Abstract
Objective
Surgical resection has been the mainstay of curative treatment of early stage lung cancer in selected patients. We evaluated survival and patterns of recurrence after surgical resection for early stage lung cancer from the ACOSOG Z0030/Alliance trial.
Methods
1018 patients in the Z0030 trial were analyzed according to clinical T stage. Differences between groups were compared using the two-sample rank test or chi-square test. Logrank test and Cox proportional hazards regression were used to compare survival and recurrence. To compare patients who underwent open versus VATS resections, propensity-score matched analysis was performed. 752 patients (66 VATS and 686 open) were classified into 5 equal-sized propensity score groups. Proportional hazards regression was used to compare these outcomes. Two-sided p-values<0.05 were considered statistically significant.
Results
There were 578 patients with cT1 tumors and 440 patients with cT2 tumors. Median follow-up was 6.7 years. Median overall survival was 9.1 years (T1) and 6.5 years (T2). Overall survival at 5 years was 72% (T1) and 55% (T2). Local recurrence-free survival at 5 years was 95% (T1) and 91% (T2) (p=0.015). Among T1 patients, 4.2% (23/542) had local recurrences, while 7.3% (30/409) of those with T2 tumors had local failure. There was no difference in the development of new primary tumors between T1 and T2 groups.
In the propensity-score matched analysis of VATS versus open lobectomy patients, there was no difference in overall survival, disease-free survival, and freedom from development of a new primary tumor.
Conclusions
Results of patients with resected early stage NSCLC from a large-scale, multicenter trial serve as benchmarks against which to compare non-surgical therapies for early stage lung cancer. Propensity-score matched analysis shows no difference in survival between the VATS and open lobectomy patients.
INTRODUCTION
To date surgical resection has been the gold standard for curative treatment of early stage lung cancer in appropriately selected patients. However, over the last decade, new technologies for treating early stage NSCLC have emerged as alternatives for patients who may be poor or marginal operative candidates. Outcomes of surgical treatment are needed to serve as reference points against which to compare the outcomes of these nonsurgical therapies in early stage lung cancer.
We performed a secondary analysis of a large-scale multicenter, randomized trial in order to determine the long-term clinical outcomes of patients undergoing surgical treatment for early stage NSCLC. ACOSOG Z0030 (Alliance) trial was a prospective, randomized, multi-institutional clinical trial that was designed to determine the effect on survival of lymph node sampling versus mediastinal lymph node dissection in patients undergoing complete resection of early stage NSCLC [1]. Once the primary endpoints of the study were reached, we secondarily analyzed the data in order to determine overall survival and patterns of recurrence. The advantages of this dataset include the rigor and uniformity with which the trial was conducted regarding eligibility criteria, staging procedures, data collection, and surgical techniques and the fact that these data were audited. The long-term results derived from this study serve as benchmark data against which to compare the results of more recent non-surgical therapies for the treatment of early stage lung cancer.
METHODS
Details of the study design, eligibility requirements, and the morbidity and mortality of patients enrolled in the Z0030 trial have been previously reported [1, 2]. The protocol was approved by a central institutional review board in addition to the institutional review board at each participating institution. All patients provided written informed consent before trial enrollment. In summary eligible patients were required to be older than 18 years of age, to have an ECOG performance status lower than 3, and a tissue diagnosis of a clinical T1 or T2, N0 or non-hilar N1, M0 NSCLC before randomization. Eligible patients had to be candidates for resection by means of pneumonectomy, lobectomy, bilobectomy or segmentectomy. The type of resection (VATS versus open) was recorded in the data set. Patients with N2 metastases were excluded from randomization.
There were 1023 eligible patients who were evaluated for the following long-term outcomes: local, locoregional, distant recurrence, disease-specific and overall survival (5 were excluded because clinical stage was not reported in the database). Thus in this study we evaluated 1018 patients by clinical T classification: 578 patients with T1 tumors and 440 patients with T2 tumors. Based on the Z0030 definitions, recurrence was defined as local if it occurred in the adjacent lung parenchyma, bronchial stump, or the hilum adjacent to the bronchial stump. It was defined as regional if it occurred in the hilum (separate from bronchial stump), mediastinum, chest wall, or ipsilateral pleura. Recurrence was defined as distant if it occurred in a separate lobe of ipsilateral lung, contralateral thorax, supraclavicular lymph nodes, or distant organ.
