ABSTRACT
Gut microbiota has been revealed to play an important role in various health conditions, and recent studies have suggested the modulation of gut microbiota as a therapeutic strategy. There is no effective treatment of norovirus infection, though vitamin A has been suggested to have an antiviral effect in an epidemiological study. We demonstrated that vitamin A significantly inhibited murine norovirus replication. Vitamin A supplementation significantly increased the abundance of Lactobacillus sp. during norovirus infection, which played a crucial role in antiviral efficacy, inhibiting murine norovirus. Therefore, we elaborated the antiviral effect of vitamin A via modulation of gut microbiota. Furthermore, we suggest a novel strategy, using potential probiotics, as having a protective and therapeutic effect on noroviral infection.
KEYWORDS: gut microbiome, IFN-β, Lactobacillus, norovirus, vitamin A
Introduction
The gut microbiota plays an important role in energy and immune homeostasis of the gastrointestinal tract.1-3 Recent studies have revealed that the modulation of gut microbiota significantly impacts various diseases including infectious diseases.4,5 For example, fecal microbial transplantation (FMT), as a gut microbiota-targeted therapy, for the treatment of Clostridium difficile, could be a good example of the application of gut microbiota modulation for preventing infectious diseases.6 In our previous study and other recent studies, metabolic improvement by commonly used drugs such as metformin, accompanied by modulation of the abundance of bacterial taxa (e.g, Akkermansia muciniphila), had a beneficial effect on obesity and type 2 diabetes.7,8 In addition, gut microbiota influences healthy immunity from viral infections such as influenza virus and hepatitis virus.9,10
Norovirus is the most common cause of acute gastroenteritis, with clinical symptoms that include diarrhea, vomiting, nausea, abdominal pain, and fever lasting one to 3 d.11 Unfortunately, there is no current treatment or vaccine effective against norovirus infection. A recent study reported that norovirus infection did not significantly alter the gut microbiota, the composition of gut microbiota was not considered in norovirus pathogenesis.12
In previous epidemiological studies, the norovirus infection rate and clinical symptoms decreased significantly with sufficient vitamin A supplementation.13 Retinoic acid, the metabolite of dietary vitamin A, plays an essential role in innate immune response against viral infection. A recent study reported that sufficient vitamin A supplementation reduced both mortality and morbidity related to infectious gastrointestinal diseases.14 Moreover, retinoic acid-inducible gene 1 (RIG-1) and melanoma differentiation-associated gene 5 (MDA5) signaling contribute to antiviral responses by activating type I interferons (IFNs).15 Previous studies have revealed that MDA5- and STAT-1-deficient mice had a defect in their immune response to murine norovirus.16,17
In the current study, we investigated the possibility that gut microbiota, modulated by vitamin A during norovirus infection, could have a potential role in the treatment of, and preventive measures against, norovirus. Our findings provide new perspectives on how vitamin A exerts an antiviral effect against norovirus, and indicate a novel therapeutic strategy for the development of antiviral agents through gut microbiota modulation.
Inhibitory effect of vitamin A on murine norovirus replication
Vitamin A administration was considered to prevent norovirus infection and improve the clinical symptoms.13 However, there has been no further study to evaluate the antiviral mechanism of vitamin A related to the control of norovirus infection. In our study, we showed that vitamin A effectively inhibited murine norovirus (Murine norovirus 1) replication in vitro and in vivo, providing evidence of the antiviral effect of vitamin A on norovirus. Further, we identified that IFN-β expression played a key role in immune responses, with vitamin A treatment. Various other studies revealed that type I and type II interferon signaling play a crucial role in antiviral immune responses.15 Jung et al. reported that simvastatin increased the replication of norovirus, indicating that IFN-α has potential as an antiviral therapy.18 Moreover, IFN-γ increased significantly in norovirus-associated diarrhea specimens, and IFN-λ determined the persistence of murine norovirus infection.19,20 In addition, RIG-1 signaling was mediated by antiviral immune responses, stimulated by vitamin A, against murine norovirus, but the antiviral effect of Lactobacillus sp was not RIG-1-dependent.
Modulation of gut microbiota by vitamin A and murine norovirus
Recent studies have indicated that norovirus replication was related to the intestinal environment, including gut microbiota.19,21 Enterobacter cloacae, a common bacteria identified in gut, was reported to facilitate norovirus infection.21 On the other hand, disruption of gut microbiota by antibiotic treatment prevented the norovirus infection and persistence.19,21 Ettayebi et al. successfully cultivated human norovirus in stem cells derived from human intestinal enteroid, a cultivation system requiring bile which is regulated by the intestinal microbiota.22 Moreover, Kernbauer et al. suggested that murine norovirus can replace the beneficial function of bacterial microbiota.23 These results strongly support our results that the modulation of gut microbiota is related to the inhibition of murine norovirus.
