SUMMARY
Female Aedes aegypti mosquitoes typically mate only once with one male in their lifetime, a behavior known as “monandry” [1]. This single mating event provisions the female with sufficient sperm to fertilize the >500 eggs she will produce during her ~4–6-week lifespan in the laboratory [2]. Successful mating induces lifetime refractoriness to subsequent insemination by other males, enforcing the paternity of the first male [3–5]. Ae. aegypti mate in flight near human hosts [6], and females become refractory to remating within seconds [1, 3, 4], suggesting the existence of a rapid mechanism to prevent female remating. In this study we implicate HP-I, an Aedes- and male-specific peptide transferred to females [7], and its cognate receptor in the female, NPYLR1 [8], in rapid enforcement of paternity. HP-I mutant males were ineffective in enforcing paternity when a second male was given access to the female within 1 hour. NPYLR1 mutant females produced mixed paternity offspring at high frequency, indicating acceptance of multiple mates. Synthetic HP-I injected into wild-type but not NPYLR1 mutant virgins reduced successful matings. Asian tiger mosquito (Ae. albopictus) HP-I peptides potently activated Ae. aegypti NPYLR1. Invasive Ae. albopictus males are known to copulate with and effectively sterilize Ae. aegypti females by causing them to reject future mates [9]. Cross-species transfer of sperm and active seminal fluid proteins including HP-I may contribute to this phenomenon. This signaling system promotes rapid paternity enforcement within Ae. aegypti, but may promote local extinction in areas where they compete with Ae. albopictus.
Keywords: Aedes aegypti mosquito, mating behavior, neuropeptides, Head Peptide-I, paternity enforcement, satyrization, male seminal fluid proteins
eTOC Blurb
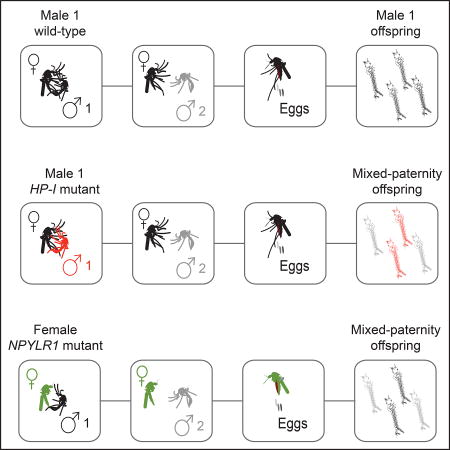
Duvall et al. describe a rapidly-acting peptide/receptor system that acts to enforce paternity in the Aedes aegypti mosquito within one hour of copulation. HP-I peptide is transferred from male to female where it acts on its cognate receptor, NPYLR1. Understanding paternity enforcement is key for vector control and interspecies competition.
RESULTS AND DISCUSSION
HP-I is an Aedes-specific, male enriched peptide that is transferred to females
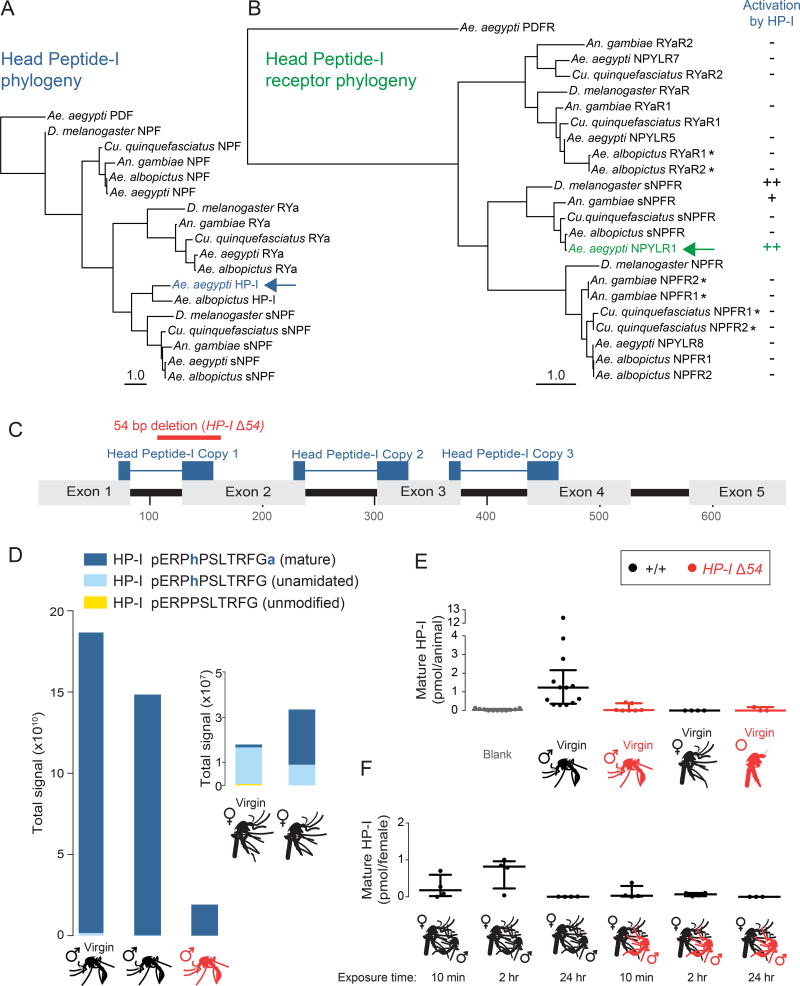
A widely used strategy to prevent female remating in insects is the transfer of biologically active male seminal proteins, produced by the male reproductive system and secreted into the ejaculatory duct along with sperm during insemination, to affect the sexual receptivity of the female [3, 10–14]. Perhaps the best-characterized male seminal fluid protein in insects is the Drosophila fly sex peptide [12], which acts on the sex peptide receptor in the female to suppress receptivity and trigger egg production [13]. In the course of investigating a role for Head Peptide-I (HP-I; so named because of its initial detection in mosquito heads [15]) in female host-seeking behavior [16], we discovered that HP-I is a factor that exerts enforcement of paternity within one hour of mating. Ae. aegypti HP-I is a member of the short neuropeptide F (sNPF) family, and likely resulted from the duplication of the sNPF gene in the Aedes lineage [17]. Based on extensive BLAST searches of genome and short-read databases, HP-I appears to be unique to Aedes species. It has a close homologue in Ae. albopictus, but no known homologues in other species (Figure 1A). The HP-I gene encodes a pre-pro-peptide (Figure 1C) that is post-translationally modified to produce three identical copies of HP-I, which are then further modified by the addition of hydroxy-proline and c-terminal amidation to produce mature HP-I (Figure 1D) [15].
Figure 1. HP-I is an Aedes-specific, Male-Enriched Peptide.
(A–B) Phylogenetic protein trees of HP-I and related peptides (pre-pro-peptides) (A) and NPYLR1 and related receptors (B), with PDF and PDFR as the out group, respectively. Branch lengths represent mean expected rate of amino acid substitution. Scale = 1.0. Pairs of receptors marked with an asterisk encode highly similar or identical proteins that are annotated as separate genes. Column at right indicates relative activation of indicated receptors by 50 µM Ae. aegypti HP-I in cell-based assays carried out in this study and previously published work [8].
(C) Structure of the HP-I gene and the position of the HP-IΔ54 mutation.
(D) Relative levels of mature and immature HP-I detected by LC-MS in whole adult mosquitoes of the indicated sex, genotype, and mating status with females shown as inset due to dramatically lower levels of HP-I (n = 1).
(E) Quantification of mature HP-I detected by LC-MS/MS in whole adult mosquitoes of the indicated sex and genotype (n = 4–13 groups of 7–10 animals).