Statistical methods
Differences between groups with regard to clinical and tumor characteristics were compared using the two-sample rank test or chi-square test as appropriate. Cumulative survival probabilities were estimated using the Kaplan-Meier method. The logrank test and Cox proportional hazards regression were used to compare survival and recurrence across groups.
As an additional analysis, we evaluated the Z0030 data set based on propensity-score matching to compare patients who underwent open versus VATS anatomic lung resections [3]. Clinical and tumor characteristics were used to build a propensity score for choice of treatments. These variables included age, gender, histology, performance status, tumor location, and clinical T classification (T1 versus T2). Propensity scores were developed to estimate the adjusted risks of perioperative outcomes associated with the approach of treatment (VATS versus open). Logistic regression was used to estimate the probability of VATS versus open given the previously listed risk factors. Patients were classified into 7 groups based on their propensity scores. Two hundred eight thoracotomy patients had lower scores than the lowest score of any VATS patient treated (group 0); 4 open lobectomy patients had higher scores than the highest VATS patient treated (group 6). Patients from these 2 groups were omitted from further analysis [3]. The remaining 752 patients (66 in the VATS group and 686 in the open lobectomy group) were classified into 5 equal-sized propensity score groups (groups 1–5). Proportional hazards regression with 5 strata (propensity score groups 1–5) was used to compare long-term outcomes between VATS and open patients. In all cases, two-sided p-values <0.05 were considered statistically significant.
RESULTS
Overall survival
There were 1018 patients who were evaluated by clinical T classification: 578 patients with T1 tumors and 440 patients with T2 tumors. The stratification by clinical T classification is shown in Table I. Median follow-up was 6.7 years in the entire cohort. The median overall survival for patients with T1 tumors was 9.1 years, while that for those with T2 tumors was 6.5 years. Overall survival and disease-free survival for clinical T1 and T2 patients are shown in Table II.
Table I.
Characteristics of patients in Z0030 trial by clinical T classification (n=1023)
| cT1 | 578 (57%) |
| cT2 | 440 (43%) |
| Pathologic stage | |
| IA | 423 (41%) |
| IB | 418 (41%) |
| IIA | 37 (4%) |
| IIB | 97 (9%) |
| IIIA | 26 (3%) |
| IIIB | 19 (2%) |
Table II.
Long term outcomes in patients with T1 and T2 tumors
| T1 (n=578) | T2 (n=440) | ||||||
|---|---|---|---|---|---|---|---|
| Median | 5 year survival (95% CI) |
Median | 5 year survival (95% CI) |
HR | 95% CI |
P | |
| Overall survival | 9.1 | 72 (68–76) | 6.5 | 55 (51–60) | 1.64 | 1.36–1.99 | <0.001 |
| Disease free survival | NA | 77 (73–81) | NA | 58 (53–64) | 1.88 | 1.49–2.38 | <0.001 |
| Local disease free survival | NA | 95 (93–97) | NA | 91 (88–94) | 1.96 | 1.14–3.37 | 0.015 |
| Local/regional disease free survival | NA | 88 (85–91) | NA | 84 (80–88) | 1.46 | 1.01–2.11 | 0.044 |
| Distant disease free survival | NA | 83 (79–86) | NA | 66 (61–71) | 1.99 | 1.53–2.61 | <0.001 |
| New primary | 9 | 83 (79–86) | NA | 84 (80–87) | 0.84 | 0.61–1.16 | 0.29 |
NA = median survival not achieved
Disease free survival (n=542 (125 events) in the T1 group and n=409 (156 events) in the T2 group): deaths are censored.
Local disease-free survival (n=542 (23 events) in the T1 group and n=409 (30 events) in the T2 group): deaths and regional/distant recurrence are censored.
Local/regional disease-free survival (n=542 (57 events) in the T1 group and n=409 (56 events) in the T2 group): deaths and distant recurrence are censored.
