Abstract
Background
Chronic stress is implicated in the development of various psychiatric illnesses including major depressive disorder. Previous reports suggest that patients with major depressive disorder have increased levels of oxidative stress, including higher levels of DNA/RNA oxidation found in postmortem studies, especially within brain regions responsible for the cognitive and emotional processes disrupted in the disorder. Here, we aimed to investigate whether unpredictable chronic mild stress in mice induces neuronal DNA/RNA oxidation in the prelimbic, infralimbic, and cingulate cortices of the frontal cortex and the basolateral amygdala and to explore potential associations with depressive-like behaviors. We expected that animals subjected to unpredictable chronic mild stress will present higher levels of DNA/RNA oxidation, which will be associated with anxiety-/depressive-like behaviors.
Methods
C57BL/6J mice were assigned to unpredictable chronic mild stress or nonstress conditions (n = 10/group, 50% females). Following five weeks of unpredictable chronic mild stress exposure, mice were tested in a series of behavioral tests measuring anxiety- and depressive-like behaviors. Frontal cortex and amygdala sections were then immunolabeled for neuronal nuclei, a marker of post-mitotic neurons and anti-8-hydroxy-2-deoxyguanosine/8-oxo-7,8-dihydroguanosine, which reflects both DNA and RNA oxidation.
Results
Levels of neuronal DNA/RNA oxidation were increased in the frontal cortex of mice subjected to unpredictable chronic mild stress (p = 0.0207). Levels of neuronal DNA/RNA oxidation in the frontal cortex were positively correlated with z-emotionality scores for latency to feed in the novelty-suppressed feeding test (p = 0.0031). Statistically significant differences were not detected in basolateral amygdala levels of neuronal DNA/RNA oxidation between nonstress- and unpredictable chronic mild stress-exposed mice, nor were correlations found with behavioral performances for this region.
Conclusion
Our results demonstrate that unpredictable chronic mild stress induces a significant increase in neuronal DNA/RNA oxidation in the frontal cortex that correlate with behavioral readouts of the stress response. A lack of DNA/RNA oxidation alterations in the basolateral amygdala suggests greater vulnerability of frontal cortex neurons to DNA/RNA oxidation in response to unpredictable chronic mild stress. These findings add support to the hypothesis that chronic stress-induced damage to DNA/RNA may be an additional molecular mechanism underlying cellular dysfunctions associated with chronic stress and present in stress-related disorders.
Keywords: chronic stress, DNA/RNA oxidation, unpredictable chronic mild stress, oxidative stress, depression
Introduction
Chronic stress is a major risk factor contributing to the development and maintenance of several psychiatric disorders, including major depressive disorder (MDD).1 Chronic stress exposure has been shown to alter neuronal function and morphology across multiple brain regions, including corticolimbic areas such as the prefrontal cortex (PFC), hippocampus, and amygdala,2–5 three brain regions highly involved in emotion regulation, fear processing, and cognition, and principally affected among MDD pathophysiological deficits.3,6 In chronic stress-exposed rodents, the frontal cortex (FC) and hippocampus undergo selective neural atrophy, whereas neuronal hypertrophy is reported in the amygdala.4,5 Indeed, chronic stress was reported to induce a reduction in the number of both dendrites and synapses in the FC and hippocampus5,7–9 and increase dendritic arborization and synaptic markers in the amygdala.10,11 These changes are believed to lead to alterations in synaptic plasticity and, consequently, behavioral deficits characteristic of disturbed emotionality and impaired cognition.12,13 Although studies employing rodent stress models have provided insight into the molecular pathways underlying some structural alterations observed in MDD, the intrinsic cellular mechanisms associated with these changes have not been fully elucidated.
Growing evidence from preclinical and clinical studies suggests that chronic physical or psychosocial stress is associated with increased cellular oxidative stress in the brain.14–16 Oxidative stress is characterized by an imbalance between the production of reactive oxygen species (ROS)/reactive nitrogen species, typically compensated by adaptive antioxidant defense systems that clear reactive molecules from the body.17 However, persistently unquenched levels of ROS ultimately lead to oxidative damage of the lipids, proteins, and DNA essential for biological homeostatic states.18 Since the effects of oxidative stress are widespread and the brain has low antioxidant capacities, neurons are particularly vulnerable to oxidative damage induced by ROS excess.19 This type of cellular stress has been implicated in various neurodegenerative disorders19 and more recently identified as a contributing factor in the pathophysiology of several psychiatric illnesses.20
In recent years, increasing evidence suggests an involvement of metabolic stress and oxidative stress in the pathophysiology of MDD.16,21,22 A recent meta-analysis reported that oxidative stress markers are increased in subjects with MDD or bipolar disorder with depressive symptoms.16 Postmortem work corroborates this data, demonstrating (a) decreased levels of glutathione, a major molecular antioxidant defense in the brain, in the PFC of patients with MDD or bipolar disorder23 and (b) increased RNA oxidation in the hippocampus of patients with MDD or bipolar disorder.24 Interestingly, changes in makers of oxidative stress have also been linked to synaptic pruning alterations in other psychiatric disorders, such as schizophrenia.25
The aforementioned supports the notion that oxidative stress, particularly DNA/RNA oxidation, may be a potential pathological hallmark of MDD. In support of this, other studies have demonstrated increased serum levels of 8-hydroxy-2-deoxyguanosine (8-OHdG, a marker of DNA oxidation)26 and 8-hydroxy-2-deoxyguanosine/8-oxo-7,8-dihydroguanosine (8-OHdG/8-oxo-G, a marker of DNA and RNA oxidation) in patients with MDD relative to nonpsychiatric controls.16,22,27 Additionally, urinary levels of the stress hormone, cortisol, were significantly associated with increased nucleic acid oxidation in patients with MDD,28 suggesting a potential link between stress hormones and oxidative stress. Notably, this link is further supported by preclinical findings demonstrating that exogenous administration of corticosterone elevates levels of DNA/RNA oxidation in rodent models of stress.29
Together, these clinical and preclinical studies suggest that chronic stress may induce elevated neuronal DNA/RNA oxidation and that these changes may contribute to the emergence of depressive symptoms or maladaptive behavioral stress responses. To address this question, we investigated (a) the impact of chronic stress on DNA/RNA oxidation in the FC and amygdala, indexed by changes in levels of the well-validated oxidative stress markers,16 8-OHdG and 8-oxo-G, respectively, and (b) whether these cellular changes are linked to the behavioral deficits induced by exposure to chronic stress. More specifically, we employed unpredictable chronic mild stress (UCMS), a well-characterized paradigm for modeling stress-related pathophysiology, including for reproducing behavioral and molecular hallmarks of depression.30,31 Indeed, mice exposed to UCMS exhibit progressive behavioral and physiologic changes analogous to the symptoms observed in human MDD32,33 and show cellular and molecular changes similar to those observed in postmortem MDD studies, including deficits in synaptic markers, impaired function of inhibitory neurons and glial cells, elevated levels of stress hormones, and decreased neuronal plasticity.34–38 Using this model, we tested the hypothesis that UCMS-exposed mice have higher levels of neuronal DNA/RNA oxidation in both the FC and basolateral amygdala (BLA), compared to nonstress-exposed mice and that these increased levels of DNA/RNA oxidation correlate with depressive-like behavioral outcomes.