(F) Quantification of mature HP-I detected by LC-MS/MS in whole adult female mosquitoes of the indicated genotype who were exposed to males of the indicated genotype for the indicated time (n = 4 groups of 7–10 females). Data in E–F are shown as median with interquartile range.
Our previous work identified neuropeptide Y-like receptor 1 (NPYLR1) as the HP-I receptor in Ae. aegypti [8]. NPYLR1 is a member of the insect sNPF receptor family (Figure 1B). To examine the specificity of HP-I, we carried out cell-based assays with three major classes of neuropeptide receptors, RYaR, sNPFR, and NPFR, from 5 insect species. We found that Ae. aegypti NPYLR1 was strongly activated by HP-I, and that HP-I did not activate RYa receptors or NPF receptors in any of these species (Figure 1B). HP-I activated more distantly related sNPFRs in D. melanogaster and An. gambiae. To test the in vivo function of HP-I, we used CRISPR-Cas9 [18] to generate a 54 bp deletion spanning intron 1 and exon 2, which is predicted to disrupt copy 1 of HP-I and introduce a frame shift (Figure 1C). These HP-IΔ54 mutants developed normally and showed no gross developmental delays or abnormalities (data not shown).
Although HP-I levels were previously reported to increase in female heads following a blood-meal [16], later studies did not detect HP-I in female tissues [19]. Naccarati et al. [7] showed that the male accessory gland is the primary source of the peptide. We used liquid chromatography mass spectrometry (LC-MS) to confirm these results and to characterize HP-I levels in the HP-I mutant. Mature HP-I was detected at very high levels in wild-type males, greatly reduced levels in mutant males, and was nearly undetectable in wild-type females (Figure 1D). This extreme sexual dimorphism was not observed for 4 other peptides also detected in all samples. The presence of residual HP-I in the mutant suggests that it is a hypomorph, perhaps due to repair of the frameshift by splicing to downstream exons encoding two of the three copies of HP-I.
Naccarati et al. [7] showed that HP-I is transferred from males to females, where it is detectable for only ~2 hours after mating. We carried out quantitative LC-MS/MS to measure levels of mature HP-I in wild-type and HP-I mutant virgin males and females (Figure 1E), and wild-type virgin females exposed to wild-type or HP-I mutant males for varying periods of time (Figure 1F). Wild-type males produced high levels of mature HP-I, mutant males produced lower levels, and mature HP-I was nearly undetectable in both wild-type and HP-I mutant females (Figure 1E). We detected high levels of HP-I in wild-type females exposed to wild-type males for 10 minutes and 2 hours, but not 24 hours (Figure 1F). There were nearly undetectable levels of HP-I in females exposed to HP-I mutant males for any amount of time.
HP-I mutant females show normal reproductive and host-seeking behavior
We next examined blood-feeding behavior and fecundity in females mated to HP-I mutant males. Females blood-fed to the same extent (Figure S1A and B), consumed blood-meals of the same size, produced eggs with the same temporal profile (Figure S1C) in the same quantity (Figure S1D), regardless of their genotype or that of the males they were exposed to. This suggests that HP-I transferred from the male during mating plays no significant role in controlling egg production, in strong contrast to the sex peptide system in Drosophila [12, 13].
HP-I was previously thought to mediate suppression of host-seeking behavior after a blood-meal [8, 16], either by being produced in females in response to a blood-meal [16] or by being transferred from the male during mating [7]. We therefore measured attraction to human host cues of wild-type and HP-I mutant females exposed to wild-type or HP-I mutant males, before and 48 hours after a blood-meal. We found no effect of the HP-I mutation on attraction to human hosts measured in the uniport olfactometer before a blood meal (Figure S1E) or suppression of host attraction 48 hours after a blood-meal (Figure S1F). These findings are consistent with our previous work showing that the HP-I receptor NPYLR1 is not required for female mosquito fecundity, host-seeking, blood-feeding, or egg-laying behaviors [8].
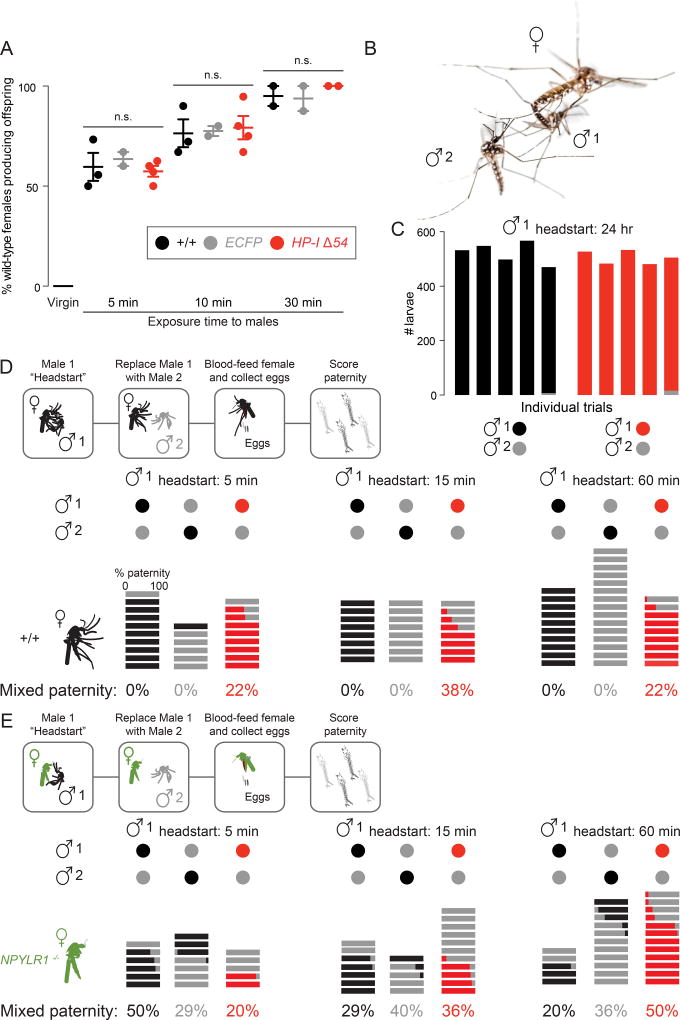
HP-I mutant males mate normally but fail to enforce paternity, and NPYLR1 mutant females produce mixed-paternity offspring
Given its enrichment in males and transfer to females during mating, we investigated a role for HP-I in mating success. HP-I mutant males, and control wild-type males and paternity marker males carrying a transgenic marker with ubiquitous expression of ECFP, were equally capable of fathering offspring when mated to wild-type females (Figure 2A). Over half of females successfully produced offspring after only 5 minutes of exposure to males of any of the three genotypes, with nearly 100% success after 30 minutes of exposure (Figure 2A). These results reinforce the observation that mating occurs very rapidly in Ae. aegypti [1, 3, 4].
Figure 2. HP-I Mutant Males Mate Normally but Fail to Enforce Paternity.
(A) Offspring produced by wild-type virgins or females exposed to males of the indicated genotype for the indicated time (mean ± SEM, n = 2 – 4 trials, 18 – 24 females per trial. Kruskal-Wallis test, n.s. = not significant).
(B) Aerial mating of wild-type Ae. aegypti. Photo: Alex Wild.
(C) Paternity enforcement in groups of 6 wild-type females exposed to 7 males of the indicated genotype for 24 hours (n = 5 trials, 6 females per trial). Data indicate the paternity of offspring.
