Abstract
Voltage-gated sodium channels are required for electrogenesis in excitable cells. Their activation, triggered by membrane depolarization, generates transient sodium currents that initiate action potentials in neurons, cardiac, and skeletal muscle cells. Cells that have not traditionally been considered to be excitable (“nonexcitable cells”), including glial cells, also express sodium channels in physiological conditions as well as in pathological conditions. These channels contribute to multiple functional roles that are seemingly unrelated to the generation of action potentials. Here, we discuss the dynamics of sodium channel expression in astrocytes and microglia, and review evidence for noncanonical roles in effector functions of these cells including phagocytosis, migration, proliferation, ionic homeostasis, and secretion of chemokines/cytokines. We also examine possible mechanisms by which sodium channels contribute to the activity of glial cells, with an eye towards therapeutic implications for CNS disease.
Keywords: sodium channels, astrocytes, microglia, sodium-calcium exchanger, EAE, multiple sclerosis
Graphical Abstract

Introduction
Voltage-gated sodium channels are heteromeric transmembrane protein complexes that are a molecular hallmark of excitable cells. Membrane depolarization triggers their activation, generating transient inward sodium currents that initiate action potentials in neurons, cardiac, and skeletal muscle cells. Sodium currents were discovered by Hodgkin and Huxley using the voltage clamp technique and reported in their groundbreaking series of papers in 1952; Catterall et al. (2005) subsequently began a molecular characterization of sodium channels from excitable membranes in the 1980’s. It is now known that there are nine pore-forming α-subunits of sodium channels, Nav1.1-Nav1.9, encoded by genes SCN1A-SCN11A (Catterall et al. 2005), which associate with one or more non-pore-forming β-subunits encoded by SCN1B-SCN4B (Brackenbury and Isom 2011), lending considerable pharmacological and electrophysiological diversity, and possibly explaining their unique tissue-specific expression patterns (Catterall et al. 2005). The canonical role of these channels in driving electrogenesis and conduction in neurons (Nav1.1, Nav1.2, Nav1.3, Nav1.6, Nav1.7, Nav1.8), myocytes (Nav1.4), and cardiomyocytes (Nav1.5) has been extensively studied and is well-characterized (Catterall 2012; Waxman 2000), with a great deal of effort being put forth investigating the pathological consequences of sodium channel dysfunction in neurological disorders including neuropathic pain (Dib-Hajj et al. 2007; Dib-Hajj et al. 2013; Wood 2007), peripheral neuropathy (Faber et al. 2012a; Faber et al. 2012b; Hoeijmakers et al. 2015), epilepsy (Helbig et al. 2008; Oliva et al. 2012), multiple sclerosis (Waxman 2006; Waxman 2008), cardiac arrhythmias such as Brugada syndrome and long QT syndrome (Liu et al. 2014; Remme 2013), and muscular disorders (Cannon 2010).
In addition to being expressed in cells capable of generating action potentials, sodium channels have also been identified in cells that have not traditionally been considered to be electrically excitable (“nonexcitable cells”), leading to speculation as to their functional role. Voltage-gated sodium channels have been documented in immune cells such as macrophages (Black et al. 2013; Carrithers et al. 2011; Carrithers et al. 2009; Carrithers et al. 2007; Schmidtmayer et al. 1994), lymphocytes (DeCoursey et al. 1985; Decoursey et al. 1987; Fraser et al. 2008; Lai et al. 2000; Lo et al. 2012), and dendritic cells (Kis-Toth et al. 2011; Zsiros et al. 2009), in addition to fibroblasts (Chatelier et al. 2012; Estacion 1991; Li et al. 2009; Munson et al. 1979), osteoblasts (Black et al. 1995b), keratinocytes (Zhao et al. 2008), epithelial cells (Wu et al. 2006; Wu et al. 2008), and others. Sodium channels contribute to multiple, varied cellular functions in these cells including phagocytosis (Carrithers et al. 2007), migration (Fraser et al. 2008; Kis-Toth et al. 2011; Wu et al. 2008), and proliferation (Wu et al. 2006). Furthermore, it is becoming increasingly recognized that the expression of sodium channels correlates with invasiveness and metastatic potential in some types of cancer cells (Patel and Brackenbury 2015; Roger et al. 2015) including prostate (Brackenbury and Djamgoz 2006; Diss et al. 2005; Fraser et al. 2003), breast (Brackenbury et al. 2007; Driffort et al. 2014; Gillet et al. 2009; Nelson et al. 2015; Yang et al. 2012), ovarian (Gao et al. 2010), melanoma (Carrithers et al. 2009) and colon (House et al. 2010; House et al. 2015).
Though glia, too, are considered nonexcitable, studies have demonstrated that these cells, including oligodendrocyte precursor (NG2+) cells, Schwann cells, Müller glia, microglia, and astrocytes also express voltage-gated sodium channels (Table 1). There is growing evidence that these channels regulate or participate in effector functions of glia through signaling mechanisms that are just beginning to be understood. The regulation of glial function by sodium channels has particular implications for the response of reactive glia to central nervous system (CNS) disease and insult. In this review, we focus on the dynamics of sodium channel expression in astrocytes and microglia and examine the evidence supporting a contribution of sodium channels to multiple functions of these cells. We also discuss mechanisms by which sodium channels may contribute to the regulation of the activity of glial cells, focusing on therapeutic implications for neurological disease.
Table 1.
Voltage-Gated Sodium Channels in Glia
Sodium channel expression in glia
The earliest indication of voltage-gated sodium channels in glia originated from patch-clamp studies on cultured astrocytes (Bevan et al. 1985) and Schwann cells (Chiu et al. 1984), which showed fast-activating, fast-inactivating currents that were blocked with the sodium channel-specific antagonists saxitoxin (STX) and tetrodotoxin (TTX). Patch-clamp recordings have since confirmed the expression of functional sodium channels in oligodendrocyte precursor cells (Chen et al. 2008; Karadottir et al. 2008; Kettenmann et al. 1991; Kressin et al. 1995; Linnertz et al. 2011; Sontheimer et al. 1989), Schwann cells (Howe and Ritchie 1990), microglia (Korotzer and Cotman 1992; Nicholson and Randall 2009; Persson et al. 2014), and astrocytes (Barres et al. 1988; Barres et al. 1989; Sontheimer et al. 1992; Sontheimer and Waxman 1992). Importantly, voltage-dependent sodium currents have been detected in astrocytes in situ within spinal cord (Chvatal et al. 1995) and hippocampal (Sontheimer and Waxman 1993) slices and in “tissue print” preparations (Barres et al. 1990). Patch clamp recordings lend the ability to distinguish between the expression of sodium channels that are sensitive to nanomolar levels of TTX (TTX-S; Nav1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.5, Nav1.7) and those that require micromolar concentrations of TTX for blockade (TTX-R; Nav1.5, Nav1.8, Nav1.9).
Microglia express a variety of voltage-gated ion channels, including sodium channels (Kettenmann et al. 2011), the predominant isoform being TTX-S Nav1.6 (Black et al. 2009; Craner et al. 2005). In vitro, microglia derived from mixed glial cultures from neonatal rats exhibit immunolabeling for Nav1.1, Nav1.5, and Nav1.6, which is most prominent (Fig. 1A), while Nav1.2, Nav1.3, Nav1.7, Nav1.8, and Nav1.9 are not detectable above background levels (Black et al. 2009). Recently, using whole-cell voltage clamp on cultured rat microglia, depolarization-induced sodium currents were elicited and then completely blocked by 0.3 μM TTX, consistent with the presence of functional TTX-S sodium channels (Persson et al. 2014) (Fig. 1B–D). Similarly, microglia within normal CNS tissues exhibit low levels of Nav1.6 immunolabeling in situ (Black and Waxman 2012).
Figure 1.
Expression of sodium channels in microglia in vitro and in vivo. (A) Iba1+ and CD11b+ (green) microglia exhibit immunolabelling for sodium channels Nav1.1, Nav1.5, and Nav1.6 (red). Microglial exhibit background levels of Nav1.2, Nav1.3, Nav1.7, Nav1.8, and Nav1.9 immunoreactivity. [Modified from Black et al. (2009)]. (B) Representative sodium current traces recorded from microglial before (black) and after (red) treatment of 0.3 μM TTX. (C) Normalized peak current-voltage relationship. (D) Voltage-dependence of activation and steady-state fast-inactivation. [Modified from Persson et al. (2014)]. E. Images of spinal cord sections from control mice immnolabeled with CD45 (green), OX-42 (blue), and Nav1.6 (red) antibodies exhibit a lack of CD45/OX-42 immunopositive cells in control tissue (top panels). Images of spinal cord sections from EAE mice show extensive infiltration of CD45 (green) and OX-42 (blue) positive cells in EAE spinal cord, with extensive co-localization of Nav1.6 and OX-42 (yellow) and Nav1.6, OX-42, and CD45 (white) in merged image of EAE spinal cord (bottom panels). (F) Microglia labeled with OX-42 (blue) and CD45 (green) in control spinal cord exhibit a non-activated morphology (top row, left) and low levels of Nav1.6 immunolabeling (middle, left). In spinal cords of mice with EAE there is a progressive transformation to a amoeboid-like appearance consistent with a phagocytic microglial phenotype (top, right) and an incremental increase in Nav1.6 immunoreactivity (middle, right). [Modified from Craner et al. (2005)].
