Abstract
Purpose
To limit corneal damage and potential loss of vision, bacterial keratitis must be treated aggressively. Innovation in antimicrobials is required due to the need for empirical treatment and the rapid emergence of bacterial resistance. Designed host defense peptides (dHDPs) are synthetic analogues of naturally occurring HDPs, which provide defense against invading pathogens. This study investigates the use of novel dHDPs for the treatment of bacterial keratitis.
Methods
The minimum inhibitory concentrations (MICs) were determined for dHDPs on both Gram-positive and -negative bacteria. The minimum biofilm eradication concentrations (MBEC) and in vitro time-kill assays were determined. The most active dHDP, RP444, was evaluated for propensity to induce drug resistance and therapeutic benefit in a murine Pseudomonas aeruginosa keratitis model.
Results
Designed HDPs were bactericidal with MICs ranging from 2 to >64 μg/mL and MBEC ranging from 6 to 750 μg/mL. In time-kill assays, dHDPs were able to rapidly reduce bacterial counts upon contact with as little as 2 μg/mL. RP444 did not induce resistance after repeated exposure of P. aeruginosa to subinhibitory concentrations. RP444 demonstrated significant efficacy in a murine model of bacterial keratitis as evidenced by a significant dose-dependent decrease in ocular clinical scores, a significantly reduced bacterial load, and substantially decreased inflammatory cell infiltrates.
Conclusions
Innovative dHDPs demonstrated potent antimicrobial activity, possess a limited potential for development of resistance, and reduced the severity of murine P. aeruginosa keratitis. These studies demonstrate that a novel dHDP may have potential to treat patients with sight-threatening bacterial keratitis.
Keywords: antimicrobial peptide, host defense peptide, bacterial keratitis, biofilm
Bacterial keratitis is an aggressive corneal infection that, without prompt and effective treatment, can lead to perforation of the cornea and blindness.1 In the United States, there are approximately 30,000 patients annually with these infections, with contact lens wear as the prime risk factor. Bacterial keratitis is presently treated with antibiotics, particularly fluoroquinolones; however, innovation in antimicrobials is needed due to the rapid emergence of bacterial resistance and pathogen resilience to biofilm formation.2–4
Pathogens causing bacterial keratitis include both Gram-positive and -negative bacteria. Gram-positive causative pathogens include Enterococcus faecium,5 Staphylococcus aureus (including methicillin-resistant Staphylococcus aureus [MRSA]),6 Staphylococcus epidermidis,6 and Streptococcus pneumonia.6 Gram-negative causative pathogens include Pseudomonas aeruginosa,6 Enterobacter aerogenes,7 and Acinetobacter baumanii.8 The most frequent pathogen in bacterial keratitis associated with contact lens wear is Gram-negative P. aeruginosa while for non–contact lens–related disease the Gram-positive bacterium S. aureus predominates.9,10
Pathogens that cause bacterial keratitis can become extremely resilient to traditional antibiotic treatment due to biofilm formation. Bacteria within biofilms are 20 to 1000 times less sensitive to antibiotics than their planktonic counterparts,11 as they are physically protected from antibiotics and the host's immune system. Hence the imperative need to find efficacious agents that are able to target biofilms and eradicate disease-causing bacteria. The increasing emergence of antibiotic resistance also highlights the need for innovative alternatives that provide rapid and complete microbicidal activity with minimal safety-related effects while exhibiting limited susceptibility to mechanisms of microbial resistance.
Designed host defense peptides (dHDPs) are synthetic peptides that are chemically derived from naturally occurring HDPs (also referred to as antimicrobial peptides), which provide the first line of defense against invading pathogens.12 HDPs possess an amphipathic α-helix or β-sheet structure and a net positive charge. These features are crucial as the peptides generally act as antimicrobial agents through electrostatic interactions with the microbial membrane, thereby perturbing the barrier function of these membranes. HDPs are effective against Gram-positive and -negative bacteria.13,14 The direct killing of bacteria via perturbation of the cell membrane infers a reduced likelihood of inducing bacterial resistance, a critical key in fighting antibiotic-resistant pathogens.15
There is an urgent and profound need for the design and clinical development of dHDPs to treat sight-threatening infectious keratitis. Here, 11 novel dHDPs were evaluated for their bactericidal effectiveness against isolates and biofilm cultures of both Gram-positive and -negative bacteria, and against drug-resistant MRSA and P. aeruginosa. The most promising of these novel peptides, RP444, was evaluated for potential to induce resistance and efficacy in a murine model of P. aeruginosa keratitis.
Methods
Designed Host Defense Peptides
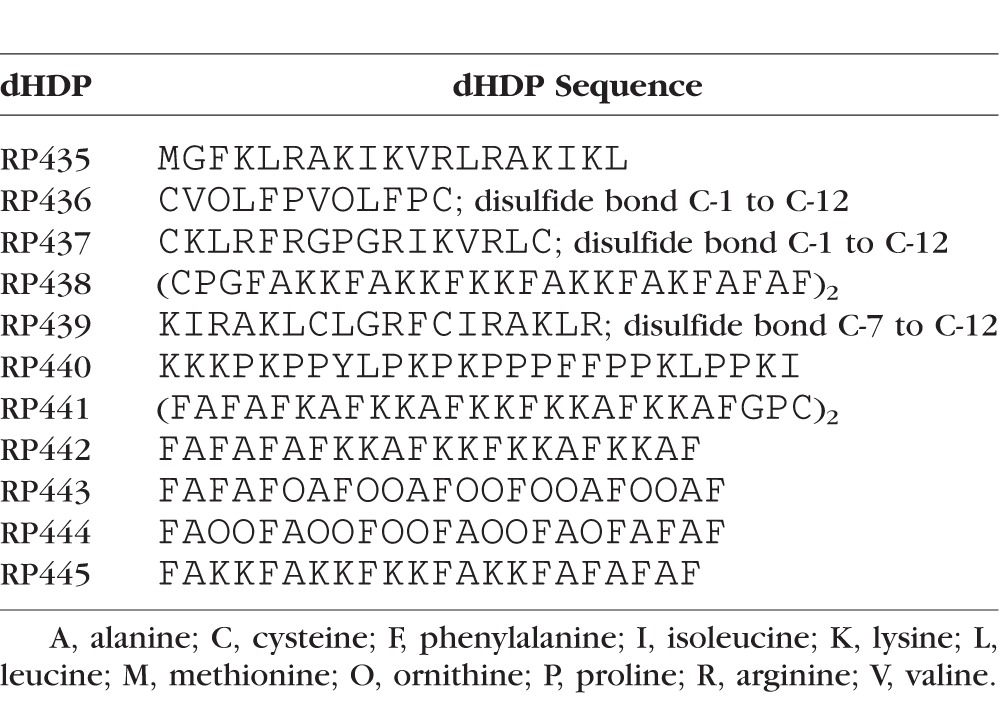
Eleven dHDPs, whose amino acid sequences are depicted in Table 1, were synthesized via traditional solid phase synthesis (CSBio, Menlo Park, CA, USA). The discovery and identification of the bactericidal cecropins and magainins and their role in providing “freedom from infection” provided the structural basis and amphipathic properties upon which these dHDPs were designed.16–19 The peptide sequences include use of nonnatural amino acids, not encoded by the Universal Genetic Code, and the specific replacement of lysine with ornithine, which has been shown to increase antibacterial activity while also enhancing proteolytic stability.20–21
Table 1.
