Graphical abstract
Keywords: Histopathological studies, Biochemical studies, Moringa peregrina, Toxicological evaluation
Highlights
-
•
M. peregrina seeds, taken orally, did not show any alternation in rats’ behavioral signals and no mortality.
-
•
M. peregrina seeds controlled sugar blood level and lowered hyperlipidaemia by lowering cholesterol and triglyceride levels.
-
•
M. peregrina seeds, improved liver and kidney functions by lowering liver enzymes and serum creatinine.
-
•
No histopathological changes were detected in the body tested organs.
-
•
The intake of different doses of M. peregrina even a high one, exhibit no organ toxicity and are safe for human use.
Abstract
Moringa is multipurpose promising tree particularly for medicinal use. With its high nutritive and pharmaceutical values, every part of the tree is suitable for human consumptions. The use of vegetate parts, seeds or extracts requires toxicological evaluations to prove and verify safety uses before being added to pharmaceutical medicine, or any other products related to human diet. In this study, Moringa peregrina seeds, grown in high elevation mountain in Saint Catherin area, were investigated and evaluated for their toxicity with respect to its topological potential through histological and biochemical studies in Albino rats. Daily doses of 0, 500, 1000 and 2000 mg/kg body weight of dry seed of M. peregrina were administered orally to 4 groups of rats for 14 days. Biochemical and histopathological results were evaluated by standard methods. Measured biochemical parameters, insulin, albumin, total protein, creatinine, urea, uric acid, Follicle-stimulating hormone, Luteinizing hormone and Testosterone, revealed normal levels compared to control group. However, measured level of blood sugars, cholesterol, triglyceride and liver enzyme, displayed significant decreases. No histopathological changes were detected in the body tested organs. In consequences, intake of different doses of M. peregrina, even high one, exhibit no organ toxicity and are safe for human use.
1. Introduction
Natural products have been known as unique source for delivering many bioactive lead compounds for drug discovery. Plants, animals and microorganisms represent unlimited reservoirs for these natural products. In Particular, plants produce various metabolites that play a major role in their adaptation to certain environment. Several thousands of these compounds have been claimed to possess medicinal properties which can be used directly or as pharmaceutical agents [1]. Therefore, the plants possess specific activity; named as “herbal plants”; have been used by folk traditions for treating several diseases a century ago and represent a renewable source of natural products and chemical entities [2].
These natural products, nowadays, continue to provide a diverse and unique source of bioactive lead compounds for drug novelty especially with renewed interest for this source which considered as promising alternative medicines [3]. Additionally, there is an emerging increase in the intake of plant formulation as alternative drugs for the strong belief that these products are safe and devoid of toxic side effects [4]. Meanwhile, their acceptability, effectiveness, affordability, safety and low cost give more attention to use them as alternative medicine [5], [6]. Moringa tree, a member of family Moringaceae, is considered among these medicinal plants which have multiuse as folk and nutritive medicine [7]. It occurs in arid desert mountains. It is currently threatened in large part of its distribution range in the Middle East and the Arabian Peninsula due to the high rate of its consumption. It is native to the southern foothills of the Himalayas in northwestern India. Today, Moringa tree is widely cultivated in tropical and subtropical areas where its young seed pods and leaves are used as vegetables. There are thirteen species of Moringa are known worldwide, M.oleifera is the most distributed species beyond its origin [8]. It has already in use for several medical purposes and the evidence for its therapeutic practices have been reported by many studies [9]. The second popular M species is M. peregrina (Forssk.) Fiori., a small tree widely adapted to different harsh ecological conditions [10], [11], [12]. It geographically distributed from tropical Africa to east India. Nearly all parts of this plant species are culturally consumed by human, in its habitats, for its nutritional value and for its taste and flavor as a vegetable.
Moringa peregrina can also be used in herbal medicine for treatment of fever, headache, malaria, constipation, abdominal pains, muscle pains, hypertension, vasodilator activity, asthma, diabetes and burns in folk medicine [10]. Further pharmacological studies have shown the ability of this M. species to be used as antioxidant [13], analgesic, anti-inflammatory [14], antiulcer, anti-hyperglycemic [15], anti-hyperlipidemic, antimicrobial activities [14], [16].
In Egypt, Moringa peregrina (Forssk.) Fiori. is the only native species occurs in Egyptian habitat among the thirteen species distributed all over the world. It is characterized by a very fast growth that can reach 3–5 m in height after 10 months of plantation time [12]. M. peregrina seeds have diminutive germination time and high seedling growth rate. Trees of M. peregrina bear 20–40 cm-long seed pods, each were containing 5–15 unwinged seeds. The seed kernel has high oil content in the range of 42–54% of its weight [17]. The Egyptian Bedouin used the seeds as a medicinal plant due to its composition of oil. It also used by pregnant woman for strengthen the muscles, health condition and facilitate the fetus delivery.
Many studies have been carried out to explore the medicinal properties of Moringa species; however, the most of the studies done were on M. oleifera and no studies were carried out for testing the toxicity and medical activates of naturally occurred M. peregerina in Saint Katherine area, South Sinai, Egypt. In the meantime, using this plant by local Bedouin often look at the medicinal benefits and neglect their toxic effects which may affect various human organs. For such purpose we collected Moringa seeds from its unique habitats in Saint Katherine area and used them to evaluate their toxicity for human uses.
2. Material and methods
2.1. Collection and identification of plant material
Fresh mature seed pots of M. peregrina trees, grown on the foot-hills and draining slopes of the higher mountains in South Sinai were collected. The plant was identified and authenticated taxonomically in Botany department, Faculty of Science, Suez Canal University. The seed pods were left to dry after then the seeds were collected from opened-dry pods. The seeds were peeled off and coat peeled-seeds were used.
2.2. Experimental animals
Twenty four young adult male albino rats, weighed between 120 and 150 g, were used in this study. They were fed with regular diet and tap water ad libitum. The rodents were housed under standard laboratory environment, and allowed to acclimatize to the laboratory environment (temperature of 28 ± 2 °C with relative humidity and naturally illuminated environment of 12/12 h day light/dark cycles) for two weeks before being used in the experiment. The experiment was performed in accordance with the internationally accepted standard ethical guidelines for laboratory animal use and care as described in the European Community guidelines [18].
