Summary
Compliance with awakening salivary sampling is important for precise measurement of the diurnal cortisol profile. During childhood and adolescence, developmental factors influence sampling upon awakening (awake0) due to school routine, sleep/wake patterns, and age related cortisol changes. In the present study, children and adolescents’ sampling compliance of awakening cortisol was evaluated using accelerometry. Children and adolescents (N = 201; 45.3% female; 8–18 years; Mage = 12.68 years, SD = 2.03) participating in the Healthy Heart Project collected saliva samples, wore a tri-axle accelerometer, and completed demographic questionnaires. Intra-class correlations derived to examine awake0 sampling compliance indicated children and adolescents were highly compliant (ICC = .98). In children, a delay in awake0 sampling was associated with a steeper diurnal slope (β = −.23, p = .037) and greater awake0 cortisol (β = .24, p = .024); this was not observed in adolescents. In summary, children and adolescents are compliant with awakening salivary sampling. Sampling delay, particularly in children, and time of awakening influenced measures of the diurnal cortisol profile. These findings inform future studies assessing the diurnal cortisol profile in children and adolescents.
Keywords: Cortisol, Compliance, Children, Adolescents, HPA axis, Methodology
1. Introduction
Compliance with salivary cortisol sampling is a requirement for the valid assessment of the diurnal cortisol profile. Cortisol levels change rapidly in the morning as part of the awakening response, when cortisol increases quickly, peaking approximately 30 min after wake-time (Young et al., 2004; Fries et al., 2009). To accurately capture the cortisol awakening response (CAR) individuals must be compliant with saliva sampling, which includes collecting a sample immediately upon waking (awake0 sample). Compliance with this initial sample is also important for other measures of the diurnal cortisol profile (e.g., diurnal slope), as they too use the awake0 sample in their calculation (Adam and Kumari, 2009; Rotenberg et al., 2012). To date, most research has exclusively focused on verifying self-reported sampling time with an objective measure of time, such as an electronic monitor that date- and time-stamps bottle opening and presumed time of saliva collection (e.g., MEMS Cap; Kudielka et al., 2003; Broderick et al., 2004). Although this research demonstrates that most adults report collecting their awake0 sample within 10 min of the time reported by the electronic monitor (Broderick et al., 2004), this method does not verify sampling compliance against actual wake-time. Electronic monitors record when the sample was taken, but cannot indicate if there was a delay between wake-time and collection of the awake0 sample (Clow et al., 2004; Dockray et al., 2008).
Emerging technology has allowed researchers to examine whether the awakening sample (awake0) is taken at wake-time. Adults’ compliance with sampling upon awakening has been investigated using accelerometry technology to detect physical movement and postural changes (Kupper et al., 2005; Dockray et al., 2008; DeSantis et al., 2010; Griefahn and Robens, 2011). Postural change from lying down (supine) to sitting up in bed or standing is deemed a valid proxy for waking in the sleep literature (Sadeh, 2011; Zeiders et al., 2011; Anders et al., 2012). In these adult accelerometry studies, only 15–19% of awake0 samples were taken without delay, whereas 82–90% were taken within 15 min of wake-time (Dockray et al., 2008; DeSantis et al., 2010). Even this short delay can be problematic as later awake0 samples result in blunted CAR and steeper diurnal slope (Kupper et al., 2005; Dockray et al., 2008; Okun et al., 2010; Griefahn and Robens, 2011). Further, Dockray and colleagues (2008) found that when there was a delay of more than 15 min between wake-time and collecting the awake0 sample, estimates of CAR were lower than when there was delay of less than 15 min. These objective, accelerometry-based findings in adults suggest there may be an acceptable period in which the awake0 sample can be collected (i.e., within 15 min) to yield reliable estimates of CAR.