Amino Acid Sequences of the dHDPs Evaluated in This Study
Bacterial Strains
Bacterial strains were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The Gram-positive bacterial strains included Enterococcus faecium ATCC 700221 (E. faecium 700221), methicillin-resistant Staphylococcus aureus ATCC 33591 (MRSA 33591), Staphylococcus epidermidis ATCC 51625 (S. epidermis 51625), Streptococcus pneumoniae ATCC 49619 (S. pneumoniae 49619), and Staphylococcus aureus ATCC 49525 (Wright).
The Gram-negative bacterial strains tested were Enterobacter aerogenes ATCC 13048 (E. aerogenes 13048), Acinetobacter baumannii Bouvet and Grimont ATCC 17978D-5 (A. baumannii 17978D-5), Pseudomonas aeruginosa ATCC 19660 (P. aeruginosa 19660), and Pseudomonas aeruginosa ATCC 27853 (P. aeruginosa 27853).
The bioluminescence strains, P. aeruginosa 19660 transfected with the Xen5 luciferase gene (here termed P. aeruginosa 19660_Xen5), and Staphylococcus aureus ATCC 49525 (Wright) transfected with the Xen36 luciferase gene (S. aureus 49525_Xen36), were obtained from PerkinElmer (Hopkinton, MA, USA).22,23
In Vitro Bactericidal Activity
The minimum inhibitory concentrations (MICs) of dHDPs were determined by broth microdilution methods approved by the Clinical and Laboratory Standards Institute.24 The minimum biofilm eradication concentrations (MBECs) were determined using the MBEC Assay system (Innovotech, Edmonton, AB, Canada).25 Briefly, the bacteria are grown on 96-well plates with pegs on the lid filled with growth medium enabling biofilm formation on the pegs. The reduction in viable bacteria living in biofilm after exposure to the dHDPs was calculated as the difference between the viable colony forming units (CFU) on the control pegs and those in the treated pegs. The MBEC values for the dHDPs are higher than the corresponding MIC values; thus any bacteria in a biofilm that were shed or dissipated during the biofilm assay have been eradicated by the MBEC level of dHDP.
Antibiotic Resistance Profiling
Subinhibitory concentrations of RP444 and gentamicin were incubated with P. aeruginosa 27853 for 24 hours. The bacteria that showed growth in the highest concentration were repassaged in fresh dilutions containing sub-MIC levels of RP444 or gentamicin. The MIC was redetermined at each time point, and this was repeated for 21 consecutive passages.
Time-Kill Bactericidal Activity
Noninvasive and real-time monitoring of dHDP bactericidal activity was performed using bioluminescent strains of P. aeruginosa and S. aureus.26,27 Cultures of P. aeruginosa 19660_Xen5 and S. aureus 49525_Xen36 were made from a single colony, which was inoculated into 5 mL sterile lysogeny broth (LB). Tubes were incubated at 37°C overnight with agitation. Cultures were diluted 1:10 into fresh LB and incubated at 37°C for 2 hours to achieve mid-logarithmic growth. Optical density measurements (OD, 600 nm) were performed on the bacterial cultures, which were then adjusted to 1 × 107 CFU/mL. Bacterial suspensions (100 μL) were plated in 96-well black-walled plates. The candidate dHDPs were 2-fold serially diluted from 128 μg/mL in LB. Each concentration was done in triplicate, with the final volume being 200 μL. Imaging was performed at select times after the addition of the dHDP, and compared to concurrently run positive control antibiotics, tobramycin and vancomycin, using an IVIS Lumina imaging system (Caliper Life Sciences, Inc., Hopkinton, MA, USA). For imaging, the 96-well plate was positioned on the stage (12.5-cm field of view), with an open emission filter, binning of 4, and f-stop 1. Photons were counted for 30 seconds, and data analysis was performed using the LivingImage software program (version 4.3, Caliper Life Sciences, Inc.).
Animals
All animals received care in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication, 1996) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All studies were approved by the Institutional Animal Care and Use Committee at the University of Houston. C57BL/6 mice, 9 to 13 weeks, were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). General anesthesia consisted of an intraperitoneal injection of ketamine 100 mg/kg and xylazine 10 mg/kg (Vedco, Inc., St. Joseph, MO, USA).
In Vivo Corneal Epithelial Wound Healing
Rapid clearance occurs following topical ocular application, due in part to test agent drainage, blinking (every 5 minutes in mice), tear film, and tear film turnover.28 Therefore, in consideration of in vitro to in vivo dosing translation, low, mid, and high doses of 2, 64, and 640 μg/mL were evaluated in a murine model of corneal wound healing. Corneal epithelial scrape wounds (2-mm diameter) were made with an Algerbrush under a dissecting microscope in the right eye.29,30 Immediately following wound creation, 5 μL RP444 (2, 64, or 640 μg/mL) or vehicle (PBS) was applied. Topical application of the drops was repeated 5 minutes later, and then again at 6, 12, and 18 hours post wound. Corneal wound areas were assessed by staining with 1.5 μL 1% sodium fluorescein (Sigma-Aldrich Corp., St. Louis, MO, USA) every 6 hours until wound closure (24 hours) and images captured with an Olympus SZX16 stereomicroscope (Center Valley, PA, USA). Wound areas were outlined and measured with ImageJ software (https://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA), and expressed as a percentage of the original wound area, at 0 hours.30 Data were analyzed using 2-way repeated measures ANOVA with Bonferroni's test for multiple comparisons, with P < 0.05 considered significant.
