Summary
Recent evidence suggests that poor sleep is a potential pathway underlying the association between stressful experiences and the diurnal cortisol profile. However, existing findings are largely limited to adults. The present study examines whether poor sleep (duration, quality) mediates the relation between stressful experiences and the diurnal cortisol profile in children and adolescents. Children and adolescents (N = 220, Mage = 12.62) provided six saliva samples over two days to derive cortisol indices (bedtime, AUCAG, AUCTG, slopeMAX). Perceived stress, stressful life events, self-reported sleep duration, and sleep quality were measured. Using bootstrapping analyses, sleep quality mediated the relation between perceived stress and AUCTG (R2 = 0.10, F(7, 212) = 3.55, p = .001; 95% BCI[0.09, 1.15]), as well as the relation between stressful life events and AUCTG (R2 = 0.11, F(7, 212) = 3.69, p = .001; 95% BCI[0.40, 3.82]). These mediation models remained significant after adjusting for sleep duration, suggesting that poor sleep quality underlies the association between stressful experiences and the diurnal cortisol profile in children and adolescents. Longitudinal data combined with objectively-measured sleep is essential to further disentangle the complex association between sleep and stress.
Keywords: Sleep, Cortisol, Stressful life events, Perceived stress, Child, Adolescent
Stress is known to alter cortisol secretion. Exposure to acute laboratory-induced stressors elicits transient increases in cortisol level (Dickerson and Kemeny, 2004). Naturally occurring stressful experiences, including perceived stress and stressful life events, have also been associated with a disrupted diurnal cortisol profile. Adults who report greater perceived stress (i.e., nonspecific, subjective appraised stress, Cohen et al., 1995) have higher cortisol awakening response, total cortisol level, and flatter diurnal slopes (Miller et al., 2007; Pruessner et al., 1999; Schulz et al., 1998). Greater stressful life events (e.g., unemployment, divorce) are associated with higher morning and evening cortisol levels and flatter diurnal slopes (Miller et al., 2007). Similar findings have been established in both children and adolescents (hereafter referred to as “youth” for parsimony). Youth who report greater stressful life events (e.g., family conflict, academic stress) have a higher cortisol awakening response and elevated afternoon cortisol levels than their less stressed counterparts (Gustafsson et al., 2010; Wolf et al., 2008). While there is convincing evidence that stressful experiences disrupt the diurnal cortisol profile, the potential pathways underlying this association remain unclear.
Sleep is one plausible pathway by which stressful experiences disrupt the diurnal cortisol profile. The sleep-wake cycle has a close, temporal association with diurnal cortisol secretion. Nocturnal sleep onset reliably exerts an inhibitory effect on cortisol secretion (Van Cauter et al., 1991), with the emergence of slow-wave sleep, especially during the first sleep cycle, coinciding with the lowest cortisol level across the 24-h period (Born and Fehm, 1998). Cortisol level then gradually increases over the night, paralleling the upsurge of REM sleep and the decline of slow-wave sleep in later sleep cycles (Somers et al., 1993). Given the modulatory effect of sleep on cortisol secretion, sleep has been proposed as one plausible pathway by which stress exposure “gets under the skin” to affect the diurnal cortisol profile (Vargas and Lopez-Duran, 2014). Moreover, bedtime/evening cortisol is thought to be regulated by the negative feedback loop of the hypothalamic—pituitary adrenal axis, which suppresses the release of corticotropin-releasing-hormone and adrenocorticotropic hormone from the anterior pituitary into the bloodstream, resulting in the reduction of cortisol secretion throughout the day (Sapolsky et al., 1984). As such, poor sleep may be implicated in the association between stressful experiences and the diurnal cortisol profile through alterations at the level of the negative-feedback loop of the hypothalamic-pituitary adrenal axis.
Stressful experiences also have an adverse physiological effect on sleep. Experimental findings based on animal studies show that chronic stress exposure results in adverse sleep architecture changes, including decreased slow-wave sleep, decreased REM sleep latency, and increased REM sleep (Adrien et al., 1991; Cheeta et al., 1997). In adults, greater report of perceived stress over the past month was associated with poorer sleep quality and shorter sleep duration (Kashani et al., 2012; Lund et al., 2010). In youth, cross-sectional findings show that greater exposure to stressful life events over the past year adversely affects both subjectively-reported and objectively-measured sleep. For instance, greater parental conflict, family stress, and high academic stress are related to shorter sleep duration, poorer sleep quality, lower sleep efficiency, and greater sleep problems (El-Sheikh et al., 2006; Roberts et al., 2011). Greater stressful life events are also associated with changes in sleep architecture, including decreased slow-wave sleep, decreased REM sleep latency, and increased REM sleep duration among youth (Williamson et al., 1995). Altogether, there is a clear association between greater stressful experiences and poorer sleep.