In contrast to the low levels of sodium channel expression exhibited by unperturbed microglia, there is a marked upregulation of Nav1.6 in microglia within the context of experimental autoimmune encephalomyelitis (EAE), an inflammatory/demyelinating model of multiple sclerosis (MS), both at the mRNA and protein levels (Fig. 1E) (Craner et al. 2005). Intriguingly, Nav1.6 expression in reactive microglia is dynamic, with upregulated expression corresponding to increasing disease severity of the animal and coincident with a morphological transformation into an amoeboid appearance in microglia in both the spinal cord and optic nerve (Fig. 1F) (Craner et al. 2005). Similarly, while microglia from human control tissue obtained at autopsy from individuals without neurological disease show minimal levels of Nav1.6 immunolabeling, microglia found within active MS lesions show robust Nav1.6 expression, along with a change in morphology from ramified to amoeboid (Craner et al. 2005). Additionally, a recent study identified the preferential accumulation of Nav1.6 within lamellipodia of ATP-activated microglia in vitro (Fig. 4A) (Persson et al. 2014). Collectively, these data suggest a phenomenon of upregulated sodium channel expression in microglia that is correlated to the extent of CNS pathology, consistent with a functional role of sodium channels in the response of reactive microglia to inflammation/demyelination.
Figure 4.

Sodium channels contribute to lamellipodia formation in ATP-stimulated microglia. (A) Nonstimulated microglia exhibit a diffuse distribution of phalloidin (green) and Nav1.6 (red) immunolabelling whereas ATP stimulation of microglia induces formation of lamellipodia that display robust immunolabelling for phalloidin and Nav1.6. Scale bar, 10 μm. (B) Nonstimulated microglia from wild-type mice display limited formation of lamellipodia (green) whereas ATP stimulation induces substantial lamellipodia formation. Treatment of ATP-stimulated microglia with 0.3 μm TTX significantly attenuates lamellipodia formation. Scale bars, 25 μm. [Modified from Persson et al. (2014)].
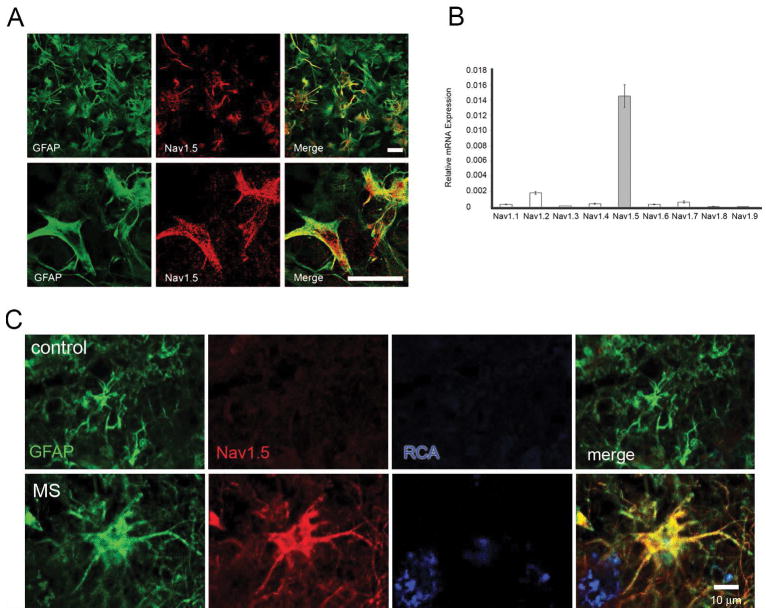
In astrocytes, the TTX-R (Rogart et al. 1989) “cardiac” isoform Nav1.5 (Black et al. 1998; Black et al. 2010; Pappalardo et al. 2014b) is the predominant sodium channel characterized (Fig. 2A,B), though expression of Nav1.2, Nav1.3 (Black et al. 1995a), and Nav1.6 (Reese and Caldwell 1999) have also been reported. Black et al. (1998) demonstrated Nav1.5 mRNA and protein in rodent astrocytes in vitro and in situ and the expression of all nine sodium channel isoforms in cultured embryonic rat astrocytes was recently examined using qPCR, demonstrating Nav1.5 mRNA levels far higher than any other sodium channel (Fig. 2B) (Pappalardo et al. 2014b).
Figure 2.
Expression of sodium channels in astrocytes in vitro and in vivo. (A) GFAP-positive cultured rat cortical astrocytes (green) exhibit prominent Nav1.5 immunolabelling (red), observed at low magnification (top panel) and at increased magnification (bottom panel). Scale bars, 25 μm. (B) RT-PCR showing relative mRNA expression of voltage-gated sodium channels (VGSC). Nav1.5 is the predominant VGSC subtype expressed in cultured rat cortical astrocytes. [Modified from Pappalardo et al. (2014b)]. (C) GFAP-positive astrocyte (green) within control human tissue obtained at autopsy does not exhibit Nav1.5 labeling. In contrast reactive astrocyte within an active MS lesion displays robust Nav1.5 immunolabeling (red). Ricinus communis agglutinin I (RCA) positive macrophages (blue) are present adjacent to the reactive astrocyte (bottom, right). [Modified from Black et al. (2010)].
In parallel to microglia, the expression of sodium channels in astrocytes has been well-documented as a dynamic process. In vitro, the density of sodium channels in astrocytes varies according to culture conditions and the extracellular milieu (Thio and Sontheimer 1993; Thio et al. 1993). Astrocytic sodium channel expression is also modulated by exposure to injury or disease. In an in vitro model of astrogliosis, in which a confluent monolayer of astrocytes was scratched linearly, MacFarlane and Sontheimer (1998) described a shift from TTX-S sodium currents to TTX-R sodium currents with properties attributed to Nav1.5 in response to the injury, and there was a significant increase in the numbers of scarring, proliferating astrocytes along the edges of the injury displaying transient sodium currents compared to control astrocytes (MacFarlane and Sontheimer 1997). Consistent with this earlier work, markedly upregulated expression of Nav1.5 was reported in reactive astrocytes along the borders of the injury in the same in vitro model (Samad et al. 2012).
A recent study examining the temporal expression of astrocytic sodium channels in monophasic and chronic-relapsing (CR) EAE models of MS showed robust Nav1.5 upregulation in affected animals that correlated with disease severity, as indicated by clinical score (Pappalardo et al. 2014a). Interestingly, Nav1.5 expression was dynamic in CR EAE, with increased levels during more severe periods of disease and attenuated expression during remissions (Pappalardo et al. 2014a). The low level of Nav1.5 expression in astrocytes of control animals suggests that Nav1.5 upregulation is part of the biological response of astrocytes to CNS insult (Pappalardo et al. 2014a). Notably, Black et al. (2010) observed the upregulation of Nav1.5 on rapid-autopsy tissue, which is not seen in normal control brains, in human scarring astrocytes in situ within acute and chronic MS lesions (Fig. 2C), surrounding new and old stroke lesions, and along the borders of CNS tumors, including gliomas and a metastatic carcinoma. Consistent with these observations, Bordey and Sonthimer (1998) reported the expression of functional sodium channels in astrocytoma cells. Finally, Nav1.5 upregulation has been observed in scarring astrocytes following a contusion spinal cord injury (SCI) (unpublished observations). Together, as in microglia, these observations suggest a commonality of upregulated astrocytic sodium channels in response to CNS tissue injury and disease.
Sodium channels regulate multiple effector functions of microglia
Despite the long-characterized expression of sodium channels in neuroglia, their functional role (Table 2) has remained elusive until recent years. Microglia are motile resident immune cells within the brain and spinal cord that normally provide surveillance to the healthy CNS and become reactive in response to tissue insult, pathogenic challenge, or signaling within the CNS. Microgliosis involves migration, phagocytosis and secretion of chemokines, cytokines, and reactive species (Colton 2009; Hanisch and Kettenmann 2007; Ransohoff and Perry 2009). To test the notion that sodium channels modulate the microglial response to inflammation/demyelination, mice were inoculated with myelin oligodendrocyte glycoprotein (MOG 35–55) to induce EAE and fed chow supplemented with phenytoin (Lo et al. 2003), a clinically-used antiepileptic drug that blocks sodium channels (Mantegazza et al. 2010). Mice receiving phenytoin chow 10 days post EAE induction had a four-fold decrease in the number of CD45/CD11b/c-positive microglia (Sedgwick et al. 1991) within spinal cords compared to untreated mice when assessed at 18 days (Fig. 3A), which was coincident with a significant improvement in clinical status (Craner et al. 2005). Additionally, Morsali et al. (2013) demonstrated significant axonal protection with the sodium channel blocker safinamide in EAE, even with administration delayed until the onset of clinical symptoms. Rats treated with high dose safinamide for 2 weeks showed greater numbers of surviving and functional axons than did controls treated with saline and 10% of safinamide-treated rats exhibited bilateral hindlimb paralysis at the end of the trial compared with 65% of controls, outcomes the authors partially attributed to reduced microglial/macrophage activation, with reduced numbers of activated microglia within the spinal cord (Morsali et al. 2013).
Table 2.
Sodium Channel Functions in Glia
| Cell type | Effect | References |
|---|---|---|
| Astrocytes | TTX: attenuates Na+/K+-ATPase activity TTX, Nav1.5 siRNA: attenuates astrogliosis in vitro; decreases [Ca2+]i response in model of astrogliosis Veratidine: increases NOS activity |
(Oka et al. 2004; Pappalardo et al. 2014b; Sontheimer et al. 1994) |
| Microglia | TTX, phenytoin: attenuate phagocytosis, migration, cytokine release TTX: attenuates lamellipodia formation, decreases active Rac-1 levels, decreases phosphorylated ERK1/2 levels, and decreases [Ca2+]i response after ATP stimulation |
(Black et al. 2009; Craner et al. 2005; Persson et al. 2014) |
| Müller glia | TTX, STX, phenytoin: inhibit ligand-induced release of glutamate | (Linnertz et al. 2011) |
| Oligodendrocyte precursor cells (NG2+) | TTX, Nav1.x siRNA: attenuate GABA-induced migration | (Tong et al. 2009) |
Figure 3.