Murine P. aeruginosa Keratitis Model
Scarified eyes were infected with 1 × 105 CFU P. aeruginosa ATCC 19660 as outlined by Kolar and colleagues,31 and dosed with 5 μL PBS or 2, 64, or 640 μg/mL RP444, beginning 8 hours post infection and continuing every 8 hours thereafter for 4 days. The clinical progression of infection was evaluated by capturing digital images using a slit-lamp biomicroscope equipped with a camera module CM 01 (Haag-Streit USA, Mason, OH, USA) on days 1, 3, and 5 post infection. The corneal infection was graded using a clinical scale of 0 to 4: 0, clear or slight opacity, partially covering the pupil; +1, slight opacity, fully covering the cornea; +2, dense opacity, partially or fully covering the pupil; +3, dense opacity fully covering the cornea; and +4, corneal perforation or phthisis.
At days 1, 3, and 5 post infection, corneas from infected eyes of 4 to 10 mice per group were harvested, and two corneas were pooled in 200 μL sterile PBS, generating two to five independent samples. The corneas were homogenized and then briefly sonicated. A 10-μL aliquot of the homogenate was diluted in sterile PBS and 10-fold serial dilutions were plated in duplicate onto Pseudomonas isolation agar plates. The plates were incubated overnight at 37°C and CFU counted.
The remainder of the homogenate was processed to quantitate the number of infiltrating inflammatory cells by myeloperoxidase (MPO) activity, a standard and well-established method in the study of infectious keratitis.32,33 Corneal homogenate (90 μL) was added to hexadecyltrimethylammonium bromide at a final concentration of 0.5% wt/vol in 50 mM phosphate buffer (pH 6.0). Samples were then freeze-thawed three times and centrifuged at 8,000g for 20 minutes at 4°C. Ten microliters supernatant was pipetted in triplicate into a microtiter plate and the reaction initiated by the addition of 90 μL 0.0167% (wt/vol) o-dianisidine dihydrochloride and 0.002% (vol/vol) H2O2 in PBS. The absorbance was measured for up to 3 hours at 450 nm. A standard curve was generated using purified MPO (Calbiochem, San Diego, CA, USA) on the same plate. Results were expressed as relative units of MPO activity per cornea (one MPO unit is proportional to 2 × 105 infiltrating neutrophils).
Data are expressed as mean ± standard error (SE). Comparisons were performed using a 1-way analysis of variance (ANOVA) followed by a post hoc Dunnett's test. A P value < 0.05 was considered statistically significant.
Results
dHDPs Exhibit Broad-Spectrum Bactericidal Activity
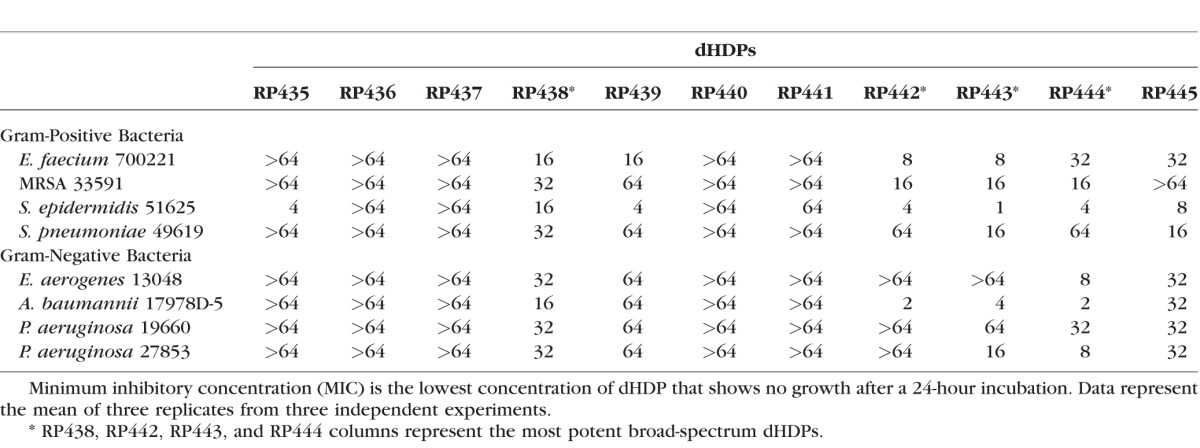
Eleven dHDPs were screened for their bactericidal effectiveness against four strains of Gram-positive and -negative bacteria, representing the most common keratitis pathogens, using the MIC assay (Table 2). RP438, RP442, RP443, and RP444 (shaded columns) were the most active peptides against both Gram-positive and -negative bacterial species and were selected for additional bactericidal screening.
Table 2.