Experimental manipulation of the sleep-wake cycle disrupts the diurnal cortisol profile. Adults undergoing partial or total experimental sleep deprivation exhibit flatter diurnal slopes and 37–45% increase in cortisol levels the subsequent evening (i.e., bedtime cortisol level; Balbo et al., 2010; Leproult et al., 1997; Spiegel et al., 1999). Selective deprivation of slow-wave sleep also stimulates increased cortisol levels (Tasali et al., 2008). These experimental findings provide causal evidence suggesting that both poor sleep quality and short sleep duration contribute to disruption of the diurnal cortisol profile. Emerging cross-sectional evidence suggests that the effect of sleep deprivation on cortisol secretion also exists in youth. Greater youth-report sleep fragmentations are associated with higher total cortisol level in children (El-Sheikh et al., 2008). Shorter sleep duration, longer sleep onset latency, lower sleep efficiency, and greater sleep fragmentation based on actigraphy-assessment are associated with higher afternoon and evening cortisol levels, higher total cortisol, and flatter diurnal slopes in children (El-Sheikh et al., 2008; Hatzinger et al., 2012; Pesonen et al., 2012; Räikkönen et al., 2010). As well, adolescent boys, but not girls, with shorter actigraphy-derived sleep duration have higher morning cortisol levels and a lower cortisol awakening response (Pesonen et al., 2014). Polysomnography-derived longer sleep onset latency, shorter sleep duration, lower sleep efficiency, and higher sleep fragmentation are related to higher morning and daytime cortisol levels (Hatzinger et al., 2008, 2010). While both poor sleep quality and short sleep duration have been linked to a disrupted diurnal cortisol profile, evidence suggests that sleep quality may be a marker of slow-wave sleep, a sleep stage thought to have important inhibitory effects on cortisol secretion (Jarrin et al., 2013; Kaneita et al., 2007). It is plausible that the quality of sleep may have greater impact on the diurnal cortisol profile than the quantity of sleep.
Based on evidence in the extant literature, there is strong support for the adverse effect of stressful experiences on the diurnal cortisol profile. Findings also suggest that sleep is one plausible pathway mediating this association. Despite emerging adult evidence suggesting poor sleep as a potential mediating pathway, this hypothesis has not been examined in youth. In the present study, the mediating role of sleep in the association between stressful experiences and the diurnal cortisol profile was examined in a cross-sectional sample of youth. It was hypothesized that poor sleep would mediate the relation between stressful experiences and the diurnal cortisol profile in youth.
1. Method
1.1. Participants
Youth aged 8–18 were recruited as part of the Healthy Heart Project at Concordia University, Montreal, Quebec. Participants were recruited using bookmarks distributed in primary and secondary schools approved by the English Montreal School Board and flyers posted in the local neighbourhood. Youth with serious psychopathology (e.g., psychosis, severe depression) or medication use known to interfere with cardiovascular or endocrine functioning were not eligible to participate. Participants (N = 220) included youth aged 8 to 18 years (M = 12.62), with the majority attending 8th grade (see Table 1 for participant demographics). Youth were predominantly Caucasian (59.5%; Black/African 9.2%; Asian 9.7%; Latino 4.1%; other/mixed 17.4%). The sample included youth across the full range of pubertal stages, with the majority in Tanner Stage IV. Most parents completed a university degree and their annual household income averaged $77,769.71CAD. The project was approved by the Concordia University Research Ethics Committee (UH2005-077).
Table 1.
Sample characteristics.
| M (n) | SD (%) | |
|---|---|---|
| Age (8–18 yrs) | 12.62 | 2.04 |
| Sex | ||
| Male | (123) | (55.90) |
| Puberty status | ||
| Tanner I | (36) | (16.4) |
| Tanner II | (36) | (11.4) |
| Tanner III | (43) | (19.5) |
| Tanner IV | (51) | (23.2) |
| Tanner V | (47) | (21.4) |
| Parent education (8–22 yrs) | 16.44 | 3.32 |
| Household income (CAD) | 77,769.71 | 51,902.65 |
| Stressful experiences | ||
| Perceived stress (0–40) | 15.43 | 6.92 |
| Stress events (0–76) | 13.70 | 7.25 |
| Stress ratio (0–100) | 19.79 | 10.26 |
| Stress intensity (0–304) | 24.31 | 19.03 |
| Sleep | ||
| Sleep duration (weekday h) | 9.12 | 1.04 |
| Subjective sleep quality (0–10) | 6.75 | 2.05 |
| Daytime sleepiness (0–32) | 14.03 | 5.40 |
| Sleep disturbance (0–105) | 41.32 | 5.24 |
| Cortisol | ||
| Bedtime cortisol (nmol/L) | 1.72 | 2.19 |
| AUCAG | 11.04 | 7.93 |
| AUCTG | 67.87 | 34.05 |
| SlopeMAX | −1.03 | 0.50 |
AUCAG = area under the curve relative to ground for awakening response.
AUCTG = area under the curve relative to ground for diurnal profile.
SlopeMAX = diurnal slope anchored at maximum sample.
1.2. Measures
1.2.1. Participant characteristics
Information about youth’s age, education level, and sex as well as parental education level and household income was obtained. Youth reported their pubertal status (adrenarche) using sex-appropriate schematic drawings of pubic hair growth corresponding to Tanner Stages I to V of pubertal development (Golding et al., 2001; Tanner, 1962). Pubertal illustrations have previously demonstrated good validity and reliability (Morris and Udry, 1980).
1.2.2. Cortisol
Youth collected six saliva samples each day over two weekdays at awakening (awake0), 30 min post-awakening (awake+30), 45 min post-awakening (awake+45), pre-lunch, pre-dinner, and bedtime. Saliva samples were collected using the Salivette sampling device (Salimetric, Inc.). Youth were instructed to not eat, drink, or brush their teeth 10 min before each sampling. They were told to place the cotton swab under their tongue for at least 30 s until the cotton swab was saturated. Each sample was initialled by parents or teachers as a measure of compliance. Previous research has demonstrated good compliance with saliva sampling protocols in youth (Rotenberg and McGrath, 2014). Youth were instructed to store saliva samples in the freezer at home until they returned them to the laboratory. Samples were stored in sub-zero freezers in the lab until they were packaged in dry ice and shipped to the University of Trier, Germany for assaying. Samples were assayed using a competitive solid phase time-resolved fluorescence immunoassay with fluorometric end point detection (DELFIA; Dressendörfer et al., 1992). The intra-assay coefficient of variation was less than 11.0%.