Sodium channel blockade reduces effector functions of microglia (A) Spinal cords from control, EAE, and phenytoin-treated EAE mice were immunostained with anti-CD45 (green) and anti-OX-42 (blue) antibodies. There is a notable increase in the number of immune cells within spinal cords from mice with EAE (middle) compared to control (left), and mice treated with phenytoin (right) exhibit a marked reduction of inflammatory infiltrate. [Modified from Craner et al. (2005)]. (B) LPS-stimulated microglia (red) exhibit marked phagocytosis of fluorescent-labeled latex beads (yellow) which is attenuated by incubation with 0.3 μm TTX and phenytoin (phen). (C) Microglia exhibit limited migration through the trans-well membrane in astrocyte-conditioned medium (ACM) only; in contrast, ATP stimulates a large number of microglia to migrate which is attenuated by both phenytoin and 0.3 μM TTX. [Modified from Black et al. (2009)].
Further investigation into the mechanisms of the beneficial effect of sodium channel blockade in EAE has demonstrated the functional role of sodium channels, particularly Nav1.6, in multiple aspects of microglial response to CNS insult including phagocytosis (Black et al. 2009; Craner et al. 2005), chemokine/cytokine release (Black et al. 2009; Morsali et al. 2013), and migration (Black et al. 2009; Persson et al. 2014). Craner et al. (2005) demonstrated that sodium channel blockade with TTX and phenytoin attenuates phagocytosis by 40% in cultured lipopolysaccharide (LPS)-stimulated microglia and demonstrated a reduction of 65% in the phagocytic ability of microglia derived from med mice, in which functional Nav1.6 channels are lacking (Kohrman et al. 1996), compared to microglia from wild-type mice. Black et al. (2009) additionally showed a 50–60% reduction in microglial phagocytic activity with TTX and phenytoin (Fig. 3B). Furthermore, TTX and phenytoin attenuated the release of multiple inflammatory cytokines and chemokines including interleukin 1-α (IL-1α), IL-1β and tumor necrosis factor α (TNF-α) from reactive microglia with minimal effects on IL-2, IL-4, IL-6, IL-10, monocyte chemotactic protein 1 (MCP-1), and transforming growth factor α (TGF-α), and safinamide administration reduced superoxide production and enhanced synthesis of the anti-oxidant glutathione in cultured microglia activated by phorbol-12-myristate-13-acetate (PMA) or LPS (Morsali et al. 2013).
A crucial and early functional response of reactive microglia is directed migration to focal sites of injury or infection within the CNS, which is a complex and highly coordinated process involving multiple cellular pathways, including transduction of external migratory signals, membrane adhesion and retraction, microglial polarization, and rearrangement of cytoskeletal proteins (Kettenmann et al. 2011). Microglial chemotaxis is also associated with pathological conditions occurring outside the CNS such as neuropathic pain (Beggs et al. 2012; Tsuda et al. 2013; Watkins et al. 2001); thus a detailed molecular mechanisms underlying this phenomenon is crucial. One of the initial structural events required for migration is the formation of lamellipodia (Bisi et al. 2013), membrane protrusions containing polymerized actin (F-actin), actin-binding proteins, Ca2+-binding molecules, and the GTP-binding signaling protein Rac (Honda et al. 2001; Ridley 1994; Ridley et al. 1992; Siddiqui et al. 2012). Rac signaling has been identified as a crucial component in the formation of lamellipodia (Hall 1998) and MAP kinases have been linked to reorganization of actin filaments and cellular motility (Huang et al. 2004). Ca2+ signaling also plays a critical role in lamellipodia protrusion and motility, as cell migration is Ca2+-dependent (Schwab et al. 2012; Wei et al. 2012). It is interesting to note that both Rac1 and MAP kinase activity are modulated by levels of intracellular Ca2+ (Aspenstrom 2004; Chuderland et al. 2008; Price et al. 2003; Wiegert and Bading 2011).
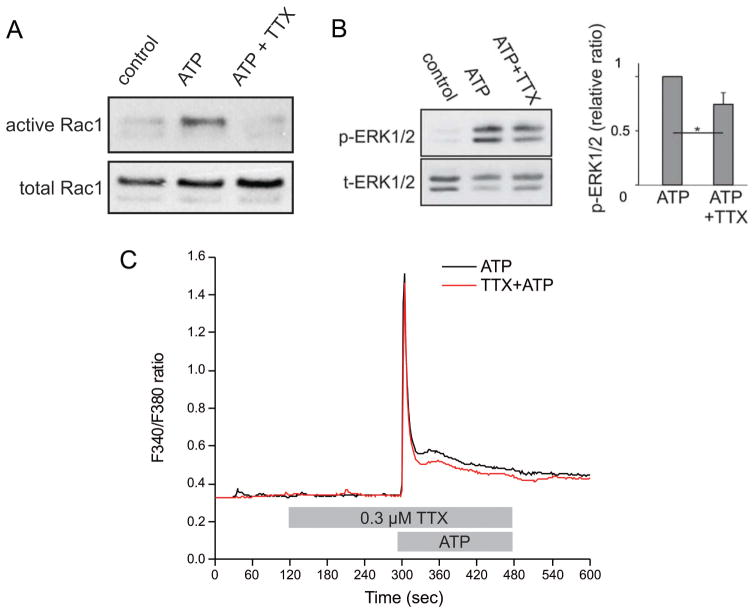
To assess the role of sodium channel activity in the pathways leading to migration, microglia were allowed to travel toward a chemoattractant in a trans-well plate in the presence or absence of sodium channel blockers (Black et al. 2009). As seen in Fig. 3C, ATP produced an almost four-fold increase in the mean number of microglia migrating through the pores of the trans-well membrane. This cell migration was significantly reduced (~50%) in the presence of phenytoin or 0.3 μM TTX (Black et al. 2009). Recently, Persson et al. (2014) demonstrated the robust expression and preferential distribution of Nav1.6 to lamellipodia of ATP-activated microglia (Fig. 4A); the ATP-induced formation of lamellipodia is decreased (~50%) by treatment with TTX (Fig. 4B) and in med mice, which lack functional Nav1.6 (Persson et al. 2014). Furthermore, sodium channel blockade with 0.3 μM TTX attenuated the ATP-induced increase in levels of active Rac1 (Fig. 5A) in addition to the ATP-induced phosphorylation of MAP kinase ERK1/2 (Fig. 5B) (Persson et al. 2014). Coincident with reduced active Rac1 and phosphorylated ERK1/2 levels, TTX additionally enhanced recovery of the Ca2+ transient in microglia following ATP stimulation (Fig. 5C), which may explain the effects of sodium channel blockade on decreasing active Rac1 and phosphorylated ERK1/2 levels (Persson et al. 2014). Collectively, these studies demonstrate an important functional role of sodium channels in regulating the behavior of reactive microglia.
Figure 5.
Sodium channels contribute to Rac1 and ERK1/2 activation and [Ca2+]i response in ATP-stimulated microglia. (A) A pull-down assay using the microglial cell line C8-B4 demonstrates that ATP induces elevated levels of activated Rac1 which is attenuated by 0.3 μM TTX. (B) Western blot analysis demonstrates that ATP stimulation of microglia increases levels of phosphorylated ERK1/2 compared to unstimulated control microglia. Treatment of ATP-activated microglia with 0.3 μM TTX significantly reduces activation of ERK1/2. (C) ATP stimulation induces a robust microglial [Ca2+]i response (black); the recovery of the [Ca2+] transient is enhanced by pretreatment with 0.3 μM TTX (red). Lines represent the ratio of fluorescent signals induced by 340 and 380 nm excitation in cells loaded with Fura-2 AM. [Modified from Persson et al. (2014)].
Sodium channels regulate multiple effector functions of astrocytes
Astrocytes serve multiple important functions in the CNS, including metabolic support of neurons, regulation of CNS ionic homeostasis, and participation in formation and maintenance of the blood-brain barrier. A standing Na+ influx in astrocytes is necessary for Na+/K+-ATPase activity and Sontheimer et al. (1994) postulated that sodium channels may provide a pathway for Na+ to enter the cell to maintain [Na+]i at levels necessary for Na+/K+-ATPase activity which, in turn, supports ionic homeostasis in the CNS, particularly in regard to K+ fluxes. Furthermore, it has been reported through 23Na MRI that sodium concentrations are elevated in acute and chronic MS lesions compared to normal appearing white matter (Inglese et al. 2010), which poses a possible clinical correlate to the suggestion of Black et al. (2010) that astrocytic Nav1.5 upregulation may provide a compensatory mechanism to maintain ionic homeostasis mediated by Na+/K+-ATPase activity within areas of CNS insult.
In addition to their many functions in the healthy CNS, an additionally crucial role for astrocytes is the response to CNS insult or disease through the incompletely understood process of reactive astrogliosis, which is a hallmark of nearly all CNS pathologies (Sofroniew 2009; Sofroniew 2015) and involves migration, proliferation, and release of multiple mediators of the immune response. While the functional ramifications of astrogliosis are complex, it is agreed that astrocytes can exert both beneficial and harmful effects, the outcome of which is determined by specific signaling cascades (Sofroniew 2009), temporal sequence of scar formation (Rolls et al. 2009), and extent and type of injury, which together determine reactive astrocyte phenotype (Zamanian et al. 2012). Though astrogliosis is associated with many beneficial functions, under certain circumstances severe forms of the process may lead to detrimental effects, such as compact scar formation, that can inhibit the regeneration of injured neurons (Silver and Miller 2004). The notion of astrocytopathies – dysfunctions of astrocytes and astrogliosis that can contribute to or be primary causes of CNS disorders, is an area of active investigation (Sofroniew 2015; Verkhratsky et al. 2013b; Verkhratsky et al. 2012).