There is a lack of research examining awake0 sampling compliance in children and adolescents. Adult findings cannot be generalized to children and adolescents due to several developmental factors that influence the diurnal cortisol profile (Rotenberg et al., 2012). Developmental factors during childhood and adolescence influence awake0 sampling (e.g., school routine, changes to sleep/wake pattern; Jessop and Turner-Cobb, 2008) as well as the cortisol response. First, when school is in-session, children and adolescents typically have a regimented morning routine (i.e., wake up, brush teeth, get dressed, eat breakfast) that ensures they catch the bus and arrive at school on time. For many, this routine occurs under pressured time constraints, which can limit their ability to accurately collect the awake0 sample immediately upon waking. In contrast to adults, this morning routine is usually less internalized and self-governed. Second, night-time sleep duration decreases and morning drowsiness increases across childhood and adolescence (Carskadon, 1990; Sadeh et al., 2000; Fallone et al., 2002; Liu et al., 2005). Fewer changes in sleep habits are observed among adults. Feeling drowsy and less alert in the morning may contribute to children and adolescents forgetting to take the sample or less precision in the collection of their awake0 sample. Relatedly, shorter sleep duration is associated with higher awake0 cortisol levels (Rotenberg et al., 2012) and flatter diurnal slope (Zeiders et al., 2011). Further, adolescents commonly experience phase-shift delay, resulting in potentially greater morning fatigue and grogginess due to the propensity to sleep-in later, despite early school start times. Thus, adolescents may be less compliant with awake0 sampling compared to younger children. Consistent with this idea, Jessop and Turner-Cobb (2008) suggest that children may collect the awake0 sample more reliably than adolescents, due to varying degrees of parental supervision. Finally, the cortisol response differs across childhood and adolescence, as total cortisol concentrations increase steadily (Lupien et al., 2001; Walker et al., 2001; Tornhage, 2002; Gunnar et al., 2009). Pubertal maturation is also associated with a flatter diurnal slope (Rotenberg et al., 2012), increased cortisol (Kiess et al., 1995; Oskis et al., 2009), and reduced CAR (Adam, 2006). Given that these developmental factors may influence awake0 sampling and the cortisol response, and in turn, that awake0 sampling is important to accurately capture CAR and the diurnal cortisol profile, it is necessary to consider whether children and adolescents are compliant with awake0 sampling.
Previous methodological studies have examined the stability of CAR and the diurnal profile in children and adolescents (see Oskis et al., 2009; Rotenberg et al., 2012). However, the methodological issue regarding awake0 compliance has yet to be examined in childhood. The goal of the present study was to evaluate children and adolescents’ compliance with collecting an awake0 sample validated against accelerometry, as an objective measure of wake-time. Based on previous findings, it was hypothesized that children and adolescents would be highly compliant with collecting an awake0 sample, with children more compliant than adolescents. The effect of a delay between wake-time and collecting the awake0 sample on measures of the diurnal cortisol profile was also examined. It was hypothesized that a greater delay would yield lower estimates of the cortisol awakening response and diurnal cortisol profile.
2. Method
2.1. Participants
Children and adolescents aged 8–18 years were recruited to take part in the larger Healthy Heart Project, a longitudinal study examining early cardiovascular risk factors, at Concordia University, Montreal, QC. Flyers, postcards, and bookmarks were distributed throughout the community and in schools approved by the Montreal English School Board. Children with serious psychopathology or prescription medication use were excluded. During the study, participants were asked to refrain from using over-the-counter medications and caffeine. Parental and adolescent informed consent and child assent were obtained. This study was approved by the Concordia University Ethics Review Committee (UH2005-077).
2.2. Measures
2.2.1. Wake-time
Children and adolescents wore an undergarment vest that contained an embedded tri-axle accelerometer for 24 h for the Healthy Heart Project protocol. The accelerometer was fitted securely around the abdomen, and differentiated supine from upright posture. Accelerometry data was processed using VivoLogic Version 3.2 (VivoMetrics Inc.) and visually inspected. Accelerometer-based wake-time was defined by the onset of a continuous upright signal.
2.2.2. Cortisol
Saliva samples were collected six times per day. Samples were collected upon awakening (awake0), +30 min post-awakening (awake30), +45 min post-awakening (awake45), before lunch, before dinner, and before bed. For the awake0 sample, children and adolescents were instructed to “sit up and remain in bed” for saliva collection. Children and adolescents recorded the date and time each sample was taken in a daily saliva collection log. Parents and/or teachers initialed each entry to verify that samples were collected at the written time. The data acquisition unit for the accelerometer contained a visible clock that was to be used for recording the time of saliva sampling. Participants were unaware that the accelerometer embedded in the vest was also synced to this clock.
