Abstract
Background
Stress is a prevailing risk factor for mood-related illnesses, wherein women represent the majority of those afflicted with major depression. Despite the growing literature suggesting that affective disorders can arise after a traumatic event is vicariously experienced, this relationship remains understudied in females at the preclinical level. Thus, the objective of the current investigation was to examine whether exposure to emotional/psychological stress (ES) mediates depression-related outcomes in female mice.
Methods
Female c57BL/6 mice (8-week old, nullparity) vicariously experienced the defeat bout of a male conspecific, by a male CD1 aggressor, for 10 consecutive days. Twenty-four h after the last stress exposure, female mice were tested in the social interaction, sucrose preference, tail suspension, or elevated plus-maze tests. Furthermore, we examined whether ketamine and chlordiazepoxide, pharmacological agents used to treat mood-related disorders in the clinical population, would reverse the ES-induced social dysfunction.
Results
When compared to controls, female mice exposed to ES displayed decreased social behavior and preference for sucrose, along with increased immobility in the tail suspension test. Also, they displayed higher levels of blood serum corticosterone, as well as decreased body weight. Lastly, the ES-induced avoidance-like phenotype was ameliorated by both ketamine and chlordiazepoxide.
Conclusions
Our data indicate that female mice exposed to ES display a behavioral- and physiologic-profile that mimics symptoms of depression in the clinical population. As such, this experimental model may be adopted to examine vicarious stress-induced mood-related disorders, as well as pharmacological antidepressant response, in a sex specific manner.
Keywords: animal model, chlordiazepoxide, depression, ketamine, psychological stress, social defeat stress
Introduction
According to the World Health Organization, depression will soon become the leading cause of disability across the globe (1). While there have been significant advancements for the treatment of numerous illnesses, such as heart disease and stroke, rapid and effective therapeutic treatments for mood-related disorders continue to fall behind. A likely reason is the complex multifactorial nature of depression, making it difficult to develop preclinical models that recapitulate the etiological factors of the disease.
Exposure to stress is a well recognized risk-factor for the development of mood-related illnesses (2). As such, numerous preclinical models have been developed to assess the deleterious effects of stress and the development of depression-related behaviors in rodents. For example, stressed animals display memory impairment, decreased sensitivity for rewards (i.e., anhedonia), weight fluctuation, as well as increased social avoidance and despair-like behavior (3). Interestingly, most experimental paradigms rely primarily on exposure to physical stressors. However, a growing body of literature suggests that emotional/psychological stress (ES), in particular, may also play a critical role in the etiology of mood-related psychopathology (4-7). As such, there is need for the development of animal models designed to tease apart the specific contributions of individual stress-subtypes (i.e., physical vs. psychological) in the mediation of a depressive-like behavioral phenotype. Importantly, such models must recapitulate symptomology of the clinical population, wherein females, when compared to males, represent the majority of those who suffer from mood-related illnesses (8).
To date, however, most rodent models of depression have been predominantly validated using male subjects. Females, on the other hand, have been primarily incorporated in designs that include social instability (9), isolation (10, 11), maternal separation (12-14), and unpredictable stressors (13-18). While these approaches have helped breach the underutilization of female subjects in preclinical stress-related studies, the need for an animal model of female depressive-like behavior that is mediated by a naturalistic stressor, is still much needed.
In recent years, the social defeat stress paradigm has been widely implemented to study the neurobiology of depression, given its high ethological and pharmacological validity, when compared to other preclinical models of stress, in both males (19) and females (20). Interestingly, the social defeat model has been recently modified to include a vicariously-observed stress condition (i.e., ES) incorporating c57BL/6 mice (21). In this paradigm, male mice exhibit depressive-like behaviors after witnessing the defeat bout of a same-sex conspecific, which resembles the behavioral profile of physically stressed (PS) mice (22, 23). Surprisingly, whether female mice will exhibit similar outcomes following vicariously experienced stress has not been investigated. As such, the objective of the current investigation is to examine whether the vicarous defeat stress (VDS) paradigm yields depression-like behaviors in female c57BL/6 mice, and if so, whether pharmacological agents used to treat depression/anxiety ameliorate the social-related alterations observed.
Methods and Materials
Animals
Description of animals (purchased from Charles River, Hollister, CA) and housing conditions are provided in Supplement 1.
Vicarious Defeat Stress (VDS) and Experimental Design
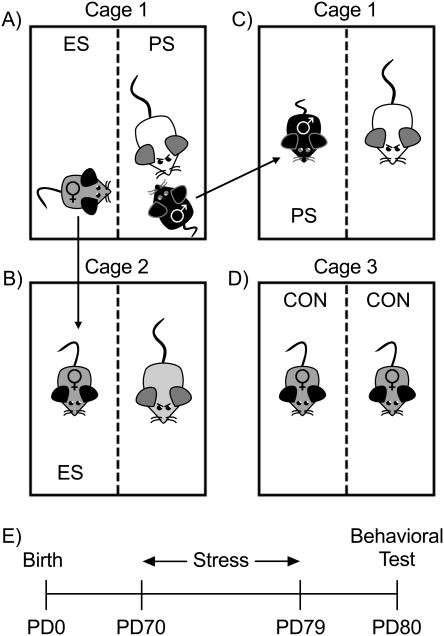
The social defeat stress paradigm was performed as previously described (24), with modifications to incorporate an emotional/psychological stress (ES) component (21) – where female c57BL/6 mice vicariously experience the defeat of a male c57BL/6 counterpart. We refer to ES as all non-physical sensory stimuli (visual, olfactory, and chemosensory) associated with indirectly experiencing the defeat of the physically stressed (PS) male mouse. To do this, CD1 retired breeders with reliable attack latencies (≤30 s on three consecutive screening tests) were housed in cages containing clear perforated Plexiglass separators, which divide the cage into two separate compartments (Fig. S1A). For each stress session (10-min/day), PS male mice were placed into the same compartment as the CD1 aggressor, while female ES mice were placed in the neighboring compartment allowing only a vicarious experience (i.e., visual, olfactory, auditory) of the physical bout (Fig. 1A). Following each session, ES-females were housed next to a novel CD1 aggressor for 24 h until the next stress episode (Fig. 1B), while male PS mice were housed for 24 h in the compartment adjacent to their respective CD1 aggressor (Fig. 1C). This procedure ensured that all experimental mice (ES and PS) were exposed to a novel CD1 aggressor each day, for 10 consecutive days (PD70-79). In the event that the CD1 aggressor exhibited severe attacks, the defeat bout was immediately interrupted (25). Non-stressed control (CON) female mice were handled daily and housed in similar cages, one on each side of the perforated Plexiglass separator (Fig. 1D). Additional control housing-conditions are described in Supplement 1. For specifics on cage sizes, dimensions of dividers, and screening for aggressive behavior in CD1 mice, we refer the reader to the standardized protocol recently published for male c57BL/6 mice (26). Immediately after the last stress episode (i.e., PD79), all c57BL/6 mice were single housed. Twenty-four h later (PD80), separate groups of experimental mice were tested on a battery of mood-related tasks (Fig. 1E). This approach was taken to avoid possible testing carry-over effects (see Table S1 for experimental groups). For the social interaction and elevated plus-maze tests, behavior was recorded via an automated video-tracking system (EthovisionXT; Noldus). Behavioral outcomes for the sucrose preference- and tail suspension- tests were scored by observers blind to stress conditions.