Methods
Animals
C57BL/6J mice (Charles River Laboratories, Saint-Constant, Quebec) were maintained under standard conditions (22 ± 1℃, 12 h light/dark cycle) with food and water ad libitum except during UCMS or behavioral testing. Experiments were performed with adult mice (8 weeks old at the start of experiment, n = 10/group, two groups, 50% females). All animal use and procedures were performed in accordance with Canadian Council on Animal Care (CCAC) and Centre for Addiction and Mental Health Animal Care Committee (ACC) guidelines.
Unpredictable Chronic Mild Stress Model
Mice were exposed to a randomized schedule of 3–4 mild stressors/day for five weeks. The UCMS protocol comprised environmental and psychosocial stressors as described in our previous publications.39,40–42 Specifically, stressors include exposure to forced bath, which consisted of placing the mouse in a rat cage (with 2 cm of water for 15 min), restraint stress consisted of placing the animal for 15–30 min in a 50-ml falcon tube with air holes at the extremity and in the cap. Other stressors included wet bedding, aversive smell (fox or bobcat urine), light cycle reversal or disruption, tilted cage, reduced space, bedding change, used bedding, no bedding, or nest-let removal. Although uncomfortable for the animals, these stressors do not individually induce distress and are classically described as mild. UCMS-exposed mice were single-housed, whereas nonstress-exposed mice were group-housed (3–4 per cage) until testing. All mice were handled regularly for weight, coat state quality, or behavioral assessment.
Behavioral Assays
Coat Quality
Quality of the animal’s coat state across seven anatomical regions (head, neck, dorsal/ventral coat, forepaw, hindpaw, and tail) was assessed once per week for five weeks. A value of 0, 0.5, or 1 (from maintained to unkempt) was assigned for each anatomical area, and a sum of all scores was calculated for each animal. Results obtained on week 5 are shown here.
Phenotyper Test
Following five weeks of UCMS exposure, on day 35, mouse behavior was assessed using the PhenoTyper apparatus (Noldus, Leesburg, VA), an observational cage that uses live video-tracking with EthoVision version 10 software. The test provides longitudinal observations of the animal’s behavioral response, before, during, and after an anxiogenic stimulus with a specific apparatus called phenotyper; hence, we opted to call the test a phenotyper test. Phenotypers are equipped with infrared camera, which detects mouse behavior in three defined zones (food, drinking, and shelter zones) during the animal’s dark cycle (7 p.m. to 7 a.m.). Four hours into the dark cycle, the animal’s response to an anxiogenic challenge of a white light applied over the food zone was measured for a 1-h period (from 11 p.m. to 12 a.m.). Time spent in the food and shelter zones before, during, and after the light challenge was analyzed in 1-h bins.
Elevated Plus Maze
On day 37, mice were tested in an elevated plus-shaped apparatus (raised 55 cm from the floor), containing two open arms (27 × 5 cm) and two closed arms (27 × 5 × 15 cm). Animals were allowed to explore the apparatus freely for 5 min under dimly illuminated conditions (∼28 lux). The number of entries and time spent in the open arms were manually scored for each animal.
Open Field Test
On day 39, exploratory behavior was measured in an open arena (∼70 × 70 cm width) for 10 min using ANY-Maze tracking software (Stoeling, IL). The amount of time spent in and number of entries into the center zone were measured.
Novelty-Suppressed Feeding Test
On day 41, following food deprivation (∼14–16 h), mice were placed in a novel arena (45 × 30 × 27 cm) with standard food pellets under dim light (28–30 lux) for up to a maximum of 12 min. The latency to feed on the pellet (in seconds) for each mouse was measured. A home cage test was performed as a control measure of appetite drive.
Forced Swim Test
On day 43, mice were assessed over 10 min swimming in a transparent tank filled with water (25 cm, 23–24℃). Immobility time was manually scored for the last 8 min for each mouse.
Sucrose Consumption Test
On day 47, following a 48-h habituation period to 1% sucrose solution, mice were fluid-deprived for ∼14 h. Total sucrose intake (ml) during a 1-h period was measured for each mouse. All bottles were switched to water, and the next night the animals were again fluid deprived (∼14 h). On the following day, water intake was measured (ml) for a 1-h period to control for changes in fluid intake.