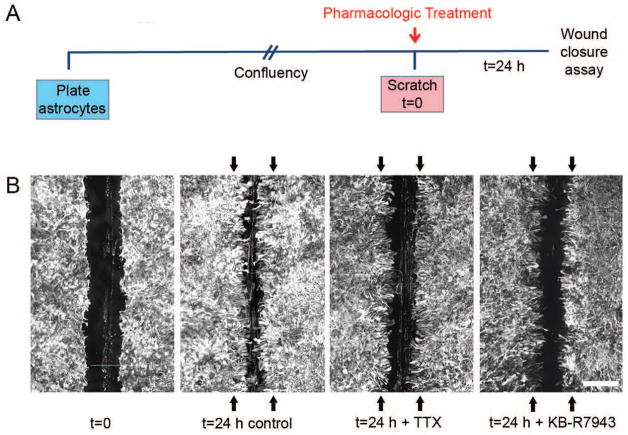
Recent work has shown that Nav1.5 plays a functional role in an in vitro model of astrogliosis similar to the previously described experiments in which TTX-R sodium currents (MacFarlane and Sontheimer 1998) and upregulation of Nav1.5 (Samad et al. 2012) have been described after injury. Embryonic rat cortical astrocytes were grown to confluency, then scratched linearly to provide a model of mechanical injury with subsequent assessment of scar formation (Fig. 6A). Twenty-four hours following mechanical injury (scratch), astrocytes extended into the injury gap, resulting in ~55% closure of the gap compared to the original wound size (Pappalardo et al. 2014b). Upon addition of 10 μM TTX (a dose which inhibits Nav1.5), the degree of closure of the gap was attenuated, with blockade of sodium channels resulting in significantly decreased closure of ~25% of the initial wound size (Fig. 6B) due to effects on both migration and proliferation (Pappalardo et al. 2014b). Given that Nav1.5 was determined by qPCR to be the predominant sodium channel isoform in vitro (Fig. 2B), the experiment was repeated with siRNA knockdown of Nav1.5. Twenty-four hours after the scratch injury, exposure to Nav1.5 siRNA reduced the amount of wound closure to ~45% compared to non-targeting (NT) siRNA (Pappalardo et al. 2014b), confirming Nav1.5 as the predominant sodium channel isoform involved. Reminiscent of the effects of microglial sodium channels on [Ca2+]i transients, Pappalardo et al. (2014b) also demonstrated that the robust [Ca2+]i response exhibited by astrocytes after injury was attenuated by pretreatment with TTX and Nav1.5 siRNA, affecting both the peak and total [Ca2+]i response (Fig. 7). Finally, it has been reported that activation of voltage-gated sodium channels by veratridine increases constitutive expression of nitric oxide synthase (NOS) in human astrocytes (Oka et al. 2004). In sum, these experiments indicate that sodium channels, particularly Nav1.5, play an important role in astrogliosis in vitro. Whether these results are applicable in vivo and whether sodium channels modulate additional effector functions of astrocytes is currently under investigation.
Figure 6.
Sodium channels contribute to astrogliosis in vitro. (A) Schematic of experimental design. Astrocytes were plated and allowed to grow until confluent, at which point they were treated with either TTX or KB-R7943 and linearly scratched. After 24 h, wound closure was analyzed and compared to initial wound size. (B) Astrocytes grow together following scratch injury, resulting in significant closure of the wound compared to the average original wound size (represented by black arrows) after a 24 h time period. Treatment with TTX or KB-R7943 attenuates closure of the scratch wound. Scale bar, 400 μm. [Modified from Pappalardo et al. (2014b)].
Figure 7.
Astrocytes display robust [Ca2+]i response after injury that is attenuated by TTX and KB-R7943. After a scratch injury, there is a marked [Ca2+]i response, which propagates through the syncytium of confluent astrocytes and slowly resolves after 2–4 min (first column). Application of TTX and KB- R7943 attenuates this [Ca2+]i response (second and third columns, respectively). Color scale represents the ratio of fluorescent signals induced by 340 and 380 nm excitation in cells loaded with Fura-2 AM. Scale bar, 50 μm. [Modified from Pappalardo et al. (2014b)].
Molecular mechanisms of sodium channel regulation of glial function
While there is considerable evidence that sodium channels contribute to the regulation of physiological functions of glia, especially with regard to the orchestrated response to CNS insult, there are presently limited data detailing the underlying mechanisms, which is an area of active investigation. It is likely there are multiple molecular pathways; thus it is useful to consider the signaling cascades that have been suggested to link the activity of sodium channels to effector functions in other nonexcitable cells (Black and Waxman 2013), such as immune cells (Lo et al. 2012) and cancer cells (House et al. 2015). For example, blockade or knockdown of Nav1.5 inhibits the sustained Ca2+ influx required for the positive selection of CD4+/CD8+ T lymphocytes (Lo et al. 2012) and invasiveness of melanoma cells seems to rely on activation of Nav1.6, which increases intracellular Ca2+ release and invadopodia formation (Carrithers et al. 2009).
Although glia do not generate action potentials under physiological conditions, these cells can exhibit excitability by way of ionic fluxes, particularly in the form of [Ca2+]i oscillations (Verkhratsky and Kettenmann 1996). Ca2+ dynamics participate in the regulation of microglial activation and many effector functions, including cell migration (Ifuku et al. 2007; Noda et al. 2013) and release of chemokines/cytokines and nitric oxide (Farber and Kettenmann 2006; Hoffmann et al. 2003; Ikeda et al. 2013). Astroglial [Ca2+]i fluxes modulate neuronal synapses, a phenomenon termed “gliotransmission” (Agulhon et al., 2008), and intracellular Ca2+ levels are critical for numerous cellular functions in astrocytes, including migration and proliferation (Parnis et al. 2013; Stanimirovic et al. 1995; Wang et al. 2010), both of which are processes involved in astrogliosis (Faulkner et al. 2004; Pappalardo et al. 2014b; Wanner et al. 2013). For example, treatment with the Ca2+ chelator BAPTA-AM inhibits astrogliosis in vitro (Pappalardo et al. 2014b) and in vivo (Gao et al. 2013), consistent with an important physiological role for the robust astroglial [Ca2+]i response seen after injury (Fig. 7) (Pappalardo et al. 2014b). Intriguingly, a recent study identified a Ca2+ signaling cascade that contributes to glial scarring in an in vitro mechanical injury model similar to that previously discussed (MacFarlane and Sontheimer 1998; Pappalardo et al. 2014b; Samad et al. 2012). Gao et al. (2013) showed that the increased [Ca2+]i transient in astrocytes after injury activates the protein kinase JNK, which phosphorylates transcription factor c-jun to facilitate GFAP upregulation and subsequent astrogliosis.
Recent work has also highlighted the importance of the contribution of [Na+]i fluctuations to glial function and homeostasis, with a prominent mechanism involving the linkage of transmembrane movements of Na+ and Ca2+ (Kettenmann et al. 2011; Kirischuk et al. 2012; Parpura and Verkhratsky 2012; Rose and Karus 2013; Verkhratsky et al. 2013a). Glutamate receptors and purinoceptors (Parpura and Verkhratsky 2012), as well as voltage-gated sodium channels, are known to play a role in glial Na+ influx, and a role for sodium channels as a driver of reverse (Ca2+-importing) Na+/Ca2+ exchange, which has been observed in multiple glial cell types including microglia (Ifuku et al. 2007; Kettenmann et al. 2011; Noda et al. 2013), astrocytes (Kirischuk et al. 1997; Paluzzi et al. 2007; Pappalardo et al. 2014b), and NG2+ cells (Tong et al. 2009), is beginning to emerge as a common theme.
The Na+/Ca2+ exchanger (NCX) operates in forward mode, transporting Na+ ions down their concentration gradient into cells and exporting Ca2+ in return or, if the Na+ electrochemical gradient is decreased or the cell is depolarized, operates in reverse mode by exporting Na+ ions in exchange for Ca2+ (Annunziato et al. 2004). Thus, sodium channel activity has the capability to increase [Ca2+]i via the reverse mode of NCX. Because the reversal potential of NCX in astrocytes is set at levels close to the resting membrane potential (Kirischuk et al. 1997; Reyes et al. 2012), it is possible that even small [Na+]i increases or depolarization can rapidly trigger reverse mode of NCX operation (Kirischuk et al. 2012; Paluzzi et al. 2007), increasing [Ca2+]i levels. Indeed, mechanical strain injury increases intracellular sodium, leading to NCX operating in reverse mode in cortical astrocytes (Floyd et al. 2005). As previously mentioned, sodium channel blockade attenuates astrogliosis in vitro, which is interestingly also reduced by blockade of the reverse (Ca2+-importing) mode of NCX. A KB-R7943 concentration of 0.5 μM selectively affects the reverse mode of NCX [IC50 = 1.1– 3.4 μmol/L for reverse mode and IC50 > 30 μmol/L for forward mode] (Iwamoto et al. 1996; Persson et al. 2013a; Persson et al. 2013b), and Pappalardo et al. (2014b) detailed an attenuation of injury-induced gliosis (Fig. 6B), affecting both astroglial proliferation and migration after treatment with 0.5 μM KB-R7943. Of note, blockade of reverse Na+/Ca2+ exchange decreased wound closure to a similar extent as both 10 μM TTX or Nav1.5 siRNA knockdown and there was no additional attenuation of gliosis with the combination of KB-R7943 + TTX, indicating possible non-redundancy in the underlying mechanisms involved (Pappalardo et al. 2014b). Furthermore, blockade of reverse NCX activity with KB-R7943 diminished the [Ca2+]i transient observed after injury to a similar extent as both TTX and Nav1.5 siRNA (Fig. 7) (Pappalardo et al. 2014b). Thus, Na+ flux through voltage-gated sodium channels, triggered by mechanical injury, elicits reverse operation of the Na+/Ca2+ exchanger, affecting cellular motility, facilitating astrogliosis in vitro (Fig. 8).
Figure 8.

Schematic of putative pathway of sodium channel contribution to intracellular Ca2+ levels and downstream pathways. Depolarization of glial membrane leads to activation of voltage-gated sodium channels (Nav) allowing influx of Na+. Increased [Na+]i induces reverse operation of the sodium-calcium exchanger (NCX), contributing to the level of [Ca2+]i. Ca2+ signaling initiates downstream effects on cellular functions. Blockade of sodium channels with tetrodotoxin (TTX) and reverse operation of NCX with KB-R7943 attenuates [Ca2+]i levels. [Modified from Persson et al. (2014)].
