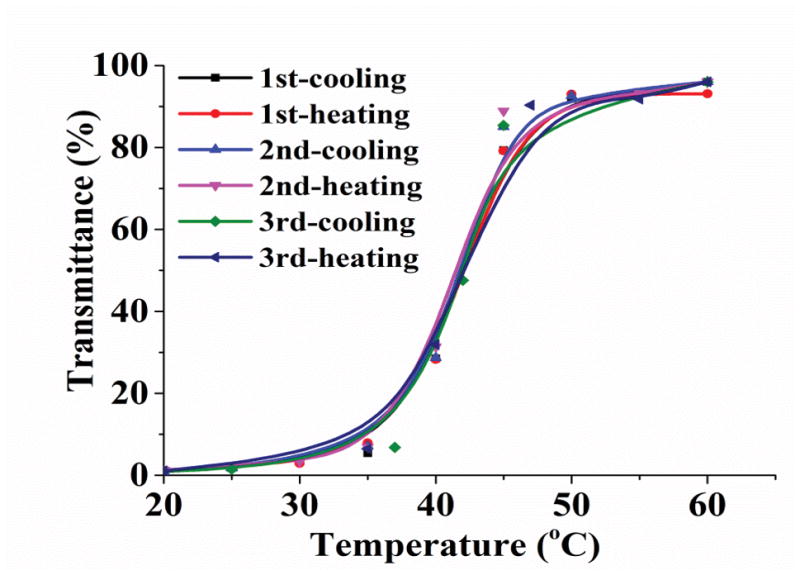
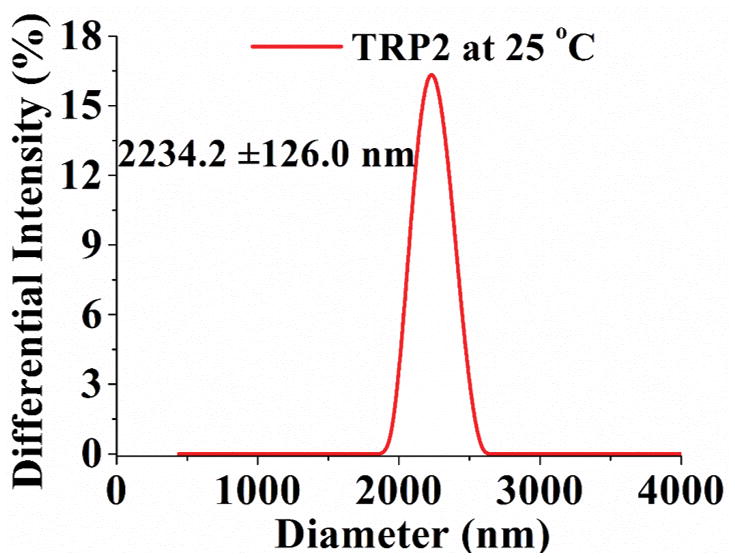
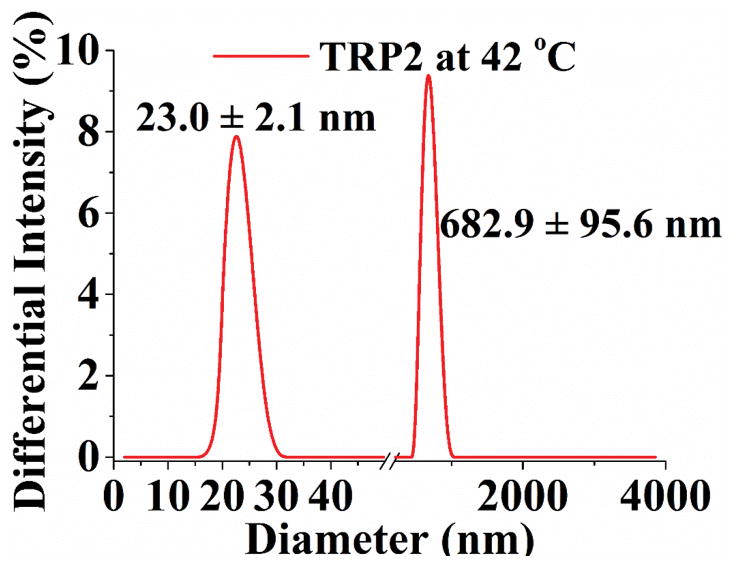
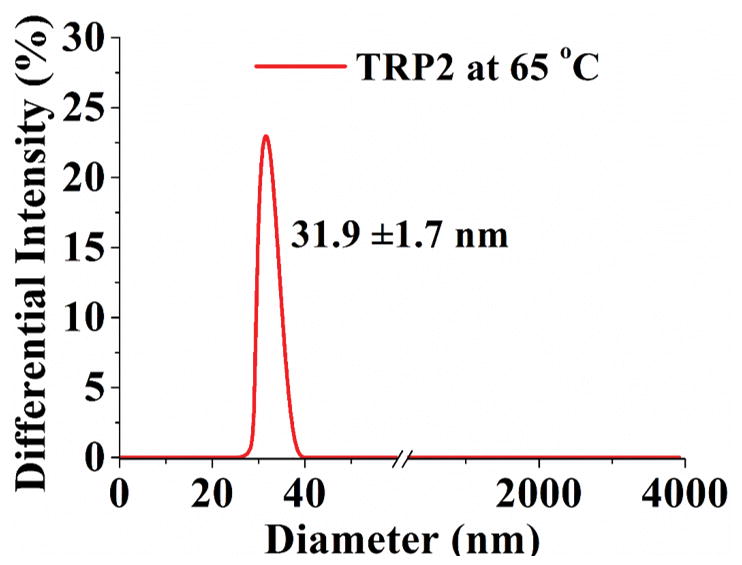
Turbidity curve and size distribution of TRP1, TRP2 and TRP3 (1 wt %) in PBS. (a) Transmittance changes with temperature for polymers with cloud points of 32°C, 42°C, and 50°C, respectively; (b) Transmittance of TRP2 solution changes during repeated heating and cooling cycles. And size distribution of TRP2 at different temperatures measured using dynamic laser scattering (DLS): (c) 25°C; (d) 42°C; (e) 65°C.
The hydrophilicity of TRPs changes with the temperature, resulting in their chain morphology change between random coils and collapsed globules, which can be detected by measuring the changes in size and size distribution of the TRP aggregates. [9] The size distribution of TRP2 in PBS was determined at different temperatures using the dynamic laser scattering (DLS) technique. The results show that TRP2 agglomerated into large particles with diameters around 2200 nm at 25°C (Figure 1c), indicating the high hydrophobicity of the polymer chains at this low temperature (forming large particles to reduce the total surface area and therefore to reduce the total surface energy). When the temperature was increased to its cloud point 42°C, the globules gradually assumed a bimodal distribution with two peaks at 23 nm and 700 nm, respectively (Figure 1d). The intensities of each of the two individual peaks at this temperature were lower than that of the one peak at 25°C, which may be resulted from the gradual disaggregation of polymer chains due to increased hydrophilicity at higher temperature. The polymer solution became transparent at 65°C and exhibited a narrow particle size distribution at 32 nm with enhanced intensity (Figure 1e). The other two TRPs (TRP1 and TRP3) also showed the similar thermo-responsive characteristics to those of TRP2 in terms of changing size distribution with temperature (Figure S3 and S4).