Z-Scoring
To improve the consistency and power of measurement across behavioral tests, z-score normalization was utilized to average behavioral outcomes reflecting similar symptom-like dimensions within each test. The formula used to calculate the z-scores was previously published in Guilloux et al.40 First, a z-score is calculated for each parameter measuring emotionality, as described below: z-score = , where x, raw scores for each measure, µ, mean scores per measure, and σ, the standard deviation. Second, when applicable, an average z score is calculated for each test if multiple emotionality parameters are measured, e.g., time and distance in the center for the open field or time and number of entries in the open arms for the Elevated Plus Maze (EPM). This step is not necessary when only one parameter of the test measure emotionality like in the novelty-suppressed feeding test (NSF), forced swim test (FST), or sucrose consumption tests. Lastly, a z-emotionality score (all behaviors) per animal is calculated as an average performance across all tests. This statistical method has previously been shown to increase the sensitivity of detecting behavioral emotionality outcomes for multiple preclinical tests.40
Immunohistochemistry
On day 50, 24 h after the last stressor, mice were sacrificed and brains were harvested and stored at −80℃. Brains were cryo-cut using a Leica cryostat microtome and collected 14 -µm coronal sections containing the PFC FC and amygdala were briefly fixed in 4% paraformaldehyde for 15 min, followed by permeabilization in 0.3% Triton-X-100-PBS solution. Sections were incubated for 1 h in 5% normal donkey serum, at room temperature. Primary antibodies used were as follows: Anti-DNA/RNA Damage antibody [15A3] (ab62623; 1:100, high affinity and specificity for 8OHdG over 8-oxo-G) and guinea pig polyclonal anti-NeuN/Fox3 (Synaptic Systems 266004; 1:100). Following overnight 4℃ incubation with primary antibodies, sections were washed in 1 × PBS and incubated with secondary antibodies: donkey anti-mouse (Jackson ImmunoResearch, Alexa Fluor488, lot # 121478; 1:100) and donkey anti-guinea pig (Cy3, lot # 20376; 1:100) for 2 h at 4℃. After three washes with PBS, slides were mounted and cover-slipped with 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI) containing mounting media vectashield (Vector Labs). Mounted sections air-dried at room temperature and were stored at 4℃ until imaging.
Image Acquisition and Quantification
Fluorescent images of all tissue sections were obtained using Nikon Eclipse Epifluorescent microscope equipped with a 12-bit Q-imaging camera, Retiga 1300B (7323). All images were taken at the 20 × objective lens, 1 × gain, and exposure time adjusted between channels. A minimum of three sections per animal and a minimum of one image per section and/or hemisphere were acquired by an experimenter blinded to the animal treatment history. Image composites were saved uncompressed for subsequent analyses. Cell counting and co-localization analysis were performed using Metamorph (Olympus applications software version 7.7.5., Richmond Hill, ON). This was used to quantify cells that were positive for either neuronal cells markers (neuronal nuclei, NeuN, red), markers for 8-OHdG/8-oxo-G (green), and/or for DAPI (blue). Initially, a “Threshold Above Local Background” was set for each channel in order to remove any potential background staining as well as to enable discrimination of cellular from noncellular staining using a built-in multiwavelength quantification function (Metamorph software). More specifically, we used a nuclei counting application module to accurately capture intensity measures and to segment “touching cells.” To perform both nuclear and cytoplasmic cell scoring, DAPI was assigned nuclear, whereas NeuN and 8-OHdG stains were both assigned nuclear and cytoplasmic compartments. This assignment closely follows understanding of the distinct biological localizations of oxidized products of both RNA and DNA, which may be cytoplasmic and/or nuclear.43 Although a study previously reported that it is possible to quantify DNA and RNA oxidation separately,24 the antibody used here does not differentiate 8-OHdG from 8-oxo-G. In addition, since 8OHdG can also occur in the cytoplasmic and not exclusively in the nuclear compartment,44 we will therefore refer to this assessment as DNA/RNA oxidation. All DAPI-positive cells were quantified and overlap with NeuN and/or 8-OHdG/8-oxo-G staining was considered for co-localization. Total number of DAPI cells co-localized or nonoverlapping with NeuN and 8-OHdG/8-oxo-G was averaged across all sections for each animal. A total of 28,266 FC NeuN + cells and 49,599 for the BLA were assessed for co-localization with 8-OHDG/8-oxo-G immunofluorescence. This corresponded to an average (±SEM) of 1413 ± 141 NeuN + cells per animal for the FC and 2479 ± 213 for the BLA. A total of 23,100 (FC) and 47,310 (BLA) were also positive for 8-OHDG/8-oxo-G, i.e., 5166 (FC) and 2289 (BLA) NeuN + cells were negative for 8-OHDG/8-oxo-G. The number of NeuN + /8-OHDG/8-oxo-G + cells per field or acquired image (corresponding to 0.25 mm2).
Statistical Analyses
All values are expressed as median and interquartile range. The potential interaction between sex and stress was analyzed by analysis of covarinance, where sex was considered as the covariate. Since this did not achieve significance, analysis of varinance (ANOVA) was used. Repeated measure ANOVA was performed for the phenotyper test where time (before, during, and after light challenge) and stress were considered as factors, followed by Scheffe post hoc analysis. Differences meeting criteria for a cut-off of p < 0.05 were considered statistically significant. Levels of neuronal DNA/RNA oxidation changes were correlated with behavioral readouts (Pearson correlation, p < 0.05).