A similar mechanism seems to exist in NG2+ cells. Tong et al. (2009) demonstrated increased intracellular Na+ and Ca2+ levels, membrane depolarizations, and enhanced migratory capacity after GABA application. Blockade or knockdown of sodium channels by siRNA significantly decreased the rise in both [Na+]i and [Ca2+]i and attenuated cell migration, and siRNA knockdown of NCX or blockade of reverse Na+/Ca2+ exchange with KB-R7943 similarly decreased [Ca2+]i and reduced cell migration (Tong et al. 2009).
Microglia also express NCX (Kettenmann et al. 2011) and it is possible that the functional contribution of sodium channels to effector functions (e.g. migration, phagocytosis) involves the close linkage between glial Na+ and Ca2+ homeostasis. Indeed, as previously mentioned, Nav1.6 blockade (with TTX) or knockout (in med mice) decreases the formation of lamellipodia in ATP-activated microglia (Fig. 4B), which is the initial step in migration (Persson et al. 2014). Sodium channel blockade additionally enhances recovery of the microglial [Ca2+]i response following ATP stimulation (Fig. 5C) and decreases levels of active Rac1 (Fig. 5A) and phosphorylated MAP kinase ERK1/2 (Fig. 5B) (Persson et al. 2014). Given that both Rac1 and MAP kinase activity are modulated by levels of intracellular Ca2+ (Aspenstrom 2004; Chuderland et al. 2008; Price et al. 2003; Wiegert and Bading 2011), it is plausible that sodium channel blockade decreases [Ca2+]i levels, resulting in attenuation of active Rac1 and phosphorylated ERK1/2 levels, subsequently inhibiting microglial migration. Thus, while the underlying mechanisms linking sodium channel activity to effector functions in glia are still incompletely understood, increasing evidence points to a link between Na+ and Ca2+ signaling, with particular implications for the activity of the Na+/Ca2+ exchanger.
Therapeutic implications and future directions
As described above, there is a growing body of evidence detailing the favorable effect of sodium channel blockade in animal models of neurological diseases including EAE and the partial blockade of voltage-gated sodium channels has been proposed as a treatment strategy for MS (Waxman 2008). Previous studies on in vivo models of multiple sclerosis have shown improved clinical status and reduction of axonal loss following treatment with a variety of voltage-gated sodium channel blockers including phenytoin (Black et al. 2007; Craner et al. 2005; Lo et al. 2002; Lo et al. 2003), lamotrigine (Bechtold et al. 2006), carbamazepine (Black et al. 2007), safinamide (Morsali et al. 2013), and flecainide (Bechtold et al. 2004; Bechtold et al. 2005; Morsali et al. 2013). Additionally, phenytoin protects spinal cord axons, reduces gray and white matter destruction surrounding the lesion, and improves functional recovery after contusion-induced SCI (Hains et al. 2004) and phenytoin, riluzole, and mexilitine have all shown to improve outcome in murine models of SCI (Ates et al. 2007).
It is likely that inhibition of sodium channel activity by state-dependent sodium channel-blocking agents protects against axonal degeneration by two or more different mechanisms: firstly, by directly blocking the persistent sodium influx which can drive reverse Na+/Ca2+ exchange, leading to irreversible axonal damage due to high [Ca2+]i levels (Bechtold and Smith 2005; Stys et al. 1992), and second, by modulating the response of immune cells and/or glial cells to neuroinflammation via sodium channel blockade. Thus, sodium channel blockade may provide effective neuroprotection through multiple mechanisms. This notion is consistent with the observations that flecainide (Bechtold et al. 2004; Morsali et al. 2013), safinamide (Morsali et al. 2013), phenytoin (Black et al. 2007; Craner et al. 2005), and carbamazepine (Black et al. 2007) reduce the abundance of reactive microglia in several types of EAE. Given the evidence that sodium channels govern microglial phagocytosis, lamellipodia extension and migration, and chemokine/cytokine release, it is plausible that the protective effect of sodium channel-blocking agents in neuroinflammation is in part attributable to the functional role of sodium channels in reactive microglia.
The possible effects of astrocytic sodium channel blockade in CNS disease are less well-studied in vivo, though immunomodulatory roles for astrocytes in the injured CNS are well-recognized (Brosnan and Raine 2013; Dong and Benveniste 2001; Nair et al. 2008; Okun et al. 2009; Sofroniew 2014). The critical role of astrocytes in orchestrating the immune response in EAE was recently reported: knockout of astrocytic transcription factor NF-κB resulted in reduced disease severity and improved functional recovery (Brambilla et al. 2009), which the authors attribute to a reduction in peripheral immune cell infiltration into the CNS due to reduced immune cell mobilization from the periphery, diminished ability of T cells to produce proinflammatory cytokines, and reduced number of total and activated microglia (Brambilla et al. 2014; Brambilla et al. 2009). Thus, it is possible that modulation of astrogliosis may contribute to the positive outcomes seen in EAE with sodium channel blockade, though more investigation is certainly warranted.
Given the central role of glia in CNS health and disease, it is clear that there is a need for further understanding of the physiologically relevant roles of glial sodium channels and characterization of molecular pathways governing the functional roles of sodium channels in these cells. There has been much work performed in cell culture, but further in vivo studies are of crucial importance for determination of the therapeutic implications of targeting glial sodium channels in neurological disorders such as MS. With the heightened focus on developing sodium channel specific blockers, it is increasingly relevant to assess the roles of individual sodium channel isoforms in glia (e.g. Nav1.6 in microglia, Nav1.5 in astrocytes). In vivo studies using targeted knockdown of specific sodium channel isoforms are necessary and an area of active investigation. A fuller understanding of the signaling cascades linking sodium channel activity to effector functions of glia should allow for the development of specific therapeutic targets in neurological disease.
Main Points.
Functional sodium channels are expressed in astrocytes and microglia and contribute to the regulation of multiple effector functions of these glia through underlying mechanisms that are beginning to be understood.
Acknowledgments
The authors thank the members of their group for valuable discussions. Work in the authors’ laboratory is supported in part by grants from the Rehabilitation Research and Development Service and Medical Research Service, US Department of Veterans Affairs (S.G.W.). The Center for Neuroscience and Regeneration Research is a collaboration between the Paralyzed Veterans of America and Yale University, Connecticut, USA. L.W.P. is supported in part by NIH Medical Scientist Training Program Training Grant T32GM007205. The authors declare no competing financial interests.
References
- Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–54. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- Aspenstrom P. Integration of signalling pathways regulated by small GTPases and calcium. Biochim Biophys Acta. 2004;1742:51–8. doi: 10.1016/j.bbamcr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Ates O, Cayli SR, Gurses I, Turkoz Y, Tarim O, Cakir CO, Kocak A. Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J Clin Neurosci. 2007;14:658–665. doi: 10.1016/j.jocn.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Barres BA, Chun LL, Corey DP. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1:10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Barres BA, Chun LL, Corey DP. Glial and neuronal forms of the voltage-dependent sodium channel: characteristics and cell-type distribution. Neuron. 1989;2:1375–88. doi: 10.1016/0896-6273(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Barres BA, Koroshetz WJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron. 1990;5:527–44. doi: 10.1016/0896-6273(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Kapoor R, Smith KJ. Axonal protection using flecainide in experimental autoimmune encephalomyelitis. Ann Neurol. 2004;55:607–16. doi: 10.1002/ana.20045. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Miller SJ, Dawson AC, Sun Y, Kapoor R, Berry D, Smith KJ. Axonal protection achieved in a model of multiple sclerosis using lamotrigine. J Neurol. 2006;253:1542–51. doi: 10.1007/s00415-006-0204-1. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Smith KJ. Sodium-mediated axonal degeneration in inflammatory demyelinating disease. J Neurol Sci. 2005;233:27–35. doi: 10.1016/j.jns.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bechtold DA, Yue X, Evans RM, Davies M, Gregson NA, Smith KJ. Axonal protection in experimental autoimmune neuritis by the sodium channel blocking agent flecainide. Brain. 2005;128:18–28. doi: 10.1093/brain/awh328. [DOI] [PubMed] [Google Scholar]
- Beggs S, Trang T, Salter MW. P2X4R+ microglia drive neuropathic pain. Nat Neurosci. 2012;15:1068–73. doi: 10.1038/nn.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Chiu SY, Gray PT, Ritchie JM. The presence of voltage-gated sodium, potassium and chloride channels in rat cultured astrocytes. Proc R Soc Lond B Biol Sci. 1985;225:299–313. doi: 10.1098/rspb.1985.0063. [DOI] [PubMed] [Google Scholar]
- Bevan S, Lindsay RM, Perkins MN, Raff MC. Voltage gated ionic channels in rat cultured astrocytes, reactive astrocytes and an astrocyte-oligodendrocyte progenitor cell. J Physiol (Paris) 1987;82:327–35. [PubMed] [Google Scholar]