Figure 1.
Schematic of the vicarious defeat stress (VDS) procedure and time-line of experimental design. (A) Diagram of the VDS paradigm where female c57BL/6 mice would witness [emotional/psychological stress (ES)] the social defeat [physical stress (PS)] of a male c57BL/6 counterpart by a CD1 male mouse (cage 1). (B) After the 10-min stress episode, the ES female mouse was moved to the cage of a novel CD1 aggressor (cage 2), (C) while the PS male mouse was moved to the adjacent compartment of the same CD1 aggressor (cage 1). (D) Non-stressed control (CON) female mice were pair-housed in similar cages, but did not experience stressors associated with the VDS paradigm. (E) Experimental timeline where adult male and female c57BL/6 mice were exposed to the VDS paradigm for 10 consecutive days (i.e., postnatal day [PD] 70-79). Twenty-four h later (PD80), separate groups of experimental mice were tested on depression-related behavioral tests.
Social Interaction Test
The social interaction test is a two-trial procedure (24), conducted under red light conditions. In the first 2.5 min session, the experimental c57BL/6 mouse is allowed to freely explore an open field arena (40 cm length × 40 cm width × 40 cm height; Fig. S1C). Along one side of the arena is a circular (7 cm diameter) wire cage (Stoelting Co., Wood Dale, IL) that remains empty during the first trial (target-absent condition). The experimental c57BL/6 mouse is then removed from the testing arena for 30 sec (into a separate holding cage), and a novel CD1 male mouse is placed into the wire cage. In the second 2.5 min trial (target-present condition), the experimental c57BL/6 mouse is reintroduced into this arena now containing a social target (unfamiliar CD1 male mouse) within the circular wire cage. In order to determine whether social interaction levels would be influenced by the strain/sex of the social target itself, a different group of ES-females was exposed to an unfamiliar female of the same strain during the social interaction test. Also, to examine the salience of the visual stimuli in mediating ES-induced avoidance, we conducted an experiment where white-opaque-dividers without holes (Fig. S1B) were used to prevent females from witnessing the resident-intruder interaction – removing visual sensory cues related to the defeat of the male conspecific. In all cases (social target being an unfamiliar male CD1, or an unfamiliar female c57BL/6 mouse) a social interaction ratio was used to evaluate the effects of stress on social behavior – where the time (sec) spent in the interaction zone (8 cm wide corridor surrounding the wire cage) in the presence of the social target is divided by the time spent in the interaction zone in the absence of the target (25, 27). Lastly, we recorded the distance traveled (cm) during the first 2.5 min of the social interaction test (target-absent condition) to examine whether basal locomotor activity or exploratory behavior was influenced by stress exposure.
Corticosterone Immunoassay and Other Behavioral Approaches
See Supplement 1 for details on the corticosterone immunoassay (28), as well as the sucrose preference, tail suspension, and elevated plus-maze behavioral tests (22).
Pharmacological Study
Because social defeat-induced avoidance is reversed by antidepressants in male c57BL/6 mice (19), we conducted an experiment to assess whether pharmacological agents used to treat depression and/or anxiety reverse the decreased social behavior observed in ES-female mice. We selected ketamine (Spectrum Chemicals, Gardena, CA) and the benzodiazepine chlordiazepoxide (Toronto Research Chemicals, North York, ON, Canada) because they have been reported to mediate antidepressant- (29) and anxiolytic-like (30) effects shortly after drug exposure, thus matching our behavioral testing time (i.e., 24 h after VDS). Ketamine and chlordiazepoxide were diluted in 9% sterile saline, and injected at a volume of 2 mg/kg. Specifically, ES female mice received three injections of ketamine (20 mg/kg, ip) prior to the social interaction test (immediately and 4 h after the tenth VDS episode, as well as 30 min before the social interaction test); whereas females administered with the benzodiazepine received a single injection of chlordiazepoxide (10 mg/kg, ip) 30 min before the social interaction test. Dose and timing of drug administration were selected based on previous work (29, 31).
Statistical Analysis
Description of statistical analyses is provided in Supplement 1.