Aggregate cortisol indices were calculated for each day of sampling using established formulae (see Rotenberg et al., 2012). Aggregate indices derived included AUCAG (i.e. cortisol awakening response), AUCTG (i.e., total cortisol level secreted throughout the day), and slopeMAX (i.e., cortisol decline over the day). Bedtime cortisol for each day of sampling was used as a single sample index. Cortisol indices were averaged across the two days.
1.2.3. Stressful life events
A questionnaire adapted from the Stressful Life Events Schedule semi-structured interview (SLES; Williamson et al., 2003) was used to identify the occurrence of life stressors across nine domains: education, work, money, housing, crime, health, deaths, romantic relationships, and other relationships. Youth endorsed the number of stressful life events they experienced over the past year and rated the intensity of the stress associated with each event endorsed on a 5-point Likert scale (0 not at all to 4 very stressful). Three total scores were derived: total number of events endorsed (stress events), percent of events endorsed (stress ratio), and total stress intensity ratings for endorsed events (stress intensity). A composite score was computed for these three sum scores, with a higher score indicating greater reported stress and events on the SLES. The original SLES has previously demonstrated good psychometric properties (Williamson et al., 2003).
1.2.4. Perceived stress
The Perceived Stress Scale (PSS) is a 10-item self-report questionnaire that measures the degree to which individuals appraise their stress level related to daily situations over the past month (Cohen et al., 1983). Youth rated items on a 5-point Likert scale (0 never to 4 very often). Items were summed for a total perceived stress score, with higher scores indicating higher levels of perceived stress. The PSS has previously demonstrated good psychometric properties (α = 0.84–0.86; Cohen et al., 1983). This measure has been used with youth (e.g., Martin et al., 1995). In the current sample, internal consistency was high (α = 0.81).
1.2.5. Sleep
Youth reported their typical bed- and wake-times on school days to derive sleep duration during school nights. Three sleep quality measures (Pediatric Daytime Sleepiness Scale, self-report sleep quality, Child’s Sleep Habits Questionnaire) were used to derive a latent variable sleep quality.
The Pediatric Daytime Sleepiness Scale (PDSS; Drake et al., 2003) is an 8-item questionnaire used to assess daytime sleepiness in youth. Youth reported the frequency to which they felt sleepy or alert during their daily routine on a 5-point Likert scale (1 never to 5 always). Items were summed to derive a total score, with higher scores indicating greater daytime sleepiness. The PDSS has previously demonstrated good psychometric properties (α = 0.80; Drake et al., 2003). In the present sample, the measure had good internal consistency (α = 0.74).
Youth rated the item “Overall, I would rate my sleep as “ on a 10-point Likert scale (1 very bad to 10 very good). Previous published studies have used this item to assess subjective sleep quality in youth (e.g., Jarrin et al., 2013).
The Child’s Sleep Habits Questionnaire (CSHQ) is a 35-item parent-report questionnaire that assesses sleep problems in children over the past month (Owens et al., 2000). This questionnaire includes eight subscales: bedtime resistance, sleep onset latency, sleep duration, sleep anxiety, sleep behaviours and night waking, sleep-disordered breathing, parasomnias, and daytime sleepiness. Parents rated the frequency of their children’s sleep problems in a typical recent week on a 3-point scale (1 usually to 3 rarely). Items were summed to derive a total score, with higher scores indicating more problems. The CSHQ has previously demonstrated good psychometric properties (Owens et al., 2000). Internal consistency of the measure for the current sample was comparable to those previously established (α = 0.59; Owens et al., 2000).
Principal components analysis, based on Varimax rotation, on the three sleep quality measures was conducted to derive the latent variable sleep quality. The analysis yielded a 1-component solution, accounting for 52.98% of the total variance, with factor loadings of −0.87 for daytime sleepiness, 0.72 for subjective sleep quality, and −0.57 for sleep disturbance. A higher score indicated better subjective sleep quality.
1.3. Procedure
Youth and their parents were scheduled for two laboratory sessions. During the initial laboratory session, informed consent was obtained from parents and youth; children younger than 14 years of age provided assent. Next, parents and youth completed questionnaires. Then, youth were provided saliva collection kits and instructed how to complete the daily log to record their sleep and saliva sampling times. During the second laboratory session, youth returned the saliva samples and daily log and were compensated for their participation in the study.
1.4. Data analyses
Analyses were conducted using SPSS 20. Data were screened to identify outliers and verify normality of distribution in cortisol, stress, and sleep variables to ensure statistical assumptions were met. Raw cortisol values were square root transformed to address non-normality. Any single samples that were either not returned or did not contain enough saliva for assay were coded as missing data. Missing cortisol values were addressed using multiple imputation (McKnight et al., 2007). Multiple imputation was conducted using data from the larger Healthy Heart Project, including subsequent cortisol samples, day of sampling, and puberty to fill in plausible values for the missing values. Missing values were imputed 20 times with re-sampling techniques to derive aggregate cortisol indices. This method was previously used in a study examining cortisol in youth (Rotenberg and McGrath, 2014). Regression analyses were conducted to examine (1) the relation between stressful experiences (i.e., perceived stress, stressful life events) and diurnal cortisol, (2) the relation between stressful experiences and sleep, and (3) the relation between sleep and diurnal cortisol. Upon establishment of these preconditions of mediation, bootstrapping analyses were used to test the mediation hypothesis (Preacher and Hayes, 2008). Indirect effects were analysed based on 1000 bootstraps and were evaluated as significant if the 95% bias-corrected confidence interval of the indirect effect does not include zero. All analyses controlled for age, sex, adrenarche, time of awakening, and socioeconomic status. Finally, in post-hoc analyses, sleep duration was added as an additional covariate to assess the mediating role of sleep quality in the relation between stressful experiences and diurnal cortisol, beyond sleep duration.