Distant disease-free survival (n=542 (94 events) in the T1 group and n=409 (126 events) in the T2 group): deaths and local/regional recurrence are censored.
New primary (n=564 (101 events) in the T1 group and n=432 (57 events) in the T2 group): deaths are censored.
The 5-year overall survival was 72% for T1 patients and 55% for T2 patients (p<0.001) (Figure 1). Disease-free survival at 5 years was 77% for patients with T1 tumors and 58% for those with T2 tumors (p<0.001). (Figure 2)
Figure 1.
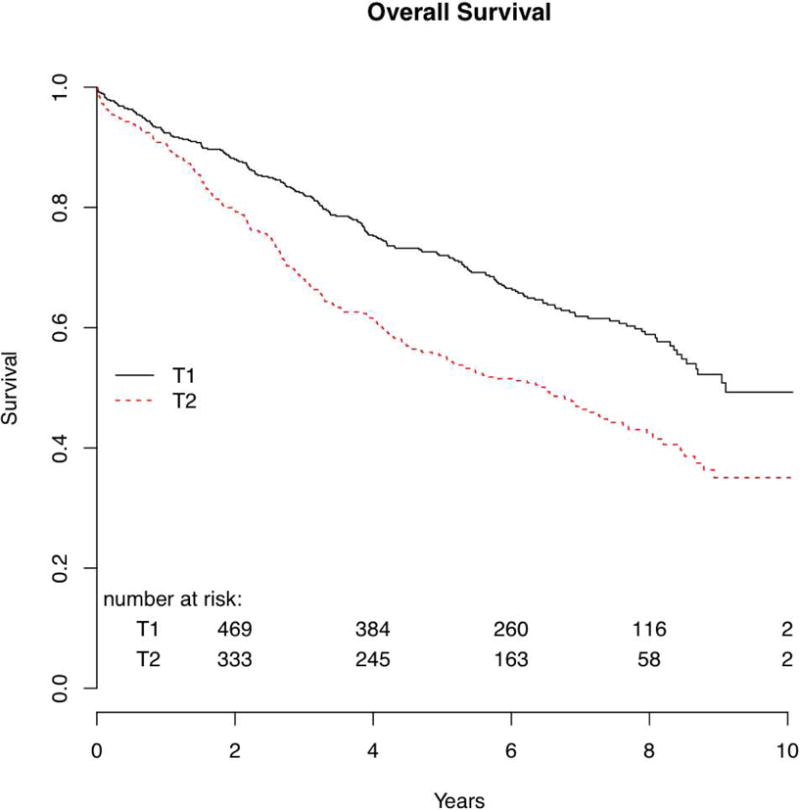
Figure 2.
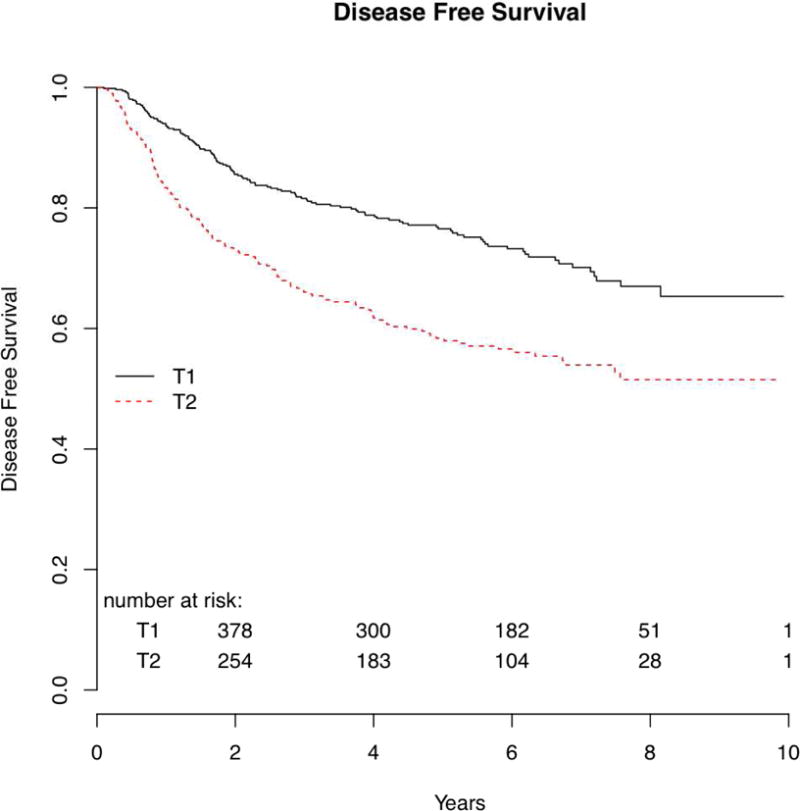
Local and_locoregional_recurrence
The 5-year local recurrence-free survival for the T1 cohort was 95% and for T2 group was 91% (p=0.015).