The inverse relationship between the larger TRP particle size and the solution temperature indicated that the hydrophilicity of polymer chains increased as temperature rose. The smaller sized peaks (23 nm at 42°C and 32 nm at 65°C) could represent single polymer chain coils or some semi-stable small aggregates of a smaller number of polymer chains. At the higher temperature of 65°C, the polymer chains became hydrophilic enough for all of them to assume the either single polymer chain coils or smaller aggregates of multiple polymer chains. To decipher the nature of these larger and smaller aggregates, static light scattering and dynamic light scattering were carried out to determine the gyration diameter (Dg, two times the radius of gyration) and hydrodynamic diameter (Dh) of the larger and smaller aggregates of TRP2 in PBS using a Zetasizer Nano ZSP (Malvern). The typical Dg/Dh ratio for a linear flexible chain coil is 1.55, the typical Dg/Dh ratio for a micellar sphere is around 0.774.[19] It was found that the Dg/Dh ratios for both the larger aggregates and the smaller aggregates of TRP2 in PBS were around 1.0 (Figure S5), indicating that these aggregates were consisted of twisted chains. At temperatures lower than the cloud point (< 42°C), a larger percentage of the aggregates were larger than the wavelength of visible light and therefore the suspension was cloudy. When the temperature rose above 65°C, all or the majority of the aggregates became smaller than the wavelength of visible light, and therefore the solution became transparent. At the relatively lower temperature (42°C), likely only the shorter polymer chains became the smaller aggregates, therefore the average size of the smaller aggregates was smaller (23 nm). At a higher temperature (65°C), because of the increase in hydrophilicity all the polymer chains became the smaller aggregates, therefore the average size of “small aggregates” became relatively larger (32 nm) than that of the smaller aggregates (23 nm) at the lower temperature of 42°C.