In our study, we characterized the gut microbiome by vitamin A administration and murine norovirus infection: 103 genera of bacteria were identified in the murine norovirus-infected mouse group during vitamin A administration, and 75 were also found in other groups. The result of principal coordinates analysis (PCoA) revealed that bacterial communities were more clearly clustered in weighted UniFrac than unweighted. Therefore, we expected that the abundance of certain bacterial taxa play an important role in the antiviral effect of vitamin A against murine norovirus infection.
The composition of the gut microbiome was changed significantly with murine norovirus infection in ICR mice, unlike the situation with Swiss Webster and inbred C57BL/6 mice.12 Murine norovirus infection significantly increased the abundance of genus Alistipes, Bacteroides, Bilophila, Corynebacterium, Desulfovibrio, Facklamia, Jeotgalicoccus, LE30, Oligella, Parabacteroides, and Yaniella. On the other hand, genus Dialister, Lactobacillus, and Veillonella decreased with murine norovirus infection. And, vitamin A administration significantly increased the abundance of genus Aggregatibacter, Allobaculum, Bifidobacterium, Dialister, and Enhydrobacter.
Most of the identified bacteria have not been studied closely until now. Among them, we observed 2 genera, Lactobacillus and Bifidobacterium, which are well-known potential beneficial microbes. First, the abundance of Lactobacillus sp decreased significantly with murine norovirus infection, whereas it recovered during vitamin A administration. In recent studies, Lactobacillus sp showed an antiviral effect on various viruses such as rotavirus and influenza A virus mediated with IFNs signaling.24,25 We also showed that the expression of IFN-β and -γ increased significantly by treatment of Lactobacillus sp in murine norovirus-infected RAW264.7 cells; IFN-β was remarkable. Therefore, the activation of IFNs by vitamin A and increased proportion of Lactobacillus sp plays a crucial role in the antiviral immune response against murine norovirus infection.
Putative probiotic candidates using modulation of gut microbiota
A recent study reported an antiviral effect on murine norovirus replication using Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv.26 In our study, Lactobacillus sp were suggested as preventive probiotics against murine norovirus. Interestingly, the abundance of Lactobacillus sp increased significantly after murine norovirus infection during vitamin A administration. Various beneficial effects of supplementation with Lactobacillus have been reported in clinical settings.27-29
The genus Lactobacillus currently contains over 180 species and the effect of Lactobacillus sp is likely to be strain-dependent.30,31 Among them, certain Lactobacillus sp have demonstrated antiviral effects against other viral infections such as rotavirus and human papillomavirus.32 Although, we did no clearly identified Lactobacillus sp in fecal material, the development of potential Lactobacillus probiotics to prevent noroviral infection needs to be studied further, with the inclusion of clinical trials.
Further study
Firstly, the mechanism by which the abundance of Lactobacillus sp in gut microbiota is modulated by vitamin A should be identified to understand the inhibition of murine norovirus by vitamin A. We showed a correlation between Lactobacillus sp, vitamin A, and murine norovirus that supports the anti-noroviral effect of modulating gut microbiota via vitamin A administration. However, that correlation is not sufficient to confirm the inhibitory effect of vitamin A on norovirus. Based on Koch's postulates, Lactobacillus sp isolated from the modulated gut microbiota after vitamin A administration should be tested for inhibition of murine norovirus. Such antiviral immune responses by modulated gut microbiota have not been identified in previous research. Fecal microbiota transplantation using gut microbiota modulated by vitamin A could provide useful information to elucidate the antiviral effect of murine norovirus inhibition.
Secondly, we could investigate the antiviral effect of vitamin A on murine norovirus independently of the modulation of gut microbiota. Although activation of IFNs by RIG-1 and MDA5 signaling plays a key role in antiviral effects against a variety of different viruses, an increase in expression levels of those genes was not observed during inhibition of murine norovirus by Lactobacillus sp15 Therefore, we could also investigate whether specific chemokines, such as chemokine (C-C motif) ligand 2 (CCL2) and interleukin 8 (IL-8), are involved in the immune response against murine norovirus infection.
Finally, murine norovirus was used as a surrogate for human norovirus due to the lack of a cell culture infection model for human norovirus. Recently, there has been the remarkable research development of the replication of human norovirus using stem cell-derived human enteroids.22 Therefore, the antiviral effect of vitamin A via modulation of gut microbiota could be better validated using this model for human norovirus in further studies.