2.3. Animal treatments and experimental protocol
The experimental protocol, designed to evaluate toxicity of Moringa seeds, was in accordance to the guideline of Diderich [19], [20], [21]. Twenty four rats were randomly divided into four groups with six rats each. Following an overnight fasting, the rats were weighted and the doses of M. peregerina seeds were calculated in reference to their body weight (BW). The groups were as follow: the control group, which received no treatment and feed normal diet; group one (G1), group 2 (G2) and group 3 (G3) were treated with Moringa seeds at doses 500, 1000 and 2000 mg/Kg BW, respectively. The seeds were crushed, after removing the outer fibrous covers, and daily administrated orally for 14 days in their diet. The rat's observation and monitoring was made for a period of 14 days for any signs of toxicity, mortality, behavior and clinical symptoms [20], [21]. On the day14th of the running experiment, following an overnight fasting, all animals in designed groups were anesthetized under chloroform vapor and blood samples were collected by cardiac puncture [22] and injected into non-heparin's bottles for biochemical investigations. Collected blood samples were allowed to clot and centrifuged according to groups; and serum was separated for the biochemical analyses. The treated groups and control group were dissected and body organs (liver, kidneys, pancreases, spleen and testes) were excised and cleaned immediately from blood by rinsing with physiological saline and then fixed in 10% buffered formalin for histopathological examination.
2.4. Biochemical analyses
Different biochemical tests were carried out using diagnostic kits to evaluate the toxicity of different doses of Moringa seeds and their effects on body function. Fasting blood glucose, expressed in mmol/L, was determined by (GOD/POD method) [23]. Serum insulin, expressed in ng/mL, was estimated by a radioimmunoassay [24]. Serum total cholesterol and triglycerides were measured colorimetric [25]. Liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) [26]; serum total protein concentration [27]. Serum Albumin was determined according to the method of Doumas, Watson [28]. Blood urea nitrogen, expressed in mg/dL, was assessed based on the cleavage of urea with urease according to the method of Karr [29]. Serum creatinine level (mg/dl) was determined colorimetric following the method of Heinegård and Tiderström [30]. Uric acid levels, expressed in mg/dL, was measured following the method of Kageyama [31]; Follicle-stimulating hormone (FSH), Luteinizing Hormone (LH) and testosterone were also measured according to manufacturer’s structure of Midgley [32]; Niswender, Midgley Jr [33] and Vermeulen, Verdonck [34], respectively.
2.5. Histopathological examination
Selected organs including pancreas, spleen, liver, kidneys, and testes of treated and control rats were fixed in 10% buffered formalin in labeled bottles [35], and processed for histological examination [36]. Tissues embedded in paraffin wax were sectioned 5 mm thick, stained with Haematoxylin and Eosin, mounted on glass slides and examined under a standard light microscope. Light microscopic examinations of multiple tissue sections, from each organ in all groups, were performed and images representative of the typical histological profile were examined.
2.6. Statistical analysis
All data obtained, expressed in mean ± standard error (SE), were statistically analyzed by the one-way ANOVA. The significance of the difference versus the control group was determined by Student’s t-test. For ranking the data, Duncan Multiple Range test was applied. Values were considered statistically significant at p ≤ 0.05. The Statistical Package for Social Sciences (SPSS) program, version 20, was used to run the statistical analyses.
3. Result
In the oral toxicity assay, all treated groups of rats at different doses of M. peregrina seeds (500, 1000 and 2000 mg/kg body weight) did not show any alternation in behavioral signals with no mortality during the time period of the study.
3.1. Biochemical studies
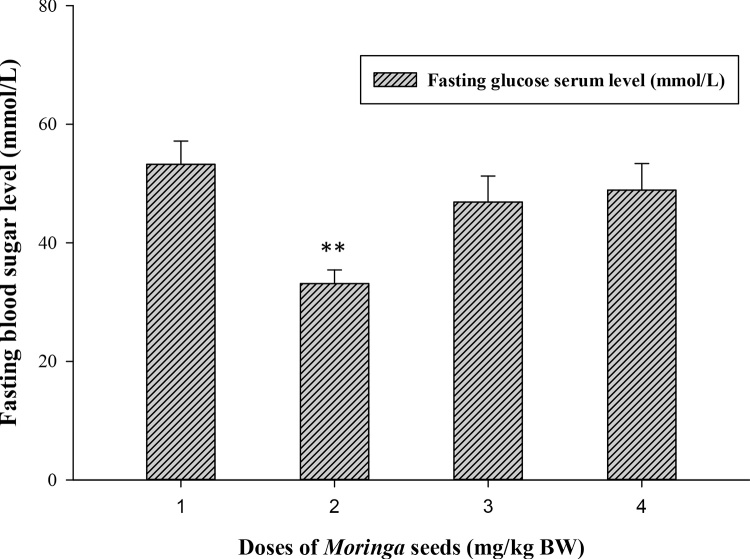
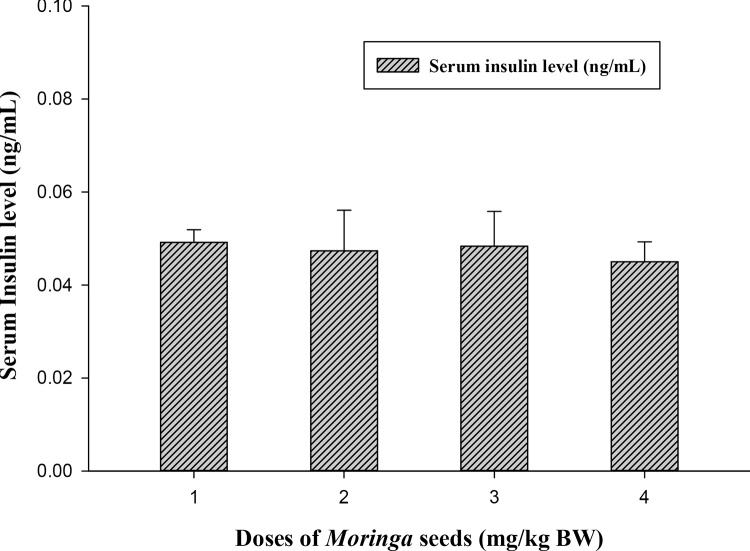
The biochemical results obtained from the oral Moringa-treated rats showed the safety use of M. peregrina seeds at all tested doses (500, 1000 and 2000 mg/kg body weight) when compared to control. For fasting serum glucose level, all Moringa-treated groups able to lower the level of blood sugar of the experimental rats (Fig. 1) and at dose 500 (mg/kg BW) highly significant decreases (33.121 ± 2.31 vs. 53.25 ± 3.92, p ≤ 0.01) was recorded when compared to control group. On the contrary, M. peregrina seeds did not significantly (p ≤ 0.05) alter the level of insulin among treated and untreated groups (Fig. 2). Although there was no significant differences among treated and control groups, Moringa dose at 2000 mg/kg BW showed the lowest level of insulin compared to control group (0.045 vs. 0.049 ng/mL; respectively).