Mesoporous silica nanoparticles (MSN) have been widely utilized in constructing various release systems due to their high loading capacity, easy surface functionalization, and high biocompatibility.[20] In this study, the temperature-dependent morphology change of aqueous TRP2 were utilized to gate the channels of MSN with the intention to achieve temperature-triggered drug release. We grafted TRP2 onto the surface of MSN through disulfide bonds (reducing agent responsive) to achieve dual responsive delivery system, MSN-TRP2, aiming for more precisely controlled release. FTIR and zeta potential measurements confirmed the successful grafting of TRP2 on the surface of MSN (Figure S6&7). Transmission electron microscopy (TEM) showed that the TRP2-grafted MSN tend to aggregate more than the as-prepared MSN, which could result from physical crosslinking of MSN by the entanglement of TRP2 chains on adjacent MSN at 25°C (Figure 2a and 2b). Dynamic laser scattering analysis showed that MSN-TRP2 at lower temperatures tended to have larger sizes and broader size distributions than MSN-TRP2 at higher temperatures (Figure 2c–e). Such changes are likely resulted from increased hydrophilicity of MSN-TRP2 at high temperatures and therefore improved dispersing ability in water.
Figure 2.
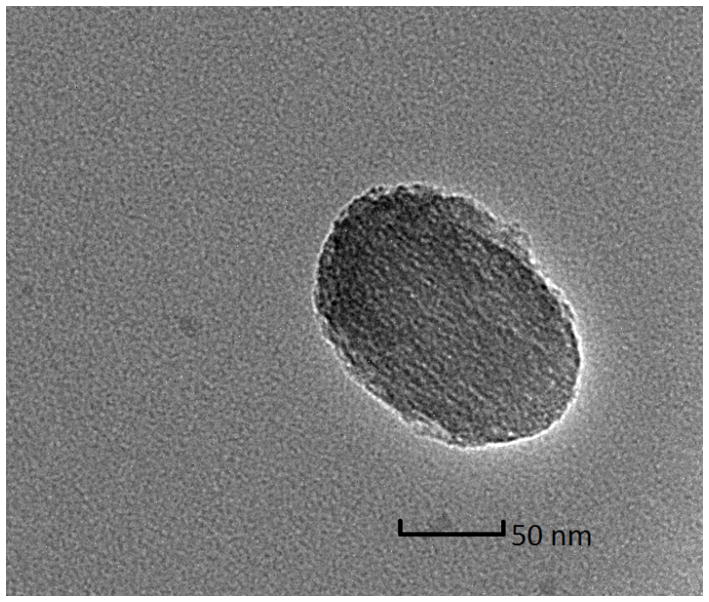