Minimum Inhibitory Concentrations (MICs, μg/mL) Against Gram-Positive and -Negative Bacteria
dHDPs Are Active Against Gram-Negative and -Positive Biofilms
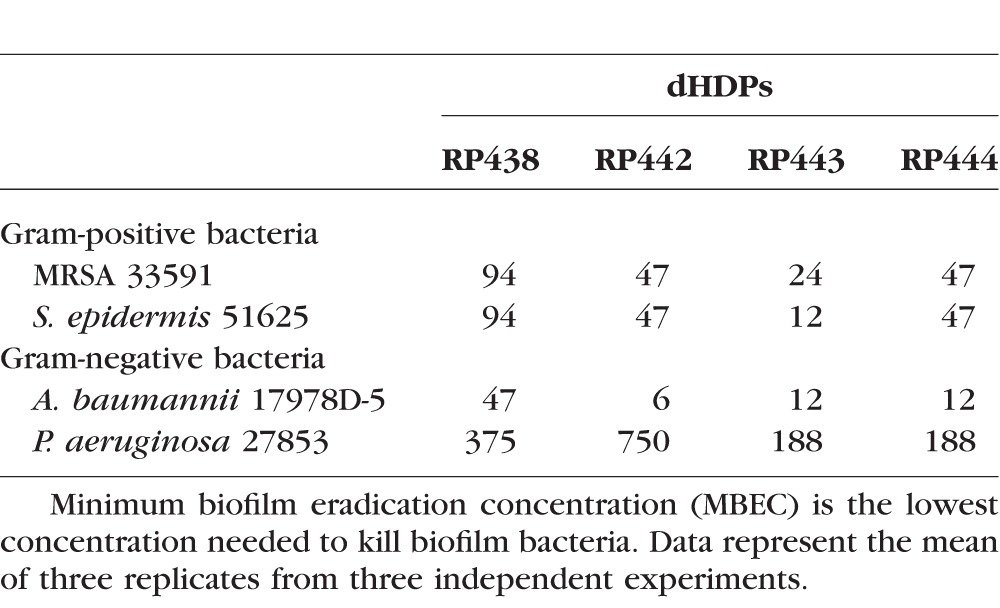
In addition to inhibiting planktonic growth, the MBEC values demonstrate effective eradication of both Gram-positive and -negative bacteria in their biofilm form (Table 3).
Table 3.
Minimum Biofilm Eradication Concentration (MBECs, μg/mL) Values Against Gram-Positive and -Negative Biofilm Bacteria
dHDPs Rapidly Eradicate P. aeruginosa and S. aureus in In Vitro Time-Kill Assays
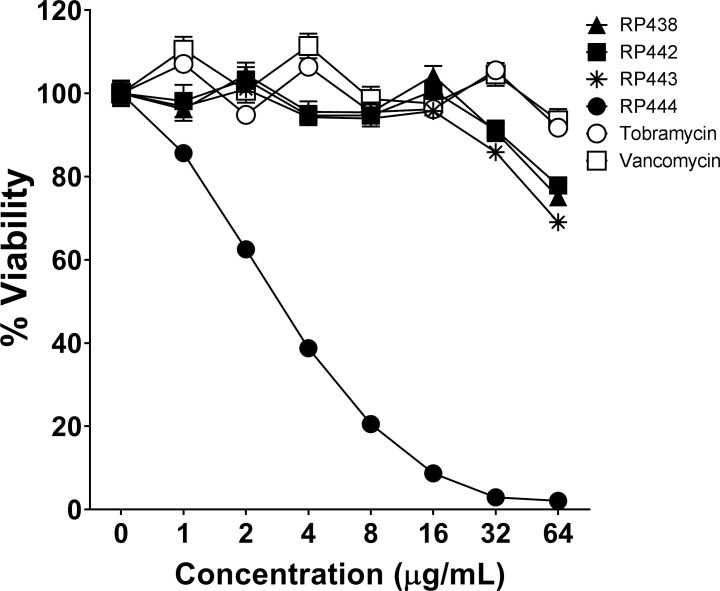
The immediate concentration-dependent bacterial eradication by RP438, RP442, RP443, RP444, tobramycin, and vancomycin on P. aeruginosa 19660_Xen5 is shown in Figure 1. RP444 killed P. aeruginosa immediately in a concentration-dependent manner, suggesting that RP444 exerts its bactericidal activity by disrupting membrane function (Fig. 1). In contrast, all other evaluated dHDPs, including tobramycin, an aminoglycoside that disrupts cell membranes and protein synthesis in Gram-negative bacteria, and vancomycin, a bactericide that acts by inhibiting cell wall synthesis in Gram-positive bacteria, exhibited no trace of antibacterial activity upon direct exposure.
Figure 1.
RP444 kills P. aeruginosa upon direct application. RP438, RP442, RP443, RP444, tobramycin, and vancomycin were applied to P. aeruginosa 19660_Xen5, and bactericidal effectiveness was assessed immediately after direct contact at time zero. The bioluminescence of viable bacteria was evaluated noninvasively with an IVIS Lumina bioimaging system. Data represent the mean ± SE of triplicate replicates from two independent experiments. For some points, the error bars are shorter than the height of the symbols.
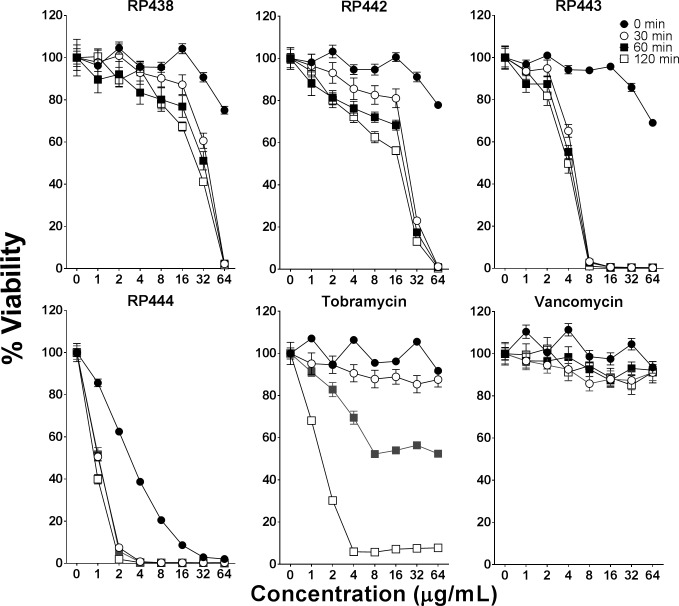
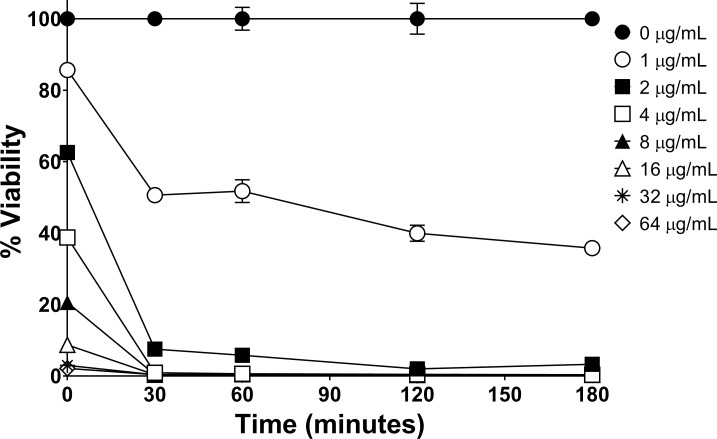
P. aeruginosa, as illustrated in Figure 2, was killed by all evaluated dHDPs following 60 minutes of exposure. The order of bactericidal effectiveness was RP444 followed by RP443, with RP442 and RP438 being equipotent. The striking immediate bactericidal activity of the dHDPs relates to direct electrostatic interaction with the bacterial walls. As anticipated, due to their respective modes of action, vancomycin showed no evidence of bactericidal activity during this short incubation time period (2 hours), with tobramycin exhibiting bactericidal effects at 60 minutes. The concentration-dependent time-kill evaluation of RP444 on P. aeruginosa 19660_Xen5 is displayed in Figure 3. P. aeruginosa was killed by RP444 within 30 minutes with as little as 2 μg/mL peptide.