2. Results
2.1. Descriptive statistics
On average, youth endorsed one in five stressors on the SLES (13.7 out of 76 events; 19.79%), with an average total stress intensity rating of 24.31 for the endorsed stressors. Consistent with previous adult studies, youth reported moderate levels of perceived stress (Cohen et al., 1983). Mean sleep disturbance scores were consistent with previous research (Owens et al., 2000). Youth reported good sleep quality and moderate daytime sleepiness. Youth reported 9.12 h of sleep during school nights, corresponding to National Sleep Foundation’s (2000) recommendation of 9 to 10 h of sleep per night for youth. Cortisol values were similar to values previously published in the pediatric literature (e.g., Oskis et al., 2009), showing a peak in cortisol secretion shortly after morning awakening, followed by a gradual decline throughout the day. Means and standard deviations for stressful experiences, sleep, and cortisol values are presented in Table 1.
2.2. Hypothesis testing
Results for the regression analyses, controlling for relevant covariates, are presented in Table 2. First, there was partial support for the relation between stressful experiences and disrupted diurnal cortisol. Greater perceived stress was associated with elevated AUCAG, AUCTG, and steeper slopeMAX, but not bedtime cortisol. There was a trend toward elevated AUCAG and AUCTG among youth who reported more stressful life events. Stressful life events were not associated with bedtime cortisol or slopeMAX. Second, there was support for the relation between stressful experiences and poor sleep. Both greater perceived stress and stressful life events were associated with poorer sleep quality. Greater perceived stress was associated with shorter sleep duration; there was a trend toward shorter sleep duration and more stressful life events. Third, there was partial support for the relation between poor sleep and disrupted diurnal cortisol. Poorer sleep quality was associated with higher AUCTG. There was a trend in the association between poor sleep quality and higher bedtime cortisol. Poorer sleep quality was not associated with AUCAG or slopeMAX. Shorter sleep duration was not associated with any cortisol values. Results from the regression analyses suggest that the preconditions of mediation were met for stressful experiences (perceived stress, stressful life events), sleep quality, and AUCTG.
Table 2.
Regression models for pre-conditions of mediation.
| Stress & cortisol | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Bedtime cortisol | AUCAG | AUCTG | SlopeMAX | |||||||||||||
|
|
|
|
|
|||||||||||||
| β | t | p | ΔR2 | β | t | p | ΔR2 | β | t | p | ΔR2 | β | t | p | ΔR2 | |
| Perceived stress | −.07 | .96 | .34 | .004 | .16 | 2.29 | .023 | .02 | .14 | 2.01 | .046 | .02 | −.15 | −2.12 | .035 | .02 |
| Stressful life events | −.03 | −.40 | .70 | .001 | .11 | 1.65 | .099 | .01 | .11 | 1.62 | .099 | .01 | −.06 | −.86 | .39 | .003 |
| Stress & sleep | ||||||||||||||||
| Sleep quality | Sleep duration | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||
| β | t | p | ΔR2 | β | t | p | ΔR2 | |||||||||
|
| ||||||||||||||||
| Perceived stress | −.56 | −9.45 | <.001 | .28 | −.14 | −2.46 | .015 | .02 | ||||||||
| Stressful life Events | −.22 | −3.21 | .002 | .04 | −.11 | −1.90 | .059 | .01 | ||||||||
| Sleep & cortisol | ||||||||||||||||
| Bedtime cortisol | AUCAG | AUCTG | SlopeMAX | |||||||||||||
|
|
|
|
|
|||||||||||||
| β | t | p | ΔR2 | β | t | p | ΔR2 | β | t | p | ΔR2 | β | t | p | ΔR2 | |
|
| ||||||||||||||||
| Sleep quality | −.13 | −1.89 | .060 | .02 | −.03 | −.42 | .67 | .001 | −.21 | −3.18 | .002 | .04 | .03 | .40 | .69 | .001 |
| Sleep duration | −.14 | 1.62 | .10 | .01 | −.03 | −.35 | .73 | .001 | −.11 | 1.29 | .20 | .01 | −.12 | −1.46 | .15 | .01 |
AUCAG = area under the curve relative to ground for awakening response.
AUCTG = area under the curve relative to ground for diurnal profile.
SlopeMAX = diurnal slope anchored at maximum sample.
All analyses controlled for age, sex, adrenarche, time of awakening, socioeconomic status.
β denotes standardized beta coefficients.
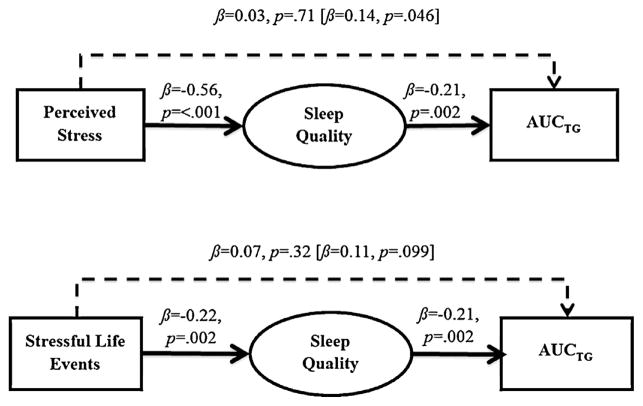
Bootstrapping analyses were conducted with sleep quality as a hypothesized mediator in the relation between stressful experiences and AUCTG. The hypothesis that poor sleep quality would mediate the relation between stressful life events and AUCTG was supported (Fig. 1). The direct effect of perceived stress on AUCTG was substantially reduced when sleep quality was included as a mediator in the analysis. This result was further supported by bootstrapping analyses, which showed that sleep quality exerted a statistically significant indirect effect on perceived stress in predicting AUCTG (R2 = 0.10, F(7, 212) = 3.55, p = .001; 95% BCI[0.09, 1.15]). Likewise, the direct effect of stressful life events on AUCTG was substantially reduced when sleep quality was included as a mediator in the analysis. Results based on bootstrapping analyses showed that sleep quality exerted a statistically significant indirect effect on stressful life events in predicting AUCTG (R2 = 0.11, F(7, 212) = 3.69, p = .001; 95% BCI[0.40, 3.82]). Overall, the mediation hypothesis that sleep quality was a mediator in the relation between perceived stress and AUCTG, and between stressful life events and AUCTG was supported.