- Bisi S, Disanza A, Malinverno C, Frittoli E, Palamidessi A, Scita G. Membrane and actin dynamics interplay at lamellipodia leading edge. Curr Opin Cell Biol. 2013;25:565–73. doi: 10.1016/j.ceb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, Cohen S, Hinson AW, Waxman SG. Glial cells have heart: rH1 Na+ channel mRNA and protein in spinal cord astrocytes. Glia. 1998;23:200–8. [PubMed] [Google Scholar]
- Black JA, Liu S, Carrithers M, Carrithers LM, Waxman SG. Exacerbation of experimental autoimmune encephalomyelitis after withdrawal of phenytoin and carbamazepine. Ann Neurol. 2007;62:21–33. doi: 10.1002/ana.21172. [DOI] [PubMed] [Google Scholar]
- Black JA, Liu S, Waxman SG. Sodium channel activity modulates multiple functions in microglia. Glia. 2009;57:1072–81. doi: 10.1002/glia.20830. [DOI] [PubMed] [Google Scholar]
- Black JA, Newcombe J, Waxman SG. Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain. 2010;133:835–46. doi: 10.1093/brain/awq003. [DOI] [PubMed] [Google Scholar]
- Black JA, Newcombe J, Waxman SG. Nav1.5 sodium channels in macrophages in multiple sclerosis lesions. Mult Scler. 2013;19:532–42. doi: 10.1177/1352458512460417. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Sodium channels and microglial function. Exp Neurol. 2012;234:302–15. doi: 10.1016/j.expneurol.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Noncanonical roles of voltage-gated sodium channels. Neuron. 2013;80:280–91. doi: 10.1016/j.neuron.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Black JA, Westenbroek R, Minturn JE, Ransom BR, Catterall WA, Waxman SG. Isoform-specific expression of sodium channels in astrocytes in vitro: immunocytochemical observations. Glia. 1995a;14:133–44. doi: 10.1002/glia.440140208. [DOI] [PubMed] [Google Scholar]
- Black JA, Westenbroek RE, Catterall WA, Waxman SG. Type II brain sodium channel expression in non-neuronal cells: embryonic rat osteoblasts. Brain Res Mol Brain Res. 1995b;34:89–98. doi: 10.1016/0169-328x(95)00141-e. [DOI] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res. 1998;32:286–303. doi: 10.1016/s0920-1211(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB. The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2007;101:149–160. doi: 10.1007/s10549-006-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz MB. Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol. 2006;573:343–56. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL. Na Channel beta Subunits: Overachievers of the Ion Channel Family. Front Pharmacol. 2011;2:53. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia. 2014;62:452–67. doi: 10.1002/glia.22616. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR. Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–40. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan CF, Raine CS. The astrocyte in multiple sclerosis revisited. Glia. 2013;61:453–465. doi: 10.1002/glia.22443. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Voltage-sensor mutations in channelopathies of skeletal muscle. J Physiol. 2010;588:1887–95. doi: 10.1113/jphysiol.2010.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers LM, Hulseberg P, Sandor M, Carrithers MD. The human macrophage sodium channel NaV1.5 regulates mycobacteria processing through organelle polarization and localized calcium oscillations. FEMS Immunol Med Microbiol. 2011;63:319–327. doi: 10.1111/j.1574-695X.2011.00853.x. [DOI] [PubMed] [Google Scholar]
- Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, Rahner C, Graham M, Waxman SG. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem. 2009;284:8114–8126. doi: 10.1074/jbc.M801892200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrithers MD, Dib-Hajj S, Carrithers LM, Tokmoulina G, Pypaert M, Jonas EA, Waxman SG. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol. 2007;178:7822–7832. doi: 10.4049/jimmunol.178.12.7822. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–89. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Chao TI, Skachkov SN, Eberhardt W, Reichenbach A. Na+ channels of Muller (glial) cells isolated from retinae of various mammalian species including man. Glia. 1994;10:173–85. doi: 10.1002/glia.440100304. [DOI] [PubMed] [Google Scholar]
- Chatelier A, Mercier A, Tremblier B, Theriault O, Moubarak M, Benamer N, Corbi P, Bois P, Chahine M, Faivre JF. A distinct de novo expression of Nav1.5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. J Physiol. 2012;590:4307–19. doi: 10.1113/jphysiol.2012.233593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PH, Cai WQ, Wang LY, Deng QY. A morphological and electrophysiological study on the postnatal development of oligodendrocyte precursor cells in the rat brain. Brain Res. 2008;1243:27–37. doi: 10.1016/j.brainres.2008.09.029. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Schrager P, Ritchie JM. Neuronal-type Na+ and K+ channels in rabbit cultured Schwann cells. Nature. 1984;311:156–7. doi: 10.1038/311156a0. [DOI] [PubMed] [Google Scholar]
- Chuderland D, Marmor G, Shainskaya A, Seger R. Calcium-mediated interactions regulate the subcellular localization of extracellular signal-regulated kinases. J Biol Chem. 2008;283:11176–88. doi: 10.1074/jbc.M709030200. [DOI] [PubMed] [Google Scholar]
- Chvatal A, Pastor A, Mauch M, Sykova E, Kettenmann H. Distinct populations of identified glial cells in the developing rat spinal cord slice: ion channel properties and cell morphology. Eur J Neurosci. 1995;7:129–42. doi: 10.1111/j.1460-9568.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craner MJ, Damarjian TG, Liu S, Hains BC, Lo AC, Black JA, Newcombe J, Cuzner ML, Waxman SG. Sodium channels contribute to microglia/macrophage activation and function in EAE and MS. Glia. 2005;49:220–9. doi: 10.1002/glia.20112. [DOI] [PubMed] [Google Scholar]
- DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-dependent ion channels in T-lymphocytes. J Neuroimmunol. 1985;10:71–95. doi: 10.1016/0165-5728(85)90035-9. [DOI] [PubMed] [Google Scholar]
- Decoursey TE, Chandy KG, Gupta S, Cahalan MD. Mitogen induction of ion channels in murine T lymphocytes. J Gen Physiol. 1987;89:405–20. doi: 10.1085/jgp.89.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Na v 1.7 and human pain disorders. Trends Neurosci. 2007;30:555–63. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The Na(V)1.7 sodium channel: from molecule to man. Nat Rev Neurosci. 2013;14:49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–73. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Driffort V, Gillet L, Bon E, Marionneau-Lambot S, Oullier T, Joulin V, Collin C, Pages JC, Jourdan ML, Chevalier S, et al. Ranolazine inhibits NaV1.5-mediated breast cancer cell invasiveness and lung colonization. Mol Cancer. 2014;13:264. doi: 10.1186/1476-4598-13-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estacion M. Characterization of ion channels seen in subconfluent human dermal fibroblasts. J Physiol. 1991;436:579–601. doi: 10.1113/jphysiol.1991.sp018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber CG, Hoeijmakers JG, Ahn HS, Cheng X, Han C, Choi JS, Estacion M, Lauria G, Vanhoutte EK, Gerrits MM, et al. Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012a;71:26–39. doi: 10.1002/ana.22485. [DOI] [PubMed] [Google Scholar]
- Faber CG, Lauria G, Merkies IS, Cheng X, Han C, Ahn HS, Persson AK, Hoeijmakers JG, Gerrits MM, Pierro T, et al. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci U S A. 2012b;109:19444–9. doi: 10.1073/pnas.1216080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Functional role of calcium signals for microglial function. Glia. 2006;54:656–65. doi: 10.1002/glia.20412. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd CL, Gorin FA, Lyeth BG. Mechanical strain injury increases intracellular sodium and reverses Na+/Ca2+ exchange in cortical astrocytes. Glia. 2005;51:35–46. doi: 10.1002/glia.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke M, Pannicke T, Biedermann B, Faude F, Reichelt W. Sodium current amplitude increases dramatically in human retinal glial cells during diseases of the eye. Eur J Neurosci. 1996;8:2662–70. doi: 10.1111/j.1460-9568.1996.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Lloyd LJ, Pani F, Chioni AM, George AJ, Djamgoz MB. T-lymphocyte invasiveness: control by voltage-gated Na+ channel activity. FEBS Lett. 2008;569:191–194. doi: 10.1016/j.febslet.2004.05.063. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Salvador V, Manning EA, Mizal J, Altun S, Raza M, Berridge RJ, Djamgoz MB. Contribution of functional voltage-gated Na+ channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: I. Lateral motility. J Cell Physiol. 2003;195:479–487. doi: 10.1002/jcp.10312. [DOI] [PubMed] [Google Scholar]
- Gao K, Wang CR, Jiang F, Wong AY, Su N, Jiang JH, Chai RC, Vatcher G, Teng J, Chen J, et al. Traumatic scratch injury in astrocytes triggers calcium influx to activate the JNK/c-Jun/AP-1 pathway and switch on GFAP expression. Glia. 2013;61:2063–77. doi: 10.1002/glia.22577. [DOI] [PubMed] [Google Scholar]
- Gao R, Shen Y, Cai J, Lei M, Wang Z. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol Rep. 2010;23:1293–9. doi: 10.3892/or_00000763. [DOI] [PubMed] [Google Scholar]