(D–E) Top: Schematic of paternity experiments with wild-type (D) or NPYLR1 mutant (E) females. Bottom: Paternity enforcement in individual wild-type females (n = 6 – 15) (D) or NPYLR1 mutant females (n = 5 – 12) (E) exposed to males of the indicated genotype for the indicated time. Each horizontal bar represents the offspring of a single female colored to indicate the paternity of her offspring. Mixed paternity of each group is indicated at the bottom.
See also Figure S1.
Female Ae. aegypti are generally monandrous [1, 3], which poses the question of how remating is rapidly suppressed, and how the paternity of the first male is enforced (Figure 2B). We used remating assays in which groups of females were sequentially exposed to males of two different genotypes, and the paternity of the offspring determined. We defined paternity enforcement as the ability of a male to father all offspring produced by a female despite her subsequent exposure to other potential mates. Previous work reported levels of remating in the field [20] and in semi-field conditions as high as 14% [21]. It is important to note that these earlier experiments used different methods to establish paternity, either microsatellite analysis of offspring [20] or detection of labeled seminal fluid derived from two different males [21]. Our laboratory work directly scored the genotype of each live offspring using the ECFP paternity marker strain.
In these experiments, offspring of groups of 6 females were pooled and scored for paternity Offspring fathered by wild-type or HP-I mutant males were non-fluorescent, and easily distinguished from their ECFP-positive half-siblings. HP-I mutant males, given a 24 hour head start, showed no deficit in enforcing their paternity (Figure 2C). This is consistent with our quantitative LC-MS/MS experiment showing that HP-I has a short half-life in females, not detectible 24 hours after mating (Figure 1F) [7]. It further suggests that additional, later-acting and long-lasting male factors enforce paternity on the scale of days to weeks [10]. In a single trial, we observed a very small number of ECFP-positive offspring among the much larger number of wild-type or HP-I mutant offspring. We cannot distinguish between the possibilities that this was a rare polyandrous event or that a female mated only with ECFP-positive Male 2.
We next asked whether HP-I is required to enforce paternity within one hour of exposure to the male. In these experiments, offspring of single females were scored individually. This method allowed us to attribute any mixed paternity offspring to acceptance of multiple mates by a single female. When Male 1 was wild-type or carried the ECFP marker, there was no mixed paternity whether the exposure time was 5, 15, or 60 minutes (Figure 2D). In experiments with wild-type and ECFP males given a 5 minute headstart, we occasionally observed offspring fathered exclusively by Male 2. We assume that these are instances where Male 1 did not mate with the female during the head start he was offered (see Figure 2A). In contrast to the monandry enforced by wild-type males, HP-I mutant males failed to enforce their paternity, and we observed offspring fathered by both males at all time points tested (Figure 2D). Based on reduced levels of HP-I detected by proteomics (Figure 1D) we believe that the HP-IΔ54 mutation is a hypomorph. It is conceivable that an HP-I protein null mutant would show more complete failure to enforce paternity. Since NPYLR1 is the only known HP-I receptor in Ae. aegypti (Figure 1B) [8], we asked if NPYLR1 is required in females to enforce male paternity. Indeed, offspring of NPYLR1 mutant females [8] showed mixed paternity at all time points tested, regardless of the genotype of Male 1 or Male 2 (Figure 2E).
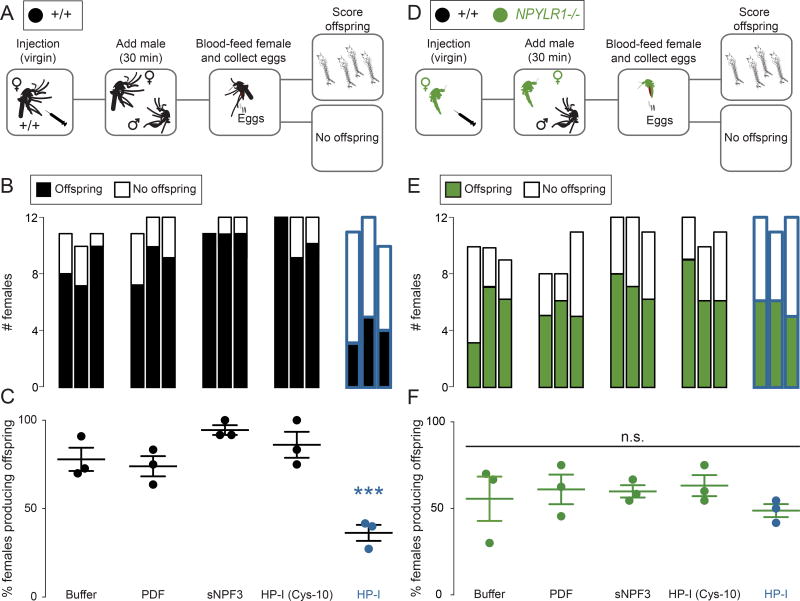
Injection of HP-I interferes with reproduction in wild-type but not NPYLR1 mutant females
If HP-I transferred from a male to a female during mating enforces his paternity, HP-I injected directly into a virgin female should interfere with subsequent mating success (Figure 3). To test this, individual wild-type (Figure 3A) and NPYLR1 mutant (Figure 3D) virgin females were injected with buffer, mature HP-I, or control peptides, and allowed to recover for 12–16 hours in groups. This extended recovery time was required because virgins tested shortly after injection did not mate regardless of the substance injected (data not shown). After recovery, injected females were exposed to wild-type males for 30 minutes, an exposure time that was sufficient for nearly all females to produce offspring (Figure 2A). >70% of females injected with buffer, inactive HP-I (Cys-10), and two other peptides successfully produced offspring. In contrast, <40% of females injected with mature HP-I produced offspring (Figure 3B–C). These results are consistent with the idea that HP-I acts as a rapidly acting signal to the female that she has already mated. We note that suppression of successful mating following HP-I injection was not complete, perhaps because the dose of injected HP-I was lower than that after mating. If the HP-I receptor NPYLR1 mediates the detection of male-derived HP-I, then NPYLR1 mutant virgins should be insensitive to HP-I injection. Although the overall success of offspring production was lower in NPYLR1 mutants injected with any peptide, HP-I had no significant effect on success in producing offspring compared to control peptide and buffer injections (Figure 3E–F).
Figure 3. Injected HP-I Interferes with Reproduction in Wild-Type but not NPYLR1 Mutant Females.
(A) Schematic of wild-type female injection experiments.
(B) Stacked bar plots of raw data for each of n=3 trials.
(C) Production of offspring by females injected with the indicated peptides (mean ± SEM, n = 3 trials with 5–12 wild-type females per trial. ANOVA with Bonferroni correction, ***p < 0.001).
(D) Schematic of NPYLR1 mutant female injection experiments.
(E) Stacked bar plots of raw data for each of n=3 trials.
(F) Production of offspring by females injected with the indicated peptides (mean ± SEM, n = 3 trials with 5–12 NPYLR1 mutant females per trial. ANOVA with Bonferroni correction, n.s. = not significant).
Proteomic data suggest that native (Figure 1F)[7] and synthetic [16] HP-I peptides are degraded by proteolysis in vivo within hours of being transferred to the female by mating or by direct injection into hemolymph. Technical limitations imposed by recovery needs of the injected females prevented us from testing their sexual receptivity within 2 hours of injection. We speculate that injected HP-I “switches” the female into a state that has longer-lasting consequences for her mating behavior, which are subsequently reinforced by later-acting and longer-lasting male accessory gland proteins. The scenario might be that sufficient amounts of injected HP-I reached NPYLR1-expressing cells shortly after injection to activate a signaling cascade that inhibits mating hours later.