The 5-year locoregional recurrence-free survival was 88% for T1 patients, 84% for T2 patients (p=0.044). The 5-year distant disease-free survival for the T1 patients was 83% and for the T2 patients was 66% (p<0.001). (Table II)
Of the 542 patients with T1 tumors assessed for recurrence, 4.2% had local recurrences and 17.3% had distant metastases. Among patients with T1 tumors who were reported to develop recurrent tumor (125 patients), 6% of total recurrences were local alone, while 75.2% of recurrences were distant in nature (Table III).
Table III.
Patterns of recurrences in Z0030 data set
| T1 (n=542) | T2 (n=409) | |
|---|---|---|
| Local | 7 (1%) | 13(3%) |
| Regional | 22(4%) | 13(3%) |
| Distant | 68(13%) | 99(24%) |
| Local/regional | 2(0.4%) | 3(0.7%) |
| Local/distant | 10(2%) | 9(2%) |
| Regional/distant | 12(2%) | 13(3%) |
| Local/regional/distant | 4(0.7%) | 5(1%) |
| Total #pts with recurrences | 125 | 156* |
Location of recurrence was not indicated for 1 subject.
Of the 409 patients with T2 tumors assessed for recurrence, 7.3% had local recurrences and 30.8% had distant metastases. Among patients with T2 tumors who developed recurrent tumor (156 patients), 8% of total recurrences were purely local, while 80.8% of recurrences included distant metastases (Table III).
New primary tumors
There was no significant difference in the numbers of patients who developed new primary tumors in comparing the T1 and T2 groups. At 5 years, 83% of patients with T1 tumors and 84% of those with T2 tumors remained free of new primary tumors (p=0.29) (Table II).
Propensity-score matched analysis of VATS versus open lobectomy patients
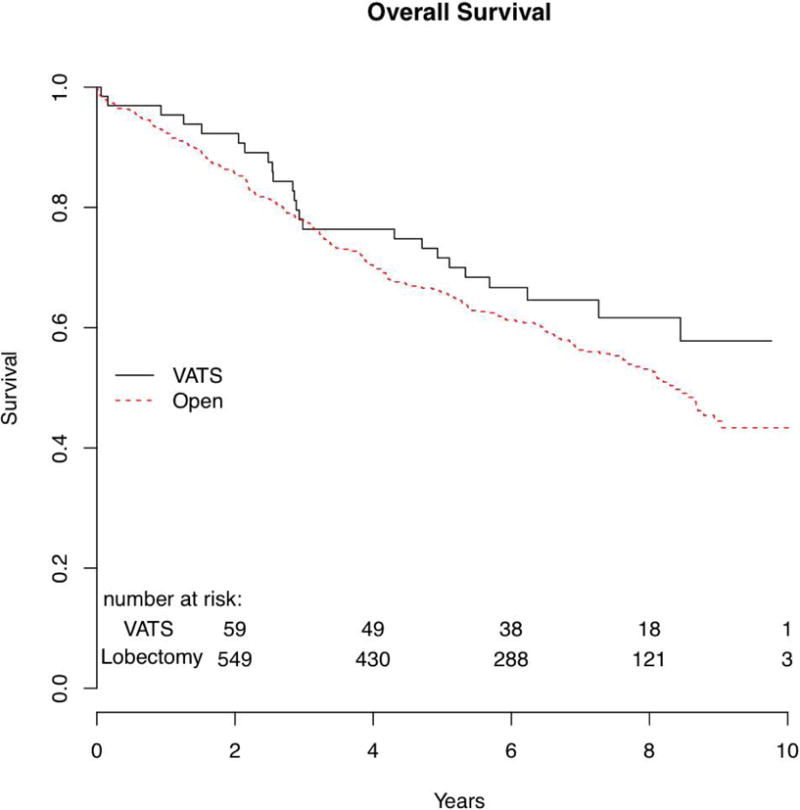