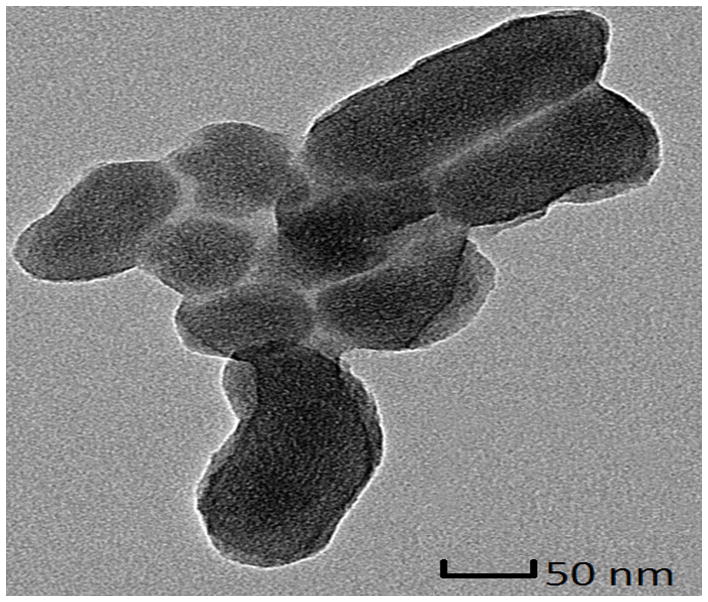
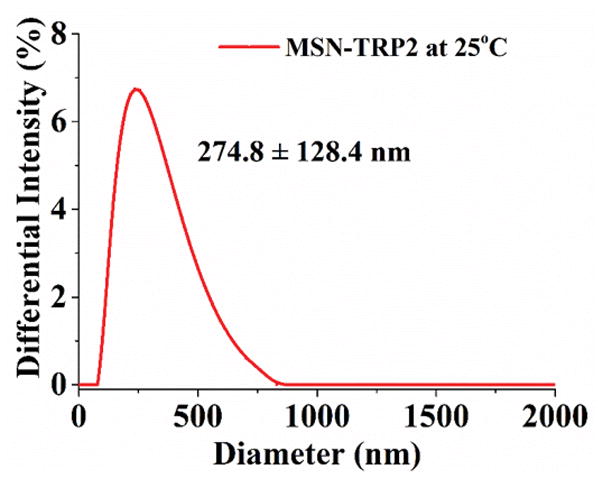
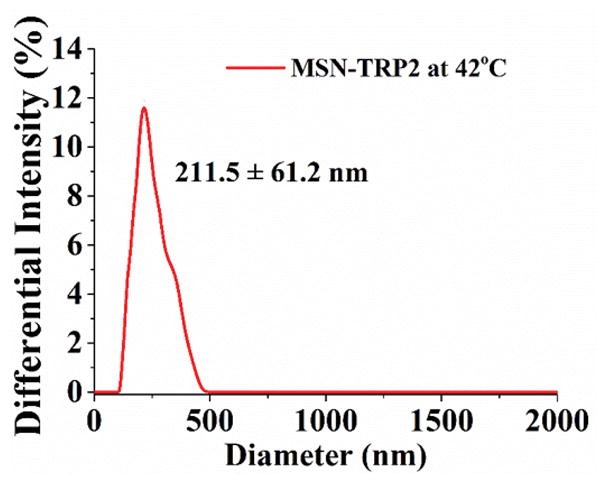
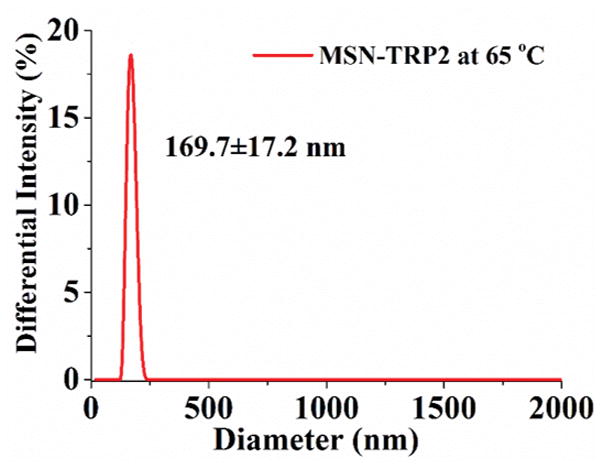
Characterization of MSN grafted with TRP2; (a) TEM image of MSN; (b) TEM image of MSN-TRP2; (c)–(e) Dynamic laser scattering analysis of MSN-TRP2 at 25°C, 42°C and 65°C in PBS (0.1 wt %).
We loaded MSN-TRP2 with rhodamine B to test the intended temperature responsive and reduction responsive release properties. The cumulative release is positively associated with the release temperature (Figure 3a). After 5 hours, less than 5% rhodamine was released at 25°C, about 20% rhodamine was released at 37°C, and about 60% rhodamine was released at 42°C. At 42°C, the release rate increase was more dramatic likely due to the globule-coil transition at this temperature. In addition, the reductive reagent dithiothreitol (DTT) further increased the release rate at both 37°C and 42°C. This additional acceleration in release rate was likely resulted from the irreversible detachment of TRP2 from MSN after the disulfide bonds were cut by DTT (as shown in Figure S8). The addition of DTT at 37°C triggered less cargo release than that at 42°C, which could be due to the hindrance of relatively more hydrophobic surface to DTT penetration at the lower temperature.
Figure 3.