Ae. albopictus HP-I peptides are potent activators of Ae. aegypti NPYLR1
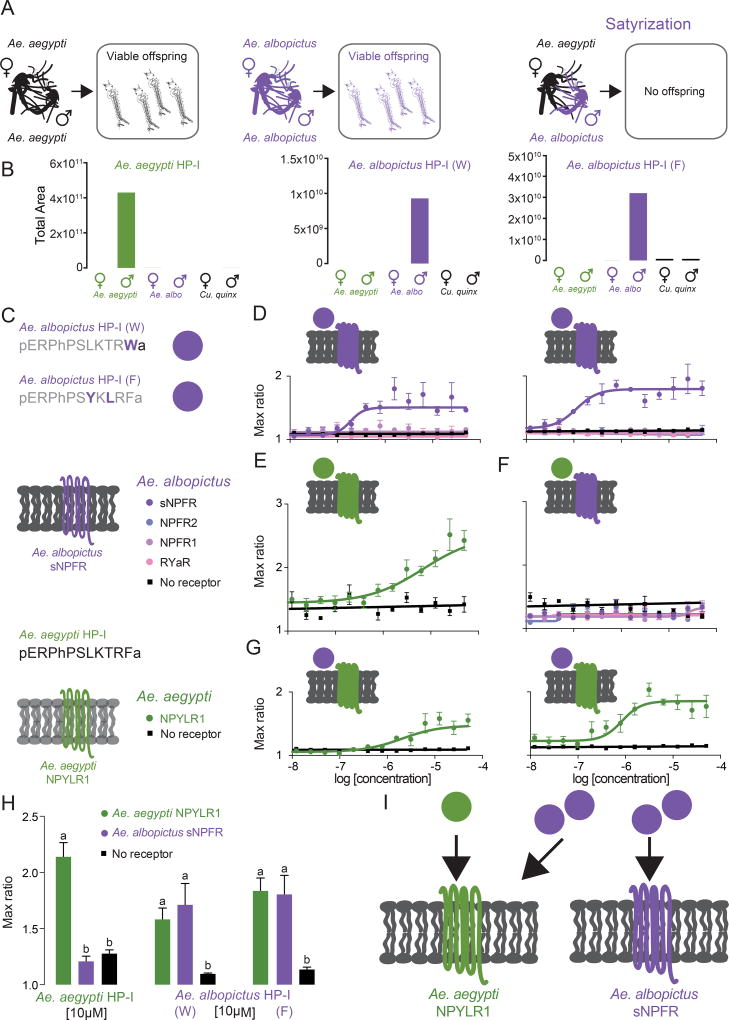
Since the 1980s, invasive Asian tiger mosquitoes (Ae. albopictus) have been displacing Ae. aegypti throughout the southern United States, and field observations have documented instances of Ae. aegypti females inseminated by Ae. albopictus males [9]. Since no offspring are generated by these non-productive pairings, this cross-species mating effectively sterilizes Ae. aegypti females by preventing subsequent mating with Ae. aegypti males. This phenomenon was first observed by Phil Lounibos and colleagues, who termed it satyrization [9] (right panel, Figure 4A). Importantly, satyrization between these two species is unidirectional. Ae. aegypti males do not sterilize Ae. albopictus females [22]. It is possible that the seminal fluid proteins that promote monandry within a species are capable of acting across species to promote satyrization. If this is the case, Ae. albopictus male-derived proteins should activate signaling pathways in Ae. aegypti females, but the cognate proteins in Ae. aegypti should fail to activate Ae. albopictus pathways.
Fig 4. Ae. albopictus HP-I Peptides are Potent Activators of Ae. aegypti NPYLR1.
(A) Schematic of normal within-species mating (left and middle) and cross-species satyrization of Ae. aegypti females by Ae. albopictus males (right).
(B) Relative levels of mature Aedes aegypti HP-I and Aedes albopictus HP-I RWamide and RFamide peptides detected by LC-MS in whole adult mosquitoes of the indicated sex and species.
(C) Legend for cell-based assay experiments in C–G, including the amino acid sequences of predicted mature HP-I in Ae. albopictus compared to Ae. aegypti HP-I.
(D) Dose-response curves of Ae. albopictus HP-I peptides (W and F) on Ae. albopictus receptors.
(E) Dose-response curve of Ae. aegypti HP-I on Ae. aegypti NPYLR1.
(F) Dose-response curves of Ae. aegypti HP-I on Ae. albopictus neuropeptide receptors.
(G) Dose-response curves of Ae. albopictus HP-I peptides (W and F) on Ae. aegypti NPYLR1.
(H) Responses to 10µM Ae. aegypti and Ae. albopictus HP-I peptides. Data in C–G are shown as max ratio (maximum fluorescence level/baseline fluorescence level) (mean ± SEM, 3 replicates. *** p < 0.0001, 1-way ANOVA followed by Tukey’s multiple comparisons test).
(I) Schematic of activity of Ae. aegypti and Ae. albopictus HP-I peptides against Ae. aegypti NPYLR1 and Ae. albopictus sNPFR.
We profiled peptides with LC/MS in both sexes in three different mosquito species to determine the presence and sequence of HP-I in vivo. We detected mature Ae. aegypti HP-I only in male Ae. aegypti samples (Figure 4B, see also Figure 1D). The Ae. albopictus HP-I gene (Figure 1A) produced two different peptides. Of these, one mature peptide is identical to Ae. aegypti HP-I with the exception of a terminal RW-amide instead of RF-amide. We refer to this peptide as HP-I (W). The second Ae. albopictus HP-I mature peptide has a terminal RF-amide, but two internal substitutions compared to Ae. aegypti HP-I (Figure 4C) We refer to this peptide as HP-I (F). We detected both Ae. albopictus peptides only in male Ae. albopictus samples, but not female, Ae. albopictus, or any Ae. aegypti samples (Figure 4B). The Culex quinquefasciatus genome is not predicted to encode HP-I, and we did not detect peptides homologous to mature Aedes HP-Is in these samples (Figure 4B).
To ask if HP-I can cross-activate receptors in the two Aedes species, we used HEK293T cell-based assays to express candidate HP-I receptors from both mosquito species and monitored receptor activation with calcium imaging by these peptide ligands. Based on its homology to Ae. aegypti NPYLR1, we predicted that Ae. albopictus sNPFR would be the HP-I receptor (Figure 1A). Indeed, both Ae. albopictus HP-I (W) and Ae. albopictus HP-I (F) activated Ae. albopictus sNPFR, but not Ae. albopictus receptors from the NPF and RYa family (Figure 4D). Consistent with our earlier published work [8], Ae. aegypti HP-I activated Ae. aegypti NPYLR1(Figure 4E). Although Ae. aegypti HP-I is a potent agonist of the HP-I receptor in its own species, this peptide did not activate any of the Ae. albopictus receptors, including the Ae. albopictus HP-I receptor, sNPFR (Figure 4F). While there was no cross-species activation of the Ae. albopictus HP-I receptor by the Ae. aegypti peptide, both of Ae. albopictus HP-Is strongly activated the Ae. aegypti HP-I receptor, NPYLR1 (Figure 4G and H). This unidirectional cross-species activity suggests that Ae. albopictus male-derived peptides may show biological activity in Ae. aegypti females in vivo. The transfer of rapid-acting and transient HP-I along with other later-acting and long-lasting mating-related peptides during cross-species mating could contribute to satyrization by Ae. albopictus males by activating the HP-I peptide system in Ae. aegypti females and inappropriately enforcing the “paternity” of the Ae. albopictus male (Figure 4I).