An additional analysis was performed to evaluate the VATS versus open lobectomy patients based on propensity-score matched groups of the Z0030 cohort. The patient demographics for this analysis are shown in Table IV. Median follow-up was 7 years for the VATS patients (n=66) and 6.7 years for the open lobectomy patients (n=686). Overall survival between the VATS and open lobectomy groups were similar. The median overall survival for the VATS group was not achieved, and the 5-year survival was 71.6% (95% CI 61.3–83.6%). The median overall survival for the open lobectomy group was 8.4 years, and the 5-year survival was 65.9% (95% CI 62.3–69.7%) (p=0.36). (Figure 3)
Table IV.
Patient demographics for subjects included in the propensity-based analysis
| VATS (n=66) | Open (N=686) | P* | |
|---|---|---|---|
| Age | 72.9; 70.9±9.7 | 68.6; 68.1±8.8 | 0.011 (0.38) |
| Gender | 0.15 | ||
| Female | 38 (57.6) | 331 (48.3) | (0.99) |
| Male | 28 (42.4) | 355 (51.8) | |
| Histology | 0.029 | ||
| Squamous cell | 10 (15.2) | 206 (30.0) | (0.99) |
| Adenocarcinoma | 45 (68.2) | 354 (51.6) | |
| Large cell | 2 (3.0) | 36 (5.3) | |
| Bronchoalveolar | 8 (12.1) | 57 (8.3) | |
| Other non-small cell | 1 (1.5) | 33 (4.8) | |
| Performance status | 0.002 | ||
| 0 | 60 (90.9) | 488 (71.1) | (0.18) |
| 1 | 5 (7.6) | 192 (28.0) | |
| 2 | 1 (1.5) | 6 (0.9) | |
| Tumor location | 0.69 | ||
| RUL | 32 (48.5) | 284 (41.4) | (0.99) |
| RML | 2 (3.0) | 44 (6.4) | |
| RLL | 8 (12.1) | 112 (16.3) | |
| LUL | 18 (27.3) | 173 (25.2) | |
| LLL | 7 (10.6) | 85 (12.4) | |
| Clinical stage | 0.26 | ||
| T1 | 44 (66.7) | 408 (59.5) | (0.89) |
| T2 | 22 (33.3) | 278 (40.5) | |
| Pathologic T-stage | 0.78 | ||
| T1 | 37 (56.1) | 328 (48.0) | (0.98) |
| T2 | 27 (40.9) | 329 (48.2) | |
| T3 | 1 (1.5) | 16 (2.3) | |
| T4 | 1 (1.5) | 10 (1.5) | |
| Pathologic N-stage | 0.5 | ||
| N0 | 61 (92.4) | 592 (86.5) | (0.65) |
| N1 | 5 (7.6) | 81 (11.8) | |
| N2 | 0 (0) | 11 (1.6) | |
| Pathologic stage | 0.54 | ||
| IA | 35 (53.0) | 297 (43.5) | (0.80) |
| IB | 25 (37.9) | 275 (40.3) | |
| IIA | 2 (3.0) | 28 (4.1) | |
| IIB | 3 (4.6) | 58 (8.5) | |
| IIIA | 0 (0) | 15 (2.2) | |
| IIIB | 1 (1.5) | 10 (1.5) |
Values are n (%) or median; mean±SD.