Concluding remarks
We first identified that vitamin A administration effectively inhibited murine norovirus replication in vitro and in vivo. Moreover, vitamin A modulated the composition of gut microbiota; the increase in the relative abundance of Lactobacillus sp was remarkable after murine norovirus infection during vitamin A administration. Finally, we demonstrated the antiviral efficacy of Lactobacillus sp from in vitro experiments of the inhibitory effect of Lactobacillus treatment on murine norovirus replication; IFN-β signaling was involved in antiviral immune response (Fig. 1). Although the identified Lactobacillus sp should be evaluated further in human, we expect that a remarkable antiviral effect on norovirus is highly likely with these potential probiotics. Furthermore, the modulation of gut microbiota could be used as a novel strategy to control various viral infectious diseases.
Figure 1.
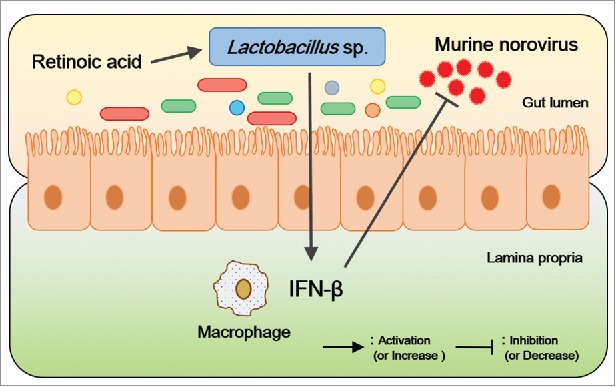
Model of murine norovirus inhibition by vitamin A. In this study, the abundance of Lactobacillus sp was considered to inhibit the murine norovirus proliferation via the upregulation of IFN-β; RA administration increased their abundance levels.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Research Foundation of Korea (NRF-2015R1A2A1A10054078 and 315067–3).
References
- [1].Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab: TEM 2015; 26:493-501; PMID:26257300; https://doi.org/ 10.1016/j.tem.2015.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 2013; 14:668-75; PMID:23778794; https://doi.org/ 10.1038/ni.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 2016; 535:75-84; PMID:27383982; https://doi.org/ 10.1038/nature18848 [DOI] [PubMed] [Google Scholar]
- [4].Kinross JM, Darzi AW, Nicholson JK. Gut microbiome-host interactions in health and disease. Genome Med 2011; 3:14; PMID:21392406; https://doi.org/ 10.1186/gm228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol 2011; 19:349-59; PMID:21684749; https://doi.org/ 10.1016/j.tim.2011.05.006 [DOI] [PubMed] [Google Scholar]
- [6].Borgia G, Maraolo AE, Foggia M, Buonomo AR, Gentile I. Fecal microbiota transplantation for Clostridium difficile infection: back to the future. Expert Opin Biol Ther 2015; 15:1001-14; PMID:26063385; https://doi.org/ 10.1517/14712598.2015.1045872 [DOI] [PubMed] [Google Scholar]
- [7].Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol 2014; 80:5935-43; PMID:25038099; https://doi.org/ 10.1128/AEM.01357-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al.. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013; 110:9066-71; PMID:23671105; https://doi.org/ 10.1073/pnas.1219451110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Robinson CM, Pfeiffer JK. Viruses and the Microbiota. Annu Rev Virol 2014; 1:55-69; PMID:25821837; https://doi.org/ 10.1146/annurev-virology-031413-085550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Karst SM. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol 2016; 14:197-204; PMID:26853118; https://doi.org/ 10.1038/nrmicro.2015.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ahmed SM, Lopman BA, Levy K. A systematic review and meta-analysis of the global seasonality of norovirus. PloS one 2013; 8:e75922; PMID:24098406; https://doi.org/ 10.1371/journal.pone.0075922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nelson Adam M, Elftman Michael D, Pinto Amelia K, Baldridge Megan, Hooper Patrick, Kuczynski Justin, et al.. Murine norovirus infection does not cause major disruptions in the murine intestinal microbiota. Microbiome 2013; 1:1; PMID:24467924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Long KZ1, Garcia C, Santos JI, Rosado JL, Hertzmark E, Dupont HL, Ko G. Vitamin A supplementation has divergent effects on norovirus infections and clinical symptoms among Mexican children. J Infect Dis 2007; 196:978-85; PMID:17763317; https://doi.org/ 10.1086/521195 [DOI] [PubMed] [Google Scholar]