Saliva samples were collected using the Salivette sampling device (Salimetric, Inc.). Participants were instructed to place the cotton swab under their tongue for at least 30 s. When saturated, it was placed back in the Salivette tube and refrigerated until returned to the laboratory. Participants were instructed not to eat, drink, or brush their teeth 10 min before taking a sample. When returned, saliva samples were stored in a sub-zero freezer until packaged in dry ice and couriered to the University of Trier, Germany, for cortisol assaying. Cortisol levels are robust to environmental conditions associated with the shipping process (Clements and Parker, 1998). Cortisol levels were determined in duplicate using a competitive solid phase time-resolved fluorescence immunoassay with fluorometric end point detection (Dressendorfer et al., 1992). The intra-assay coefficients of variation were less than 11%.
Untransformed cortisol values were used to derive area under the awakening response relative to ground (AUCAG), dynamic increase of the awakening response (AUCI), area under the diurnal profile relative to ground (AUCTG), and diurnal slope. The diurnal slope was determined by standard linear regression and was anchored to the awakening sample (Slopeawake) and the maximum sample (Slopemax; for formulae, see Rotenberg et al., 2012).
2.3. Procedure
Children and their parents were scheduled for two laboratory visits. During the first visit, participants and their parents completed demographic and health questionnaires. Children and adolescents were fitted with the undergarment vest, instructed on the use of the Salivette sampling device, and provided saliva collection kits. Participants were unaware that their time of awakening could be verified. Saliva samples and accelerometry data were collected concurrently, on the same weekday. During the second visit, participants returned the saliva samples and accelerometer. Participants were debriefed and received compensation for their time.
2.4. Sample exclusion criteria
Of the initial 241 participants who were recruited, participants who did not have accelerometery data due to equipment malfunction (n = 30), did not collect any saliva samples (n = 7), or whose data were extreme outliers (>6 SD; n = 3) were excluded from all subsequent analyses.
2.5. Data analyses
Of the remaining 201 participants, missing data were observed across the saliva samples (awake0 9.0%, awake30 6.0%, awake45 5.0%, lunch 9.0%, dinner 9.5%, bed 14.4%). Since data are not likely to be “missing completely at random” (MCAR), complete case analysis may lead to biased results. Thus, multiple imputation was informed by data from the larger Healthy Heart Project (e.g., subsequent cortisol samples, day of sampling, puberty) to fill in plausible values for the missing values. Missing data analyses were guided by previous recommendations (Little, 1988; McKnight et al., 2007). Missing values were imputed 20 times with re-sampling techniques so that measures of the cortisol awakening response (AUCAG, AUCI) and diurnal cortisol profile (AUCTG, Slopeawake, Slopemax) could be derived. Analyses were performed in both original and imputed datasets.
To test the hypotheses, two analyses were conducted. First, intra-class correlation (ICC) analyses were used to examine compliance with collecting an awake0 sample at wake-time. Second, multivariable linear regression, controlling for wake-time, was used to evaluate the effect of a delay in collecting the awake0 sample on six cortisol measures (AUCAG, AUCI, AUCTG, Slopeawake, Slopemax, awake0). Sampling delay was defined as the absolute difference between the accelerometer-based wake-time and awake0 sampling (see Table 1). Models were tested separately for children, adolescents, and total participants. Participants were grade-stratified to account for school start times (Children = 3–6th Grades, Primary school; Adolescents = 7–11th Grades, Secondary school). Analyses were conducted using IBM SPSS Statistics software (Version 20).
Table 1.
Demographic information.