Results
Mice Subjected to UCMS Paradigm Show Anxiety-/Depressive-Like Behaviors in the Phenotyper Test and the NSF
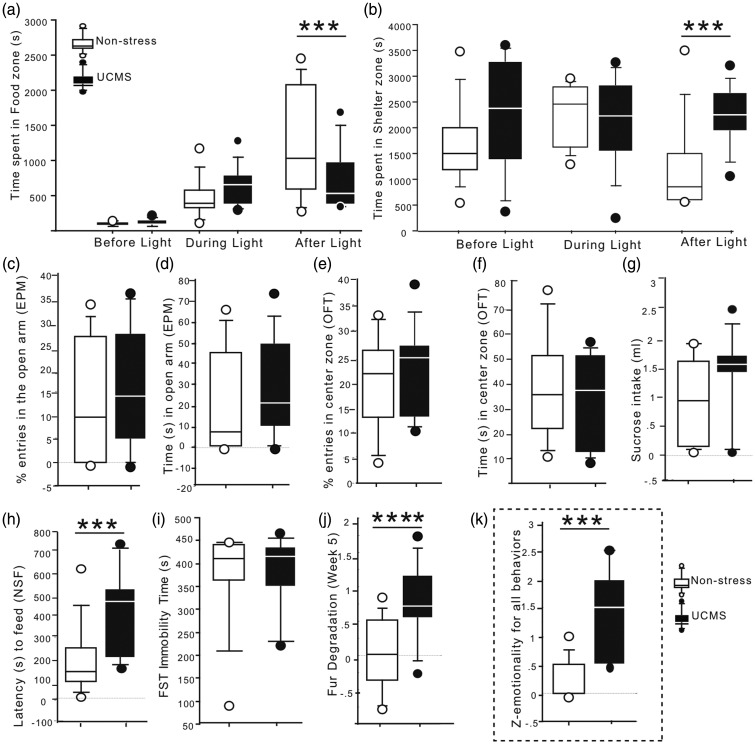
Home cage-like behavior of UCMS and nonstress mice was monitored throughout the dark cycle. Four hours after the start of the test, a spotlight challenge is applied over the food zone for 1 h. Time spent in the food or shelter zone before, during, or after the challenge was measured to assess the animal’s anxiogenic response to the challenge. Analysis of baseline home-cage behavior revealed no significant differences in the amount of time spent in the food and shelter zone in the hours before or during light challenge between nonstress- and UCMS-exposed mice. However, repeated measures ANOVA revealed a significant interaction between stress and time (F2,54 = 3.371, p = 0.041, Figure 1(a)). Specifically, post hoc analysis demonstrated that UCMS-exposed mice avoided, or spent a significantly reduced amount of time, in the food zone 1 h following light challenge, compared to the hours before and during the challenge, when compared to nonstress-exposed mice (p < 0.001 and p = 0.002, respectively). In addition, there was a significant between-group differences for time spent in the shelter zone (stress main effect: F1,54 = 4.225; p = 0.044), wherein UCMS animals spent more time in the shelter zone than nonstress-exposed mice (post hoc analysis, p = 0.008, Figure 1(b)). Analysis of the locomotor activity in the phenotyper tests also demonstrated no changes in the locomotor activity between groups (nonstress: 431.8 m ± 60.7, UCMS: 515.1 m ± 66.8 per hour; p = 0.4).
Figure 1.
Effects of UCMS in tests measuring anxiety/depressive-like behaviors. Performances of UCMS or nonstress animals were measured in the phenotyper test where in time spent (in seconds) in the (a) food zone and (b) shelter zone the hours before, during and after the light challenge was assessed. UCMS and nonstress mice percent entries (c) or time in the open arms (d) in the elevated plus maze (EPM) as well as percent entries (e) or time spent (f) in the center of the arena in the open field test was also measured. (g) No changes in sucrose consumption was found in UCMS-exposed mice when compared to nonstress control group. (h) UCMS-exposed mice exhibit significantly greater latency to feed in the novelty-suppressed feeding test compared to nonstress mice. (i) Nonstress and UCMS mouse groups spent similar amount of time immobile in the forced swim test (FST). (j) UCMS-exposed mice show significantly poorer fur coat quality than nonstress mice. (k) Z-emotionality score calculated from all behavioral tests measured revealed a significant increase in behavioral emotionality in the UCMS mouse group compared to nonstress group. Results are illustrated in box plot (median and interquartile) with circles for animals with the lowest or highest performances in each test. **p < 0.01, ***p < 0.001, and ****p < 0.0001 when compared to nonstress control group.
In the elevated plus maze, UCMS-exposed mice showed no significant changes in the percentage of entries or time spent in the open arms (p = 0.625 and p = 0.515, respectively, Figure 1(c) and (d)). In the open field, no between-group differences were found for the number of entries (p = 0.522, Figure 1(e)) or time spent in the center zone (p = 0.559, Figure 1(f)). No significant between-group differences were found for sucrose or water intake in the sucrose consumption test (p = 0.174, Figure 1(g)) or time spent immobile in the forced swim test (p = 0.927, Figure 1(i)). In the novelty-suppressed feeding test, UCMS-exposed mice showed a significant increase for latency to feed in the novel environment (p = 0.009, Figure 1(h)) and no significant change in latency to feed in home-cage conditions between groups (nonstress: 22.9 s ± 4.8, UCMS = 36.3 s ± 18.5; p = 0.09). In addition, there was a significant deterioration of coat state quality in the UCMS-exposed group when compared to nonstress-exposed mice (p < 0.0001, Figure 1(j)). Overall, UCMS induced an increase in emotionality, indexed by a z-score calculated from all behavioral tests measuring emotional-like behavior, including the phenotyper, elevated plus maze, open field, forced swim, sucrose consumption, and novelty-suppressed feeding tests (p = 0.006, Figure 1(k)).