Concluding remarks
In Ae. aegypti mosquitoes, mating occurs rapidly in the context of the human host with competing males in close proximity [6], and it is advantageous for males to quickly induce their mate to reject future suitors. Our results show that HP-I acts to enforce paternity shortly after mating. Long-term monandry is HP-I-independent, and likely relies on other male seminal proteins and sperm [3, 5, 11, 23]. The mechanisms by which rapid-acting and transient HP-I works with other later-acting and long-lasting male seminal proteins to enforce paternity in Ae. aegypti remain to be discovered. It will be important to determine in what cells the HP-I receptor, NPYLR1, is expressed in the female. The Drosophila sex peptide receptor is expressed in sensory neurons in the female reproductive tract that project to the abdominal ganglion of the ventral nerve cord [24]. These neurons relay mating information to central circuits to trigger long-term changes in sexual receptivity [25]. The sex peptide system exerts its control over female reproduction over a relatively long time-scale, starting between 4–12 hours after mating [12, 13], and is not permanent. Wild-type Drosophila females will remate with additional males within 5–10 days of initial mating [26, 27]. Because the Ae. aegypti HP-I system acts more rapidly than the sex peptide system, it may use a local circuit within the female mosquito reproductive tract to sense HP-I in seminal fluid and switch off receptivity on a rapid time scale. Indeed analysis of previously published RNAseq data [28] showed enriched expression of NPYLR1 in female ovaries and abdominal tip, a tissue that includes the female reproductive tract.
Monandry can be exploited by other species, as suggested by the observation that Ae. albopictus males can satyrize Ae. aegypti females. Our discovery that Ae. albopictus HP-I can activate Ae. aegypti NPYLR1 in vitro suggests that male peptides from one species have may have the capacity to cross-activate receptors in another. It is intriguing to note that just as Ae. aegypti males cannot satyrize Ae. albopictus females, Ae. aegypti HP-I does not activate the Ae. albopictus HP-I receptor. Because HP-I is unlikely to be the sole factor that enforces lifetime monandry in either species, additional long-acting factors transferred from Ae. albopictus males must be biologically active in Ae. aegypti females and the mechanisms of satyrization remain a fertile area for ongoing studies. Both of these species are invasive disease vectors that pose an increasing threat to public health. The release of genetically modified sterile males [29], relies on monandry to be effective because sterile males father no offspring, but they make females refractory to subsequent mating. This reduces vector mosquito populations, and thus disease transmission.
We describe a rapid-acting peptide/receptor system that enforces paternity in the dengue and Zika vector mosquito, Aedes aegypti, within one hour of copulation. We show that a male-derived peptide called HP-I transferred from the male during mating acts on the cognate receptor NPYLR1 in the female to enforce his paternity. Using CRISPR-Cas9 to generate HP-I mutants, we show that this peptide-receptor signaling system breaks down when HP-I is mutated in males and NPYLR1 is mutated in females. Ae. albopictus HP-I is capable of activating the Ae. aegypti HP-I receptor, but the opposite is not true. This is consistent with the observation that satyrization is unilateral in that Ae. aegypti males do not suppress remating of Ae. albopictus females. We speculate that the HP-I system is part of the mechanism that allows an invasive species to displace local populations. Further exploration of the mechanisms of paternity enforcement will enable more effective application of genetic control strategies, and better understanding of the natural dynamics of interspecies competition.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents should be directed to and will be fulfilled by the lead contact, Leslie Vosshall (leslie.vosshall@rockefeller.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mosquito rearing and maintenance
Aedes aegypti wild-type laboratory strains (Orlando) and mutant strains were maintained and reared at 25–28°C, 70–80% relative h umidity with a photoperiod of 14 hours light:10 hours dark (lights on at 7 a.m.) as previously described in [30]. Briefly, eggs were hatched in deoxygenated, deionized water with powdered Tetramin fish food and larvae were fed Tetramin tablets (Tetra) until pupation. Adult mosquitoes were housed with siblings in BugDorm-1 (Bugdorm) or custom cages and provided constant access to 10% sucrose. Adult females were blood-fed on mice for stock maintenance, on human subjects for HP-I mutant generation, and on human subjects or sheep blood delivered via Glytube membrane feeders [31] for egg-laying and host-seeking experiments. Mosquitoes were sexed and sorted under cold anesthesia (4°C). Female mosquitoes were fasted for 14 –24 hours in the presence of a water source prior to behavioral experiments. Blood-feeding procedures with live hosts were approved and monitored by The Rockefeller University Institutional Animal Care and Use Committee and Institutional Review Board, protocols 15772 and LV-0652, respectively. Human subjects gave their written informed consent to participate.
HP-I mutant generation
The HP-I gene was mutated using CRISPR-Cas9 methods as previously described [18]. In brief, a 23 nucleotide guide RNA was designed to target the HP-I gene (target sequence with PAM underlined: AAAGACACGTTTCGGACGTTCGG). Purified guide RNA (25 ng/µl), Cas9 mRNA (300 ng/µl), and a DNA plasmid containing a homologous recombination sequence including a fluorescent marker (700 ng/µl) were injected into 1,131 pre-blastoderm stage Ae. aegypti embryos (Orlando strain) by the University of Maryland Insect Transformation Facility. 222 G0 animals survived, for a final hatch rate of 19.6%. G0 pupae were sexed and separated into male and female groups prior to eclosion. Male and female G0 adults were outcrossed to wild-type Orlando animals in batches of 20 G0 and 20 wild-type mates. F1 animals were screened for fluorescence to detect insertion of the fluorescent marker, but none were recovered. We therefore screened F1 animals for insertions or deletions at the HP-I locus. 174 F1 adults were intercrossed in groups of 3 females and 3 males and analyzed with Illumina MiSeq for insertions/deletions surrounding the cut site. Animals were pooled into groups of 3 for genomic DNA extractions and MiSeq amplicon generation. PCR primers used to generate MiSeq amplicons (LD15F CGAGGATCAACGTTAGTGTCATATA; LD15R GAGCCGAGCGCTTTTCCATTATGTC) generating a 170bp wild-type product. HP-IΔ54 mutation was selected for isolation due to isolation of stable lines from both male and female founders and ease of genotyping. The HP-IΔ54 mutant was genotyped by generating PCR products using the following primers: Forward (5’-CGTTAGTGTCATATAGTTGATTTTT-3’), Reverse (5’-TACTGACTCTGAGCCGAGCGCTTTT-3’). These products were readily discriminable on a 2% agarose gel (wild-type: 170 bp vs mutant: 116 bp). Genotypes were confirmed by Sanger DNA sequencing (Genewiz). Mutants were blood-fed on human subjects until a stable line was generated, and subsequently maintained by blood-feeding on mice.
ECFP paternity marker strain generation
A genetically modified strain generated for an unrelated study (C.J. McMeniman, unpublished) was utilized as a paternity marker because it expressed high levels of ubiquitous ECFP in larvae, had normal capacity to produce offspring (Figure 2A), and showed wild-type levels of paternity enforcement (Figure 2D and E). A zinc-finger nuclease (ZFN) targeting Ae. aegypti AAEL002167 was produced by the CompoZr Custom ZFN Service (Sigma-Aldrich Life Science). The nucleotide sequence of the ZFN binding and wild-type heterodimeric Fok1 endonuclease sites for this ZFN pair are denoted in upper case and lower case letters, respectively: 5’-CCACACTTCTGGATTCCATtcgtaGGATGGGGAGTAGCA-3’. Homologous recombination was used to insert a poly-Ubiquitin-ECFP cassette (pSL1180-HR-PUbECFP, Addgene plasmid #47917) into the locus. Full details of strain generation are available upon request from C.J.M.
NPYLR1 mutant strain
Experiments using NPYLR1 mutants used in this study carried an 8 bp deletion (NPYLR1Δ8) as previously described [8]. Strains were genotyped prior to use to confirm the presence of the allele in a homozygous state [8].