Results
ES Decreases Social Behavior in Female and Male c57BL/6 Mice
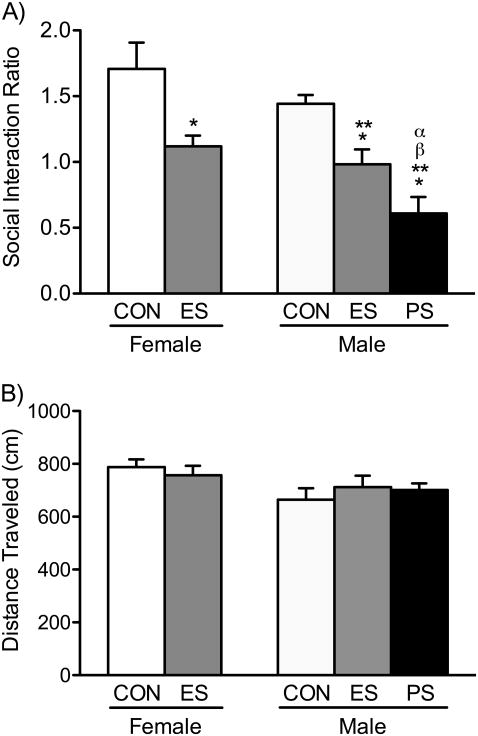
As an initial experiment, we examined whether or not ES exposure decreases social behavior in female c57BL/6 mice, in a similar fashion as in male mice of the same strain (21). The effects of VDS on social behavior, in female and male c57BL/6 mice (n=11/group), are shown in Figure 2. A one-way ANOVA indicated that the time spent in the interaction zone, with a novel CD1 male aggressor as a social target, was influenced by stress exposure (F4,50=11.16, p<0.01). Specifically, Figure 2A demonstrates how ES-female mice (left panel), displayed a significantly lower social interaction ratio, when compared to non-stressed CON-female mice (Tukey, p<0.05). Similarly, within the male groups (right panel), both ES- and PS-mice displayed significantly lower social interactions when compared to male CON's (Tukey, p<0.05). Not surprisingly, PS-males displayed a significantly lower interaction ratio when compared to all other experimental groups (Tukey, p<0.05, respectively). No differences between the female-CON and male-CON groups were observed (p>0.05). Importantly, when assessing distance traveled (cm) during the first 2.5 min interaction trial (target-absent condition), no differences were noted between the groups (F4,50=1.77, p>0.05), thus, indicating that stress exposure did not influence general locomotor activity or exploratory behavior (Fig. 2B). Furthermore, in a separate group of mice, we examined whether females exposed to ES would display altered social interaction levels if they were exposed to the CD1 aggressor for 24 h, after witnessing the defeat bout of the PS-male conspecific (i.e., overnight). Figure S2A shows that, when compared to the CON-female group, these ES-females also display decreases in social interaction (t20=2.18, p<0.05), indicating that VDS exposure results in lowered sociability regardless of whether the ES-female stays overnight with the CD1 aggressor, or not.
Figure 2.
Vicarious social defeat stress decreases social behavior in female and male c57BL/6 mice. (A) Female and male mice exposed to emotional/psychological stress (ES) spent significantly less time in the interaction zone, when compared to respective same-sex non-stressed controls (CON) (p<0.05). Similarly, when compared to male-CONs, male mice exposed to physical stress (PS) displayed decreases in social interaction. (B) No differences in locomotor activity were evident across the different experimental groups. Data are presented as mean + SEM (n=11/group). *Indicates p<0.05 when compared to the female-CON group. **Indicates p<0.05 when compared to the male-CON group. αIndicates p<0.05 when compared to the female-ES group. βIndicates p<0.05 when compared to the male-ES group.
Housing Conditions Do Not Influence Baseline Levels of Social Interaction
To examine if differences in baseline levels of social interaction would be observed as a function of housing/chemosensory conditions, we conducted a separate experiment where CON-females were exposed to the bedding of PS-male mice for 10 consecutive days (n=12). Also, a separate group of CON-female mice was paired-housed with CD1 male mice (as in Fig. 1D), thus, removing the visual aspects of physical defeat stress in the presence of the aggressor (n=10). In Figure S2B, a one-way ANOVA indicated that, when compared to the original CON-female group (Fig. 2A), these housing/chemosensory conditions do not influence baseline levels of social interaction (F2,30=1.37, p>0.05).
ES-induced Avoidance is Influenced by Visual Cues, but not Social Target Strain/Sex
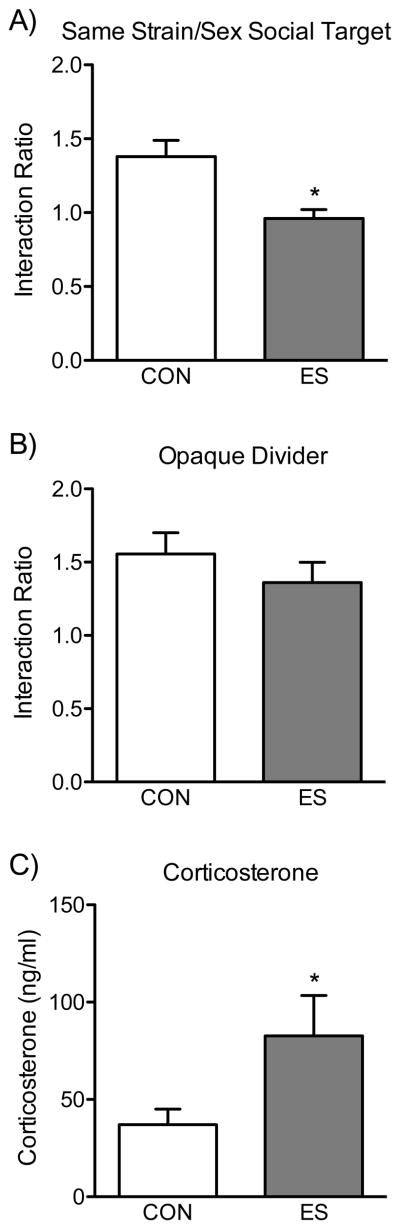
As additional controls for the development of avoidance-like behavior, we examined whether social behavior would be influenced by the strain/sex of the social target during the social interaction test, as well as the role that visual cues play during VDS exposure. Figure 3A shows that the ES-induced reduction in social behavior is exhibited even when the social target is an age-matched unfamiliar c57BL/6 female counterpart (t20=3.33, p<0.05), indicating generalized avoidance (n=11/group). However, as depicted in Figure 3B, when an opaque divider is used to block visual cues during the VDS episodes, ES-females do not develop avoidance-like behavior (n=10/group), since no differences in social interaction ratios were observed between the groups (t18=0.97, p>0.05).