- Gillet L, Roger S, Besson P, Lecaille F, Gore J, Bougnoux P, Lalmanach G, Le Guennec JY. Voltage-gated Sodium Channel Activity Promotes Cysteine Cathepsin-dependent Invasiveness and Colony Growth of Human Cancer Cells. J Biol Chem. 2009;284:8680–8691. doi: 10.1074/jbc.M806891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Lo AC, Waxman SG. Sodium channel blockade with phenytoin protects spinal cord axons, enhances axonal conduction, and improves functional motor recovery after contusion SCI. Exp Neurol. 2004;188:365–77. doi: 10.1016/j.expneurol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Helbig I, Scheffer IE, Mulley JC, Berkovic SF. Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 2008;7:231–45. doi: 10.1016/S1474-4422(08)70039-5. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JG, Faber CG, Merkies IS, Waxman SG. Painful peripheral neuropathy and sodium channel mutations. Neurosci Lett. 2015;596:51–9. doi: 10.1016/j.neulet.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Kann O, Ohlemeyer C, Hanisch UK, Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J Neurosci. 2003;23:4410–9. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–82. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–67. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House CD, Wang BD, Ceniccola K, Williams R, Simaan M, Olender J, Patel V, Baptista-Hon DT, Annunziata CM, Gutkind JS, et al. Voltage-gated Na+ Channel Activity Increases Colon Cancer Transcriptional Activity and Invasion Via Persistent MAPK Signaling. Sci Rep. 2015;5:11541. doi: 10.1038/srep11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JR, Ritchie JM. Sodium currents in Schwann cells from myelinated and non-myelinated nerves of neonatal and adult rabbits. J Physiol. 1990;425:169–210. doi: 10.1113/jphysiol.1990.sp018098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004;117:4619–28. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- Ifuku M, Farber K, Okuno Y, Yamakawa Y, Miyamoto T, Nolte C, Merrino VF, Kita S, Iwamoto T, Komuro I, et al. Bradykinin-induced microglial migration mediated by B1-bradykinin receptors depends on Ca2+ influx via reverse-mode activity of the Na+/Ca2+ exchanger. J Neurosci. 2007;27:13065–73. doi: 10.1523/JNEUROSCI.3467-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Tsuno S, Sugiyama T, Hashimoto A, Yamoto K, Takeuchi K, Kishi H, Mizuguchi H, Kohsaka S, Yoshioka T. Ca(2+) spiking activity caused by the activation of store-operated Ca(2+) channels mediates TNF-alpha release from microglial cells under chronic purinergic stimulation. Biochim Biophys Acta. 2013;1833:2573–85. doi: 10.1016/j.bbamcr.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Inglese M, Madelin G, Oesingmann N, Babb JS, Wu W, Stoeckel B, Herbert J, Johnson G. Brain tissue sodium concentration in multiple sclerosis: a sodium imaging study at 3 tesla. Brain. 2010;133:847–57. doi: 10.1093/brain/awp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–6. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Blankenfeld GV, Trotter J. Physiological properties of oligodendrocytes during development. Ann N Y Acad Sci. 1991;633:64–77. doi: 10.1111/j.1749-6632.1991.tb15596.x. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35:497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kis-Toth K, Hajdu P, Bacskai I, Szilagyi O, Papp F, Szanto A, Posta E, Gogolak P, Panyi G, Rajnavolgyi E. Voltage-gated sodium channel Nav1.7 maintains the membrane potential and regulates the activation and chemokine-induced migration of a monocyte-derived dendritic cell subset. J Immunol. 2011;187:1273–80. doi: 10.4049/jimmunol.1003345. [DOI] [PubMed] [Google Scholar]
- Kohrman DC, Smith MR, Goldin AL, Harris J, Meisler MH. A missense mutation in the sodium channel Scn8a is responsible for cerebellar ataxia in the mouse mutant jolting. J Neurosci. 1996;16:5993–9. doi: 10.1523/JNEUROSCI.16-19-05993.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotzer AR, Cotman CW. Voltage-gated currents expressed by rat microglia in culture. Glia. 1992;6:81–8. doi: 10.1002/glia.440060202. [DOI] [PubMed] [Google Scholar]
- Kressin K, Kuprijanova E, Jabs R, Seifert G, Steinhauser C. Developmental regulation of Na+ and K+ conductances in glial cells of mouse hippocampal brain slices. Glia. 1995;15:173–87. doi: 10.1002/glia.440150210. [DOI] [PubMed] [Google Scholar]
- Lai ZF, Chen YZ, Nishimura Y, Nishi K. An amiloride-sensitive and voltage-dependent Na+ channel in an HLA-DR-restricted human T cell clone. J Immunol. 2000;165:83–90. doi: 10.4049/jimmunol.165.1.83. [DOI] [PubMed] [Google Scholar]
- Li GR, Sun HY, Chen JB, Zhou Y, Tse HF, Lau CP. Characterization of multiple ion channels in cultured human cardiac fibroblasts. PLoS One. 2009;4:e7307. doi: 10.1371/journal.pone.0007307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnertz R, Wurm A, Pannicke T, Krugel K, Hollborn M, Hartig W, Iandiev I, Wiedemann P, Reichenbach A, Bringmann A. Activation of voltage-gated Na(+) and Ca(2)(+) channels is required for glutamate release from retinal glial cells implicated in cell volume regulation. Neuroscience. 2011;188:23–34. doi: 10.1016/j.neuroscience.2011.04.058. [DOI] [PubMed] [Google Scholar]
- Liu M, Yang KC, Dudley SC., Jr Cardiac sodium channel mutations: why so many phenotypes? Nat Rev Cardiol. 2014;11:607–15. doi: 10.1038/nrcardio.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AC, Black JA, Waxman SG. Neuroprotection of axons with phenytoin in experimental allergic encephalomyelitis. Neuroreport. 2002;13:1909–12. doi: 10.1097/00001756-200210280-00015. [DOI] [PubMed] [Google Scholar]
- Lo AC, Saab CY, Black JA, Waxman SG. Phenytoin protects spinal cord axons and preserves axonal conduction and neurological function in a model of neuroinflammation in vivo. J Neurophysiol. 2003;90:3566–71. doi: 10.1152/jn.00434.2003. [DOI] [PubMed] [Google Scholar]
- Lo WL, Donermeyer DL, Allen PM. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat Immunol. 2012;13:880–7. doi: 10.1038/ni.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Electrophysiological changes that accompany reactive gliosis in vitro. J Neurosci. 1997;17:7316–29. doi: 10.1523/JNEUROSCI.17-19-07316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Spinal cord astrocytes display a switch from TTX-sensitive to TTX-resistant sodium currents after injury-induced gliosis in vitro. J Neurophysiol. 1998;79:2222–6. doi: 10.1152/jn.1998.79.4.2222. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–24. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- Morsali D, Bechtold D, Lee W, Chauhdry S, Palchaudhuri U, Hassoon P, Snell DM, Malpass K, Piers T, Pocock J, et al. Safinamide and flecainide protect axons and reduce microglial activation in models of multiple sclerosis. Brain. 2013;136:1067–82. doi: 10.1093/brain/awt041. [DOI] [PubMed] [Google Scholar]
- Munson R, Jr, Westermark B, Glaser L. Tetrodotoxin-sensitive sodium channels in normal human fibroblasts and normal human glia-like cells. Proc Natl Acad Sci U S A. 1979;76:6425–9. doi: 10.1073/pnas.76.12.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Frederick TJ, Miller SD. Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci. 2008;65:2702–20. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M, Yang M, Dowle AA, Thomas JR, Brackenbury WJ. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol Cancer. 2015;14:13. doi: 10.1186/s12943-014-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson E, Randall AD. Na(v)1.5 sodium channels in a human microglial cell line. J Neuroimmunol. 2009;215:25–30. doi: 10.1016/j.jneuroim.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Noda M, Ifuku M, Mori Y, Verkhratsky A. Calcium influx through reversed NCX controls migration of microglia. Adv Exp Med Biol. 2013;961:289–94. doi: 10.1007/978-1-4614-4756-6_24. [DOI] [PubMed] [Google Scholar]
- Norenberg W, Illes P, Gebicke-Haerter PJ. Sodium channel in isolated human brain macrophages (microglia) Glia. 1994;10:165–72. doi: 10.1002/glia.440100303. [DOI] [PubMed] [Google Scholar]
- Nowak L, Ascher P, Berwald-Netter Y. Ionic channels in mouse astrocytes in culture. J Neurosci. 1987;7:101–9. doi: 10.1523/JNEUROSCI.07-01-00101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien BJ, Caldwell JH, Ehring GR, Bumsted O’Brien KM, Luo S, Levinson SR. Tetrodotoxin-resistant voltage-gated sodium channels Na(v)1.8 and Na(v)1.9 are expressed in the retina. J Comp Neurol. 2008;508:940–51. doi: 10.1002/cne.21701. [DOI] [PubMed] [Google Scholar]
- Oh Y, Black JA, Waxman SG. The expression of rat brain voltage-sensitive Na+ channel mRNAs in astrocytes. Brain Res Mol Brain Res. 1994;23:57–65. doi: 10.1016/0169-328x(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Oka M, Wada M, Yamamoto A, Itoh Y, Fujita T. Functional expression of constitutive nitric oxide synthases regulated by voltage-gated Na+ and Ca2+ channels in cultured human astrocytes. Glia. 2004;46:53–62. doi: 10.1002/glia.10359. [DOI] [PubMed] [Google Scholar]
- Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res Rev. 2009;59:278–92. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva M, Berkovic SF, Petrou S. Sodium channels and the neurobiology of epilepsy. Epilepsia. 2012;53:1849–59. doi: 10.1111/j.1528-1167.2012.03631.x. [DOI] [PubMed] [Google Scholar]
- Paluzzi S, Alloisio S, Zappettini S, Milanese M, Raiteri L, Nobile M, Bonanno G. Adult astroglia is competent for Na+/Ca2+ exchanger-operated exocytotic glutamate release triggered by mild depolarization. J Neurochem. 2007;103:1196–207. doi: 10.1111/j.1471-4159.2007.04826.x. [DOI] [PubMed] [Google Scholar]
- Pappalardo LW, Liu S, Black JA, Waxman SG. Dynamics of sodium channel Nav1.5 expression in astrocytes in mouse models of multiple sclerosis. Neuroreport. 2014a;25:1208–15. doi: 10.1097/WNR.0000000000000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappalardo LW, Samad OA, Black JA, Waxman SG. Voltage-gated sodium channel Nav 1.5 contributes to astrogliosis in an in vitro model of glial injury via reverse Na+ /Ca2+ exchange. Glia. 2014b;62:1162–75. doi: 10.1002/glia.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnis J, Montana V, Delgado-Martinez I, Matyash V, Parpura V, Kettenmann H, Sekler I, Nolte C. Mitochondrial exchanger NCLX plays a major role in the intracellular Ca2+ signaling, gliotransmission, and proliferation of astrocytes. J Neurosci. 2013;33:7206–19. doi: 10.1523/JNEUROSCI.5721-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V, Verkhratsky A. Homeostatic function of astrocytes: Ca(2+) and Na(+) signalling. Transl Neurosci. 2012;3:334–344. doi: 10.2478/s13380-012-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel F, Brackenbury WJ. Dual roles of voltage-gated sodium channels in development and cancer. Int J Dev Biol. 2015 doi: 10.1387/ijdb.150171wb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AK, Estacion M, Ahn H, Liu S, Stamboulian-Platel S, Waxman SG, Black JA. Contribution of sodium channels to lamellipodial protrusion and Rac1 and ERK1/2 activation in ATP-stimulated microglia. Glia. 2014;62:2080–95. doi: 10.1002/glia.22728. [DOI] [PubMed] [Google Scholar]