Higher Levels of Neuronal DNA/RNA Oxidation Is Found in the FC of Mice Subjected to UCMS
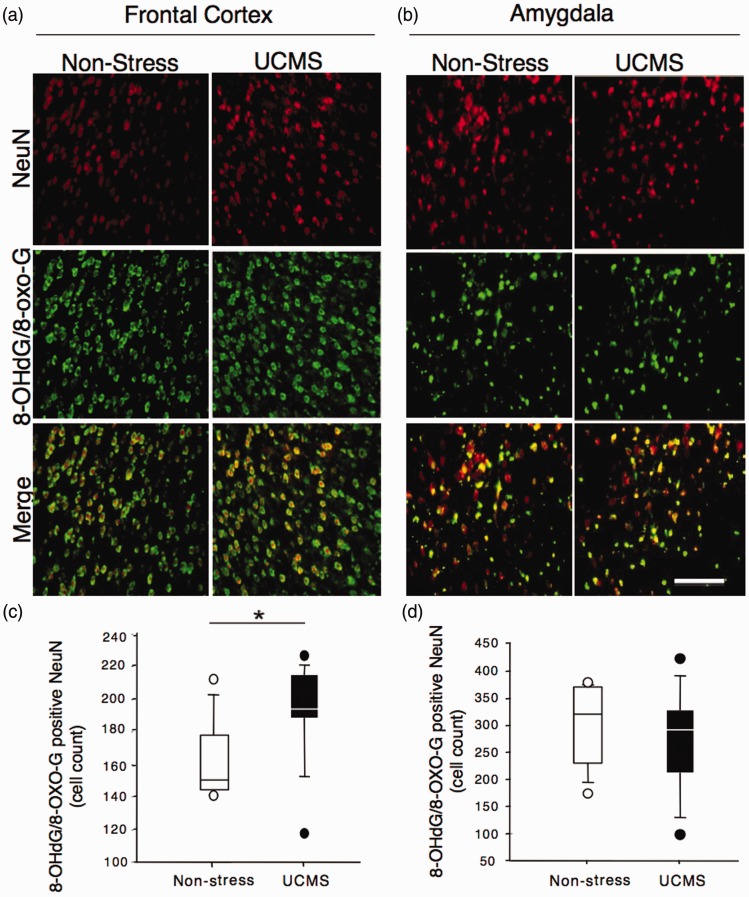
We next tested the hypothesis that UCMS exposure in mice may induce an increase in DNA/RNA oxidation levels in the FC and BLA. Quantitative analysis of the number of 8-OHdG/8-oxo-G cells co-labeled with NeuN was performed using immunohistochemistry and fluorescence microscopy techniques. Statistical analysis revealed a significant increase in the number of cells co-expressing 8-OHdG/8-oxo-G and NeuN in the FC of mice subjected to UCMS when compared to nonstress mice (p = 0.0207, Figure 2(a) and (c)). However, no significant differences were found for the number of cells co-labeled with 8-OHdG/8-oxo-G and NeuN in the BLA of UCMS-exposed compared to nonstress mouse group (p = 0.553, Figure 2(b) and (d)).
Figure 2.
Effects of UCMS on DNA/RNA oxidation in frontal cortex (FC) and basolateral amygdala (BLA). Representative immunostaining for NeuN (red) and 8-OHdG/8-oxo-G (green) and co-staining (merge) in the FC (a) or BLA (b) of nonstress mice and mice subjected to UCMS. The original magnification was × 20. Scale bar represents 50 µm. Quantitative analysis of the number of NeuN/8-OHdG/8-oxo-G co-labeled cells using Metamorph count nuclei software revealed a significant increase in neuronal DNA/RNA oxidation in the FC (c) and no change in the BLA (d). *p < 0.05 compared to nonstress animal group.
Increased Latency to Feed in the NSF Test Is Associated With Higher Levels of Neuronal DNA/RNA Oxidation in the FC
We next performed correlational analyses between the number of cells co-expressing 8-OHdG/8-oxo-G and NeuN in either the FC or BLA using both the z-emotionality score obtained from all behavioral tests, and results from the NSF, because of the group differences in this test. We found a robust positive correlation between the number of cells co-expressing 8-OHdG/8-oxo-G and NeuN in the FC and latency to feed in the NSF test (Figure 3(a); r2 = 0.393, p = 0.0031). Mice with a higher number of cells co-expressing 8-OHdG/8-oxo-G and NeuN had a greater latency to feed in the novel environment during the NSF test. This correlation was not conserved when groups were split, partly due to the small sizes of the sample. Individual group correlations were as follows: for UCMS (r2 = 0.256, p = 0.1125) and nonstress (r2 = 0.288, p = 0.1406) groups. However, no correlation was found for latency to feed in home cage (r2 = 0.011; p = 0.662), suggesting that these results were not driven by changes in normal appetitive drive, but rather an emotionality-like state. No significant correlation was observed for the number of cells co-expressing 8-OHdG/8-oxo-G and NeuN in the BLA and behavioral performances in the NSF (r2 = 0.063, p = 0.287, Figure 3(b)). Upon individual group correlations, there was expectedly no difference between BLA neuronal DNA/RNA oxidation and behavioral performances in the NSF test (UCMS: r2 = 0.113, p = 0.354; nonstress: r2 = 0.037, p = 0.605). No significant correlation was observed between the number of cells co-expressing 8-OHdG/8-oxo-G and NeuN in the FC or the BLA and the integrated z-emotionality scores for combined behavioral tests (Figure 3(c) and (d)). Individual group correlations did not reveal significant association between overall z-emotionality and number of cells co-labeled with NeuN and 8-OHdG/8-oxo-G in the FC (UCMS: r2 = 0.004, p = 0.875; non stress: r2 = 0.003, p = 0.0889) or in the BLA (UCMS: r2 = 0.017, p = 0.729; nonstress: r2 = 0.001, p = 0.95).