Figure 3.
Effects of social-target sex and strain, as well as visual stimuli, on social behavior in female c57BL/6 mice exposed to the vicarious defeat stress (VDS) model. (A) When compared to controls (CON, n=11), female mice exposed to emotional/psychological stress (ES, n=11) spent significantly less time in the interaction zone when exposed to a same sex/strain social target during the “target present” condition of the social interaction test (p<0.05). (B) Using opaque dividers to block visual stimuli during VDS exposure prevented the development of avoidance behavior, since no differences in the time spent in the interaction zone were observed between the groups (p>0.05, n=10 per group). (C) Twenty-four h after VDS exposure, ES-female mice (n=6) exhibited higher levels of serum corticosterone, when compared to CON (n=8) mice (p<0.05). Data are presented as mean + SEM. *Indicates p<0.05 when compared to CON.
ES Increases Corticosterone
The effects of ES exposure on blood plasma corticosterone are shown in Figure 3C. A t-test indicated that 40 min after the last exposure to VDS, females in the ES condition (n=6) displayed significantly higher levels of blood serum corticosterone when compared to the CON (n=8) group (t12=2.20, p<0.05).
ES Decreases Body Weight
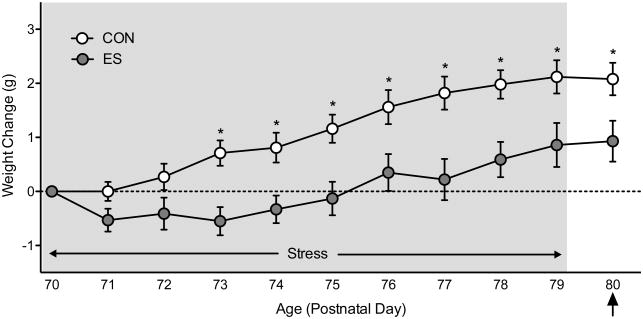
Figure 4 shows the effects of 10 days of VDS on body weight change. Body weight change was calculated by subtracting the daily-recorded weight of the animal from the weight on the initial day of VDS exposure (i.e., PD70); thus, a positive number would indicate an increase in overall body weight, while a negative number would indicate body weight decrease. A mixed-design repeated measures ANOVA indicated that weight changed as a function of stress (main effect: F1,20=7.99, p<0.05), day (main effect: F10,200=42.80, p<0.05), and their interaction (F10,200=4.21, p<0.05). Post hoc comparisons indicated that ES-female mice displayed significantly lower body weight, when compared to CONs, by the fourth day of stress exposure (PD73-79; p<0.05, respectively). Weight remained significantly lower 24 h after the last day of stress (PD80; p<0.05).
Figure 4.
Effects of vicarious defeat stress (VDS) on body weight. When compared to non-stressed female controls (CON), female mice exposed to emotional/psychological stress (ES) displayed significantly lower body weight as of day four of exposure (postnatal day 73). Body weight remained significantly lower 24 h after the last day of VDS exposure (postnatal day 80). Arrow indicates day of behavioral testing. Data are presented as weight change in grams (mean + SEM). *Indicates p<0.05 when compared to CON.
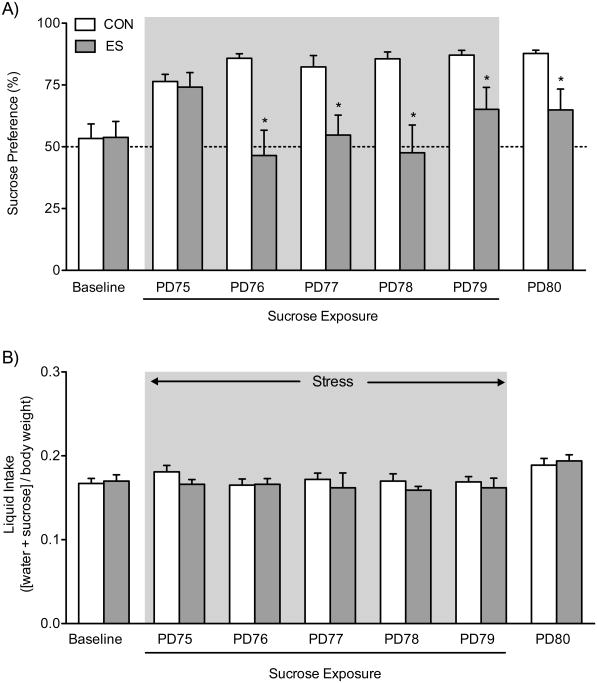
ES Decreases Preference for a Sucrose Solution
Figure 5 shows the effects of VDS on sucrose preference. A two-way repeated measures ANOVA, with stress (between measure) and day of sucrose exposure (repeated measure) as independent variables, indicated that sucrose preference was influenced by stress (main effect: F1,18=51.88, p<0.01), day of sucrose exposure (main effect: F6,108=2.93, p<0.05), and their interaction (F6,108=2.73, p<0.05). Specifically, Figure 5A demonstrates that when compared to CONs, the ES group displayed a decrease in sucrose preference from PD76 to PD79 (p<0.05, respectively; n=10/group). This decrease in sucrose preference by ES-female mice was exhibited 24 h (PD80) post stress exposure (p<0.05). Conversely, exposure to VDS did not influence total fluid intake (water+sucrose/[body weight]; F6,108=0.95, p>0.05; Fig. 5B).
Figure 5.
Effects of the vicarious defeat stress (VDS) paradigm on sucrose preference in female c57BL/6 mice. (A) When compared to non-stressed controls (CON, n=10), female mice exposed to emotional/psychological stress (ES, n=10) displayed a decrease in preference for a 1% sucrose solution during the last 4-days of defeat [postnatal day (PD) 76-79]; gray area). This decrease in preference for sucrose remained 24 h after the last episode of stress (PD 80). (B) No differences in total liquid intake ([sucrose+water]/body weight) were observed between the groups. Data are presented as mean + SEM. *Indicates p<0.05 when compared to CON.