Fig. 1.
Effect of Moringa peregrina seeds on fasting blood sugar levels (FBS, mmol/L) compared to control group. Doses of Moringa seeds (mg/kg body weight) used: 1, control, no treatment; 2, 500; 3, 1000 and 4, 2000.**p ≤ 0.01, vs. control group; n = 6 in each group; values are in mean ± SE.
Fig. 2.
Effect of Moringa peregrina seeds on serum insulin levels (ng/mL) compared to control group. Doses of Moringa seeds (mg/kg body weight) used: 1, control; 2, 500; 3, 1000 and 4, 2000. n = 6 in each group; values are in mean ± SE.
Cholesterol and triglyceride showed different concentration patterns in different Moringa-treated rats where cholesterol, at Moringa dose 1000 mg/kg body weight, recorded the highest ability for lowering the cholesterol (131.72 ± 8.64) at significant level of p ≤ 0.01 (Table 1). However, Triglyceride level reported high significant (p ≤ 0.01) decline at all Moringa doses and reached its lowest value at dose 500 mg/kg BW (78.68 ± 21.36) followed by Moringa doses at 1000 and 2000 (mg/kg BW) in sequence (Table 1).
Table 1.
Biochemical measured parameters of Cholesterol, Triglyceride, liver enzymes (ALT and AST), Albumin and Total protein concentrations in blood serum of Albino rats orally administered by Moringa seeds at different doses, 500, 1000 and 2000 mg/kg body weight, compared to untreated (Control) group. Measured parameters are in mean (±SE).
| Treatment | Measured parameters (Mean ± SE)* |
||||||
|---|---|---|---|---|---|---|---|
| (mg/kg BW) | Cholesterol (mg/dL) | Triglyceride (mg/dL) | ALT (IU/L) | AST (IU/L) | AST/ALT | Albumin (g/dL) | Total Protein (g/dL) |
| Control | 191.26 ± 8.81 c | 143.97 ± 12.93b | 70.21 ± 6.36 b | 174.18 ± 8.82 b | 2.5/1 | 3.29 ± 0.33 | 3.89 ± 0.48 |
| 500 | 164.39 ± 3.11 b | 78.68 ± 8.72 a | 24.19 ± 4.16 a | 152.37 ± 7.06ab | 6.0/1 | 3.83 ± 0.14 | 4.13 ± 0.36 |
| 1000 | 131.72 ± 8.64 a | 91.76 ± 8.65 a | 39.33 ± 2.65 a | 145.80 ± 3.11 a | 3.7/1 | 4.11 ± 0.41 | 4.84 ± 0.64 |
| 2000 | 153.45 ± 2.78 ab | 93.70 ± 8.07 a | 33.96 ±2.92 a | 145.43 ± 6.23 a | 4.3/1 | 3.57 ± 0.17 | 3.98 ± 0.24 |
Means with the same letter, per each column, are not significantly different according to Duncan’s multiple test, p ≤ 0.05, n = 6.
The result of liver enzymes revealed the significant (p ≤ 0.001) effect of Moringa seeds on lowering the level of Alanine aminotransferase enzyme (ALT) and Aspartate aminotransferase (AST) compared to the control group (Table 1). The reduction of ALT was significantly pronounced at dose 500 mg/kg BW and recorded 24.19 ± 4.16 (IU/L). The higher doses of M. seeds were also significantly decreased the ALT level in serum by 0.7 and 1.72 time less than control group for 1000 and 2000 mg/kg BW, respectively (Table 1). Moringa seed-supplement efficiently reduced the AST level to 145.43 ± 6.23 U/L (p ≤ 0.001) compared to untreated control (174.18 ± 8.82 U/L). AST/ALT ratio showed normal level and not exceeded the upper normal limits (Table 1).
Urea, uric acid and creatinine levels in serum, expressed as mg/dL, were clearly demonstrated the positive influence of Moringa seeds on treated rats where there were decreases in their levels compared to control group. However, no significant differences were recorded among groups for each test (p ≤ 0.193, 0.153 and 0.154, respectively, Table 2). Tested rats administered different doses of Moringa seeds also showed a nonsignificant increase in serum total protein level when compared to that of the control group (4.13 ± 0.36, 4.84 ± 0.64, 3.98 ± 0.24 and 3.98 ± 0.24 for 500, 1000 and 2000 mg/kg vs. 3.89 ± 0.48, respectively). Similarly, the effect of M. seeds on serum albumin revealed a positive influence on increasing the level of serum albumin to 4.11 ± 0.41 vs. 3.29 ± 0.33 for rate administrated Moringa seeds at dose 1000 mg/kg BW and untreated group respectively. However, no significant difference was detected among treated and untreated-control groups (Table 2).
Table 2.