METHOD DETAILS
Phylogenetic trees
Predicted protein sequences of pre-pro-peptide and receptor genes were downloaded from VectorBase or UniProt and aligned with MUSCLE [32]. Maximum likelihood phylogenetic trees for pre-pro-peptides and receptors were constructed with RaxML [33] using the PROTGAMMAJTT model, testing nodes with the rapid bootstrap analysis (100 replicates). The outgroup was PDF and PDFR for the peptide and receptor tree, respectively. Trees were visualized with Interactive tree of life [34]. Peptide genes (accession numbers): Ae. aegypti PDF (AAEL001754), Ae. aegypti NPF (AAEL002733), Ae. aegypti RYa (AAEL011702), Ae. aegypti sNPF (AAEL012542), Ae. albopictus NPF (AALF017136), Ae. albopictus RYa (GAPW01005454.1), Ae. albopictus sNPF (LOC109427954), Ae. albopictus HP-I (LOC109398455), An. gambiae NPF (AGAP004642), An. gambiae RYa (AGAP006765), An. gambiae sNPF (A0SIF1), Cu. quinquefasciatus NPF (JX317645.1), Cu. quinquefasciatus RYa (CPIJ008988), Cu. quinquefasciatus sNPF (CPIJ009049), D. melanogaster NPF (CG10342), D. melanogaster RYa (CG40733), D. melanogaster sNPF (CG13968). Receptor genes (accession numbers): Ae. aegypti NPYLR1 (U5N0U1), Ae. aegypti NPYLR5 (U5N1G6), Ae. aegypti NPYLR7 (U5N1H5), Ae. aegypti NPYLR8 (U5N0V1), Ae. aegypti PDFR (AAEL009024), Ae. albopictus sNPFR (AALF002670), Ae. albopictus RYaR1 (AALF021539), Ae. albopictus RYaR2 (AALF003651), Ae. albopictus NPFR1 (AALF023252), Ae. albopictus NPFR2 (AALF007614), An. gambiae sNPFR (A0SIF2), An. gambiae RYaR1 (AGAP000351), An. gambiae RYaR2 (AGAP000115), An. gambiae NPFR1 (AGAP004122), An. gambiae NPFR2 (AGAP004123), C. quinquefasciatus sNPFR (CPIJ013069), C. quinquefasciatus RYaR1 (CPIJ019394), C. quinquefasciatus RYaR2 (CPIJ018504), C. quinquefasciatus NPFR1 (CPIJ018265), C. quinquefasciatus NPFR2 (CPIJ006984), D. melanogaster sNPFR (CG7395), D. melanogaster RYaR (CG5811), D. melanogaster NPFR (CG1147).
Peptide synthesis
Mature Ae. aegypti HP-I (pERPhPSLKTRFa), Ae. aegypti HP-III (pERPPSLKTRFa), and Ae. aegypti HP-I [Cys10] (pERPh PSLKTRC) were synthesized by The Rockefeller University Proteomics Resource Center. Ae. albopictus HP-I peptides (pERPhPSLKTRWa and pERPhPSYKLRFa), Ae. aegypti sNPF-3 (APSQRLRWa) and Ae. aegypti PDF (NSELNSLLSLPKKLNDAa) were synthesized by Bachem. Two stable isotope versions of Ae. aegypti HP-I were synthesized by The Rockefeller University Proteomics Resource Center: pERPhPS(13C6 15N1)LKTRFa (+7 Da HP-I “medium”) and pERP(Arg-13C5 15N1)hPS(13C6 15N1)LKTRFa (+13 Da HP-I “heavy”).
Neuropeptide receptor cloning
Ae. aegypti NPYLR-expressing plasmids were previously described [8]. Full-length cDNAs for An. gambiae, Ae. albopictus, and Cu. quinquefasciatus receptors were synthesized by GenScript and subcloned into the XhoI-NotI sites of the pME18s vector for transfection and expression in mammalian cells under the SV40 promoter. Gene names (accession numbers) for the receptors used in this study are: An. gambiae RYaR2 (AGAP000115), Ae. aegypti NPYLR7 (AAEL008296), Cu. quinquefasciatus RYaR2 (CPIJ018504), D. melanogaster RYaR (CG5811), An. gambiae RYaR1 (AGAP000351), Cu. quinquefasciatus RYaR1 (CPIJ01934), Ae. aegypti NPYLR5 (AAEL017049), Ae. albopictus RYaR1 (AALF021539), Ae. albopictus RYa2 (AALF003651), D. melanogaster sNPFR (CG7395), An. gambiae sNPFR (AGAP012378, A0SIF2), Cu. quinquefasciatus sNPFR (CPIJ013069), Ae. albopictus sNPFR (AALF002670), Ae. aegypti NPYLR1 (AAEL013505), D. melanogaster NPFR (CG1147), An. gambiae NPFR1 (AGAP004122), An. gambiae NPFR2 (AGAP004123), Cu. quinquefasciatus NPFR1 (CPIJ018265), Cu. quinquefasciatus NPFR2 (CPIJ006984), Ae. aegypti NPYLR8 (AAEL010626), Ae. albopictus NPFR1 (AALF023252), Ae. albopictus NPFR2 (AALF007614). In three cases, we encountered receptor genes with separate annotation entries that encode highly similar (Ae. albopictus RYaR1 S31P and P32 L) or identical (A. gambiae NPFR1 and 2 and Cu. quinquefasciatus NPFR1 and NPFR2) proteins. In these cases, we selected one for expression analysis. Genes (accession numbers): Ae. albopictus RYaR1 (AALF021539) [not RYa2 (AALF003651)], An. gambiae NPFR2 (AGAP004123) [not NPYR1 (AGAP004122)], Cu. quinquefasciatus NPFR1 (CPIJ006984) [not NPFR2 (CPIJ018265)].
Cell-based assays
HEK-293T cells were maintained using standard protocols in a Thermo Scientific FORMA Series II – Water Jacketed CO2 incubator. Cells were transiently transfected with 1 µg each of plasmid expressing GCaMP6s, Gqα15, and a test receptor using Lipofectamine 2000 (Invitrogen). Transfected cells were seeded into 384 well plates, and incubated overnight in DMEM media supplemented with Fetal Bovine Serum (Invitrogen) at 37°C and 5% CO2. Cells were imaged in reading buffer [Hanks’s Balanced Salt Solution (GIBCO) + 20 mM HEPES (Sigma-Aldrich)] using GFP-channel fluorescence of a Hamamatsu FDSS-6000 kinetic plate reader at The Rockefeller University High-Throughput Screening Resource Center. Compounds were prepared at 3× concentration in reading buffer in a 384-well plate (Greiner Bio-one). Plates were imaged every 1 sec for 5 min. 10 µl of compound was added to each well containing cells in 20 µl of reading buffer after 30 sec of baseline fluorescence recording. Fluorescence was normalized to baseline, and responses were calculated as max ratio (maximum fluorescence level/baseline fluorescence level). 3 replicate plates were analyzed for each experiment.