ES Increases Sensitivity to Inescapable Stress
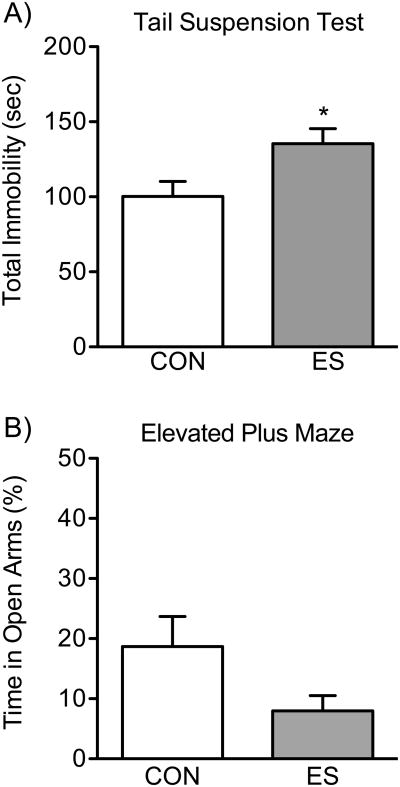
VDS increases sensitivity to inescapable stress (i.e., despair), as per the tail suspension test, in female c57BL/6 mice (Figure 6A). A t-test revealed significant mean differences as a function of ES exposure on the time spent immobile during the last 5-min of the test (t18=2.46, p<0.05). Specifically, when compared to CONs (n=9), ES-females (n=11) spent significantly more time in the immobile position (p<0.05).
Figure 6.
Effects of vicarious defeat stress (VDS) on despair- and anxiety-related behavior in female c57BL/6 mice. (A) Exposure to VDS increased sensitivity to the tail suspension test. Specifically, 24 h after stress exposure, female mice exposed to emotional/psychological stress (ES, n=11) displayed increased total immobility (p<0.05), when compared to controls (CON, n=9). (B) When exposed to the anxiogenic environment of the elevated plus-maze, 24 h post VDS exposure, ES female mice (n=11) spent significantly less time in the open arms of the maze, when compared to CON (n=12; p<0.05). Data are presented as mean + SEM. *Indicates p<0.05 when compared to CON.
Effects of ES on the Elevated Plus-maze
Figure 6B shows the effects of VDS on the anxiogenic environment of the elevated plus-maze. While ES females (n=11) displayed decreased time in the open arms of the maze, when compared to CONs (n=12), a t-test indicated that this difference did not reach statistical significance (t21=1.86, p=0.07).
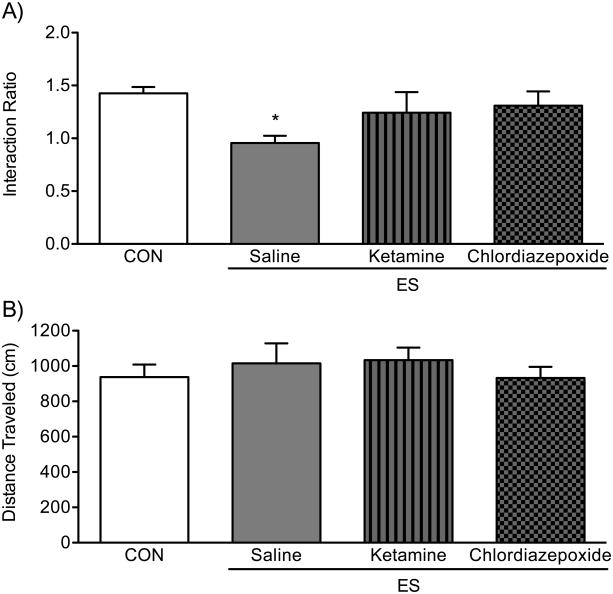
Ketamine and Chlordiazepoxide Ameliorate ES-induced Decreases in Social Behavior
Figure 7 shows the effects of ketamine and chlordiazepoxide on social behavior after exposure to ES in female mice. A one-way ANOVA indicated that social behavior was influenced by drug treatment (F3,40=3.22, p<0.05). Specifically, when compared to CONs (n=18), the females exposed to ES and administered with saline (ES-saline; n=8) displayed significantly lower interaction ratios (Tukey, p<0.05). Conversely, no differences between CONs and ES-females receiving either ketamine (n=8) or chlordiazepoxide (n=10) were observed (p>0.05, respectively), indicating that both drugs ameliorated the ES-induced decrease in social behavior. No differences in distance traveled (cm), as a function of drug administration, during the target-absent condition of the social interaction test, were detected between the groups (F3,40=1.15, p>0.05; Fig. 7B).
Figure 7.
Ketamine (20 mg/kg) and chlordiazepoxide (10 mg/kg) ameliorate the emotional/psychological stress (ES)-induced avoidance behavioral phenotype in the social interaction test. ES females receiving saline (n=8) displayed significantly lower interaction ratios when compared to non-stressed controls (CON, n=18). No differences in social behavior were detected between the CON group and the ES-females receiving ketamine (n=8), or the group of ES-females administered with chlordiazepoxide (n=10). Data are presented as mean + SEM. *Indicates p<0.05 when compared to CON group.
Discussion
The purpose of the present investigation was to examine whether depression-related outcomes would be evident in female mice after exposure to VDS (26). To achieve this, we exposed female c57BL/6 mice to ES by witnessing the defeat bout of a male conspecific for 10 consecutive days. Twenty-four h later, separate groups of female mice were tested on a collection of behavioral tests that are designed to evaluate social functioning (social interaction), anhedonia (sucrose preference), despair (tail suspension test), anxiety (elevated plus-maze), and HPA (hypothalamus-pituitary-adrenal)-axis activation (corticosterone). Collectively, our data indicate that exposure to ES, in female mice, is a significant stressor that induces behavioral and physiologic responses that recapitulate depression-like symptomology.