Biochemical measured parameters of Urea, Uric acid and creatinineconcentrations in blood serum of Albino rats orally administered by Moringa seeds at different doses, 500, 1000 and 2000 mg/kg body weight, comparedto untreated control group. Measured parameters are in means (±SE).
| Treatment | Measured parameters (Mean ± SE) |
||
|---|---|---|---|
| Urea (mg/dL) | Uric acid (mg/dL) | creatinine (μmol/L) | |
| Control | 90.54 ± 15.10 | 2.53 ± 0.62 | 0.88 ± 0.19 |
| 500 | 70.08 ± 7.83 | 1.55 ± 0.45 | 0.76 ± 0.18 |
| 1000 | 84.79 ± 4.36 | 1.64 ± 0.84 | 0.46 ± 0.12 |
| 2000 | 89.83 ± 10.83 | 1.64 ± 0.41 | 0.43 ± 0.13 |
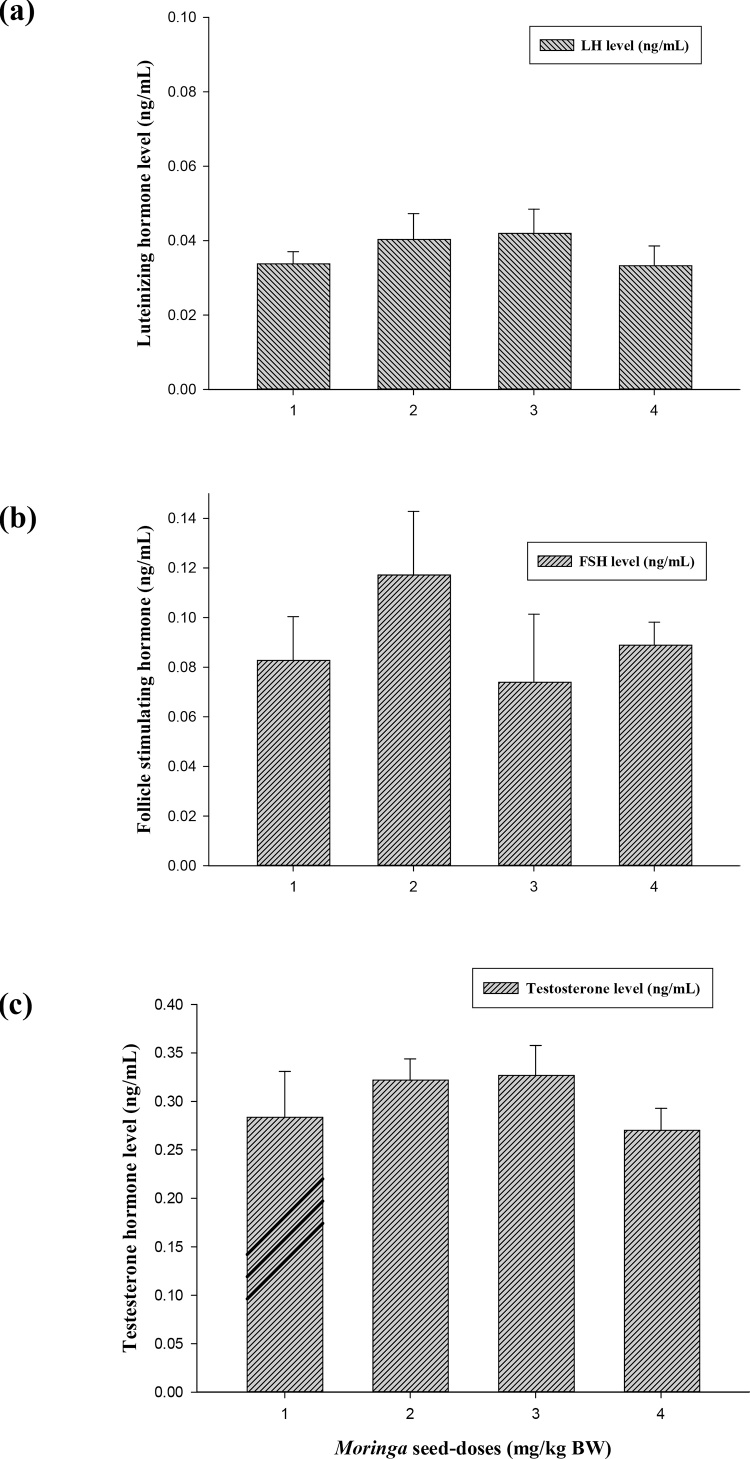
The effect of Moringa seeds on male sex hormones is shown in Fig. 3(a–c). The administration of M. seeds enhanced the levels of LH, FSH and Testosterone hormones compared to control group. The increments in hormonal levels were not significantly different at p ≤ 0.05. In addition, it was noticeable that the effect of M. seeds was higher in all hormones detected at does 1000 mg/kg BW, where it recorded 0.042, 0.12 and 0.33 for LH, FSH and Testosterone, respectively.
Fig. 3.
Effect of Moringa peregrina seeds on serum sex hormones (ng/mL) of LH (a), FSH (b) and Testosterone (c). Doses of Moringa seeds (mg/kg body weight) used: 1, control; 2, 500; 3, 1000 and 4, 2000. n = 6 in each group; values are in mean ± SE.
3.2. Histopathological findings
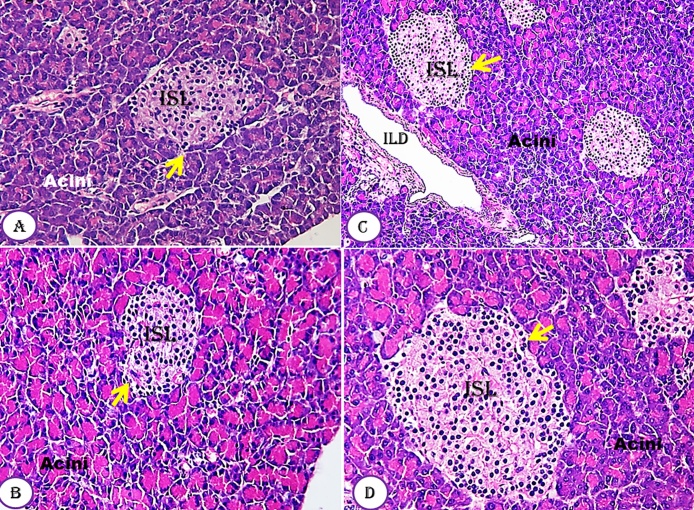
The histology of the pancreas of untreated and treated groups with Moringa seeds at all doses used (Fig. 4A–D) revealed the normal architecture of the pancreas cells which include prominent Langerhans islets, acini cells and ducts. The pancreas showed profuse islet of Langerhans interspersed within the pancreatic exocrine acini. The islets appeared lightly stained than the surrounding acini cells, with intact normal interlobular connective tissue and interlobular duct. Each endocrine islet consisted of lightly stained polygonal cells arranged in cords separated by a network of blood capillaries. However, the exocrine acini cells were characterized by their basal basophile and apical acidophilic cells. No area of cell necrosis was observed.