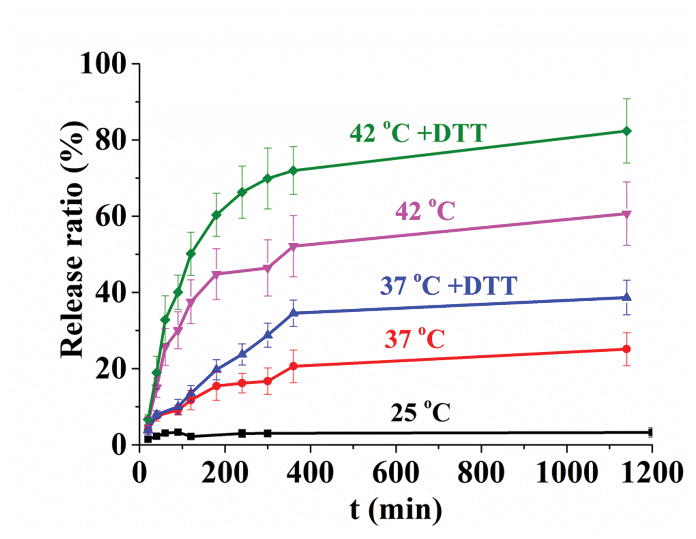
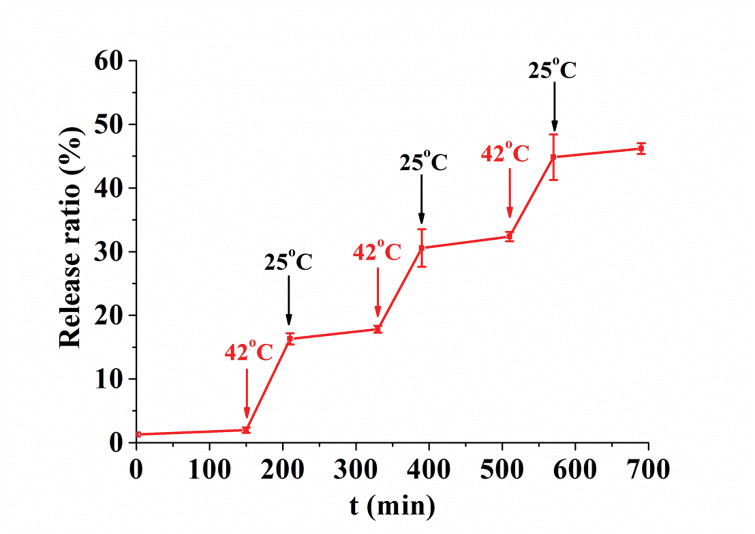
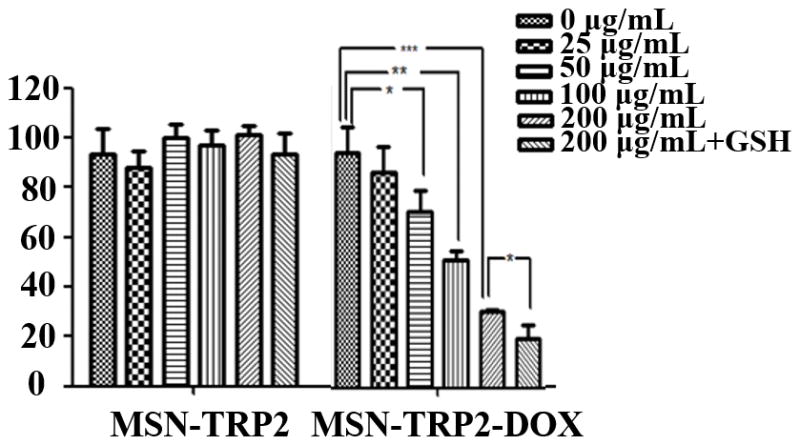
Release profiles and cell toxicity of MSN-TRP2 loaded with rhodamine B or DOX in response to stimuli in vitro. (a) Release curve of MSN-TRP2 in PBS at 25°C, 37°C, 37°C with DTT, 42°C and 42°C with DTT; (b) Controlled release of MSN-TRP2 with temperature switch between 25 °C and 42 °C. And toxicity of to breast cancer cells, SK-BR-3, at 37 °C (c) and 42 °C (d).
In order to evaluate TRP2’s ability to act as a temperature-controlled gate, we examined the release profile of rhodamine from MSN-TRP2 in PBS with cyclic temperature switching between 25°C and 42°C. The release rate of rhodamine from MSN-TRP2 could be turned higher or lower by simply adjusting the temperature, thus, allowing for temperature-controlled dosing for multiple times (Figure 3b).