Figure 2.
dHDPs exhibiting concentration-dependent killing of P. aeruginosa. The bactericidal effectiveness of RP438, RP442, RP443, RP444, tobramycin, and vancomycin was evaluated against P. aeruginosa 19660_Xen5 following 0, 30, 60, and 120 minutes' incubation. The bioluminescence of viable cells was quantitated noninvasively with an IVIS Lumina bioimaging system. Data represent the mean ± SE of triplicate replicates from two independent experiments. For some points, the error bars are shorter than the height of the symbols.
Figure 3.
RP444 quickly kills P. aeruginosa in a concentration-dependent manner. Time-kill and concentration-dependent killing of P. aeruginosa 19660_Xen5 by RP444 was evaluated. The bioluminescence of viable cells was quantitated with an IVIS Lumina imaging system. Data represent the mean ± SE of triplicate replicates from two independent experiments. For some points, the error bars are shorter than the height of the symbols.
The bactericidal activities of dHDPs, tobramycin, and vancomycin against S. aureus 49525_Xen36 are depicted in Figure 4 following 30 minutes' incubation time. RP442, RP443, and RP444 were effective at killing S. aureus, while neither tobramycin nor vancomycin exhibited any bactericidal effect during this exposure time.
Figure 4.
RP442, RP443, and RP444 exhibit antibacterial activity against S. aureus. The concentration-dependent profile of dHDPs, tobramycin, and vancomycin against S. aureus 49525_Xen36 following 30 minutes. The bioluminescence of viable cells was quantitated with an IVIS Lumina imaging system. Data represent the mean ± SE of triplicate replicates from two independent experiments. For some points, the error bars are shorter than the height of the symbols.
P. aeruginosa Did Not Develop Resistance Against RP444
P. aeruginosa did not become resistant to RP444 after 21 rounds of selection whereas gentamicin did, as evidenced by growing at 1024 times the MIC after 21 days (Fig. 5).
Figure 5.
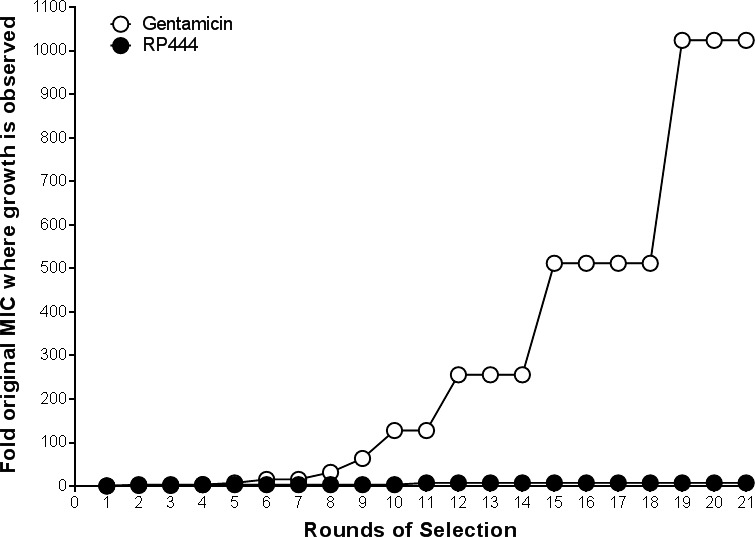
P. aeruginosa did not develop resistance against RP444. After 21 days of culturing P. aeruginosa ATCC 27853 with gentamicin, resistance was observed as 1024 times the MIC was needed for growth to occur whereas no resistance was observed with RP444. Assay was performed in duplicate; means are shown.
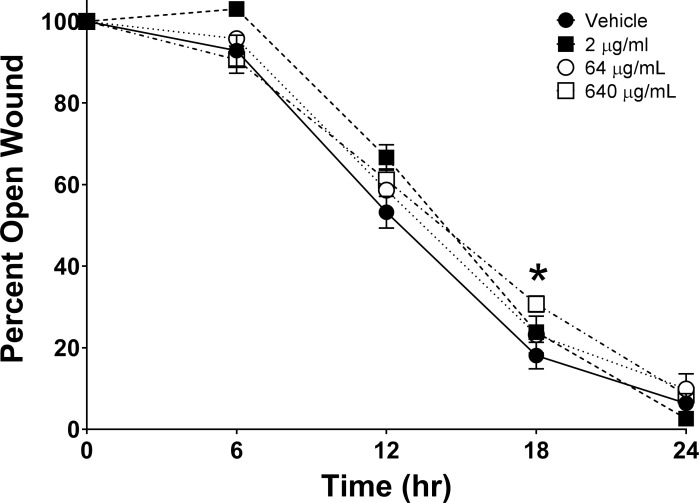
RP444 Did Not Impede In Vivo Corneal Epithelial Wound Healing
RP444 did not affect corneal epithelial wound healing in an in vivo murine wound healing model, with results obtained from two independent experiments (Fig. 6). No statistically significant difference in closure at 24 hours following topical ocular RP444 administration was observed. Hence, risks associated with delayed healing would not be anticipated following RP444 application.