Liquid chromatography and mass spectrometry
Targeted LC-MS/MS was used to analyze HP-I in 7–10 day-old wild-type Aedes aegypti or HP-IΔ54 mutant mosquitoes. Virgin males and females were obtained by sexing animals as pupae and housing them exclusively with same-sex siblings until proteomic sample preparation. Non-virgin males were group-housed with female siblings from eclosion until proteomic sample preparation. In mating experiments, 10 virgin females who had never taken a blood-meal were exposed in bucket cages at 25–28°C, 70–80% relative humidity to 11 sexually mature males for 10 min, 2 hr, or 24 hr. Males were removed, and females were immediately processed for proteomic analysis. Targeted LC-MS/MS was used to detect Ae. aegypti HP-I, and Ae. albopictus HP-I peptides in 7–10 day-old wild-type Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus animals. Males and females were group-housed with siblings from eclosion until proteomic sample preparation. Whole animals 7–10 days post-eclosion were boiled in groups of 7–10 for 5 min at 100°C in 150 µl of MilliQ water. The water fraction was decanted into a separate tube and set aside. Extraction solution (150 µl 0.25% acetic acid) was added to the carcasses along with two stable isotope versions of HP-I (1 ng/mosquito of HP-I “medium” and 10 ng/mosquito of HP-I “heavy”), and tissue was homogenized using a Kontes pellet pestle grinder (Sigma-Aldrich). The water and acid fractions were centrifuged separately at 4°C for 30 min, and then supernatants combined and passed through a Microcon 10-kDa-molecular weight cutoff filter (Millipore, Merck KGaA) by centrifuging at 4°C for 30 min. Samples were spun to dryness in an Eppendorf Speedvac and resuspended in 20 µL 0.1% TFA/2% acetonitrile. 9 µL of each sample were separated by reversed phase (Acclaim 120 C18, 3um, 120A 2.1mm × 150mm, Thermo Fisher) coupled to an Orbitrap XL (Thermo Fisher Scientific) operated in positive mode. MS spectra were acquired at a resolution of 60,000@m/z 400 and the triply charged endogenous mature HP-I (m/z 409.9034), in addition to +7 Da (HP-I “medium”) (m/z 412.2425) and +13 Da (HP-I “heavy”) (m/z 414.2471) stable isotope versions of HP-I, was continually targeted by MS/MS and measured in the ion trap. Peptides were isolated using a window of 2.0 m/z. Peptides were eluted at 200 µL/min, increasing from 7% Buffer B/93% Buffer A to 35% Buffer B/65% Buffer A over a period of 13 min (Buffer A: 0.1% formic acid; Buffer B: 0.1% formic acid in acetonitrile). Between each sample, the column was cleaned for 3 minutes in 90% Buffer B /10% Buffer A. The column was then conditioned for 5 minutes with 100% Buffer A. All solvents were HPLC grade. Both MS and MS/MS signals were extracted and analyzed using Skyline [35]. “Total Area” integrates the values of signal vs retention time for a given peptide. We are integrating the signal as a function of time to yield area, which is a proxy for peptide amount. To estimate recovery in quantitative proteomics experiments, signals of the spiked-in stable isotope-labeled HP-I peptide were compared to a dilution series of measurements of known amount of the stable isotope-labeled HP-I peptide.
Behavioral Assays
Glytube blood-meal feeding
For experiments in Figure S1, Figure 2, and Figure 3, females were fed sheep blood in groups of 20–50 using Glytube membrane feeders exactly as described [31]. Glytubes were placed on top of mesh on the mosquito cage, and females were allowed to feed through the mesh for 15 min. In Figure S1A n=trials (with 25–40 females/trial). Fed females were scored by eye for engorgement of the abdomen and weighed to confirm feeding status. In the rare cases that females scored as partially fed they were counted as non-fed and discarded.
Egg-laying assays
Groups of 7 to 14 day-old female mosquitoes were fed sheep blood using Glytube membrane feeders [31]. Immediately after blood-feeding, individual mosquitoes were placed in plastic Drosophila vials (25 mm diameter, 95 mm long) containing 5 ml water and a Whatman filter paper (55 mm diameter; GE Healthcare) folded into a cone to act as an oviposition substrate. For egg-laying timecourse experiments in Figure S1C, vials were scored for visible eggs at 12 hr intervals. At 144 hr post-blood-meal, filter papers were removed, and eggs were manually counted by eye for each individual female. Rare cases in which a female died before laying eggs were excluded from analysis.
Uniport olfactometer
Host-seeking behavior was measured using a uniport olfactometer exactly as described [8]. Briefly, groups of 15–20 females, aged 7–14 days were loaded into small plastic canisters with mesh covering both openings obtained from the World Health Organization Vector Control Research Unit (Penang, Malaysia). Canisters were attached to a 1 m long plastic tube (19 cm diameter) that led to an attraction trap (14 cm long, 5 cm diameter), followed by a sealed chamber in which a human volunteer inserted a forearm. Humidified room air was carbon-filtered (Donaldson Ultrac-A) supplemented to a final concentration of 5% CO2 using flow-meters (Cole Parmer). Mosquito-loaded canisters were attached to the olfactometer and given 5 minutes to acclimate prior to a 5 minute host-seeking testing. Mosquitoes were scored as attracted if they flew through the 1 m tube into the attraction trap within the trial period. Trials were performed at least 3 different days, experimental groups were randomized and non-fed controls were run each day. In figure S1E and F n=trials (15–20 females/trial).
Mating and paternity assays
Mosquitoes were separated by sex at the pupal stage and sex was confirmed within 24 hr of eclosion. Females were separated into small groups (n = 7–10) and housed in 473 ml paper soup cups (Webstaurant Store) overnight 25–28°C, 70–80% relative humidity. 6 females were exposed to 7 males for the indicated times, using an aspirator (John W. Hock Company) to introduce and remove males from the soup cups. For remating experiments, Male 2 was exposed to the female for 30 min at 25–28°C, 70–80% relative humidity. Immediately after assay termination, animals were anesthetized at 4°C and separated by sex. Females were allowed to recover overnight at 25–28°C, 70–80% relative humidity, blood-fed, and offered an oviposition substrate in a vial or a cup to lay eggs. In remating assays, larvae were screened for ECFP fluorescence 3–4 days after hatching by transferring them to a wet filter paper, counting the total number of larvae manually by eye, and scoring the number of ECFP-positive animals using a CFP filter on a Nikon SMZ-1500 upright microscope. In Figures 2A and C n=trials (with 6 – 24 females/trial) and in Figures 2D and E and Figure 3B–F n=individual females. Rare cases in which a female failed to blood feed or died before laying eggs were excluded from analysis.
Peptide Injections
Twelve ~12-day-old female mosquitoes of each genotype were anesthetized at 4°C for 30 min, and placed on an acrylic grid for injection at 4°C. Animals were injected in the thorax with 150 nl of each solution using a Drummond Nanoject II (Catalogue #3-000-204) attached to 3.5″ pipettes (Drummond, catalogue #3-000-203-G/X) pulled on a micropipette puller (Sutter Instruments Co., Model P-97). Peptides were dissolved at 500 µM in buffer (1× Ca+2/Mg+2-free PBS; Lonza, catalogue #17517Q). Mosquitoes were allowed to recover in groups for 12–16 hours at 25–28°C, 70–80% relative humidity with access to water, and then mated and blood-fed as described above. Eggs were collected, hatched individually and scored by eye for the presence of larvae 3–4 days after hatching. There was no difference in the number of viable offspring produced by uninjected wild-type and NPYLR1 females (Mann-Whitney test). Three independent injections were performed.
QUANTIFICATION AND STATISTICAL ANALYSIS
All statistical analysis was performed using Prism (Graphpad Software). Data with non-normal distribution are shown as median with interquartile range and data with normal distribution are shown as mean with SEM. One-way ANOVA was used to compare more than 2 groups of normally distributed datasets and Kruskal Wallis test was used to compare more than 2 groups for non-normally distributed datasets. Post-hoc tests included Bonferroni’s test when multiple comparisons were made from data produced from different conditions and Tukey’s multiple comparison when equal sample sizes were compared. Details of statistical methods are reported in the figure legends.