| Total Sample (n = 201) M (SD) |
Children (n = 75) M (SD) |
Adolescents (n = 126) M (SD) |
|
|---|---|---|---|
| Characteristic | |||
| Age (years) | 12.67 (2.03) | 10.61 (1.01) | 13.90 (1.38) |
| Female | 45.3% | 38.7% | 49.2% |
| BMI percentile | 64.88 (25.90) | 65.41 (26.92) | 64.56 (25.38) |
| Adrenarche (Tanner stage) | 3.25 (1.42) | 1.97 (0.99) | 4.07 (0.97) |
| Parental education (years) | 16.43 (3.33) | 16.61 (3.50) | 16.32 (3.23) |
| Household income ($CAD) | 79,949 (51,499) | 76,250 (49,806) | 82,157 (52,557) |
| School in-session | 78.1% | 77.3% | 78.6% |
| Cortisol levels | |||
| Awake0 | 10.68 (7.03) | 11.16 (7.76) | 10.40 (6.58) |
| AUCAG | 11.31 (5.65) | 11.80 (6.56) | 11.02 (5.04) |
| AUCI | 2.68 (5.45) | 2.48 (5.91) | 2.80 (5.17) |
| AUCTG | 70.96 (42.87) | 69.14 (48.08) | 72.05 (39.61) |
| Diurnal slope | |||
| Slopeawake | −0.56 (0.50) | −0.60 (0.58) | −0.53 (0.45) |
| Slopemax | −1.07 (0.59) | −1.10 (0.58) | −1.04 (0.60) |
| Compliance measures | |||
| Absolute sampling delay (min) | 10.06 (19.93) | 8.93 (17.65) | 10.74 (21.21) |
| 0–4 min delay | 56.3% | 53.5% | 57.9% |
| 5–9 min delay | 22.4% | 25.3% | 20.6% |
| 10–14 min delay | 9.5% | 10.7% | 8.7% |
| >15 min delay | 11.9% | 10.7% | 12.7% |
| Real-time sampling delay (min) | 1.75 (22.27) | −4.48 (19.29) | 5.45 (23.15) |
Note: Awake0 = initial cortisol value at wake-time. AUCAG = area under the curve relative to ground, for awakening response. AUCI = area under the curve relative to increase, for awakening response. AUCTG = area under curve relative to ground, for entire diurnal profile. Slopeawake = diurnal slope anchored to awake using regression. Slopemax = diurnal slope anchored to max sample using regression.
3. Results
Participant demographics for children, adolescents, and the total sample are presented in Table 1. Overall, the majority of participants were 13 years old, of normal body mass (5–85th BMI percentile: n = 140; 70%), in the third stage of adrenarche (pubic hair growth), and Caucasian (68.2%; Black 10.0%, Asian 8.5%, Latino 5.5%, Other/mixed 6.0%). Compared to adolescents, children were in a lower stage of adrenarche (t (183) = −14.22, p < .001), and collected their awake0 sample earlier (t (199) = 2.12, p = .036); no other significant differences were observed. Most saliva samples were collected while school was in-session (78.1%). Parents initialed the daily saliva collection log as a compliance check for 97% of participants’ sampling entries. Cortisol measures were normally distributed (see Table 1).
To test the first hypothesis that children and adolescents would be compliant with collecting an awake0 sample, intra-class correlation (ICC) analyses were conducted. Mean accelerometer-derived wake-time was nearly identical to self-reported collection time of the awake0 sample (07:31 −1:24 h vs. 07:31 −1:23 h, respectively). All participants were highly compliant with awake0 sampling (ICC = .98). (Results did not differ between original and imputed data; only imputed data reported for parsimony.) Children and adolescents were similarly highly compliant (ICC = .94 vs .98, respectively).
To test the second hypothesis that a greater delay would yield lower estimates of CAR and the diurnal cortisol profile, after controlling for wake-time, multivariable linear regression analyses were conducted (see Table 2). The absolute delay between wake-time and collecting the awake0 sample was 10.06 ± 19.93 min. The majority of participants (88.1%) collected the awake0 sample within 15 min of wake-time (Children 89.3%, Adolescents 87.3%, see Table 1). For both children and adolescents, earlier wake-time was associated with significantly greater AUCAG, AUCI, and AUCTG, and a steeper Slopemax (see Table 2). While the absolute sampling delay was not associated with age (r = .00, p = .950), the effect of a delay on estimates of CAR and the diurnal cortisol profile differed between children and adolescents. Specifically, after controlling for wake-time and age, longer absolute sampling delay only among children was significantly associated with steeper Slopeawake and greater awake0 cortisol level. Absolute sampling delay among adolescents was not associated with any measure of the diurnal cortisol profile. (Sex, adrenarche, time of sampling [school year vs summer holiday], and race were not associated with compliance, and thus, not included in the models.)
Table 2.