Figure 6.
RP444 treatment does not affect in vivo wound closure. Mice were wounded and treated with vehicle (PBS) or RP444 (2, 64, or 640 μg/mL) every 6 hours for 24 hours. Corneal wound areas were assessed by fluorescein staining every 6 hours. The percentage of wound remaining open was determined by comparison to the original wound area. Data represent mean ± SE and were analyzed with 2-way repeated measures ANOVA and Bonferroni's correction for multiple comparisons. *P < 0.05; statistically significant difference between vehicle and RP444 640 μg/mL treatment; n = 7–10 mice/group.
RP444 Reduced the Severity of Murine P. aeruginosa Keratitis
RP444 demonstrated significant in vivo activity in reducing clinical scores and corneal opacity in a P. aeruginosa model of bacterial keratitis (Fig. 7). Clinical scores were not different among the groups on day 1 post infection. However, scores were lower in treated animals on day 3 post infection, although they were not significantly different (data not shown). A dose-dependent decrease in ocular clinical scores was observed on day 5 post infection with scores of 2.56 ± 0.176, 1.80 ± 0.374, 1.56 ± 0.176, and 1.20 ± 0.200 obtained for the PBS control and 2, 64, and 640 μg/mL groups, respectively. All RP444-treated groups yielded lower clinical scores compared to the control group, with the data for the 64 and 640 μg/mL RP444-treated groups significantly less than for the control group (P < 0.001 and 0.0001 for 64 and 640 μg/mL RP444, respectively).
Figure 7.

RP444 significantly reduced ocular disease in a murine P. aeruginosa keratitis model. Data are mean clinical scores ± SE from two independent experiments 5 days post infection with n = 9, 5, 9, and 10 mice for 0, 2, 64, and 640 μg/mL groups, respectively. **P < 0.01 and ***P < 0.001; statistically significant difference among control and RP444 treated. The right side of the figure shows representative photographs of the treated infected right eyes compared to the uninfected left eye, illustrating a reduction in pathology (opacity) with RP444 treatment.
RP444 Reduced Bacterial Burden in Murine P. aeruginosa Keratitis
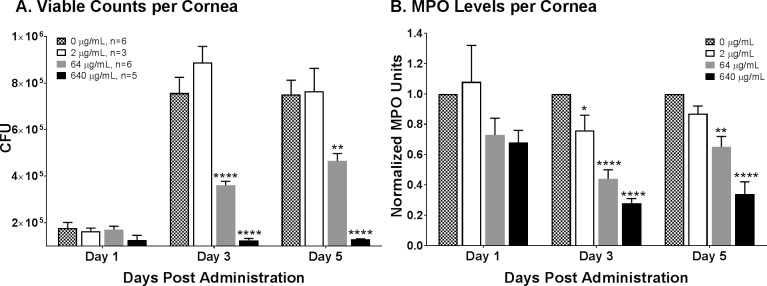
RP444 demonstrated statistically significant in vivo efficacy in reducing corneal bacterial burden in the murine P. aeruginosa model of bacterial keratitis. The number of viable bacteria recovered from the 64 and 640 μg/mL peptide-treated groups on day 3 (P < 0.001 and P < 0.001) and day 5 post infection (P < 0.01 and P < 0.001; Fig. 8A) was significantly lower than from the PBS-treated control. There was no difference in the number of viable bacteria between the control and 2 μg/mL peptide-treated groups at all time points.
Figure 8.
RP444 topical treatment reduces bacterial load and inflammatory cell infiltration in a murine P. aeruginosa keratitis model. (A) Viable bacterial counts in infected corneas treated with RP444 at 64 and 640 μg/mL were significantly lower than in PBS-treated animals at days 3 and 5 post infection. (B) A dose-dependent decrease in inflammatory cell infiltration, as measured by myeloperoxidase (MPO) activity, was observed in RP444-treated animals at days 3 and 5 post infection. Data are from three to six independent samples generated in one (2 and 640 μg/mL) or two experiments (64 μg/mL). Statistical significance was *P < 0.5, **P < 0.01, and ***P < 0.001.
RP444 Reduced Inflammatory Cell Infiltration in Murine P. aeruginosa Keratitis
RP444 demonstrated significant in vivo efficacy in reducing inflammatory cell infiltration in the P. aeruginosa model of bacterial keratitis (Fig. 8B). At day 1 post infection there was no significant difference between the vehicle- and RP444-treated corneas at any of the three concentrations. At day 3 post infection, MPO activity in corneas treated with 2, 64, or 640 μg/mL RP444 was dose-dependently lower than for treatment with the PBS vehicle (P < 0.05, P < 0.001, and P < 0.001 for 2, 64, and 640 μg/mL RP444, respectively). At day 5 post infection, MPO activity was lower in corneas treated with RP444, reaching statistical significance in the 64 and 640 μg/mL RP444-treated groups (P < 0.001).