Fig. 4.
Effect of Moringa peregrina seeds on pancreas tissue architecture compared to control group. A-D, TS in pancreas tissue showing normal lobular architecture, Islets of Langerhans (ISL, yellow arrows) surrounded by the pancreatic acini and duct. The pancreatic acini clearly showed normal basal basophilic and apical acidophilic cells with no cell boundary. 100 X, for A–C; 200 X for D. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
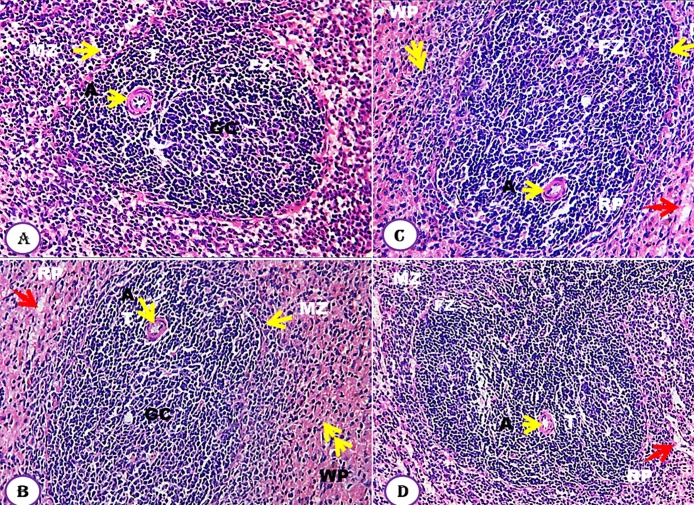
The effect of Moringa seeds, at different doses, on spleen compared to untreated control group showed normal spleen tissues (Fig. 5A–D). The tissues are clearly differentiated into white pulps (WP) and the red pulps (RP). The architecture of the white pulps displayed normal rounded scattered follicles with an arteriole on one side called central arterioles on which cells are arranged around the arteriole and classified into four zones: Thymus dependent zone which contains lymphocyte cells around the arteriole; Germinal center is second zone and appeared as lightly-stained areas in the center of secondary lymphoid follicles; Follicular zone is third zone that consists of B-Lymphocytes and Marginal zones, which exist at the periphery, is the fourth zone which was easily distinguished (Fig. 5).
Fig. 5.
Effect of Moringa peregrina seeds on spleen histology compared to control group. A-D, TS in spleen tissue showing normal tissue architecture, white pulp (WP, double yellow arrows); red pulp (RP, red arrow); zones of white pulp are normally represented, germinal central (GC), arteriole (A, arrow head), follicle zone (FZ) and marginal zone (MZ). No abnormalities in these tissues were observed. A, control group; B, group administered 500 mg/kg BW; C, group administered 1000 mg/kg BW and D, group administered 200 mg/kg BW. 100X. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
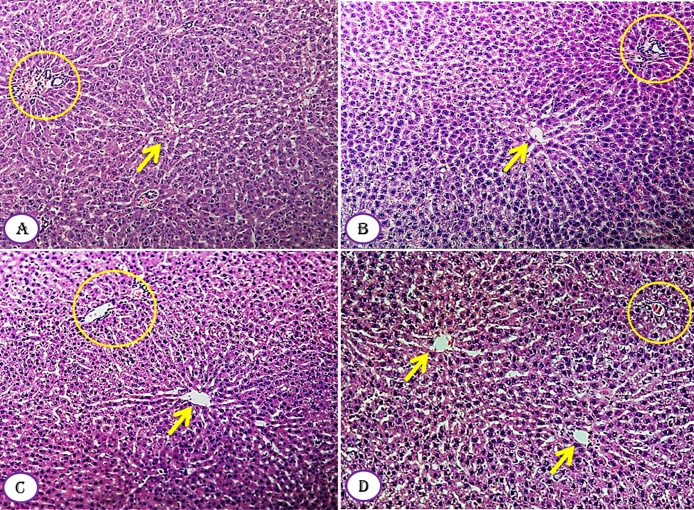
The results of histopathological examination of the liver of control and Moringa-treated rats showed no hepatic inflammation, degeneration, vacuolization and necrosis (Fig. 6A–D). The microscopically examined liver tissue also revealed the presence of normal hepatic parenchyma with the plates of hepatocytes being separated by sinusoids. The central vein was observable and had normal appearance (Fig. 6). The portal triad, artery, vein and bile duct branches were also seen and showed normal tissue morphology. In general, for all treatment does of M. seeds compared to control group showed that the liver tissues, as a whole, are healthy and have normal microscopically structural appearance (Fig. 6).
Fig. 6.