Because an association between depression and impaired social functioning is well recognized (32), and social avoidance is a behavioral endophenotype recapitulated by preclinical social stress models (19, 26), we first examined the impact of ES on the social interaction test. We found that ES-females spent less time interacting with a novel social target, regardless of the social target's sex and/or strain (Figs. 2A, 3A, S2A), when compared to CONs – demonstrating a generalized decrease in social behavior. This behavioral response is comparable to that of male mice experiencing similar psychosocial stressors (21), or PS itself (Fig. 2A). Yet, here, we extend these findings to female c57BL/6 mice, which display avoidance as a result of indirectly experiencing the defeat bout of a male conspecific – an effect that is prevented when visual cues are blocked (Fig. 3B).
Furthermore, since the reduced ability to experience pleasure (i.e., anhedonia) is a core symptom of depression, we also examined if female mice exposed to ES would display decreased preference for sucrose. We found that, when compared to CONs, ES-female mice displayed significantly lowered preference for sucrose as of the seventh day of the VDS procedure, an effect that remained 24 h after stress exposure (Fig. 5A). This finding appears to be sex dependent, since previous work suggests that ES-male c57BL/6 mice do not develop a decrease in preference for sucrose 24 h after VDS (21). Importantly, although ES-females displayed decreases in body weight (Fig. 4), no differences in total liquid intake were observed between the groups (Fig. 5B), which is indicative of an anhedonia-like state (33) – an effect that extends face validity to the VDS model in female c57BL/6 mice, specifically. Hence, our findings indicate that this paradigm induces a negative affect state in female mice, per decreases in social behavior and sucrose preference, that may lend translational significance to the clinical population, where women report depressive symptoms as a result of emotional distress (34).
In addition to social dysfunction and anhedonia-like behavior, ES increased sensitivity to subsequent stressors designed to assess mood-related behavior. Here, when compared to CONs, ES-female mice displayed increased total immobility in the tail suspension test (Fig. 6A). Conversely, when examining behavioral responses to the anxiogenic environment of the elevated plus-maze, ES-female mice did not display a significant reduction in the percent time spent in the open arms of the maze. This contrasts males that have experienced PS or ES (21), highlighting sex-dependent differences to escapable versus inescapable stressors (35). It appears that 24 h post VDS, both ES-female and -male c57BL/6 mice display decreases in social behavior, along with increased sensitivity to despair measures. Interestingly, females also display an anhedonia-like behavioral profile (decreased sucrose preference), without changes in exploratory behavior in the elevated plus-maze. Conversely, ES-male mice do not display a decrease in sucrose preference at this time point – yet, display an anxiogenic profile in the elevated plus-maze (21). Together, these findings suggest that the VDS paradigm is a powerful tool that may dissect the ES-induced neurobiological factors underlying specific mood-related syndromes (anhedonia vs. anxiety) as a function of sex. This is a critical factor when developing preclinical models of affective disorders, given that men and women exhibit different symptoms and coping mechanisms to episodes of depression (36).
Extensive clinical research indicates there are differences in the levels of stress hormones between depressed patients and their age-matched controls, pointing to a role of a dysregulated HPA-axis in the development of mood-related illnesses (37, 38). Thus, we examined how VDS would influence blood serum levels of corticosterone in ES-females. Here, we found that witnessing the defeat bout of a male conspecific is salient enough to activate the HPA axis, as indicted by elevated levels of corticosterone when compared to female controls (Fig. 3C). Indeed, previous work in rodents has supported the link between elevated corticosterone and the development of depressive-like symptomology (39). However, an important distinction is that most of these previous studies have implemented artificial stressors over extended periods of time that are unnatural to the animal (i.e., restrain, foot-shock, etc.). While these studies have been important in highlighting the strong link between stress and depression-related behaviors, such experimental approaches do not mimic the nature of stress in humans, in which social and emotional stressors predominate. Thus, we postulate that the female VDS model better recapitulates critical behavioral (social avoidance, anhedonia, despair) and physiologic (increased corticosterone and weight-change) endophenotypes that may allow researchers to uncover the neurobiological factors of ES-induced mood-related illnesses.
To examine whether pharmacological agents commonly used to treat mood-related disorders ameliorate the depression-related behaviors induced by VDS, we conducted an experiment where separate groups of ES-females were administered with ketamine or chlordiazepoxide prior to the examination of social behavior. We selected the social interaction test given that it is a behavior associated with both depressive- and anxiogenic-like characteristics with high pharmacological validity (19, 40). Specifically, decreases in social behavior, post defeat stress, are reversed by both traditional (fluoxetine; selective serotonin reuptake inhibitor) and novel antidepressants (ketamine; noncompetitive NMDA receptor antagonist), but not by anti-anxiety drugs (chlordiazepoxide; benzodiazepine), in male mice (19, 41). Here, we found that both ketamine and chlordiazepoxide ameliorated the ES-induced reductions of social behavior in female mice (Fig. 7). The finding that chlordiazepoxide reversed ES-induced social avoidance in females, unlike previously reported in PS-males (19), is likely due to experimental differences that include: type of stress (ES vs. PS), sex (female vs. male), as well as dose (10 vs. 2.5 mg/kg) and regimen (1-day vs. 28-days), between the studies. This finding suggests that the female VDS paradigm is sensitive to both traditional anti-anxiety drugs, as well as to rapid-acting non-traditional antidepressants, providing pharmacological-validity to this model.
The neurobiological factors that underlie the female ES-induced behavioral dysfunction, and pharmacological reversal of these alterations, are unknown. Nevertheless, previous work in ES-male mice suggests that adaptations in signaling molecules within reward-related brain regions, such as the nucleus accumbens (NAcc) and ventral tegmental area (VTA), may underlie the social avoidance phenotype (21, 27). Specifically, ES alters extracellular signal-regulated protein kinase (ERK)-signaling within these brain regions. ERK is a signaling molecule that has been highly implicated in regulating responses to both affect- (42-44) and reward-related behaviors (45, 46). For example, within the NAcc of male mice, ES increases ERK2 gene expression (27), while decreasing ERK-related signaling transcripts within the VTA (21). Whether these molecular alterations, within the VTA-NAcc circuitry, modulate depression-like behavioral responses in ES-female mice is yet to be determined. Interestingly, a recent report utilizing subchronic variable stress in female c57BL/6 mice found a similar depressive-like behavioral profile as the ES-females in our study (i.e., decreased sucrose preference, increased corticosterone, without changes in explorative behavior in the plus-maze). Furthermore, they also reported gene alterations within the NAcc of female mice that matched those found in postmortem tissue of female humans with depression (47), thus highlighting this brain circuitry as a potential target to examine the neurobiological factors that underlie psychological stress-induced depression.