Discussion
Designed host defense peptides are synthetic analogues of naturally occurring HDPs that provide the first line of defense against invading pathogens.34 Endogenous HDPs were initially recognized for their microbiocidal activity but are now recognized as critical immune effector and regulatory molecules that guard against infections and support healing, while suppressing inflammation.13,35,36 To date, the experimental therapeutic benefit of HDPs for bacterial keratitis has been mixed. COL-1, a 20–amino acid peptide, at doses up to 50 μg/mL, was investigated in a rabbit model of P. aeruginosa keratitis.37 COL-1 did not exhibit antimicrobial activity and induced corneal toxicity.37 The COL-1 dosing regimen entailed administration beginning 12 to 14 hours post infection and then every 15 minutes for the first hour, followed by dosing every hour for the next 9 hours. Then on days 2 through 4, dosing was every hour for 10 hours. More recently, the topical application of OH-CATH30 was shown to be efficacious in a rabbit de-epithelialization P. aeruginosa keratitis model when administered as 10 hourly doses of 1 mg/mL, 8 hours post infection.38 Additionally, an amphibian skin-derived esculentin, Esc-1a(1-21)NH2, was shown to reduce bacterial load and improve ocular clinical scores in a murine P. aeruginosa-induced keratitis model following topical administration at 88 μg/mL at 5 hours post infection and continuing three times a day for 5 days.39
Here, 11 dHDPs were shown to possess broad-spectrum antimicrobial activity following assessment of bactericidal effectiveness against isolates of both Gram-positive and -negative bacteria, and against drug-resistant pathogens (MRSA and P. aeruginosa). Empirical antimicrobial management of ocular infections is needed so an antimicrobial treatment possessing broad-spectrum activity with activity against recalcitrant biofilm is critical to limit the potential threat of corneal damage and vision loss.1,6 Four of these dHDPs (RP438, RP442, RP443, and RP444) were evaluated in additional in vitro assays that demonstrated their effectiveness in eradicating bacteria in biofilm. Biofilms are sophisticated colonies of microorganisms encased in a dense extracellular matrix enabling the bacteria to be extremely virulent and resilient, hence the imperative need to find efficacious agents that are able to encroach biofilms and kill bacteria.40 Preclinical studies indicate that mature biofilms are a common resilient feature of keratitis and need to be considered when developing therapeutic agents.41
Bacterial eradication should7 be the primary goal of antibiotic treatment, with bacterial load the main determinant of therapeutic outcome.42 Rapid, precise healing at the corneal surface is critical for restoration of the cornea's important protective and optical functions. Additionally, the rapid elimination of the infective organism should limit the emergence of resistance and the spread of infection. MIC and MBEC values, though useful for bacterial screening, provide no information on the time course of antimicrobial activity or the distinction between bacteriostatic and bactericidal mechanisms of action. Antimicrobial agents that disrupt cell membranes or interfere with essential enzyme function are likely to be bactericidal, whereas agents that inhibit ribosomal function are most likely bacteriostatic. An innovative time-kill assay using P. aeruginosa and S. aureus transfected with luciferase reporter genes was utilized to enable real-time evaluation of the effects of dHDPs and the antimicrobial agents tobramycin and vancomycin on bacteria viability.
The in vitro time-kill assays demonstrated that RP444 quickly killed bacteria, particularly P. aeruginosa, at relatively low doses (2 μg/mL) that were not cytotoxic to ocular cells as evidenced in the corneal epithelial wound study, which utilized repeat dosing of RP444 concentrations up to 640 μg/mL. The rapid destruction of the bacterial cells by potent broad-spectrum topical anti-infectives, thought to be primarily due to peptide–lipid interactions, imply a theoretical reduced likelihood of developing bacterial resistance.6,7 This was supported by RP444's lack of induction of resistance following repeated incubation with P. aeruginosa. Given the favorable therapeutic index with selective disruption of bacterial cells over the in vivo murine corneal epithelial cells, and its rapid bactericidal activity, RP444 was selected for evaluation of its therapeutic potential in a murine model of bacterial keratitis.
The topical application of 64 and 640 μg/mL RP444, at a dosing volume of 5 μL, reduced the severity of murine P. aeruginosa keratitis. This is reflected in an improved clinical score, reduced recovery of viable bacteria, and reduced immune cell infiltration.
In summary, a topically applied antimicrobial peptide, RP444, has been identified that exhibits rapid bactericidal activity, broad-spectrum effectiveness against isolates and biofilm, selective targeting of bacterial cell walls, and reduced likelihood of developing bacterial resistance. Further preclinical evaluation is under way to evaluate RP444 as a potential therapeutic for the treatment of sight-threatening bacterial keratitis.
Acknowledgments
Supported by National Institutes of Health Small Business Innovation Research (SBIR) Grant 1R43EYO24463-01 and the University of Houston (UHCO) Core Grant EY07551.
Disclosure: L.E. Clemens, Riptide Bioscience, Inc. (E), P; J. Jaynes, Riptide Bioscience, Inc. (I), P; E. Lim, None; S.S. Kolar, None; R.Y. Reins, None; H. Baidouri, None; S. Hanlon, None; A.M. McDermott, None; K.W. Woodburn, Riptide Bioscience, Inc. (C), P
References
- 1. American Academy of Ophthalmology Corneal/External Disease Panel. Preferred Practice Pattern® Guideline. Bacterial Keratitis-Limited Revision. San Francisco, CA: American Academy of Ophthalmology; 2011. Available at: www.aao.org/ppp. Accessed November 20, 2017. [Google Scholar]
- 2. Fernandes M, Vira D, Medikonda R, Kumar N. . Extensively and pan-drug resistant Pseudomonas aeruginosa keratitis: clinical features, risk factors, and outcome. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 315– 322. [DOI] [PubMed] [Google Scholar]
- 3. Vazirani J, Wurity S, Ali MH. . Multidrug-resistant Pseudomonas aeruginosa keratitis: risk factors, clinical characteristics, and outcomes. Ophthalmology. 2015; 122: 2110– 2114. [DOI] [PubMed] [Google Scholar]
- 4. Chang VS, Dhaliwal DK, Raju L, Kowalski RP. . Antibiotic resistance in the treatment of Staphylococcus aureus keratitis: a 20-year review. Cornea. 2015; 34: 698– 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rau G, Seedor JA, Shah MK, Ritterband DC, Koplin RS. . Incidence and clinical characteristics of enterococcus keratitis. Cornea. 2008; 27: 895– 899. [DOI] [PubMed] [Google Scholar]
- 6. Asbell PA, Sanfilippo CM, Pillar CM, DeCory HH, Sahm DF, Morris TW. . Antibiotic resistance among ocular pathogens in the United States: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015; 133: 1445– 1454. [DOI] [PubMed] [Google Scholar]