Figure 3.
Correlations between UCMS-induced changes in DNA/RNA oxidation in frontal cortex (FC) and basolateral amygdala (BLA) and behavior. (a) Correlation between numbers of 8-OHdG/8-oxo-G positive NeuN cells in the FC and latency to feed in the NSF test was significant (p = 0.0031). (b) No significant correlation was found between the number of 8-OHdG/8-oxo-G positive NeuN cells in the BLA and latency to feed in the NSF test. The z-emotionality scores for all behavioral tests and levels of DNA/RNA oxidation (8-OHdG/8-oxoG positive NeuN cells) in (c) the FC or the (d) BLA did not correlate.
Discussion
The results of the present study suggest that chronic stress induces increased DNA/RNA oxidation in FC neurons of mice, and that these changes may correlate with emotionality-like behavioral deficits. More precisely, UCMS- and nonstress-exposed mice were tested in various behavioral assays measuring anxiety-/depressive-like behaviors. UCMS-exposed mice exhibited increased anxiety-like behaviors, as demonstrated from results in both the phenotyper test (i.e., avoidance of the food zone and favoring the shelter zone after a light challenge) and the NSF test (i.e., increased latency to feed in a novel environment). UCMS also induced a significant deterioration of coat quality. Although we failed to observe an effect of UCMS exposure in the EPM, open field, sucrose consumption, and FST tests, overall, UCMS-exposed mice had a significantly elevated behavioral emotionality z-score when compared to nonstress-exposed mice. At the cellular level, using immunohistochemistry and fluorescence microscopy, we demonstrated that UCMS exposure induces a significant increase in neuronal DNA/RNA oxidation as indexed by an increased number of cells co-labeled with 8-OHdG/8-oxo-G and NeuN in the FC. Although correlations between the number of 8-OHdG/8-oxo-G and NeuN co-labeled cells and overall behavioral emotionally were not found in the FC or the BLA, there was a significant positive correlation between the number of cortical 8-OHdG/8-oxo-G and NeuN co-labeled cells and the latency to feed in a novel environment during the NSF test. No changes in the number of 8-OHdG/8-oxo-G and NeuN co-labeled cells, or potential correlation with behavioral outcomes, were found in the BLA following UCMS exposure, suggesting that neurons of the FC may have a greater vulnerability to DNA/RNA oxidation in response to chronic stress.
Previous work using the UCMS model has reported increased “behavioral emotionality” using one, or a series of, classical behavioral tests that measure anxiety-, helplessness-, and anhedonia-like deficits, including previous studies from our laboratory.35,36,39,40 These tests provide rapid readouts (5–10 min), depend highly on experimenter and experimental conditions, and were originally designed to assay or screen for anxiolytic- or antidepressant-like activity of pharmacological compounds.45–47 Therefore, they often provide inconsistent readouts, characterized by broad within-group variability, leading to small effect sizes when sex is considered as a covariate.31 Sucrose intake or preference is thought to be a more reliable readout of the behavioral effects of UCMS, especially in rats.31 However, in this experimental mouse cohort, we were unable to detect changes in sucrose consumption between groups. It has been shown that certain chronic stress conditions can increase rodent’s consumption of sucrose48 and can precipitate weight gain.49 Although notorious for its intricacy, UCMS is a widely adopted paradigm mainly due to its construct validity in modeling stress-related pathophysiology.33,39 Thus, we consider the possibility that the observed discrepancies between behavioral tests or readouts may be accounted for by the inadequacy or inconsistency of a behavioral test to detect the effects of stress. In this context, we recently developed a test designed for the assessment of anxiety-like behavior in a home-cage-like apparatus allowing the application of a light challenge. This automated test vastly decreases potential confounds introduced by the investigator, reducing bias during the test and allowing for a longer detection period for detailed behavioral characterization.50 Under these conditions, UCMS-exposed animals avoid the food zone during the hour following a light challenge, suggesting that UCMS exposure impairs the animal’s ability to “cope” or respond to natural drives (e.g., feeding) after an acute aversive stimulus. Overall, we confirmed here that chronic exposure to stress induces a physical response as assessed by deterioration of coat state,51 anxiety-like deficits assessed in the classical NSF test33,46 and extend these findings to a novel test measuring avoidance/anxiety-like behavior, the phenotyper test.
Growing evidence implicates metabolic and oxidative stress as potential cellular mechanisms underlying diverse pathologies induced by chronic stress. Indeed, chronic stress results in various cellular impairments in the FC, including cortical pyramidal neuron atrophy,9,52 GABA neuron dysfunction,42 astroglial loss,35,53 and increased markers of microglia and inflammatory responses.54 Each of these processes has been directly linked to the expression of anxiety-/depressive-like phenotypes in rodents.35,39,54,55 The molecular mechanisms mediating the cellular effects of stress in the brain include activation of the hypothalamic–pituitary–adrenal axis, impairment of cellular homeostasis56 as well as excitotoxicity,57 dysregulation of mitochondrial function,58 and induction of oxidative stress.29,59 Here, we report an elevation of neuronal expression of 8-OHdG/8-oxo-G, a robust maker of DNA/RNA oxidation, in the FC of mice subjected to UCMS. In the BLA, however, no increase in neuronal expression of 8-OHdG/8-oxo-G was found. This latter finding is not surprising since, contrary to the FC, chronic stress enhances dendritic growth and causes hypertrophy and hyperactivity in the amygdala.11,60 Altogether, our results provide additional evidence to support the hypothesis of an involvement of DNA/RNA oxidation in neuronal dysfunction observed in the FC in response to chronic stress and suggest that the FC, but not the BLA has a particular vulnerability to stress. Selective FC vulnerability to chronic stress has been reported in previous studies demonstrating that even uncontrollable mild stress can cause a rapid and robust loss of prefrontal cognitive abilities as well as morphological synaptic reorganization when the stress exposure is sustained.61 DNA/RNA oxidation in response to stress can be a critical element for the cellular integrity of the FC and may represent a potential mechanism underlying downstream cellular deficits observed in animal models and further in patients with MDD.