Wake-time and sampling delay standardized regression coefficients (β) for cortisol measures.
| Total sample (n = 201) | Children (n = 75) | Adolescents (n = 126) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Wake-time | Sampling delay | Wake-time | Sampling delay | Wake-time | Sampling delay | |
| Awake0 | −.10 | .14† | −.14 | .24 * | −.08 | .08 |
| AUCAG | −.24 * | .03 | −.24 * | .05 | −.26 * | .03 |
| AUCI | −.14† | −.08 | −.18 | −.13 | −.14 | −.06 |
| AUCTG | −.17 * | −.00 | −.20 | −.02 | −.19 * | .01 |
| Diurnal slope | ||||||
| Slopeawake | .05 | −.12 | .04 | −.23 * | .05 | −.06 |
| Slopemax | .22 * | −.02 | .16 | −.05 | .24 * | .00 |
Note: β = standardized beta coefficients. Regression models include age, wake-time, and sampling delay; β not shown for age. Wake-time was determined by accelerometer. Sampling delay = absolute difference between wake-time and time of awake0 sample. Awake0 = initial cortisol value at wake-time. AUCAG = area under the curve relative to ground, for awakening response. AUCI = area under the curve relative to increase, for awakening response. AUCTG = area under curve relative to ground, for entire diurnal profile. Slopeawake = diurnal slope anchored to awake using regression. Slopemax = diurnal slope anchored to max sample using regression. Bold values indicate statistical significance.
p < .05.
p < .06.
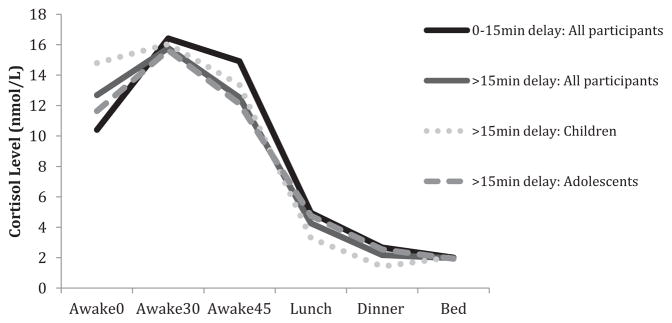
Two post hoc analyses were conducted. First, absolute sampling delay was dichotomized by delay greater than 15 min (see Fig. 1). Results were parallel to those with continuous data (All participants: Awake0 β = .12, Slopeawake β = −.11; Children: Awake0 β = .19, Slopeawake β = −.16). Second, analyses were conducted using the real-time sampling delay. The real-time delay indicated that relative to wake-time, children collected their awake0 sample later than adolescents (Children: −4.5 min ± 19.3 min; Adolescents: 5.4 min ±23.2 min; t (199) = 3.12, p = .002). Results using the real-time delay were consistent with those of the absolute delay (All participants: Awake0 β = .07, Slopeawake β =−.09; Children: Awake0 β = .32, Slopeawake β = −.31). Altogether, the post hoc analyses indicate that sampling delay, regardless if dichotomized or calculated as absolute or real-time, is associated awake0 and Slopeawake only among children.
Figure 1.
Diurnal cortisol profile over one day based on a sampling delay of either 0–15 min or greater than 15 min.
4. Discussion
Compliance with awake0 sampling is necessary for the precise measurement of the diurnal cortisol profile. Several adult studies have used objective measures of wake-time to determine compliance with awake0 sampling. Sampling compliance has not been previously examined in children and adolescents, despite developmental factors that may influence the awake0 sampling (e.g., school routine, sleep/wake pattern). In the present study, children and adolescents were found to be similarly, highly compliant; 88.1% of the awake0 samples were collected within 15 min of accelerometer-verified waking. These findings are consistent with previous adult studies in which 82–90% of respondents collected the awake0 sample within 15 min of wake-time (Dockray et al., 2008; DeSantis et al., 2010).