Effect of Moringa peregrina seeds on liver tissue architecture compared to control group. A–D, TS in classic hepatic lobule, showing normal architecture of central vein (arrow) with hepatocytes arranged in plates or cords radiating from central vein and separated by blood sinusoids, each cord formed of two or more rows of hepatocytes inclosing between them bile canaliuli. Portal vein, hepatic artery and bile duct (liver triad), represented in yellow circle, are observed between hepatic lobules. A, control group; B, group administered 500 mg/kg BW; C, group administered 1000 mg/kg BW and D, group administered 200 mg/kg BW. 100X. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
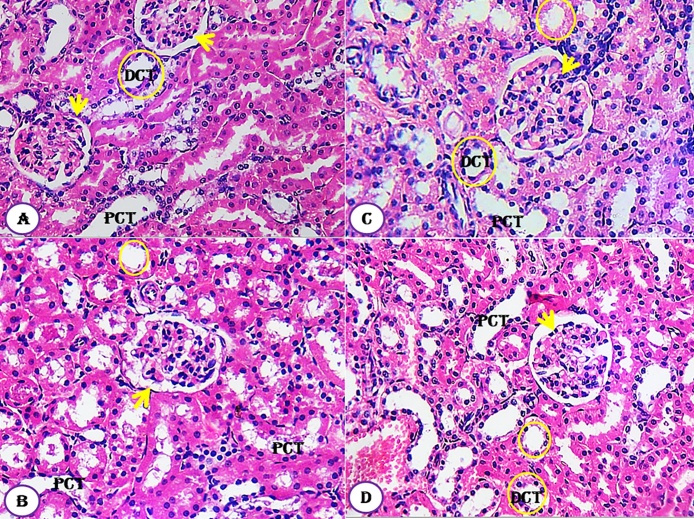
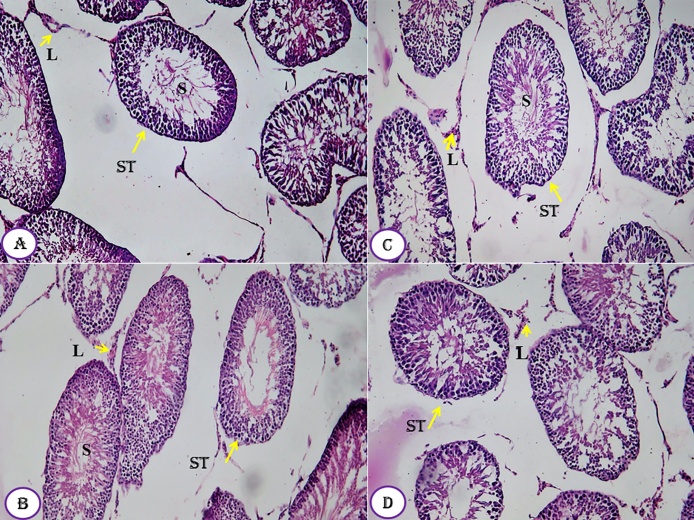
The kidney tissues of all groups, designed in this study, showed normal healthy feature where the renal histological structures of renal parenchyma are being illustrated histologically as represented in the photomicrographs (Fig. 7A–D). The cortical structures especially the glomerulus as a whole reflected quite well defined constituting elements. The neighboring renal tubules, both proximal and distal convoluted, were clearly observable and have normal architecture (Fig. 7). Similarly, the histological architectures of testicular tissues of the control and treated animals showed normal structure (Fig. 8A–D). The seminiferous tubules were well defined and the interstitial tissues were also defined. Similarly, the seminiferous tubule epithelium was well distinct and had a normal histoarchitecture. Differential stages for spermatogenesis development were clearly observed (Fig. 8). In meantime, spermatozoa in the lumen of seminiferous tubules showed increment in number compared to control group.
Fig. 7.
Effect of Moringa peregrina seeds on kidney histological architecture compared to control group. A-D, T.S. of kidney, showing normal architecture of glomerulus (arrow); proximal convoluted tubule(PCT) and distal convoluted tubule (DCT, yellow circle). A, control group; B, group administered 500 mg/kg BW; C, group administered 1000 mg/kg BW and D, group administered 200 mg/kg BW. 100X. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 8.
Effect of Moringa peregrina seeds, at different doses, on tests histology compared to control group, A-D, T.S. of tests, showing normal seminiferous tubules (ST) in between there are leydg cells (L). Sertoli cells and spermatogenic cells, laying inside the seminiferous tubules, are also observed. A, control group; B, group administered 500 mg/kg BW; C, group administered 1000 mg/kg BW and D, group administered 200 mg/kg BW. 100X.
4. Discussion
The uses of herbal plants as a source of folk medicines in primary health care have become popular globally as a safer drug because of their natural source. Moringa species have been widely uses for their nutritional and medical values in different countries. In mean time, Egyptian Moringa peregrina, naturally occurred in Sinai Peninsula, is in use by local Bedouin, and nowadays it is used on a large scale but there is a lack of proven scientific studies on their toxicity and adverse effect on local and large scale. Therefore, the present study aimed to evaluate the toxicity of Moringa peregrina seeds in low and high doses that could be used for further pharmaceutical studies. Based on the results obtained there was no negative effects of Moringa seeds, daily administrated orally, for all treated groups of rats at different doses (500, 1000 and 2000 mg/kg body weight) were observable. After an observation period of 2 weeks following seed administration, no mortality or any alternations in appearance or behavioral signals occurred during the time period of the study. These results proved the safety use of Moringa seeds orally. Our results are in agreement with study done by kahilo, Kamal [37] who found that no changes in animal behavior in the male rats received orally M. peregrina-oil at dose 3000 mg/kg BW.
Based on the biochemical assays, our results confirmed that administration of M. seeds have safety beneficial uses where serum blood sugar levels exhibited significant lower level (p ≤ 0.01), at all doses used, compared to control group. These result was aligned with nonsignificant slight increase in serum insulin, for treated groups, which may considered as sensitive monitoring for blood sugar compared to control group. This suggestion corroborates the histological report of the pancreas which showed normal architecture and no damage to islets of Langerhans. The results further showed significant decrease in cholesterol and triglyceride which endorse the beneficial effect of M. seeds even at high doses. When M. seeds at different doses, in particular 1000 mg/kg BW, was administered to normal rats, anti-hyperglycemic and anti-hyperlipidaemic response were seen compared to control levels. These effects of various doses of M. seeds are credited to their ability to restore the function of pancreatic tissues by causing an increase in the insulin output [38] and/or by inhibiting the intestinal absorption of glucose or by the facilitation of metabolites in insulin-dependent processes [39], [40].
In reference to data obtained, Moringa peregrina seeds did not produce any deleterious effects on the spleen tissue; there however may lead to an improvement in the structural architecture that may be coded or transformed into improvement on physiological functioning of the tissue. This suggestion could be confirmed due the important role of spleen tissue in body immunity [41]. Similarly, liver enzymes and its structure proved the positive effect of M. seeds at all doses where there were significant decrease in ALT and AST when compared to control group. Low dose of M. seeds (500 mg/kg BW) was more pronounced for lowering the ALT, vice versa for AST where higher doses (1000 and 2000 mg/kg BW) recorded the lowest level when compared to control group. These results reflect the normal liver architecture.
For kidney function,Moringa seed-supplements maintain healthy condition for kidney tissues where there were nonsignificant decrease in the urea, uric acid, creatinine and nonsignificant increase total protein and Albumin levels in serum of M. groups compared to control group. In addition, rat treated with all the doses of M. peregrina did not show any morphological changes in the kidney cells. Our data recorded is in agreement to kahilo, Kamal [37] who found that normal liver and kidney histological structure to rats received Moringa peregrina seeds oil (3000 mg/kg BW).