It is important to note that we did not determine the phase of the estrous cycle, across the female mice utilized in our experiments, when behavioral testing was conducted. Thus, it is possible that the normal fluctuations in estradiol/progesterone may have affected both the corticosterone and behavioral responses observed – particularly, when the social target was a male-, versus a female-mouse, during the social interaction test. As such, future work will be needed to thoroughly examine whether or not the estrous cycle may play a role in the depression-related phenotype induced by VDS.
Given the increased rates of mood-related illnesses across the globe, particularly depression, it is imperative to develop preclinical models that allow researchers to uncover the neurobiological mechanisms that underlie the disorder. Both sex and stress are common risk factors in the development of depression, thus, we propose that the VDS model can be used to experimentally dissect the individual contributions that specific social stressors (i.e. psychological), as a function of sex (i.e., female), can contribute in the expression of depression-related behavior. Collectively, we propose that our data provides face-, ethological-, and pharmacological-validity to the VDS paradigm as a model of ES-induced depression in a sex-specific manner.
Supplementary Material
Figure S1. Photograph of defeat cages, with wood shavings bedding, used during the vicarious social defeat stress paradigm (Ancare®, N40 Large Mouse Cage and wire top). (A) Defeat mouse cage with fitted clear plexiglass divider with 1 cm diameter perforated holes. (B) Defeat mouse cage with fitted white opaque divider without holes. (C) Photograph of squared open field arena with a white circular wire cage (Stoelting Co.® Mouse Stranger Enclosure #60452), used during the social interaction test.
Figure S2. (A) Social interaction ratio of female mice exposed to emotional/psychological stress (ES) that spent 24 h with CD1‐male aggressor (overnight), which physically defeated a c57BL/6‐male conspecific (PS). After 10 days of vicarious defeat stress (VDS), the ES‐ female group spent significantly less time in the interaction zone, when compared to controls (CON; n=11; female house‐paired with another female), 24 hours post last stress exposure. (B) Housing and chemosensory conditions do not influence baseline levels of social interaction in CON female mice (i.e., females that do not experience VDS). Specifically, no differences in time spent in the social interaction zone were evident between females CONs (house‐paired with separate female) and females housed in similar conditions but also exposed to the bedding of physically stressed (PS) male mice (n=12), or pair‐housed with an unfamiliar CD1 male mouse (n=10). Note: CON group in sections A and B are the same group (Table S1, Cohort 1). *p>0.05.
Table S1. Experimental groups.
Acknowledgments
This work was supported by a grant from the National Institute of General Medical Sciences (1SC2GM109811) to SDI. IG and DOS were supported by the SMART-MiND summer research program at UTEP. We would like to thank Miguel A. Arenivar for excellent technical assistance.
Footnotes
Conflict of Interest: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferrari AJ, Norman RE, Freedman G, Baxter AJ, Pirkis JE, Harris MG, et al. The Burden Attributable to Mental and Substance Use Disorders as Risk Factors for Suicide: Findings from the Global Burden of Disease Study 2010. PLoS One. 2014;9:e91936. doi: 10.1371/journal.pone.0091936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cougle JR, Resnick H, Kilpatrick DG. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behav Res Ther. 2009;47:1012–1017. doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard EB, Kuhn E, Rowell DL, Hickling EJ, Wittrock D, Rogers RL, et al. Studies of the vicarious traumatization of college students by the September 11th attacks: effects of proximity, exposure and connectedness. Behav Res Ther. 2004;42:191–205. doi: 10.1016/S0005-7967(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 7.Schlenger WE, Caddell JM, Ebert L, Jordan BK, Rourke KM, Wilson D, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans' Reactions to September 11. JAMA. 2002;288:581–588. doi: 10.1001/jama.288.5.581. [DOI] [PubMed] [Google Scholar]
- 8.Reeves WC, Strine TW, Pratt LA, Thompson W, Ahluwalia I, Dhingra SS, et al. Mental illness surveillance among adults in the United States. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002) 2011;60(Suppl 3):1–29. [PubMed] [Google Scholar]
- 9.Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, et al. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 10.Smith AS, Birnie AK, French JA. Social isolation affects partner-directed social behavior and cortisol during pair formation in marmosets, Callithrix geoffroyi. Physiol Behav. 2011;104:955–961. doi: 10.1016/j.physbeh.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 12.Hill RA, Klug M, Kiss Von Soly S, Binder MD, Hannan AJ, van den Buuse M. Sex-specific disruptions in spatial memory and anhedonia in a “two hit” rat model correspond with alterations in hippocampal brain-derived neurotrophic factor expression and signaling. Hippocampus. 2014;24:1197–1211. doi: 10.1002/hipo.22302. [DOI] [PubMed] [Google Scholar]
- 13.Bai M, Zhang L, Zhu X, Zhang Y, Zhang S, Xue L. Comparison of depressive behaviors induced by three stress paradigms in rats. Physiol Behav. 2014;131:81–86. doi: 10.1016/j.physbeh.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Llorente R, Miguel-Blanco C, Aisa B, Lachize S, Borcel E, Meijer OC, et al. Long term sex-dependent psychoneuroendocrine effects of maternal deprivation and juvenile unpredictable stress in rats. J Neuroendocrinol. 2011;23:329–344. doi: 10.1111/j.1365-2826.2011.02109.x. [DOI] [PubMed] [Google Scholar]