- Persson AK, Kim I, Zhao P, Estacion M, Black JA, Waxman SG. Sodium channels contribute to degeneration of dorsal root ganglion neurites induced by mitochondrial dysfunction in an in vitro model of axonal injury. J Neurosci. 2013a;33:19250–61. doi: 10.1523/JNEUROSCI.2148-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AK, Liu S, Faber CG, Merkies IS, Black JA, Waxman SG. Neuropathy-associated Nav1.7 variant I228M impairs integrity of dorsal root ganglion neuron axons. Ann Neurol. 2013b;73:140–5. doi: 10.1002/ana.23725. [DOI] [PubMed] [Google Scholar]
- Price LS, Langeslag M, ten Klooster JP, Hordijk PL, Jalink K, Collard JG. Calcium signaling regulates translocation and activation of Rac. J Biol Chem. 2003;278:39413–21. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Reese KA, Caldwell JH. Immunocytochemical localization of NaCh6 in cultured spinal cord astrocytes. Glia. 1999;26:92–96. doi: 10.1002/(sici)1098-1136(199903)26:1<92::aid-glia10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Remme CA. Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J Physiol. 2013;591:4099–116. doi: 10.1113/jphysiol.2013.256461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes RC, Verkhratsky A, Parpura V. Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro. 2012:4. doi: 10.1042/AN20110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Membrane ruffling and signal transduction. Bioessays. 1994;16:321–7. doi: 10.1002/bies.950160506. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rogart RB, Cribbs LL, Muglia LK, Kephart DD, Kaiser MW. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989;86:8170–4. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger S, Gillet L, Le Guennec J-Y, Besson P. VOLTAGE-GATED SODIUM CHANNELS AND CANCER: IS EXCITABILITY THEIR PRIMARY ROLE? Frontiers in Pharmacology. 2015:6. doi: 10.3389/fphar.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Rose C, Karus C. Two sides of the same coin: sodium homeostasis and signaling in astrocytes under physiological and pathophysiological conditions. Glia. 2013 doi: 10.1002/glia.22492. [DOI] [PubMed] [Google Scholar]
- Samad OA, West LE, Black JA, Waxman SG. 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience; 2012. Nav1.5 in astrocytes contributes to glial scarring. [Google Scholar]
- Schaller KL, Krzemien DM, Yarowsky PJ, Krueger BK, Caldwell JH. A novel, abundant sodium channel expressed in neurons and glia. J Neurosci. 1995;15:3231–42. doi: 10.1523/JNEUROSCI.15-05-03231.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtmayer J, Jacobsen C, Miksch G, Sievers J. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: membrane currents. Glia. 1994;12:259–67. doi: 10.1002/glia.440120403. [DOI] [PubMed] [Google Scholar]
- Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A. 1991;88:7438–42. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TA, Lively S, Vincent C, Schlichter LC. Regulation of podosome formation, microglial migration and invasion by Ca(2+)-signaling molecules expressed in podosomes. J Neuroinflammation. 2012;9:250. doi: 10.1186/1742-2094-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist. 2014;20:160–72. doi: 10.1177/1073858413504466. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Astrogliosis. Cold Spring Harb Perspect Biol. 2015;7:a020420. doi: 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Black JA, Ransom BR, Waxman SG. Ion channels in spinal cord astrocytes in vitro. I. Transient expression of high levels of Na+ and K+ channels. J Neurophysiol. 1992;68:985–1000. doi: 10.1152/jn.1992.68.4.985. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Fernandez-Marques E, Ullrich N, Pappas CA, Waxman SG. Astrocyte Na+ channels are required for maintenance of Na+/K(+)-ATPase activity. J Neurosci. 1994;14:2464–75. doi: 10.1523/JNEUROSCI.14-05-02464.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H, Minturn JE, Black JA, Ransom BR, Waxman SG. Two types of Na(+)-currents in cultured rat optic nerve astrocytes: changes with time in culture and with age of culture derivation. J Neurosci Res. 1991;30:275–87. doi: 10.1002/jnr.490300202. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Perouansky M, Hoppe D, Lux HD, Grantyn R, Kettenmann H. Glial cells of the oligodendrocyte lineage express proton-activated Na+ channels. J Neurosci Res. 1989;24:496–500. doi: 10.1002/jnr.490240406. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Waxman SG. Ion channels in spinal cord astrocytes in vitro. II. Biophysical and pharmacological analysis of two Na+ current types. J Neurophysiol. 1992;68:1001–11. doi: 10.1152/jn.1992.68.4.1001. [DOI] [PubMed] [Google Scholar]
- Sontheimer H, Waxman SG. Expression of voltage-activated ion channels by astrocytes and oligodendrocytes in the hippocampal slice. J Neurophysiol. 1993;70:1863–73. doi: 10.1152/jn.1993.70.5.1863. [DOI] [PubMed] [Google Scholar]
- Stanimirovic DB, Ball R, Mealing G, Morley P, Durkin JP. The role of intracellular calcium and protein kinase C in endothelin-stimulated proliferation of rat type I astrocytes. Glia. 1995;15:119–30. doi: 10.1002/glia.440150204. [DOI] [PubMed] [Google Scholar]
- Stys PK, Waxman SG, Ransom BR. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na(+)-Ca2+ exchanger. J Neurosci. 1992;12:430–9. doi: 10.1523/JNEUROSCI.12-02-00430.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio CL, Sontheimer H. Differential modulation of TTX-sensitive and TTX-resistant Na+ channels in spinal cord astrocytes following activation of protein kinase C. J Neurosci. 1993;13:4889–97. doi: 10.1523/JNEUROSCI.13-11-04889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio CL, Waxman SG, Sontheimer H. Ion channels in spinal cord astrocytes in vitro. III. Modulation of channel expression by coculture with neurons and neuron-conditioned medium. J Neurophysiol. 1993;69:819–31. doi: 10.1152/jn.1993.69.3.819. [DOI] [PubMed] [Google Scholar]
- Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, Duan S. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–28. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Beggs S, Salter MW, Inoue K. Microglia and intractable chronic pain. Glia. 2013;61:55–61. doi: 10.1002/glia.22379. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–52. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Noda M, Parpura V, Kirischuk S. Sodium fluxes and astroglial function. Adv Exp Med Biol. 2013a;961:295–305. doi: 10.1007/978-1-4614-4756-6_25. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Rodriguez JJ, Steardo L. Astrogliopathology: A Central Element of Neuropsychiatric Diseases? Neuroscientist. 2013b doi: 10.1177/1073858413510208. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, Nedergaard M. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro. 2012:4. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Yang CM. Calmodulin kinase II-dependent transactivation of PDGF receptors mediates astrocytic MMP-9 expression and cell motility induced by lipoteichoic acid. J Neuroinflammation. 2010:7. doi: 10.1186/1742-2094-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–86. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–5. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Waxman SG. The neuron as a dynamic electrogenic machine: modulation of sodium-channel expression as a basis for functional plasticity in neurons. Philos Trans R Soc Lond B Biol Sci. 2000;355:199–213. doi: 10.1098/rstb.2000.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7:932–41. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Mechanisms of disease: sodium channels and neuroprotection in multiple sclerosis-current status. Nat Clin Pract Neurol. 2008;4:159–69. doi: 10.1038/ncpneuro0735. [DOI] [PubMed] [Google Scholar]
- Wei C, Wang X, Zheng M, Cheng H. Calcium gradients underlying cell migration. Curr Opin Cell Biol. 2012;24:254–261. doi: 10.1016/j.ceb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49:296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Williamson AV, Compston DA, Randall AD. Analysis of the ion channel complement of the rat oligodendrocyte progenitor in a commonly studied in vitro preparation. Eur J Neurosci. 1997;9:706–20. doi: 10.1111/j.1460-9568.1997.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Wood JN. Ion channels in analgesia research. Handb Exp Pharmacol. 2007:329–58. doi: 10.1007/978-3-540-33823-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WK, Li GR, Wong HP, Hui MK, Tai EK, Lam EK, Shin VY, Ye YN, Li P, Yang YH, et al. Involvement of Kv1.1 and Nav1.5 in proliferation of gastric epithelial cells. J Cell Physiol. 2006;207:437–44. doi: 10.1002/jcp.20576. [DOI] [PubMed] [Google Scholar]
- Wu WK, Li GR, Wong TM, Wang JY, Yu L, Cho CH. Involvement of voltage-gated K+ and Na+ channels in gastric epithelial cell migration. Mol Cell Biochem. 2008;308:219–26. doi: 10.1007/s11010-007-9631-2. [DOI] [PubMed] [Google Scholar]
- Yang M, Kozminski DJ, Wold LA, Modak R, Calhoun JD, Isom LL, Brackenbury WJ. Therapeutic potential for phenytoin: targeting Na(v)1.5 sodium channels to reduce migration and invasion in metastatic breast cancer. Breast Cancer Res Treat. 2012;134:603–15. doi: 10.1007/s10549-012-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Barr TP, Hou Q, Dib-Hajj SD, Black JA, Albrecht PJ, Petersen K, Eisenberg E, Wymer JP, Rice FL, et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain. 2008;139:90–105. doi: 10.1016/j.pain.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Zsiros E, Kis-Toth K, Hajdu P, Gaspar R, Bielanska J, Felipe A, Rajnavolgyi E, Panyi G. Developmental switch of the expression of ion channels in human dendritic cells. J Immunol. 2009;183:4483–92. doi: 10.4049/jimmunol.0803003. [DOI] [PubMed] [Google Scholar]