The effect of M. seeds in enhancing male fertility is clearly manifested in all the treated M. groups compared with the control. However, rats administered with medium doses 1000 mg/kg BW of the plant seeds showed the highest nonsignificant increase in FSH, LH and testosterone levels. The normal of histoarchitectural of testes tissues was the clear indication of confirming the spermatogenic efficacy of M. peregrina seeds in male albino rats. The process of spermatogenesis and accessory reproductive organ function are hormonal dependent. FSH in male rats are crucial for the establishment of Sertoli cell population, which is directly related to sperm production [42], [43]. However, LH stimulates spermatogenesis indirectly via stimulation of Leydig cells to secrete testosterone, which acts also on Sertoli cells [44]. M. peregrina seeds were able to induce Spermatozoa density in the lumen of seminiferous tubules of rats testes. The observed increment may reflect the possible antioxidant effect of M. seeds [13], [37]. Moreover, the chemical constituent of M. seeds contain polyunsaturated fatty acids [45], [46] that may also play an important role in increasing the fertility and reproduction [47], [48].
The above mentioned results of the current study on the biochemical analyses (AST, urea, creatinine, urea, uric acid, albumin, total protein, FSH, LH and testosterone) and the histopathological studies of (pancreas, spleen, liver, kidney and testes) may therefore suggest that M. peregrina seeds are safe in use for food grade substance in the rats with no deleterious effect. Meanwhile, it is suggested to be used as alternative natural drug (herbal medicine) for lowering blood sugar (anti-hyperglycemic) and triglyceride and cholesterol (anti-hyperlipidaemic).
5. Conclusion
In summary, based on the biochemical measured parameters and histological observations evaluated on this study, we conclude that Egyptian M. peregrina seeds have a broad safety potential for therapeutic use, since no mortality or any sign of toxicity observed. Therefore, it can be used on large scale without fear of its side effects. Also, from the current investigation we suggested that taken Egyptian M. peregrina seeds in meals will help to control hyperglycaemia and hyperlipidaemia for people suffering of high risk to diabetes and high blood pressure. The above mentioned results represented in this study will benefit our future studies of M. peregrina seeds, as they will be used as an alternative dose in treatment and control liver enzymes and kidney damage. Dose declaration above the doses used, in our study, should have further studies and be incorporated into regulatory guidance to have optimal benefits as natural drug.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgments
The authors express their thanks to El-Sayed, animal house keeper for his great help during time course of the experiment.
Contributor Information
Heba N.Gad EL-Hak, Email: heba_nageh@hotmail.com.
Abdel Raouf A. Moustafa, Email: raoufmoustafa2@hotmail.com.
Samira R. Mansour, Email: samirarmansour@hotmail.com, samirarmansour@yahoo.com.
References
- 1.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.-M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., Rollinger J.M., Schuster D., Breuss J.M., Bochkov V., Mihovilovic M.D., Kopp B., Bauer R., Dirsch V.M., Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotech. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules (Basel, Switzerland) 2016;21 doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veeresham C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Tech. Res. 2012;3:200–201. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olaniyan J.M., Muhammad H.L., Makun H.A., Busari M.B., Abdullah A.S. Acute and sub-acute toxicity studies of aqueous and methanol extracts of Nelsonia campestris in rats. J. Acute Dis. 2016;5:62–70. [Google Scholar]
- 5.Baker B. 2017. Alternative Medicine, CQ Researcher; pp. 741–764. [Google Scholar]
- 6.Pandey S. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid.-Based Compl. and Altern. Med. 2013;2013:12. doi: 10.1155/2013/376327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saini R.K., Sivanesan I., Keum Y.-S. Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6:203. doi: 10.1007/s13205-016-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bisby F.A., Roskov Y., Orrell T., Nicolson D., Paglinawan L., Bailly N., Kirk P., Bourgoin T., Baillargeon G., Ouvrard D. Species 2000 & ITIS catalogue of life. digital resource. Digital Resour. 2010;2010(July) [Google Scholar]
- 9.Fahey J.W. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. part 1. Trees J. 2005;1 [Google Scholar]
- 10.Asghari G., Palizban A., Bakhshaei B. Quantitative analysis of the nutritional components in leaves and seeds of the Persian Moringa peregrina (Forssk.) Fiori. Pharmacogn. Res. 2015;7:242–248. doi: 10.4103/0974-8490.157968. (PMid:26130935 PMCid:PMC4471650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moustafa A.A., Zaghloul M.S., Dadamouny M.A. Egypt, Lamber; German: 2013. Population Ecology of Moringa Peregrina Growing in Southern Sinai. [Google Scholar]
- 12.Moustafa A.A., Zaghloul M.S., Dadamouny M.A. 2013. Population Ecology of Moringa Peregrina Growing in Southern Sinai. (Egypt) [Google Scholar]
- 13.Dehshahri S., Wink M., Afsharypuor S., Asghari G., Mohagheghzadeh A. Antioxidant activity of methanolic leaf extract of Moringa peregrina (Forssk.) Fiori. Res. Pharmac. Sci. 2012;7:111–118. [PMC free article] [PubMed] [Google Scholar]
- 14.Koheil M.A., Hussein M.A., Othman S.M., El-Haddad A. Anti-inflammatory and antioxidant activities of Moringa peregrina Seeds. Free Radicals Antioxid. 2011;1:49–61. [Google Scholar]
- 15.El-Alfy T.S., Ezzat S.M., Hegazy A.K., Amer A.M.M., Kamel G.M. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori. (family: moringaceae) growing in Egypt. Pharmacogn. Mag. 2011;7:109–115. doi: 10.4103/0973-1296.80667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majali I., Althunibat O., Qaralleh H. Antimicrobial and immunomodulatory activities of Moringa peregrine-MINIREVIEW. J. Bas. and Appl. Res. 2015;1(2):55–61. [Google Scholar]