- 15.Benoit JD, Rakic P, Frick KM. Prenatal stress induces spatial memory deficits and epigenetic changes in the hippocampus indicative of heterochromatin formation and reduced gene expression. Behav Brain Res. 2014;281c:1–8. doi: 10.1016/j.bbr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grassi D, Lagunas N, Calmarza-Font I, Diz-Chaves Y, Garcia-Segura LM, Panzica GC. Chronic unpredictable stress and long-term ovariectomy affect arginine-vasopressin expression in the paraventricular nucleus of adult female mice. Brain Res. 2014;1588:55–62. doi: 10.1016/j.brainres.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 17.McFadden LM, Paris JJ, Mitzelfelt MS, McDonough S, Frye CA, Matuszewich L. Sex-dependent effects of chronic unpredictable stress in the water maze. Physiol Behav. 2011;102:266–275. doi: 10.1016/j.physbeh.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Zaidan H, Leshem M, Gaisler-Salomon I. Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biol Psychiatry. 2013;74:680–687. doi: 10.1016/j.biopsych.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, et al. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren BL, Vialou VF, Iñiguez SD, Alcantara LF, Wright KN, Feng J, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iñiguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, et al. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17:247–255. doi: 10.3109/10253890.2014.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sial OK, Warren BL, Alcantara LF, Parise EM, Bolanos-Guzman CA. Vicarious social defeat stress: Bridging the gap between physical and emotional stress. J Neurosci Methods. 2016;258:94–103. doi: 10.1016/j.jneumeth.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP, et al. Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci. 2014;36:250–260. doi: 10.1159/000362875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iñiguez SD, Aubry A, Riggs LM, Alipio JB, Zanca RM, Flores-Ramirez FJ, et al. Social defeat stress induces depression-like behavior and alters spine morphology in the hippocampus of adolescent male C57BL/6 mice. Neurobiol Stress. 2016;5:54–64. doi: 10.1016/j.ynstr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parise EM, Alcantara LF, Warren BL, Wright KN, Hadad R, Sial OK, et al. Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol Psychiatry. 2013;74:750–759. doi: 10.1016/j.biopsych.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frussa-Filho R, Barbosa-Junior H, Silva RH, Da Cunha C, Mello CF. Naltrexone potentiates the anxiolytic effects of chlordiazepoxide in rats exposed to novel environments. Psychopharmacology (Berl) 1999;147:168–173. doi: 10.1007/s002130051157. [DOI] [PubMed] [Google Scholar]
- 31.Heredia L, Torrente M, Colomina MT, Domingo JL. Assessing anxiety in C57BL/6J mice: a pharmacological characterization of the open-field and light/dark tests. J Pharmacol Toxicol Methods. 2014;69:108–114. doi: 10.1016/j.vascn.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Bosc M. Assessment of social functioning in depression. Compr Psychiatry. 2000;41:63–69. doi: 10.1016/s0010-440x(00)90133-0. [DOI] [PubMed] [Google Scholar]
- 33.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 34.Piccinelli M, Simon G. Gender and cross-cultural differences in somatic symptoms associated with emotional distress. An international study in primary care. Psychol Med. 1997;27:433–444. doi: 10.1017/s0033291796004539. [DOI] [PubMed] [Google Scholar]
- 35.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 36.Martin LA, Neighbors HW, Griffith DM. The experience of symptoms of depression in men vs women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry. 2013;70:1100–1106. doi: 10.1001/jamapsychiatry.2013.1985. [DOI] [PubMed] [Google Scholar]
- 37.Sachar EJ, Baron M. The biology of affective disorders. Ann Rev Neurosci. 1979;2:505–517. doi: 10.1146/annurev.ne.02.030179.002445. [DOI] [PubMed] [Google Scholar]
- 38.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(Suppl 1):S26–28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 39.Johnson SA, Fournier NM, Kalynchuk LE. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav Brain Res. 2006;168:280–288. doi: 10.1016/j.bbr.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 40.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagot RC, Cates HM, Purushothaman I, Vialou V, Heller EA, Yieh L, et al. Ketamine and Imipramine Reverse Transcriptional Signatures of Susceptibility and Induce Resilience-Specific Gene Expression Profiles. Biol Psychiatry. 2017;81:285–295. doi: 10.1016/j.biopsych.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, et al. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN. Aberrant extracellular signal-regulated kinase (ERK)1/2 signalling in suicide brain: role of ERK kinase 1 (MEK1) Int J Neuropsychopharmacol. 2009;12:1337–1354. doi: 10.1017/S1461145709990575. [DOI] [PubMed] [Google Scholar]
- 44.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 45.Iñiguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolaños-Guzmán CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav Brain Res. 2010;214:460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- 47.Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, et al. Sex Differences in Nucleus Accumbens Transcriptome Profiles Associated with Susceptibility versus Resilience to Subchronic Variable Stress. J Neurosci. 2015;35:16362–16376. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Photograph of defeat cages, with wood shavings bedding, used during the vicarious social defeat stress paradigm (Ancare®, N40 Large Mouse Cage and wire top). (A) Defeat mouse cage with fitted clear plexiglass divider with 1 cm diameter perforated holes. (B) Defeat mouse cage with fitted white opaque divider without holes. (C) Photograph of squared open field arena with a white circular wire cage (Stoelting Co.® Mouse Stranger Enclosure #60452), used during the social interaction test.
Figure S2. (A) Social interaction ratio of female mice exposed to emotional/psychological stress (ES) that spent 24 h with CD1‐male aggressor (overnight), which physically defeated a c57BL/6‐male conspecific (PS). After 10 days of vicarious defeat stress (VDS), the ES‐ female group spent significantly less time in the interaction zone, when compared to controls (CON; n=11; female house‐paired with another female), 24 hours post last stress exposure. (B) Housing and chemosensory conditions do not influence baseline levels of social interaction in CON female mice (i.e., females that do not experience VDS). Specifically, no differences in time spent in the social interaction zone were evident between females CONs (house‐paired with separate female) and females housed in similar conditions but also exposed to the bedding of physically stressed (PS) male mice (n=12), or pair‐housed with an unfamiliar CD1 male mouse (n=10). Note: CON group in sections A and B are the same group (Table S1, Cohort 1). *p>0.05.
Table S1. Experimental groups.