Fig. 1.
Figure depicting the mediating role of sleep quality in the relation of perceived stress with AUCTG (top panel) and stressful life events with AUCTG (bottom panel). Estimates of total effect of perceived stress and stressful life events on AUCTG are presented in brackets above dashed line with adjacent values representing estimates of the total indirect effect of perceived stress and stressful life events on AUCTG through sleep quality.
Post-hoc analyses were conducted to explore whether sleep quality mediated the association between stressful experiences and AUCTG when sleep duration was included as an additional covariate in the mediational analyses. Results based on bootstrapping analyses demonstrated that sleep quality exerted a statistically significant indirect effect on perceived stress in predicting AUCTG (R2 = 0.11, F(8, 211) = 3.15, p = .002; 95% BCI[0.06, 1.14]), even after controlling for sleep duration. Although greater stressful life events were no longer associated with elevated AUCTG when sleep duration was included as a covariate (β = 0.11, p = .12), the direct effect of stressful life events on AUCTG was still substantially reduced when sleep quality was included as a mediator in the analysis (β = 0.06, p = .37). Results based on bootstrapping analyses also demonstrated that sleep quality exerted a statistically significant indirect effect on stressful life events in predicting AUCTG (R2 = 0.11, F(8, 211) = 3.25, p = .002; 95% BCI[0.38, 4.19]).
3. Discussion
There is emerging evidence to support the contribution of poor sleep as a pathophysiological pathway underlying the adverse effect of stressful experiences on the diurnal cortisol profile. Previous cross-sectional and experimental findings demonstrate the adverse effects of stressful experiences on sleep, which subsequently disrupts the diurnal cortisol profile. Accordingly, it was hypothesized that the association between stressful experiences and diurnal cortisol would be mediated by sleep.
3.1. Pre-conditions of mediation
First, greater stressful experiences were associated with a disrupted diurnal cortisol profile. Greater stressful life events were associated with higher cortisol awakening response, albeit the observed effect sizes were smaller than existing pediatric studies (e.g., Gustafsson et al., 2010; Wolf et al., 2008). Consistent with existing adult findings, greater perceived stress was associated with higher cortisol awakening response and total cortisol level (Pruessner et al., 1999; Schulz et al., 1998). Contrary to existing adult findings, greater perceived stress was associated with a steeper (as opposed to flatter) diurnal slope in youth. Previous findings showed that greater stress exposure is related to higher bedtime cortisol and flatter diurnal slopes only among adults who were exposed to early adversities (Hanson and Chen, 2010). However, future research is required to further elucidate this hypothesis in youth.
Second, consistent with pediatric studies based on polysomnography- and actigraphy-assessments of sleep, stressful life events were associated with both poorer sleep quality and shorter sleep duration (El-Sheikh et al., 2006; Sadeh et al., 2000). Further, consistent with findings from adult studies, greater perceived stress was associated with both poorer sleep quality and shorter sleep duration (Kashani et al., 2012; Lund et al., 2010). Interestingly, perceived stress accounted for substantially more variance in sleep quality than sleep duration, suggesting that the quality, not the quantity of youth’s sleep is more susceptible to the adverse effects of perceived stress.
Third, poorer sleep quality was associated with a disrupted diurnal cortisol profile. Specifically, sleep quality was associated with higher bedtime cortisol and total cortisol level; no association was found for cortisol awakening response and diurnal slope. The current results corroborate experimental adult studies, which demonstrated higher bedtime/evening cortisol levels following sleep restriction protocols (Leproult et al., 1997). Bedtime and evening cortisol levels are thought to be regulated by the negative feedback loop of the hypothalamic—pituitary adrenal axis, which suppresses the release of corticotropin-releasing-hormone and adrenocorticotropic hormone from the anterior pituitary into the bloodstream, resulting in the reduction of cortisol secretion throughout the day (Sapolsky et al., 1984). Further, sleep quality has been postulated to be an indirect marker of restorative slow-wave sleep (Edinger et al., 2000). Given that poor sleep quality was related to higher bedtime cortisol in the current study, this suggests that sleep may affect the diurnal cortisol profile through alterations at the level of the negative-feedback loop of the hypothalamic-pituitary adrenalaxis.
3.2. Mediation hypothesis
There was partial support for the hypothesis that poor sleep quality is a plausible pathophysiological pathway by which stressful experiences “get under the skin” to disrupt the diurnal cortisol profile in youth. Specifically, poor sleep quality partially accounted for the association between perceived stress and stressful life events with higher total cortisol level. Experimentally-induced stress is known to reduce the amount of time spent in slow-wave sleep (Cheeta et al., 1997), a sleep stage thought to be associated with subjective sleep quality and to have strong regulatory control over cortisol secretion during sleep (Edinger et al., 2000). As such, the inhibitory effects of sleep on cortisol secretion are likely suppressed following exposure to stressful experiences, leading to a disrupted diurnal cortisol profile. This hypothesis was supported even when sleep duration was controlled, suggesting that the relation between stressful experiences and diurnal cortisol in youth is driven by the quality, not the quantity of sleep. It is noted that pubertal development and age of pubertal onset are associated with widespread neurobiological, physiological, and social changes known to adversely affect sleep quality in youth (e.g., Colrain and Baker, 2011). More research is required to further elucidate the complex association between sleep, stress, and diurnal cortisol across pubertal stages and age onset of pubertal development.