Chi-square test for categorical variables and two-sample t-test (rank sum test) for continuous variables. P-value in parentheses are Cochran-Mantel-Haenszel tests for categorical variables or linear models for continuous variables demonstrating that patient characteristics were similar across treatment groups after adjusting for propensity score groups.
Figure 3.
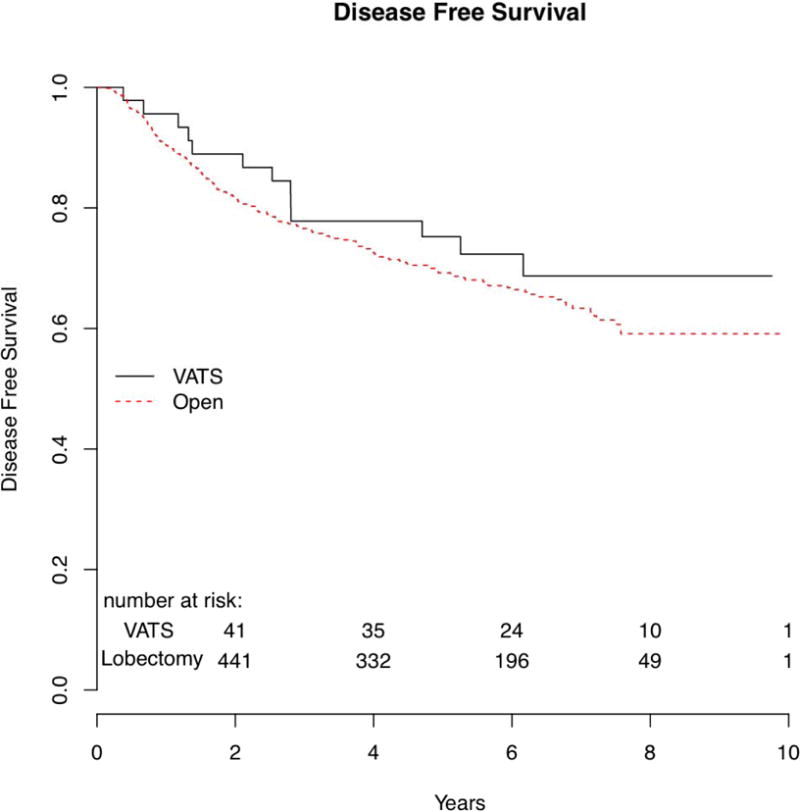
There was no difference in disease-free survival between the two groups. (Figure 4) There were 13 (20%) subjects in the VATS group who had a recurrence and 193 (28%) subjects in the open lobectomy group. Median disease-free survival was not achieved in either the VATS or open lobectomy groups. There was no difference in 5-year disease-free survival: 75.2% in the VATS group, and 69.2% in the open lobectomy group (p=0.55). Locoregional recurrence-free survival was similar between the two groups. The 5-year locoregional disease-free survival was similar, 82.0% in the VATS group, 86.1% in the open lobectomy group (p=0.58). Distant recurrence-free survival was also similar between the two groups. The 5-year distant recurrence-free survival was 87.4% in the VATS group, 75.3% in the open lobectomy group (p= 0.20).
Figure 4.
There was no statistical difference between the two groups in the time to development of a new primary tumor. The new primary tumor-free survival at 5 years was 87.8% in the VATS group and 81.7% in the open lobectomy group. There were 7 (11%) subjects in the VATS group who had a new primary tumor, of which 4 were of lung origin. There were 118 (17%) subjects in the open lobectomy group who had a new primary tumor, of which 34 were of lung origin.
DISCUSSION
To date the ACOSOG Z0030 (Alliance) trial is the largest prospective randomized trial of surgically resected patients with early stage NSCLC in the United States. Thus the outcomes of resected clinical T1 and T2 NSCLC reported in this study provide important points of reference with which to compare results of non-surgical treatments, which are being more often considered as alternatives to surgery in both inoperable and operable patients. These therapies are difficult to compare due to lack of uniformity in staging, pathological confirmation, patient selection, interpretations of post-treatment response, and nonstandard treatment protocols (eg fractionation and dose prescription regarding SBRT). Additionally, the radiographic definition of successful local control in patients treated with nonsurgical ablation is broad, including partial response, stable disease, as well as complete response by either CT or PET/CT scan. For example, an important consideration in evaluation of SBRT outcomes is that local control often refers to the radiographic response in the tumor bed alone, while local control in the surgical literature includes failure in the ipsilateral lobe, hilum, and ipsilateral/contralateral mediastinum.