Early risers had greater cortisol awakening response as well as higher concentration throughout the day (i.e., AUCAG, AUCI, AUCTG), which is consistent with previous adult findings (Edwards et al., 2001; Frederenko et al., 2004); although one study found no association (Kunz-Ebrecht et al., 2004). In children, a delay between the accelerometer-based wake-time and awake0 sampling was associated with greater awake0 cortisol levels and a steeper diurnal decline (Slopeawake), after accounting for age and wake-time. These findings were robust even when the delay was dichotomized at 15 min. No association was observed for adolescents. It is plausible that the delay in children’s collection of the awake0 sample resulted in a greater awake0 sample due to the morning rise. Consequently, the diurnal slope is steeper because the calculation of the diurnal slope relies on the awake0 sample. These findings coincide with several adult studies that found a sampling delay resulted in greater awake0 cortisol levels, regardless if measured by objective (accelerometry, polysomnograpy) or subjective measures of wake-time (Dockray et al., 2008; DeSantis et al., 2010; Okun et al., 2010).
When the real-time sampling delay was examined rather than an absolute delay, children took their samples later than adolescents, relative to their wake time. Children collected the saliva sample 4 min after waking; whereas, adolescents collected the saliva sample 5 min before waking. Wake-time was defined as upright posture as verified by a continuous accelerometer-signal. This unanticipated difference may be attributable to the sampling strategy. It is plausible that adolescents took their saliva sample immediately upon waking, before sitting up. In comparison, children may have sat-up in bed to retrieve the sampling device or simply required more time to prepare the sampling device. Our observed results were inconsistent with Jessop and Turner-Cobb’s (2008) hypothesis that children collect salivary samples more reliably than adolescents due to a higher degree of parental-control. In the present study, parents were involved in the salivary cortisol collection (i.e., initialed entries in daily log) for both children and adolescents, and both groups were highly compliant with collecting an awake0 sample.
This study has four limitations. First, wake-time was determined using an accelerometer, which examines movement rather than waking. A more precise measure, such as polysomnography (simultaneous recording of brain wave activity, eye movement) would yield an exact measure of waking. However, accelerometery is considered a valid proxy of wake-time and is used extensively in the field of sleep research (Sadeh, 2011; Zeiders et al., 2011; Anders et al., 2012). Additionally, sleeping with the accelerometer did not interfere with the quality of the participant’s sleep, as 75% of the participants reported having an average night sleep or better. Second, a small number (8.9%) of the awake0 sample was missing. These data were missing completely at random, which suggests that the missing samples are not related to other variables in this study (i.e., age, sex, wake-time; Little, 1988). Results were identical for the original and imputed datasets. Third, data collection was completed within a single day, which yields greater measurement error. Several measures of the diurnal cortisol profile are less stable with only one day of measurement and necessitate at least three days to yield moderate stability (Rotenberg et al., 2012). Fourth, the current study used self-reported sampling time to examine children and adolescents’ compliance with collecting the awake0 sample. Participants were instructed to record the time they took the awake0 sample immediately upon awakening, before getting out of bed. A separate entry was not recorded for time of awakening; thus, the nature of the wording precludes teasing apart the assessment of subjective wake time and awake0 sampling time. Previous researchers have highlighted the advantage of using an electronic device (e.g., MEMs caps) to capture sampling time (Kudielka et al., 2003). Future research should include multiple days of measurement, use a time-stamped device to monitor sampling precision, incorporate a synchronized measure of objective awakening (e.g., polysomnography; cf., Griefahn and Robens, 2011), and consider whether sampling compliance differs within clinical populations.
Despite these limitations, this study has several strengths including the use of an accelerometer that was synced with a time display, the involvement of parents in saliva collection, and the concurrent collection of accelerometery data and salivary cortisol samples. As well, participants were unaware that the awake0 sample would be verified with the accelerometer. Most studies rely on self-reported time of awakening; therefore, this study design permitted an ecologically valid test of sampling compliance in typical research practice. It will also be important for future studies to consider children’s compliance with recommended instructions for collecting saliva (e.g., refrain from eating, drinking, brushing teeth; Hanrahan et al., 2006).
In conclusion, children and adolescents demonstrated high sampling compliance with the awake0 sample. Sampling delay, particularly in children, and time of awakening influenced measures of the diurnal cortisol profile. Children had a longer sampling delay that accounted for higher awake0 cortisol levels and a steeper diurnal slope. Adolescents’ sampling delay was not associated with any cortisol measures. Studies examining the diurnal cortisol profile strive for optimal cortisol measurement, as the diurnal slope is a common health indicator (cf. Adam, 2006). While researchers examining the diurnal cortisol profile in children and adolescents should consider using self-reported salivary sampling logs; it is important to note that future research should also account for experimental design issues (e.g., sampling compliance check) and analytical considerations (e.g., controlling for sampling delay, wake time) when planning diurnal cortisol profile studies in children and adolescents.