The mediating effect of poor sleep quality was present for total cortisol level. This suggests that the overall diurnal cortisol profile, rather than the cortisol awakening response or single sample measures (i.e., bedtime cortisol) is affected by stressful experiences through sleep. These findings are consistent with extant literature demonstrating that stressful experiences and sleep affect different aspects of the diurnal cortisol profile. Exposure to stressful experiences mainly disrupts the cortisol awakening response (Pruessner et al., 1999; Schulz et al., 1998), while poor sleep mainly disrupts evening and bedtime cortisol levels (Leproult et al., 1997; Spiegel et al., 1999). Given that total cortisol level was the sole index to capture both the awakening response and bedtime/evening cortisol levels of the diurnal cortisol profile, it is credible that the mediation hypothesis was only found for total cortisol level.
3.3. Limitations and future directions
There were five methodological limitations in the present study that merit discussion. First, the present study was based on cross-sectional data. It is acknowledged that causal inferences about the mediation hypothesis cannot be drawn; experimental or longitudinal studies are required to tease apart potential causal pathways. It is possible that other models may better explain the data (e.g., poor sleep leading to both increased stressful experiences and disrupted diurnal cortisol; Hori et al., 2011). Nevertheless, the current study provides empirical groundwork supporting a plausible neuroendocrinological relation between stressful experiences, sleep, and diurnal cortisol among youth. Second, while recommended salivary cortisol sampling protocols were used (MacArthur Network, 2000), previous studies with youth have demonstrated that bedtime cortisol and diurnal slope require at least 3 to 7 days to establish stability (Rotenberg et al., 2012; Oskis et al., 2009). Two days of sampling may have been insufficient to detect associations (i.e., accounting for some of the null findings) and precluded the examination of the dynamic relation between sleep and diurnal cortisol. Reciprocal and bidirectional relations between sleep and diurnal cortisol across multiple days have been previously observed during later adolescence (Buckley and Schatzberg, 2005; Steiger et al., 2013; Zeiders et al., 2011). Third, the majority of participants were Caucasian, which limits the generalizability of the current findings to other ethnoracial groups. Previous studies have established that both sleep (e.g., Stamatakis et al., 2007) and diurnal cortisol (e.g., DeSantis et al., 2007) differ across ethnoracial groups; future studies should evaluate the current model across diverse racial and ethnic samples. Fourth, the timing, duration, and nature of stressful experiences were not assessed, which may account for the lack of association between stressful life events with bedtime cortisol and diurnal slope. In a meta-analytic review, Miller et al. (2007) found that the effects of stressful experiences on diurnal cortisol depend on the chronicity and proximity of the stressor, with stronger associations for chronic, ongoing stressors. Similarly, Dickerson and Kemeny (2004) found that experimental stress task that are both uncontrollable and socially threatening produced the highest cortisol response and took the longest time for cortisol to return to baseline levels. Existing pediatric studies include wide-ranging stress measures that vary based on nature of the stressor (e.g., stress events, life adversities, perceived stress, daily hassles), life domain area (e.g., family, friendship, school), and informant (e.g., child self-report, parent-report); as such, the field would benefit from improved standardization in the conceptualization and measurement of stress during childhood and adolescence. Future researchers should also consider using ecological momentary assessment (i.e., daily logs) for day-to-day fluctuations in stressors and sleep characteristics to more precisely evaluate the interrelations between stress, sleep, and cortisol. Fifth, the use of subjective report of sleep quality precludes the delineation of specific sleep stages and parameters (e.g., sleep efficiency, fragmentation) that are fundamental to the disruption of the diurnal cortisol profile. Importantly, replication of the current findings should include objective sleep measures (e.g., polysomnography, actigraphy) to comprehensively assess this multidimensional construct.
4. Conclusions
Taken together, the present study suggests that poor sleep is one potential pathophysiological pathway underlying the association between stressful experiences and diurnal cortisol in youth. Further, the association between stressful experiences and the diurnal cortisol profile in youth were linked to the quality, not the quantity of sleep. These findings provide empirical support for a plausible neuroendocrinological relation between stress experiences, sleep, and diurnal cortisol among children and adolescents.
Acknowledgments
Role of the funding source
This work was made possible through funding support from the Canadian Institutes of Health Research Operating Grants (J.J. McGrath MOP89886 and OCO79897), New Investigator Award (J.J. McGrath MSH95353), and Fonds de recherche Québec santé bourse de formation maîtrise (J. Ly).
We thank the participants and their families of the Healthy Heart Project and the research assistants and project coordinators of the Pediatric Public Health Psychology Laboratory. Special thanks to Natasha Hunt, Sabrina Giovanniello, and Neressa Noel for their continued dedication.
Footnotes
Conflict of interest statement
None declared.