The Z0030 cohort included patients who were clinically staged as having stage I NSCLC, although ultimately it represented those who were N2 and hilar N1 node-negative on initial nodal sampling prior to being randomized into the trial. In spite of this, 18% were found to have a pathologic stage more advanced than stage I, with 13% harboring occult N1 disease and 2% with occult N2 disease. Recent studies evaluating patients with clinical stage I NSCLC who underwent surgical resection report 29–35% pathologic upstaging at surgery [4, 5]. In the absence of pathologic staging, patients undergoing nonsurgical treatments are subject to clinical under-staging. Yet, studies of these latter treatments report low rates of local failure; for example, RTOG 0236 (which enrolled patients with cT1-2N0 NSCLC for treatment by SBRT) reported a 3-year primary tumor control of 97.6% and local control of 90.6% [6]. One rationale behind such low rates of local failure may be that studies performed in patients deemed medically inoperable due to other comorbidities underrepresent actual rates of failure on account of censoring subjects who die of non-cancer causes without documentation of recurrence.
Thus far, absence of long-term follow-up in studies involving non-surgical treatments so far precludes formation of any guidelines for potentially operable candidates with early-stage NSCLC. Long-term follow-up is required since local recurrence has been shown to occur at the site of primary tumor after an extended period following treatment (10 years) [7, 8]. Recent data from the completed RTOG 0618 trial which evaluated operable patients undergoing SBRT for early stage NSCLC report local failure (primary tumor plus involved lobe failure) rates of 19.2% at 2 years [9]. In contrast, within the Z0030 cohort the local failure rate was 4% in the T1 patients and 7% in the T2 patients at a median follow-up of 6.7 years. Because the outcomes in this paper evaluated the Z0030 cohort by clinical T1 and T2 classification (without regard to eventual pathologic stage), they represent important points of reference for comparison with outcomes of non-surgical treatment in patients of similar early stage.
Our analysis of the Z0030 data shows that the long-term outcomes between patients who underwent VATS lobectomy versus open lobectomy for early-stage lung cancer are similar. There was no difference in overall survival, disease-free survival, or survival based on pattern of recurrence. There was no difference in time to locoregional recurrence between the VATS and open lobectomy groups. Based on the given data, the conclusion that VATS provides local control at least equivalent to that provided by thoracotomy is validated. Concern has been raised that VATS lobectomy for clinical T1-2 N0 NSCLC may lead to less complete N1 lymph node evaluation and lower rates of N1 upstaging as compared to open lobectomy. Such detection of nodal involvement may extend the potential benefits of adjuvant chemotherapy to patients who otherwise would be offered none. The results of this study are unable to address this concern since the rate of pN1 involvement was low in both VATS and open lobectomy groups (8% and 12%, respectively, p=0.49).
In the past, those who doubted the validity of the VATS lobectomy approach raised concerns that small lung lesions representative of synchronous primary tumors or metastatic disease may miss the opportunity for detection by bimanual palpation at the time of initial operation. This study shows that the numbers of patients who develop second primary tumors are not different between the VATS and open lobectomy approach. This finding is in agreement with the conclusions of a recent single-institution study that showed similar incidence of second primary tumors following lobectomy by VATS versus open technique[10].
The limitations of this study include the fact that PET/CT was not required for entry into the trial; thus clinical staging by this means was not uniformly used. Data collected in follow-up was limited by return of data forms by the participating institutions. VATS sample size for the propensity-matched analysis was limited. At the time that the trial was conducted, adjuvant chemotherapy was not the standard of care for node-positive disease or tumors of size 4cm or larger [11]. These nonetheless do not undermine the relevance of the reported outcomes in surgically resected patients with early stage NSCLC.