Diverse cellular and molecular mechanisms mediate the effects of physical and/or psychosocial stress, including increased glucocorticoids, glutamate, or cytokines, and reductions of trophic support.16,57,62 Several of these changes have been linked to increased ROS production, resulting in cellular damage to proteins, lipids, and DNA, thereby affecting both peripheral and cerebral cellular function.62 For example, López-López et al.15 demonstrated that UCMS concomitantly increased serum corticosterone levels, decreased the levels of glutathione in the pancreas, and increased the level of superoxide dismutase in the pancreas. These changes were linked to UCMS-induced systemic inflammation and protein oxidation.15 In humans, similar correlation between peripheral cortisol levels and urinary 8-OHdG and 8-oxo-G levels were described in elderly patients.28 Recently, a meta-analysis reported that oxidative stress markers, including 8-OHdG, are increased in subjects with MDD or bipolar disorder with depressive symptoms, suggesting that 8-OHdG levels might be a potential biomarker of depressive states.16,63 However, translating peripheral changes in DNA oxidation levels to a cerebral increase in neuronal damage has been challenging, and there is a need for large-scale and longitudinal studies. In support to these peripheral findings, postmortem work showed increased oxidative stress using other makers, including decreased levels of glutathione in the PFC of patients with MDD or bipolar disorder,23 and increased RNA oxidation in the hippocampus of patients with MDD.24
The aforementioned studies, together with our findings, provide evidence of increased DNA/RNA damage in response to chronic stress. Moreover, collective data support the involvement of oxidative damage to nucleic acids in the pathophysiology of age-related illnesses, such as Alzheimer’s disease.64 A meta-analysis and meta-regression study revealed that persons with a history of MDD are at increased risk of developing Alzheimer’s disease.65 Given that chronic stress is implicated in the development and progression of MDD,1 and that DNA/RNA oxidation increases in response to chronic stress in patients with MDD, decreasing neuronal and systemic DNA/RNA oxidation might reduce symptom severity and slow illness progression.
These studies are to be taken with caution since they focus on a specific subset of oxidative stress markers and may not be generalizable to different markers or brain regions.66 Additionally, we demonstrated a positive correlation between DNA/RNA oxidation in the FC with one of our anxiety-like measurements (latency to feed in the NSF) between groups that did not reach significance criteria when correlations were performed within groups. Also, we did not correct for multiple comparisons because of the small sample size used in the study. Nonetheless, our results demonstrate that UCMS induces an increase in neuronal DNA/RNA oxidation levels in the FC, which might indicate that induction of oxidative stress by chronic stress may be selective to some cellular populations with a greater vulnerability to stress, e.g., neurons versus glial cells or GABAergic interneurons versus glutamatergic pyramidal neurons. Further studies evaluating the levels of DNA/RNA oxidation in glial cells or in a specific subpopulation of neurons in both rodent models of stress and postmortem MDD brains will provide further insight into the cellular mechanisms underlying chronic stress and stress-related illnesses.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a NARSAD (National Alliance for Research on Schizophrenia and Depression) Young Investigator Award (PI: Banasr). Dr Andreazza is funded by Canadian Institute of Health Research (MOP-133439), Ontario Mental Health Foundation (OMHF 498567), and Ministry of Research and Innovation of Canada (ERA-14-10-022).
References
- 1.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997; 48: 191–214. [DOI] [PubMed] [Google Scholar]
- 2.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009; 10: 423–433. [DOI] [PubMed] [Google Scholar]
- 3.Hunter RG, McEwen BS. Stress and anxiety across the lifespan: structural plasticity and epigenetic regulation. Epigenomics. 2013; 5: 177–194. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev Neurobiol. 2012; 72: 878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magariños AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995; 69: 83–88. [DOI] [PubMed] [Google Scholar]
- 6.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010; 35: 2–16. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS. Possible mechanisms for atrophy of the human hippocampus. Mol Psychiatry. 1997; 2: 255–262. [DOI] [PubMed] [Google Scholar]
- 8.Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005; 15: 1714–1722. [DOI] [PubMed] [Google Scholar]
- 9.Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004; 60: 236–248. [DOI] [PubMed] [Google Scholar]
- 10.Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003; 965: 290–294. [DOI] [PubMed] [Google Scholar]
- 11.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007; 27: 2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2016; 126: 890–909. [DOI] [PubMed] [Google Scholar]
- 14.Lucca G, Comim CM, Valvassori SS, et al. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem Int. 2009; 54: 358–362. [DOI] [PubMed] [Google Scholar]
- 15.López-López AL, Jaime HB, Escobar Villanueva Mdel C, Padilla MB, Palacios GV, Aguilar FJ. Chronic unpredictable mild stress generates oxidative stress and systemic inflammation in rats. Physiol Behav. 2016; 161: 15–23. [DOI] [PubMed] [Google Scholar]
- 16.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015; 51: 164–175. [DOI] [PubMed] [Google Scholar]
- 17.Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997; 82: 291–295. [DOI] [PubMed] [Google Scholar]
- 18.Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011; 35: 676–692. [DOI] [PubMed] [Google Scholar]
- 19.Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999; 222: 236–245. [DOI] [PubMed] [Google Scholar]
- 20.Ng F, Berk M, Dean O, et al. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008; 11: 851–876. [DOI] [PubMed] [Google Scholar]
- 21.Andreazza AC, Kauer-Sant’Anna M, Frey BN, et al. Oxidative stress markers in bipolar disorder: a meta-analysis. J Affect Disord. 2008; 111: 135–144. [DOI] [PubMed] [Google Scholar]