References
- Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol Behav. 1991;49:257–262. doi: 10.1016/0031-9384(91)90041-l. http://dx.doi.org/10.1016/0031-9384(91)90041-L. [DOI] [PubMed] [Google Scholar]
- Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo—pituitary—adrenal axis activity. Int J Endocrinol. 2010;2:1–16. doi: 10.1155/2010/759234. http://dx.doi.org/10.1155/2010/759234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Fehm HL. Hypothalamus—pituitary—adrenal activity during human sleep: a coordinating role for the limbic hippocampal system. Exp Clin Endocrinol Diabetes. 1998;106:153–163. doi: 10.1055/s-0029-1211969. http://dx.doi.org/10.1055/s-0029-1211969. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic—pituitary—adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90(5):3106–3114. doi: 10.1210/jc.2004-1056. http://dx.doi.org/10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Cheeta S, Ruigt G, van Proosdij J, Willner P. Changes in sleep architecture after chronic mild stress. Biol Psychiatry. 1997;41:419–427. doi: 10.1016/S0006-3223(96)00058-3. http://dx.doi.org/10.1016/S0006-3223(96)00058-3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Underwood LG. Strategies for measuring stress in studies of psychiatric and physical disorders. In: Cohen S, Kessler RC, Underwood LG, editors. Measuring Stress: A Guide for Health and Social Scientists. Oxford University Press; New York, NY: 1995. pp. 3–28. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Social Behav. 1983;24:385–396. http://dx.doi.org/10.2307/2136404. [PubMed] [Google Scholar]
- Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychology review. 2011;21(1):5–21. doi: 10.1007/s11065-010-9155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J Adolesc Health. 2007;41(1):3–13. doi: 10.1016/j.jadohealth.2007.03.006. http://dx.doi.org/10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. http://dx.doi.org/10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26:455–458. [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. http://dx.doi.org/10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Fins AI, Glenn DM, Sullivan RJ, Jr, Bastian LA, Marsh GR, et al. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68:586–593. http://dx.doi.org/10.1037/0022-006X.68.4.586. [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Keller PS, Granger DA. Children’s objective and subjective sleep disruptions: links with afternoon cortisol levels. Health Psychol. 2008;27:26–33. doi: 10.1037/0278-6133.27.1.26. http://dx.doi.org/10.1037/0278-6133.27.1.26. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Mize J, Acebo C. Marital conflict and disruption of children’s sleep. Child Dev. 2006;77:31–43. doi: 10.1111/j.1467-8624.2006.00854.x. http://dx.doi.org/10.1111/j.1467-8624.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- Golding J, Pembray M, Jones R. ALSPAC: the Avon longitudinal study of parents and children. I Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Anckarsäter H, Lichtenstein P, Nelson N, Gustafsson PA. Does quantity have a quality all its own? Cumulative adversity and up- and down-regulation of circadian salivary cortisol levels in healthy children. Psychoneuroendocrinology. 2010;35:1410–1415. doi: 10.1016/j.psyneuen.2010.04.004. http://dx.doi.org/10.1016/j.psyneuen.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Hanson MD, Chen E. Daily stress, cortisol, and sleep: the moderating role of childhood psychosocial environments. Health Psychol. 2010;29:394–402. doi: 10.1037/a0019879. http://dx.doi.org/10.1037/a0019879. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Perren S, Stadelmann S, Von Wyl A, Von Klitzing K, et al. Electroencephalographic sleep profiles and hypothalamic—pituitary—adrenocortical (HPA)-activity in kindergarten children: Early indication of poor sleep quality associated with increased cortisol secretion. J Psychiatr Res. 2008;42:532–543. doi: 10.1016/j.jpsychires.2007.05.010. http://dx.doi.org/10.1016/j.jpsychires.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Perren S, Stadelmann S, Von Wyl A, Von Klitzing K, et al. Sleep actigraphy pattern and behavioral/emotional difficulties in kindergarten children: Association with hypothalamic—pituitary—adrenocortical (HPA) activity. J Psychiatr Res. 2010;44:253–261. doi: 10.1016/j.jpsychires.2009.08.012. http://dx.doi.org/10.1016/j.jpsychires.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Perren S, von Wyl A, Stadelmann S, von Klitzing K, Holsboer-Trachsler E. Pre-schoolers suffering from psychiatric disorders show increased cortisol secretion and poor sleep compared to healthy controls. J Psychiatr Res. 2012;46(5):590–599. doi: 10.1016/j.jpsychires.2012.01.018. http://dx.doi.org/10.1016/j.jpsychires.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Hori H, Teraishi T, Sasayama D, Ozeki Y, Matsuo J, Kawamoto Y, et al. Poor sleep is associated with exaggerated cortisol response to the combined dexamethasone/CRH test in a non-clinical population. J Psychiatr Res. 2011;45(9):1257–1263. doi: 10.1016/j.jpsychires.2011.04.001. http://dx.doi.org/10.1016/j.jpsychires.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Jarrin DC, McGrath JJ, Drake CL. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Int J Obes. 2013;37:552–558. doi: 10.1038/ijo.2013.4. http://dx.doi.org/10.1038/ijo.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneita Y, Ohida T, Osaki Y, Tanihata T, Minowa M, Suzuki K, Hayashi K. Association between mental health status and sleep status among adolescents in Japan: a nationwide cross-sectional survey. J Clin Psychiatry. 2007;68(9):1426–1435. doi: 10.4088/jcp.v68n0916. http://dx.doi.org/10.4088/jcp.v68n0916. [DOI] [PubMed] [Google Scholar]
- Kashani M, Eliasson A, Vernalis M. Perceived stress correlates with disturbed sleep: a link connecting stress and cardiovascular disease. Stress. 2012;15:45–51. doi: 10.3109/10253890.2011.578266. http://dx.doi.org/10.3109/10253890.2011.578266. [DOI] [PubMed] [Google Scholar]
- Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–870. [PubMed] [Google Scholar]
- Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46:124–132. doi: 10.1016/j.jadohealth.2009.06.016. http://dx.doi.org/10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- MacArthur Network. Salivary Cortisol Measurement. 2000 Retrieved from 〈 http://www.macses.ucsf.edu/research/allostatic/salivarycort.php〉.