Acknowledgments
We thank the participants and their families of the Healthy Heart Project, the teachers and principals of the Montreal English School Board, and the research assistants and study coordinators of the Pediatric Public Health Psychology Laboratory at Concordia University. Special thanks to Natasha Hunt and Sabrina Giovanniello for their continued dedication. This work was made possible through funding support from the Canadian Institute of Health Research (CIHR) Operating Grants (MOP89886 and OCO79897), New Investigator Award (J.J. McGrath MSH95353), Canada Graduate Scholarships Master’s Award (S. Rotenberg), and Health Professional Student Research Award (S. Rotenberg).
Footnotes
Conflict of interest
All authors declare that they have no conflict of interest.
Role of the funding source
The funding source had no role in the design, analysis or presentation of this study.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34:1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Anders T, Iosif AM, Schwichtenberg AJ, Tang K, Goodlin-Jones B. Sleep and daytime functioning: a short-term longitudinal study of three preschool age comparison groups. Am J Intellect Dev Disabil. 2012;117:275–290. doi: 10.1352/1944-7558-117.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patient and healthy volunteers. Psychoneuroendocrinology. 2004;29:636–650. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Patterns of sleep and sleepiness in adolescents. Pediatrician. 1990;17:5–12. [PubMed] [Google Scholar]
- Clements AD, Parker CR. The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology. 1998;23:613–616. doi: 10.1016/s0306-4530(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Mendelsohn KA, Doane LD. Concordance between self-reported and objective wakeup times in ambulatory salivary cortisol research. Int J Behav Med. 2010;17:74–78. doi: 10.1007/s12529-009-9053-5. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33:77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev. 2002;6:287–306. doi: 10.1053/smrv.2001.0192. [DOI] [PubMed] [Google Scholar]
- Frederenko I, Wust S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Griefahn B, Robens S. Cortisol awakening response – are sampling delays of 15 min acceptable? Int J Psychophysiol. 2011;82:202–205. doi: 10.1016/j.ijpsycho.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Appl Nurs Res. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jessop DS, Turner-Cobb JM. Measurement and meaning of salivary cortisol: a focus on health and disease in children. Stress. 2008;11:1–14. doi: 10.1080/10253890701365527. [DOI] [PubMed] [Google Scholar]
- Kiess W, Meidert A, Dressendorfer RA, Schriever K, Kessler U, Kounig A, Schwarz HP, Strasburger CJ. Salivary cortisol levels throughout childhood and adolescence: relation with age, pubertal stage, and weight. Pediatr Res. 1995;37:502–506. doi: 10.1203/00006450-199504000-00020. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Kupper N, de Geus EJC, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115:241–249. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- Lupien S, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- McKnight PE, McKnight KM, Sidani S, Figueredo AJ. Missing Data: A Gentle Introduction. The Guilford Press; New York: 2007. [Google Scholar]
- Okun ML, Krafty RT, Buysse DJ, Monk TH, Renyolds CF, III, Begley A, Hall M. What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology. 2010;35:460–468. doi: 10.1016/j.psyneuen.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34:307–316. doi: 10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ, Roy-Gagnon MH, Tu MT. Stability of the diurnal cortisol profile in children and adolescents. Psychoneuroendocrinology. 2012;37:1981–1989. doi: 10.1016/j.psyneuen.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36:291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Tornhage CJ. Reference values for morning salivary cortisol concentrations in healthy school-aged children. J Pediatr Endocrinol Metab. 2002;15:197–204. doi: 10.1515/jpem.2002.15.2.197. [DOI] [PubMed] [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Dev Psychopathol. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson J, Lightman SL. Cortisol pulsatility and its role in stress regulation and health. Front Neuroendocrinol. 2004;25:69–76. doi: 10.1016/j.yfrne.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Adam EK. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rythms in late adolescence. J Adolesc Health. 2011;48:566–571. doi: 10.1016/j.jadohealth.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]