- 22.Palta P, Samuel LJ, Miller ER, et al. Depression and oxidative stress. Psychosom Med. 2014; 76: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gawryluk JW, Wang J-F, Andreazza AC, et al. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol. 2011; 14: 123–130. [DOI] [PubMed] [Google Scholar]
- 24.Che Y, Wang J-F, Shao L, Young T. Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. J Psychiatry Neurosci. 2010; 35: 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barron H, Hafizi S, Andreazza A, Mizrahi R. Neuroinflammation and oxidative stress in psychosis and psychosis risk. Int J Mol Sci. 2017; 18: 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med. 2006; 68: 1–7. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen A, Krogh J, Miskowiak K, et al. Systemic oxidatively generated DNA/RNA damage in clinical depression: associations to symptom severity and response to electroconvulsive therapy. J Affect Disord. 2013; 149: 355–362. [DOI] [PubMed] [Google Scholar]
- 28.Joergensen A, Broedbaek K, Weimann A, et al. Association between urinary excretion of cortisol and markers of oxidatively damaged DNA and RNA in humans. PLoS One. 2011; 6: e20795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zafir A, Banu N. Modulation of in vivo oxidative status by exogenous corticosterone and restraint stress in rats. Stress. 2009; 12: 167–177. [DOI] [PubMed] [Google Scholar]
- 30.Papp M, Willner P, Muscat R, et al. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Brain Res Rev. 1991; 104: 255–259. [DOI] [PubMed] [Google Scholar]
- 31.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005; 52: 90–110. [DOI] [PubMed] [Google Scholar]
- 32.Wiborg O. Chronic mild stress for modeling anhedonia. Cell Tissue Res. 2013; 354: 155–169. [DOI] [PubMed] [Google Scholar]
- 33.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006; 175: 43–50. [DOI] [PubMed] [Google Scholar]
- 34.Lin LC, Sibille E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry. 2015; 20: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008; 64: 863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duric V, Banasr M, Licznerski P, et al. A negative regulator of MAP kinase causes depressive behavior. Nat Med. 2010; 16: 1328–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang HJ, Voleti B, Hajszan T, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012; 18: 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin LC, Sibille E. Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front Pharmacol. 2013; 4: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soumier A, Sibille E. Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology. 2014; 39: 2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guilloux JP, Seney M, Edgar N, et al. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011; 197: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sibille E, Wang Y, Joeyen-Waldorf J, et al. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009; 166: 1011–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Northoff G, Sibille E. Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry. 2014; 19: 966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong Q, Lin CL. Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci. 2010; 67: 1817–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000; 275: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 45.Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009; 161: 359–369. [DOI] [PubMed] [Google Scholar]
- 46.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005; 29: 771–783. [DOI] [PubMed] [Google Scholar]
- 47.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997; 8: 523–532. [DOI] [PubMed] [Google Scholar]
- 48.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007; 23: 887–894. [DOI] [PubMed] [Google Scholar]
- 49.Dallman MF, Pecoraro N, Akana SF, et al. Chronic stress and obesity: a new view of ‘comfort food’. Proc Natl Acad Sci. 2003; 100: 11696–11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aarts E, Maroteaux G, Loos M, et al. The light spot test: measuring anxiety in mice in an automated home-cage environment. Behav Brain Res. 2015; 294: 123–130. [DOI] [PubMed] [Google Scholar]
- 51.Nollet M, Le Guisquet A-M, Belzung C. Models of depression: unpredictable chronic mild stress in mice. Curr Protoc Pharmacol. 2013. Chapter 5: Unit 5.65. [DOI] [PubMed] [Google Scholar]
- 52.Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol. 2008; 585: 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Czeh B, Simon M, Schmelting B, et al. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006; 31: 1616–1626. [DOI] [PubMed] [Google Scholar]
- 54.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008; 105: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ota KT, Liu RJ, Voleti B, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med. 2014; 20: 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McEwen BS, Wingfield JC. Allostasis and allostatic load. In: George Fink (ed.) Encyclopedia of Stress. Elsevier/Academic Press, 2010, pp. 135–141.
- 57.McIntosh LJ, Hong KE, Sapolsky RM. Glucocorticoids may alter antioxidant enzyme capacity in the brain: baseline studies. Brain Res. 1998; 791: 209–214. [DOI] [PubMed] [Google Scholar]
- 58.Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. 2009; 106: 3543–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McIntosh LJ, Sapolsky RM. Glucocorticoids may enhance oxygen radical-mediated neurotoxicity. Neurotoxicology. 1996; 17: 873–882. [PubMed] [Google Scholar]
- 60.Wood GE, Young LT, Reagan LP, McEwen BS. Acute and chronic restraint stress alter the incidence of social conflict in male rats. Horm Behav. 2003; 43: 205–213. [DOI] [PubMed] [Google Scholar]
- 61.Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009; 10: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996; 141: 201–206. [DOI] [PubMed] [Google Scholar]
- 63.Szebeni A, Szebeni K, DiPeri TP, et al. Elevated DNA oxidation and DNA repair enzyme expression in brain white matter in major depressive disorder. Int J Neuropsychopharmacol. 2017; 20: 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lovell MA, Soman S, Bradley MA. Oxidatively modified nucleic acids in preclinical Alzheimer’s disease (PCAD) brain. Mech Ageing Dev. 2011; 132: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006; 63: 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fontella FU, Siqueira IR, Vasconcellos APS, et al. Repeated restraint stress induces oxidative damage in rat hippocampus. Neurochem Res. 2005; 30: 105–111. [DOI] [PubMed] [Google Scholar]