- Martin RA, Kazarian SS, Breiter HJ. Perceived stress, life events, dysfunctional attitudes, and depression in adolescent psychiatric inpatients. J Psychopathol Behav Assess. 1995;17(1):81–95. [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic—pituitary—adrenocortical axis in humans. Psychol Bull. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. http://dx.doi.org/10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- McKnight PE, McKnight KM, Sidani S, Figueredo AJ. Missing data: A gentle introduction. Guilford Press; 2007. [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. How Much Sleep Do We Really Need? 2000 Retrieved from 〈 http://www.sleepfoundation.org/article/how-sleep-works/how-much-sleep-do-we-really-need〉.
- Oskis A, Loveday C, Hucklebridge F, Thorn L, Clow A. Diurnal patterns of salivary cortisol across the adolescent period in healthy females. Psychoneuroendocrinology. 2009;34:307–316. doi: 10.1016/j.psyneuen.2008.09.009. http://dx.doi.org/10.1016/j.psyneuen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The children’s sleep habits questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- Pesonen AK, Martikainen S, Kajantie E, Heinonen K, Wehkalampi K, Lahti J, Räikkönen K. The associations between adolescent sleep, diurnal cortisol patterns and cortisol reactivity to dexamethasone suppression test. Psychoneuroendocrinology. 2014;49:150–160. doi: 10.1016/j.psyneuen.2014.07.005. http://dx.doi.org/10.1016/j.psyneuen.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Pesonen AK, Kajantie E, Heinonen K, Pyhälä R, Lahti J, Jones A, et al. Sex-specific associations between sleep problems and hypothalamic—pituitary—adrenocortical axis activity in children. Psychoneuroendocrinology. 2012;37:238–248. doi: 10.1016/j.psyneuen.2011.06.008. http://dx.doi.org/10.1016/j.psyneuen.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. http://dx.doi.org/10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. http://dx.doi.org/10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Matthews KA, Pesonen AK, Pyhälä R, Paavonen EJ, Feldt K, et al. Poor sleep and altered hypothalamic—pituitary—adrenocortical and sympatho—adrenal—medullary system activity in children. J Clin Endocrinol Metab. 2010;95:2254–2261. doi: 10.1210/jc.2009-0943. http://dx.doi.org/10.1210/jc.2009-0943. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Xing Y. Restricted sleep among adolescents: prevalence, incidence, persistence, and associated factors. Behav Sleep Med. 2011;9:18–30. doi: 10.1080/15402002.2011.533991. http://dx.doi.org/10.1080/15402002.2011.533991. [DOI] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ. Sampling compliance for cortisol upon awakening in children and adolescents. Psychoology. 2014;40:69–75. doi: 10.1016/j.psyneuen.2013.10.002. http://dx.doi.org/10.1016/j.psyneuen.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg S, McGrath JJ, Roy-Gagnon MH, Tu MT. Stability of the diurnal cortisol profile in children and adolescents. Psychoneuroendocrinology. 2012;37:1981–1989. doi: 10.1016/j.psyneuen.2012.04.014. http://dx.doi.org/10.1016/j.psyneuen.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Dev Psychol. 2000;36(3):291–301. doi: 10.1037//0012-1649.36.3.291. http://dx.doi.org/10.1037//0012-I549.36.3.29I. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Nat Acad Sci USA. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz P, Kirschbaum C, Pruessner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998;14:91–97. http://dx.doi.org/10.1002/(SICI)1099-1700(199804)14:2<91::AID-SMI765>3.0.CO;2-S. [Google Scholar]
- Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. http://dx.doi.org/10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Early report Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. http://dx.doi.org/10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17(12):948–955. doi: 10.1016/j.annepidem.2007.07.096. http://dx.doi.org/10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger A, Dresler M, Kluge M, Schüssler P. Pathology of sleep, hormones and depression. Pharmacopsychiatry. 2013;46(S 01):S30–S35. doi: 10.1055/s-0033-1337921. http://dx.doi.org/10.1055/s-0033-1337921. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at Adolescence. Blackwell; Oxford: 1962. [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Nat Acad Sci USA. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. http://dx.doi.org/10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Investig. 1991;88:934–942. doi: 10.1172/JCI115396. http://dx.doi.org/10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas I, Lopez-Duran N. Dissecting the impact of sleep and stress on the cortisol awakening response in young adults. Psychoneuroendocrinology. 2014;40:10–16. doi: 10.1016/j.psyneuen.2013.10.009. http://dx.doi.org/10.1016/j.psyneuen.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Ryan ND, Shiffrin TP, Lusky JA, Protopapa J, et al. The stressful life events schedule for children and adolescents: development and validation. Psychiatry Res. 2003;119:225–241. doi: 10.1016/s0165-1781(03)00134-3. http://dx.doi.org/10.1016/S0165-1781(03)00134-3. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Dahl RE, Birmaher B, Goetz RR, Nelson B, Ryan ND. Stressful life events and EEG sleep in depressed and normal control adolescents. Soc Biol Psychiatry. 1995;37:859–865. doi: 10.1016/0006-3223(94)00240-4. http://dx.doi.org/10.1016/0006-3223(94)00240-4. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Nicholls E, Chen E. Chronic stress, salivary cortisol, and alpha-amylase in children with asthma and healthy children. Biol Psychol. 2008;78:20–28. doi: 10.1016/j.biopsycho.2007.12.004. http://dx.doi.org/10.1016/j.biopsycho.2007.12.0044. [DOI] [PubMed] [Google Scholar]
- Zeiders KH, Doane LD, Adam EK. Reciprocal relations between objectively measured sleep patterns and diurnal cortisol rhythms in late adolescence. J Adolesc Health. 2011;48(6):566–571. doi: 10.1016/j.jadohealth.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]