DATA AND SOFTWARE AVAILABILITY
Data file containing all of the raw data in this paper is available for download at bioRxiv: https://www.biorxiv.org/content/early/2017/05/09/136150.figures-only
Supplementary Material
Highlights.
HP-I is a male-specific peptide transferred to females to rapidly enforce paternity
HP-I mutant males fail to enforce their paternity
NPYLR1 mutant females produce offspring fathered by multiple males
Ae. albopictus HP-I activates NPYLR1 and may contribute to cross-species competition
Acknowledgments
We thank Laura Harrington, Kevin Lee, Jeff Liesch, Laura Kramer, Phil Lounibos, Lindy McBride, Mariana Wolfner, Nilay Yapici, and members of the Vosshall Lab for discussion and comments on the manuscript. Ben Matthews and Molly Liu helped generate the HP-IΔ54 mutant strain. Gloria Gordon and Libby Mejia provided expert assistance with mosquito rearing. Stable isotope HP-I was synthesized by Henry Zebroski. This research was supported in part by grants from the National Institutes of Health (UL1RR024143 to The Rockefeller University, R01 DC014247 to L.B.V.). L.B.D. was supported by a Rockefeller University Women & Science Fellowship, and by a Postdoctoral Fellowship from the American Philosophical Society. L.B.V. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: L.B.D. carried out all experiments with the exception of the LC-MS/MS experiments in Figure 1, which were carried out in collaboration with H.M., and the HP-I injections in Figure 3, which were carried out by N.B. C.J.M. generated the ECFP paternity marker strain. L.B.D. and L.B.V. designed the experiments, interpreted the results, and with the other co-authors composed the figures and wrote the paper.
The authors declare no conflicts of interest.
References
- 1.Degner E, Harrington L. Polyandry depends on postmating time interval in the dengue vector Aedes aegypti. Am J Trop Med Hyg. 2016;94:780–785. doi: 10.4269/ajtmh.15-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naksathit AT, Scott TW. Effect of female size on fecundity and survivorship of Aedes aegypti fed only human blood versus human blood plus sugar. J Am Mosq Control Assoc. 1998;14:148–152. [PubMed] [Google Scholar]
- 3.Craig G. Mosquitoes: female monogamy induced by male accessory gland substance. Science. 1967;156:1499–1501. doi: 10.1126/science.156.3781.1499. [DOI] [PubMed] [Google Scholar]
- 4.Gwadz R, Craig GJ, Hickey W. Female sexual behavior as the mechanism rendering Aedes aegypti refractory to insemination. Biol. Bull. 1971;140:201–214. doi: 10.2307/1540069. [DOI] [PubMed] [Google Scholar]
- 5.Helinski ME, Deewatthanawong P, Sirot LK, Wolfner MF, Harrington LC. Duration and dose-dependency of female sexual receptivity responses to seminal fluid proteins in Aedes albopictus and Ae. aegypti mosquitoes. J Insect Physiol. 2012;58:1307–1313. doi: 10.1016/j.jinsphys.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartberg WK. Observations on the mating behaviour of Aedes aegypti in nature. Bull World Health Organ. 1971;45:847–850. [PMC free article] [PubMed] [Google Scholar]
- 7.Naccarati C, Audsley N, JN K, Kim J, Howell G, Kim Y, Isaac R. The host-seeking inhibitory peptide, Aea-HP-1, is made in the male accessory gland and is transferred to the female during copulation. Peptides. 2012;34:150–157. doi: 10.1016/j.peptides.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liesch J, Bellani LL, Vosshall LB. Functional and genetic characterization of neuropeptide Y-like receptors in Aedes aegypti. PLoS Negl Trop Dis. 2013;7:e2486. doi: 10.1371/journal.pntd.0002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tripet F, Lounibos LP, Robbins D, Moran J, Nishimura N, Blosser EM. Competitive reduction by satyrization? Evidence for interspecific mating in nature and asymmetric reproductive competition between invasive mosquito vectors. Am J Trop Med Hyg. 2011;85:265–270. doi: 10.4269/ajtmh.2011.10-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs M, Craig G, Hiss E. The biochemical basis of female monogamy in mosquitoes. I. Extraction of the active principle from Aedes aegypti. Life Sci. 1968;7:835–839. doi: 10.1016/0024-3205(68)90114-8. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs M, Craig GJ, Despommier D. The protein nature of the substance inducing female monogamy in Aedes aegypti. J Insect Physiol. 1969;15:701–709. [Google Scholar]
- 12.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 14.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto S, Brown MR, Crim JW, Vigna SR, Lea AO. Isolation and primary structure of neuropeptides from the mosquito, Aedes aegypti, immunoreactive to FMRFAmide antiserum. Insect Biochem Mol Biol. 1989;3:277–283. [Google Scholar]
- 16.Brown MR, Klowden MJ, Crim JW, Young L, Shrouder L, Lea A. Endogenous regulation of mosquito host-seeking behavior by a neuropeptide. J Insect Physiol. 1994;40:399–406. [Google Scholar]
- 17.Nässel DR, Wegener C. A comparative review of short and long neuropeptide F signaling in invertebrates: Any similarities to vertebrate neuropeptide Y signaling? Peptides. 2011;32:1335–1355. doi: 10.1016/j.peptides.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Kistler KE, Vosshall LB, Matthews BJ. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 2015;11:51–60. doi: 10.1016/j.celrep.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Predel R, Neupert S, Garczynski SF, Crim JW, Brown MR, Russell WK, Kahnt J, Russell DH, Nachman RJ. Neuropeptidomics of the mosquito Aedes aegypti. J Proteome Res. 2010;9:2006–2015. doi: 10.1021/pr901187p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson JB, Jameson SB, Gloria-Soria A, Wesson DM, Powell J. Evidence of limited polyandry in a natural population of Aedes aegypti. Am J Trop Med Hyg. 2015;93:189–193. doi: 10.4269/ajtmh.14-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helinski ME, Valerio L, Facchinelli L, Scott TW, Ramsey J, Harrington LC. Evidence of polyandry for Aedes aegypti in semifield enclosures. Am J Trop Med Hyg. 2012;86:635–641. doi: 10.4269/ajtmh.2012.11-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bargielowski IE, Lounibos LP. Satyrization and satyrization-resistance in competitive displacements of invasive mosquito species. Insect Sci. 2016;23:162–174. doi: 10.1111/1744-7917.12291. [DOI] [PubMed] [Google Scholar]
- 23.Sirot LK, Hardstone MC, Helinski ME, Ribeiro JM, Kimura M, Deewatthanawong P, Wolfner MF, Harrington LC. Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Negl Trop Dis. 2011;5:e989. doi: 10.1371/journal.pntd.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng K, Palfreyman MT, Hasemeyer M, Talsma A, Dickson BJ. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron. 2014;83:135–148. doi: 10.1016/j.neuron.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron. 2009;61:511–518. doi: 10.1016/j.neuron.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Manning A. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature. 1962;194:252–253. [Google Scholar]
- 27.Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Matthews BJ, McBride CS, DeGennaro M, Despo O, Vosshall LB. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics. 2016;17:32. doi: 10.1186/s12864-015-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis. 2010;10:295–311. doi: 10.1089/vbz.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa-da-Silva AL, Navarrete FR, Salvador FS, Karina-Costa M, Ioshino RS, Azevedo DS, Rocha DR, Romano CM, Capurro ML. Glytube: a conical tube and parafilm M-based method as a simplified device to artificially blood-feed the dengue vector mosquito, Aedes aegypti. PLoS One. 2013;8:e53816. doi: 10.1371/journal.pone.0053816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nuc Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nuc Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.