In summary, as non-surgical treatments are more commonly utilized in treatment of early stage NSCLC, a critical evaluation of outcomes should be performed. The survival data and recurrence patterns following surgical treatment of clinical T1 and T2 lung cancers in the Z0030 cohort serve as benchmarks against which the outcomes of ablative techniques such as SBRT must be compared.
Table V.
Long-term outcomes in propensity matched groups of patients undergoing VATS and open lobectomies
| VATS (n=66) | Lobectomy (n=686) | ||||||
|---|---|---|---|---|---|---|---|
| Median | 5 year survival (95% CI) |
Median | 5 year survival (95% CI) |
HR | 95% CI |
P | |
| Overall survival | NA | 71.6% (61.3%–83.6%) |
8.4 yrs | 65.9% (62.3%–69.7%) |
1.22 | 0.8–1.87 | 0.36 |
| Disease free survival | NA | 75.2% (63.5%–89.1%) |
NA | 69.2% (65.4%–73.3%) |
1.19 | 0.67–2.10 | 0.55 |
| Local disease free survival | NA | 88.0% (78.6%–98.5%) |
NA | 92.6% (90.2%–95.0%) |
0.58 | 0.23–1.50 | 0.26 |
| Local/regional disease free survival | NA | 82.0% (71.5%–94.1%) |
NA | 86.1% (83.1%–89.2%) |
0.81 | 0.39–1.70 | 0.58 |
| Distant disease free survival | NA | 87.4% (77.6%–98.4%) |
NA | 75.3% (71.7%–79.1%) |
1.65 | 0.77–3.55 | 0.20 |
| New primary | NA | 87.8% (79.6%–96.8%) |
9.0 | 81.7% (78.3%–85.3%) |
1.71 | 0.79–3.72 | 0.17 |
NA = median survival not achieved
Disease free survival (n=47 in the VATS group and n=652 in the lobectomy group). Deaths are censored.
Local disease free (n=47 in the VATS group and n=652 in the lobectomy group). Deaths and regional/distant recurrence are censored.
Local/regional disease free (n=47 in the VATS group and n=652 in the lobectomy group). Deaths and distant recurrence are censored.
Distant disease free (n=47 in the VATS group and n=652 in the lobectomy group). Deaths and local/regional recurrence are censored.
New primary (n=64 in the VATS group and n=673 in the lobectomy group). Deaths are censored.
Suggested Ultra-mini Abstract.
Recurrence-free and overall survival following surgical treatment of early stage lung cancer from the ACOSOG (Alliance) Z0030 trial serve as benchmarks against which the outcomes of ablative techniques such as SBRT must be compared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Darling GE, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg. 2011;141(3):662–70. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen MS, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81(3):1013–9. doi: 10.1016/j.athoracsur.2005.06.066. discussion 1019-20. [DOI] [PubMed] [Google Scholar]
- 3.Scott WJ, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010;139(4):976–81. doi: 10.1016/j.jtcvs.2009.11.059. discussion 981-3. [DOI] [PubMed] [Google Scholar]
- 4.Crabtree TD, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2010;140(2):377–86. doi: 10.1016/j.jtcvs.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree T, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033) J Thorac Cardiovasc Surg. 2013;145(3):692–9. doi: 10.1016/j.jtcvs.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman R, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andratschke N, et al. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol. 2011;101(2):245–9. doi: 10.1016/j.radonc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Matsuo Y, et al. Preliminary report of late recurrences, at 5 years or more, after stereotactic body radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2012;7(2):453–6. doi: 10.1097/JTO.0b013e31823c5b29. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman RD, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. ASCO Meeting Abstracts. 2013;31(15_suppl):7523. [Google Scholar]
- 10.Flores RM, et al. Patterns of recurrence and incidence of second primary tumors after lobectomy by means of video-assisted thoracoscopic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg. 2011;141(1):59–64. doi: 10.1016/j.jtcvs.2010.08.062. [DOI] [PubMed] [Google Scholar]
- 11.Strauss GM, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043–51. doi: 10.1200/JCO.2008.16.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]