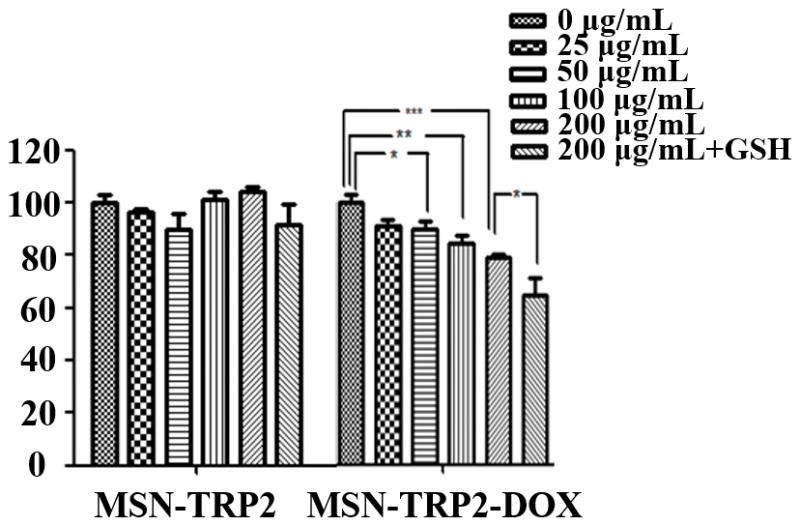
To test the feasibility of MSN-TRP2 as an anti-cancer drug carrier, we prepared the dually responsive drug delivery system MSN-TRP2 and loaded with doxorubicin hydrochloride (DOX). The cytotoxicity of the released DOX from the DOX-loaded MSN-TRP2 (MSN-TRP2-DOX) was evaluated using breast cancer cells, SK-BR-3, at 37°C and 42°C with varying particle concentrations. Since DTT has high cytotoxicity, we choose a non-toxic reagent, reduced glutathione (GSH), to evaluate the cytotoxicity of released DOX to cancer cells.
The control vehicle MSN-TRP2 had little toxicity to cancer cells at concentrations up to 200 μg/mL at either temperature (Figure 3c&d). There was no statistically significant difference in cell viability between 37°C and 42°C when there were no particles (0 μg/mL, Figure 3c&d). MSN-TRP2-DOX showed more effective cytotoxicity to the cancer cells when triggered with the higher temperature and GSH. The cytotoxicity was dependent on MSN-TRP2-DOX particle concentration as well as temperature. In the presence of MSN-TRP2-DOX, the cell viability was lower at 42°C than at 37°C and was further decreased when GSH was used in the culture. Increased cell toxicity in the presence of GSH suggests that GSH was able to successfully break disulfide bonds and detach TRP2 from the surface of MSN, achieving more DOX release from MSN-TRP2, which was corroborated by the morphology changes of MSN-TRP2 during the addition of reductive molecules (Figure S8).
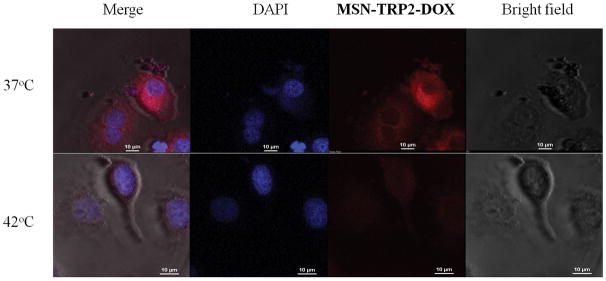
Various cells can internalize MSNs, which help to increase drug efficiency and lower the necessary dose. [21] We examined the SK-BR-3 cancer cells’ uptake of MSN-TRP2-DOX using confocal microscopy. MSN-TRP2 had positively charged surface (Figure S7), which should help the particle uptake by cells. Indeed, the MSN-TRP2-DOX particles could efficiently enter cancer cells (Figure 4a). Cells exhibited enlarged nuclei and died in the presence of MSN-TRP2-DOX at 42°C. The images have low fluorescence intensity due to temperature triggered release of DOX (red) from the particles to the medium. DOX was not significantly released from the MSN-TRP2-DOX at 37°C, therefore the cells showed higher fluorescent intensity at 37°C than at 42°C. The temperature dependence of cell viability in the presence of MSN-TRP2-DOX was further quantified experimentally. Two groups of cancer cells were cultured with MSN-TRP2-DOX at 37°C for 12 h to ensure efficient uptake of MSN-TRP2-DOX (Figure S9). The cells were washed with PBS twice to remove extracellular particles, and then placed in fresh culture medium. One group was cultured for additional 12 hours at 37°C while another group was cultured for additional 12 hours at 42°C. The viability of cells cultured at 42°C was much lower than that cultured at 37°C (Figure 4b). These results demonstrated that MSN-TRP2 could achieve intracellular drug delivery when triggered by higher temperature.
Figure 4.