- 17.Afsharypuor S., Asghari G., Mohagheghzadeh A., Dehshahri S. Volatile constituents of the seed kernel and leaf of Moringa peregrina (Forssk.) Fiori, Agricolt. Cultivated in Chabahar (Iran) Iran. J. Pharm. Sci. 2010;6:141–144. [Google Scholar]
- 18.Taylor K., Gordon N., Langley G., Higgins W. 2008. Estimates for Worldwide Laboratory Animal Use in 2005. [DOI] [PubMed] [Google Scholar]
- 19.Diderich R. The OECD chemicals programme, in: risk assessment of chemicals. Springer. 2007:623–638. [Google Scholar]
- 20.Bhattacharya R., Gujar N., Singh P., Rao P., Vijayaraghavan R. Toxicity of alpha-ketoglutarate following 14-days repeated oral administration in Wistar rats. Cell. Mol. Biol. (Noisy-le-Grand, France) 2011;543(Suppl):Ol1543–Ol1549. [PubMed] [Google Scholar]
- 21.Asare G.A., Gyan B., Bugyei K., Adjei S., Maham R., Otu-Nayarko L., Addo P., Wiredu E.K., Nyarko A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012;139(139):265–272. doi: 10.1016/j.jep.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Dacie J., Lewis S. ELBS with Churchill Livingstone. 7th edition. Longman group UK; 1991. Practical haematology. [Google Scholar]
- 23.Hönes J., Müller P., Surridge N. The technology behind glucose meters: test strips. Diabetes Technol. Ther. 2008;10:S-10–S-26. [Google Scholar]
- 24.Nakagawa S., Nakayama H., Sasaki T., Yoshino K., Yu Y.Y., Shinozaki K., Aoki S., Mashimo K. A simple method for the determination of serum free insulin levels in insulin-treated patients. Diabetes. 1973:590–600. doi: 10.2337/diab.22.8.590. [DOI] [PubMed] [Google Scholar]
- 25.Fossati P., Principe L. Serum tryglicerides determined colorymetricaly with an enzyme of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1982;28:2077–2080. [Google Scholar]
- 26.Huang X.-J., Choi Y.-K., Im H.-S., Yarimaga O., Yoon E., Kim H.-S. Aspartate aminotransferase (AST/GOT) and alanine aminotransferase (ALT/GPT) detection techniques. Sensors. 2006;6:756–782. [Google Scholar]
- 27.Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. [PubMed] [Google Scholar]
- 28.Doumas B.T., Watson W.A., Biggs H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 29.Karr W.G. A method for the determination of blood urea nitrogen. J. Lab. Clin. Med. 1924;9:329–333. [Google Scholar]
- 30.Heinegård D., Tiderström G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta. 1973;43:305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- 31.Kageyama N. A direct colorimetric determination of uric acid in serum and urine with uricase-catalase system. Clin. Chim. Acta. 1971;31:421–426. doi: 10.1016/0009-8981(71)90413-x. [DOI] [PubMed] [Google Scholar]
- 32.Midgley A.R. Radioimmunoassay for human follicle-stimulating hormone. J. Clin. Endocrinol. Metab. 1967;27:295–299. doi: 10.1210/jcem-27-2-295. [DOI] [PubMed] [Google Scholar]
- 33.Niswender G.D., Midgley A.R., Jr, Monroe S.E., Reichert L.E., Jr Radioimmunoassay for rat luteinizing hormone with antiovine LH serum and ovine LH-131I. Proc. Soc. Exp. Biol. Med. 1968;128:807–811. doi: 10.3181/00379727-128-33129. [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen A., Verdonck L., Kaufman J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J. Clinic Endocrin. Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 35.Fox C.H., Johnson F.B., Whiting J., Roller P.P. Formaldehyde fixation. J. Histochem. Cytochem. 1985;33:845–853. doi: 10.1177/33.8.3894502. [DOI] [PubMed] [Google Scholar]
- 36.Troyer D. Biorepository standards and protocols for collecting, processing, and storing human tissues. Tiss. Proteom. 2008;19:3–22. doi: 10.1007/978-1-60327-047-2_13. (0) [DOI] [PubMed] [Google Scholar]
- 37.kahilo K.A., Kamal T., Elsayed N., Shukry M., Dishesh D., Abdalla Hussein M. Fatty acid composition and acute toxicity study on moringa peregrina fixed oil in albino rats intern. J. Pharma Sci. 2015;5:1282–1288. [Google Scholar]
- 38.Floyd J.C., Jr., Fajans S.S., Knope R.F., Conn J.W. Evidence that insulin release is the mechanism for experimentally induced leucine hypoglycemia in man. J. Clin. Invest. 1963;42:1714. doi: 10.1172/JCI104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia W., Gao W.-Y., Xiao P.-G. Antidiabetic drugs of plant origin used in China: compositions, pharmacology, and hypoglycemic mechanisms. Zhongguo Zhong Yao Za Zhi=Zhongguo Zhongyao Zazhi=China J Chinese Materia Medica. 2003;28:108–113. [PubMed] [Google Scholar]
- 40.Malviya N., Jain S., Malviya S. Antidiabetic potential of medicinal plants. Acta Pol. Pharm. 2010;67:113–118. [PubMed] [Google Scholar]
- 41.Shaw A.C., Joshi S., Greenwood H., Panda A., Lord J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orth J.M. FSH-induced Sertoli cell proliferation in the developing rat is modified by β-endorphin produced in the testis. Endocrinology. 1986;119:1876–1878. doi: 10.1210/endo-119-4-1876. [DOI] [PubMed] [Google Scholar]
- 43.Plant T.M., Marshall G.R. The functional significance of FSH in spermatogenesis and the control of its secretion in male primates. Endocr. Rev. 2001;22:764–786. doi: 10.1210/edrv.22.6.0446. [DOI] [PubMed] [Google Scholar]
- 44.Babu S.R., Sadhnani M., Swarna M., Padmavathi P., Reddy P. Evaluation of FSH, LH and testosterone levels in different subgroups of infertile males. Ind. J. Clin. Bioch. 2004;19:45–49. doi: 10.1007/BF02872388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Somali M., Bajneid M., Al-Fhaimani S. Chemical composition and characteristics of Moringa peregrina seeds and seeds oil. J. Am. Oil Chem. Soc. 1984;61:85–86. [Google Scholar]
- 46.Tsaknis J. Characterisation of Moringa peregrina Arabia seed oil. Grasas Aceites. 1998;49:170–176. [Google Scholar]
- 47.Santos J., Bilby T., Thatcher W., Staples C., Silvestre F. Long chain fatty acids of diet as factors influencing reproduction in cattle. Reprod. Domest. Anim. 2008;43:23–30. doi: 10.1111/j.1439-0531.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 48.Wathes D.C., Abayasekara D.R.E., Aitken R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]