- [14].Thornton KA, Mora-Plazas M, Marin C, Villamor E. Vitamin A deficiency is associated with gastrointestinal and respiratory morbidity in school-age children. J Nutr 2014; 144:496-503; PMID:24500929; https://doi.org/ 10.3945/jn.113.185876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev 2009; 227:75-86; PMID:19120477; https://doi.org/ 10.1111/j.1600-065X.2008.00737.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McCartney SA, Thackray LB, Gitlin L, Gilfillan S, Virgin HW, Colonna M. MDA-5 recognition of a murine norovirus. PLoS Pathog 2008; 4:e1000108; PMID:18636103; https://doi.org/ 10.1371/journal.ppat.1000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mumphrey SM, Changotra H, Moore TN, Heimann-Nichols ER, Wobus CE, Reilly MJ, Moghadamfalahi M, Shukla D, Karst SM. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol 2007; 81:3251-63; PMID:17229692; https://doi.org/ 10.1128/JVI.02096-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jung K, Wang Q, Kim Y, Scheuer K, Zhang Z, Shen Q, Chang KO, Saif LJ. The effects of simvastatin or interferon-alpha on infectivity of human norovirus using a gnotobiotic pig model for the study of antivirals. PloS One 2012; 7:e41619; https://doi.org/ 10.1371/journal.pone.0041619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 2015; 347:266-9; PMID:25431490; https://doi.org/ 10.1126/science.1258025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ko G, Jiang ZD, Okhuysen PC, DuPont HL. Fecal cytokines and markers of intestinal inflammation in international travelers with diarrhea due to Noroviruses. J Med Virol 2006; 78:825-8; PMID:16628572; https://doi.org/ 10.1002/jmv.20630 [DOI] [PubMed] [Google Scholar]
- [21].Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinjé J, et al.. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014; 346:755-9; PMID: 25378626; https://doi.org/ 10.1126/science.1257147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng XL, Qu L, et al.. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016; 353:1387-93; PMID:27562956; https://doi.org/ 10.1126/science.aaf5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 2014; 516:94-8; PMID:25409145; https://doi.org/ 10.1038/nature13960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kang JY, Lee do K, Ha NJ, Shin HS. Antiviral effects of Lactobacillus ruminis SPM0211 and Bifidobacterium longum SPM1205 and SPM1206 on rotavirus-infected Caco-2 cells and a neonatal mouse model. J Microbiol 2015; 53:796-803; https://doi.org/ 10.1007/s12275-015-5302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nakayama Y, Moriya T, Sakai F, Ikeda N, Shiozaki T, Hosoya T, Nakagawa H, Miyazaki T. Oral administration of Lactobacillus gasseri SBT2055 is effective for preventing influenza in mice. Sci Rep 2014; 4:4638; PMID:24717726; https://doi.org/ 10.1038/srep04638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hoang PM, Cho S, Kim KE, Byun SJ, Lee TK, Lee S. Development of Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv as a preventive probiotic against murine norovirus infection. Appl Microbiol Biotechnol 2015; 99:2793-803; PMID:25487889; https://doi.org/ 10.1007/s00253-014-6257-7 [DOI] [PubMed] [Google Scholar]
- [27].Martinez RC, Franceschini SA, Patta MC, Quintana SM, Gomes BC, De Martinis EC, Reid G. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol 2009; 55:133-8; PMID:19295645; https://doi.org/ 10.1139/W08-102 [DOI] [PubMed] [Google Scholar]
- [28].Schaeffer EM. Re: lactobacilli vs antibiotics to prevent urinary tract infections: a randomized, double-blind, noninferiority trial in postmenopausal women. J Urol 2013; 189:1332-3; PMID:23561348; https://doi.org/ 10.1016/j.juro.2012.12.056 [DOI] [PubMed] [Google Scholar]
- [29].Dinleyici EC, Dalgic N, Guven S, Metin O, Yasa O, Kurugol Z, Turel O, Tanir G, Yazar AS, Arica V, et al.. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. Jornal de pediatria 2015; 91:392-6; https://doi.org/ 10.1016/j.jped.2014.10.009 [DOI] [PubMed] [Google Scholar]
- [30].Maassen CB, Claassen E. Strain-dependent effects of probiotic lactobacilli on EAE autoimmunity. Vaccine 2008; 26:2056-7; PMID:18378048; https://doi.org/ 10.1016/j.vaccine.2008.02.035 [DOI] [PubMed] [Google Scholar]
- [31].Drago L, Nicola L, Iemoli E, Banfi G, De Vecchi E. Strain-dependent release of cytokines modulated by Lactobacillus salivarius human isolates in an in vitro model. BMC Res Notes 2010; 3:44; PMID:20184725; https://doi.org/ 10.1186/1756-0500-3-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hanson L, VandeVusse L, Jerme M, Abad CL, Safdar N. Probiotics for Treatment and Prevention of Urogenital Infections in Women: A Systematic Review. J Midwifery Womens Health 2016; 61:339-55; https://doi.org/ 10.1111/jmwh.12472 [DOI] [PubMed] [Google Scholar]