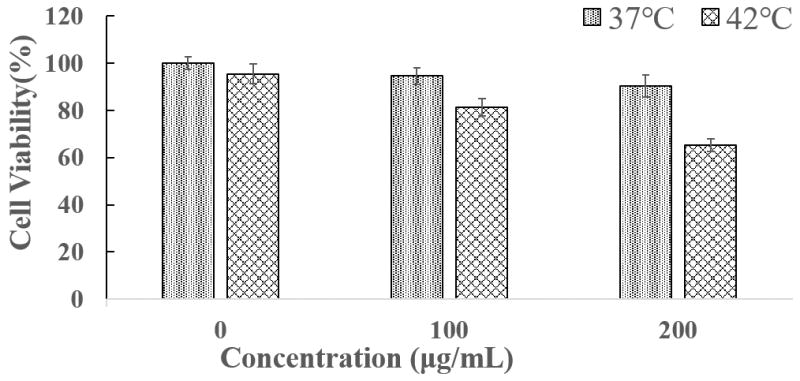
Confocal microscopy images of cellular uptake for MSN-TRP2-DOX (200 μg/mL) at different temperature (a) and cell viability of cancer cells at 37°C and 42°C after internalized the MSN-TRP2-DOX particles (b).
Since many technologies have been developed to safely achieve local temperature increase, the newly developed MSN-TRP dually responsive drug delivery system is promising for local temperature-triggered and reducing agent-triggered drug delivery, including as a targeted local cancer therapy. Therefore, it is worthwhile to evaluate this dually triggered release system in animal studies for potential future clinical applications.
Conclusions
In summary, we synthesized three UCST-type polymers, TRP1, TRP2, and TRP3, with respective cloud points of 32°C, 42°C, and 50°C. Then a dually responsive delivery system was established by grafting TRP2 on the surface of MSN (MSN-TRP2) through disulfide bonds. This system was demonstrated to be able to release hydrophobic drugs over an extended duration of time in a controlled fashion, and its release rate could repeatedly respond to temperature changes and could respond to reducing agents. When loaded with DOX, the MSN-TRP2 particles could achieve intracellular delivery and cargo release when triggered by the temperature changes and reductive agents. The released anti-cancer drug (DOX) from this dually responsive vehicle was toxic to breast cancer cells in a dose-dependent and dually responsive manor. Therefore, the new UCST polymer grafted MSN represent an innovative temperature and reduction reagent dually responsive intracellular delivery system for anti-cancer therapy.
Supplementary Material
Acknowledgments
We thank the NIH of USA (R01HL136231, R01HL114038, R01DE022327, R42TR001711) and NNSF of China (Grants 21476077) for financial support. This work was done in the Polymeric Biomaterials and Tissue Engineering Laboratory at the University of Michigan, where M Hei was a joint PhD student and partially supported by a fellowship from the Chinese Scholarship Council.
Footnotes
Conflicts of interest
There are no conflicts to declare.
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
References
- 1.a) Sriraman S, Aryasomayajula B, Torchilin V. Tissue Barriers. 2014;2:e29528(1–10). doi: 10.4161/tisb.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Matsumura Y, Maeda H. Cancer Research. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 2.Hoffman A. Journal of Controlled Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]; b) MacEwan S, Chilkoti A. Angewandte Chemie International Edition. 2017;56:6712–6733. doi: 10.1002/anie.201610819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Sun T, Zhang Y, Pang B, Hyun D, Yang M, Xia Y. Angewandte Chemie International Edition. 2014;53:12320–12364. doi: 10.1002/anie.201403036. [DOI] [PubMed] [Google Scholar]; b) Faraji A, Wipf P. Bioorganic & Medicinal Chemistry. 2009;17:2950–2962. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 4.Brannon-Peppas L, Blanchette J. Advanced Drug Delivery Reviews. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 5.a) Hirsch L, Stafford R, Bankson J, Sershen S, Rivera B, Price R, Hazle J, Halas N, West J. Proceedings of the National Academy of Sciences. 2003;23:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Stauffer P. International Journal of Hyperthermia. 2005;21:731–744. doi: 10.1080/02656730500331868. [DOI] [PubMed] [Google Scholar]; c) Shi J, Kantoff PW, Wooster R, Farokhzad OC. Nature Reviews Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Valerio M, Cerantola Y, Eggener SE, Lepor H, Polascik TJ, Villers A, Emberton M. European Urology. 2017;71:17–34. doi: 10.1016/j.eururo.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 6.a) Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. Critical Reviews in Oncology/Hematology. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]; b) Zhao Q, Fujiwara Y, Kondo T. Free Radical Biology & Medicine. 2006;40:1131–1143. doi: 10.1016/j.freeradbiomed.2005.10.064. [DOI] [PubMed] [Google Scholar]