- 7. Liu C, Ji J, Li S,et al. . Microbiological isolates and antibiotic susceptibilities: a 10-year review of culture-proven endophthalmitis cases. Curr Eye Res. 2016; 27: 1– 5. [DOI] [PubMed] [Google Scholar]
- 8. Shin KY, Cho KJ. . Clinical features of Acinetobacter baumannii keratitis. J Korean Ophthalmol Soc. 2015; 56: 607. [Google Scholar]
- 9. Stapleton F, Carnt N. . Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond). 2012; 26: 185– 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robertson DM. . The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis. Eye Contact Lens. 2013; 39: 67– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elder MJ, Stapleton F, Evans E, Dart JK. . Biofilm-related infections in ophthalmology. Eye (Lond). 1995; 9 pt 1: 102– 109. [DOI] [PubMed] [Google Scholar]
- 12. Zasloff M. . Antimicrobial peptides of multicellular organisms. Nature. 2002; 415: 389– 395. [DOI] [PubMed] [Google Scholar]
- 13. Gordon YJ, Romanowski EG, McDermott AM. . A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res. 2005; 30: 505– 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gee ML, Burton M, Grevis-James A,et al. . Imaging the action of antimicrobial peptides on living bacterial cells. Sci Rep. 2013; 3: 1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hamoen LW, Wenzel M. . Editorial: antimicrobial peptides - interaction with membrane lipids and proteins. Front Cell Dev Biol. 2017; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arrowood MJ, Jaynes JM, Healey MC. . In vitro activities of lytic peptides against the sporozoites of Cryptosporidium parvum. Antimicrob Agents Chemother. 1991; 35: 224– 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christensen B, Fink J, Merrifield RB, Mauzerall D. . Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988; 85: 5072– 5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duclohier H, Molle G, Spach G. . Antimicrobial peptide magainin I from Xenopus skin forms anion-permeable channels in planar lipid bilayers. Biophys J. 1989; 56: 1017– 1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westerhoff HV, Juretic D, Hendler RW, Zasloff M. . Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci U S A. 1989; 86: 6597– 6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berthold N, Czihal P, Fritsche S,et al. . Novel apidaecin 1b analogs with superior serum stabilities for treatment of infections by gram-negative pathogens. Antimicrob Agents Chemother. 2013; 57: 402– 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laverty G, Gorman SP, Gilmore BF. . The potential of antimicrobial peptides as biocides. Int J Mol Sci. 2011; 12: 6566– 6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. PerkinElmer. Technical Data Sheet 119243-Xen 36-Rev-1. Available at: http://www.perkinelmer.com/Content/TDLotSheet/119243-%20Xen36.pdf. Accessed February 1, 2016.
- 23. PerkinElmer. Technical Data Sheet 119228-Xen05-Rev-1. Available at: https://www.perkinelmer.com/Content/TDLotSheet/119228-%20Xen05.pdf. Accessed February 1, 2016.
- 24. Clinical and Laboratory Standards Institute, CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. CLSI document M07-A9. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 25. Ali L, Khambaty F, Diachenko G. . Investigating the suitability of the Calgary Biofilm Device for assessing the antimicrobial efficacy of new agents. Bioresour Technol. 2006; 97: 1887– 1893. [DOI] [PubMed] [Google Scholar]
- 26. Rocchetta HL, Boylan CJ, Foley JW,et al. . Validation of a noninvasive, real-time imaging technology using bioluminescent Escherichia coli in the neutropenic mouse thigh model of infection. Antimicrob Agents Chemother. 2001; 45: 129– 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. . Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003; 187: 1717– 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vezina M. . Comparative ocular anatomy in commonly used laboratory animals. : Weir AB, Collins M, . Assessing Ocular Toxicology in Laboratory Animals. New York: Humana Press; 2013: 1– 21. [Google Scholar]
- 29. Li Z, Burns AR, Smith CW. . Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest Ophthalmol Vis Sci. 2006; 47: 1947– 1955. [DOI] [PubMed] [Google Scholar]
- 30. Reins RY, Hanlon SD, Magadi S, McDermott AM. . Effects of topically applied vitamin D during corneal wound healing. PLoS One. 2016; 11: e0152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kolar SS, Luca V, Baidouri H, Mannino G, McDermott AM, Mangoni ML. . Esculentin-1a(1-21)NH2: a frog skin-derived peptide for microbial keratitis. Cell Mol Life Sci. 2015; 72: 617– 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cole N, Hume E, Khan S,et al. . Interleukin-4 is not critical to pathogenesis in a mouse model of Pseudomonas aeruginosa corneal infection. Curr Eye Res. 2005; 30: 535– 542. [DOI] [PubMed] [Google Scholar]
- 33. McClellan SA, Ekanayaka SA, Li C, Jiang X, Barrett RP, Hazlett LD. . Thrombomodulin protects against bacterial keratitis, is anti-inflammatory, but not angiogenic. Invest Ophthalmol Vis Sci. 2015; 56: 8091– 8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwab U, Gilligan P, Jaynes J, Henke D. . In vitro activities of designed antimicrobial peptides against multidrug-resistant cystic fibrosis pathogens. Antimicrob Agents Chemother. 1999; 43: 1435– 1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dutta P, Das S. . Mammalian antimicrobial peptides: promising therapeutic targets against infection and chronic inflammation. Curr Top Med Chem. 2016; 16: 99– 129. [DOI] [PubMed] [Google Scholar]
- 36. Gallo RL. . Sounding the alarm: multiple functions of host defense peptides. J Invest Dermatol. 2008; 128: 5– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mannis MJ. . The use of antimicrobial peptides in ophthalmology: an experimental study in corneal preservation and the management of bacterial keratitis. Trans Am Ophthalmol Soc. 2002; 100: 243– 271. [PMC free article] [PubMed] [Google Scholar]
- 38. Li SA, Liu J, Xiang Y, Wang YJ, Lee WH, Zhang Y. . Therapeutic potential of the antimicrobial peptide OH-CATH30 for antibiotic-resistant Pseudomonas aeruginosa keratitis. Antimicrob Agents Chemother. 2014; 58: 3144– 3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mangoni ML, Luca V, McDermott AM. . Fighting microbial infections: a lesson from amphibian skin-derived esculentin-1 peptides. Peptides. 2015; 71: 286– 295. [DOI] [PubMed] [Google Scholar]
- 40. Bispo PJ, Haas W, Gilmore MS. . Biofilms in infections of the eye. Pathogens. 2015; 4: 111– 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saraswathi P, Beuerman RW. . Corneal biofilms: from planktonic to microcolony formation in an experimental keratitis infection with Pseudomonas aeruginosa. Ocul Surf. 2015; 13: 331– 345. [DOI] [PubMed] [Google Scholar]
- 42. Ball P, Baquero F, Cars O,et al. . Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence. J Antimicrob Chemother. 2002; 49: 31– 40. [DOI] [PubMed] [Google Scholar]