- 7.a) Ward M, Georgiou T. Polymers. 2011;3:1215–1242. [Google Scholar]; b) Roy D, Brooks W, Sumerlin B. Chemical Society Reviews. 2013;42:7214–7243. doi: 10.1039/c3cs35499g. [DOI] [PubMed] [Google Scholar]
- 8.a) You Y, Kalebaila K, Brock S, Oupicky D. Chemistry of Materials. 2008;20:3354–3359. [Google Scholar]; b) Malachowski K, Breger J, Kwag H, Wang MO, Fisher JP, Selaru FM, Gracias DH. Angewandte Chemie International Edition. 2014;53:8045–8049. doi: 10.1002/anie.201311047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Wang X, Qiu X, Wu C. Macromolecules. 1998;31:2972–2976. [Google Scholar]; b) Haraguchi K, Li H. Angewandte Chemie International Edition. 2005;44:6500–6504. doi: 10.1002/anie.200502004. [DOI] [PubMed] [Google Scholar]
- 10.a) Gibson M, O’Reilly R. Chemical Society Reviews. 2013;42:7204–7213. doi: 10.1039/c3cs60035a. [DOI] [PubMed] [Google Scholar]; b) Zhang L, Wang L, Guo B, Ma PX. Carbohydrate Polymers. 2014;103:110–118. doi: 10.1016/j.carbpol.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 11.a) Bae Y, Lambert S, Soane D, Prausnitz J. Macromolecules. 1991;24:4403–4407. [Google Scholar]; b) Seuring J, Agarwal S. Macromolecular Rapid Communications. 2012;33:1898–1920. doi: 10.1002/marc.201200433. [DOI] [PubMed] [Google Scholar]
- 12.a) Schulz D, Peiffer D, Agarwal P, Larabee J, Kaladas J, Handwerker L, Garner R. Polymer. 1986;27:1734–1742. [Google Scholar]; b) Chen L, Honma Y, Mizutani T, Liaw DJ, Gong J, Osadaa Y. Polymer. 2000;41:141–147. [Google Scholar]
- 13.a) Glatzel S, Laschewsky A, Lutz J. Macromolecules. 2011;44:413–415. [Google Scholar]; b) Liu F, Seuring J, Agarwal S. Polymer of Chemistry. 2013;4:3123–3131. [Google Scholar]; c) Shimada N, Ino H, Maie K, Nakayama M, Kano A, Maruyama A. Biomacromolecules. 2011;12:3418–3422. doi: 10.1021/bm2010752. [DOI] [PubMed] [Google Scholar]
- 14.Seuring J, Agarwal S. Macromolecules. 2012;45:3910–3918. [Google Scholar]
- 15.Li W, Huang L, Ying X, Jian Y, Hong Y, Hu F, Du Y. Angewandte Chemie International Edition. 2015;54:3126–3131. doi: 10.1002/anie.201411524. [DOI] [PubMed] [Google Scholar]
- 16.a) Cheng R, Meng F, Deng C, Klok H, Zhong Z. Biomaterials. 2013;34:3647–3657. doi: 10.1016/j.biomaterials.2013.01.084. [DOI] [PubMed] [Google Scholar]; b) Zhuang J, Gordon M, Ventura J, Li L, Thayumanavan S. Chemical Society Reviews. 2013;42:7421–7435. doi: 10.1039/c3cs60094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.a) Byrne J, Betancourt T, Brannon-Peppas L. Advanced Drug Delivery Reviews. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]; b) Akhtar M, Ahamed M, Alhadlaq H, Alrokayan S, Kumar S. Clinica Chimica Acta. 2014;436:78–92. doi: 10.1016/j.cca.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 18.a) Yu S, Ding J, He C, Cao Y, Xu W, Chen X. Advanced Healthcare Materials. 2014;3:752–760. doi: 10.1002/adhm.201300308. [DOI] [PubMed] [Google Scholar]; b) Liu L, Liu P. Frontiers of Materials Science. 2015;9:211–226. [Google Scholar]
- 19.Liu J, Sondjaja HR, Tam KC. Langmuir. 2007;23:5106–5109. doi: 10.1021/la063365r. [DOI] [PubMed] [Google Scholar]
- 20.a) Sun R, Wang W, Wen Y, Zhang X. Nanomaterials. 2015;5:2019–2053. doi: 10.3390/nano5042019. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Vallet-Regí M, Balas F, Acros D. Angewandte Chemie International Edition. 2007;46:7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 21.a) Vivero-Escoto J, Slowing I, Trewyn B, Lin VS. Small. 2010;6:1952–1967. doi: 10.1002/smll.200901789. [DOI] [PubMed] [Google Scholar]; b) Lee C, Cheng S, Huang I, Souris JS, Yang C, Mou C, Lo L. Angewandte Chemie International Edition. 2010;49:8214–8219. doi: 10.1002/anie.201002639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.