Abstract
Over the past 30 years, image-guided placement of gastrostomies and cecostomies for gastrointestinal decompression has developed into a safe and effective treatment for symptomatic bowel obstruction. Gastrostomies and cecostomies relieve patient symptoms, can prevent serious complications such as colonic perforation, and may bridge patients to more definitive treatment for the underlying cause of obstruction. This article will review the history of decompressive gastrostomies and cecostomies as well as the indications, contraindications, technique, complications, and outcomes of these procedures.
Keywords: palliative care, gastrostomy, cecostomy, bowel obstruction, interventional radiology
Bowel obstruction is a common condition that results in significant patient distress, morbidity, and mortality. Postoperative adhesions are the leading cause of bowel obstruction in industrialized countries. Other nonmalignant causes of obstruction include hernias and inflammatory disorders. 1 Malignant bowel obstruction occurs in 3 to15% of patients with cancer and can be mechanical or functional in etiology. 2 3 Compression of the gastrointestinal tract by extrinsic or intrinsic tumor or metastases may result in mechanical obstruction, while tumor infiltration of the nerves involved in intestinal motility or secondary ileus can result in functional obstruction. 3 Whatever the cause, patients with nonmalignant or malignant obstruction often present with debilitating symptoms of abdominal pain, nausea, and vomiting. When colonic obstruction is present, patients are at risk for perforation and an associated 30 to 50% mortality rate. 4 5
Percutaneous gastrostomies (PG) and percutaneous cecostomies (PC) decompress the gastrointestinal system at different segments of the alimentary tract, relieving pressure and offering a pathway for gastric and bowel contents to exit the body. Patients experience symptomatic relief from obstructive symptoms and when colonic obstruction is present, the risk of perforation decreases. Gastrostomies and cecostomies offer other advantages to the terminal patient, allowing for resumption of oral intake, obviating the need for nasogastric tube placement, allowing for patient discharge home, and decreasing the rate of re-admittance after discharge. 2 This article reviews the history, indications and contraindications, preparation, technique, potential complications, and outcomes of decompressive gastrostomy and cecostomy placement.
Decompressive Gastrostomy
Gastrostomy catheters were initially developed to supply enteral nutrition to patients who cannot tolerate oral intake or in whom intake is insufficient to meet nutritional requirements. Placement of a gastrostomy was considered a surgical procedure; however, reports of fluoroscopy-guided gastrostomy placement were published in major radiology journals by the early 1980s. 6 7 Over the past 30 years, the use of decompressive gastrostomies has developed into a safe and effective treatment for decompression of bowel obstruction with associated relief of symptoms.
Indications for Decompressive Percutaneous Gastrostomy
Patients with bowel obstruction presenting with abdominal pain, gastrointestinal distention, nausea, and vomiting may benefit from decompressive gastrostomy. 3 8 9 The primary goal of placement is to remove gastrointestinal secretions and gas, resulting in symptomatic relief for patients with terminal illness and progressive debilitating conditions with associated obstruction. 8 9 10 11 12
Gastrostomies also obviate the need for long-term nasogastric tube placement, a source of discomfort and a risk factor for the development of nasopharyngeal and oropharyngeal erosions and infections. 13 In end-of-life or chronically debilitated patients, placement of a gastrostomy does not prevent the administration of other palliative treatments for obstruction. This is particularly important in the case of malignant disease where patients may still undergo palliative interventions to relieve causes of obstruction, such as chemotherapy and radiation. 10 Some patients resume oral intake and can then release ingested contents through the gastrostomy allowing for the continued enjoyment of food. 14 Patients who receive gastrostomies go home with the catheters in place, decreasing isolation resulting from hospitalization, especially important for terminal patients. 13
Contraindications to Decompressive Percutaneous Gastrostomy
Absolute contraindications to gastrostomy placement include uncorrectable coagulopathy, bacterial peritonitis, and bowel ischemia. Relative contraindications include recent gastrointestinal hemorrhage from either peptic ulcer disease with a large, identifiable vessel or esophageal varices. In these cases, before gastrostomy placement it is recommended that a 72-hour delay be instituted after cessation of bleeding. 15 The presence of varices and portal hypertension are relative contraindications to gastrostomy placement, as they confer a risk of extensive hemorrhage. Placement of gastrostomy catheters in patients with ventriculoperitoneal shunts may confer a greater risk of ascending meningitis, which should be taken into consideration when weighing the risks and benefits of gastrostomy placement. 16 17 Fever and active infection outside of the abdominal cavity are not absolute contraindications to catheter placement and practices vary by geography and institution. 15
Tumor infiltration of the stomach, peritoneal carcinomatosis, ascites, prior gastric surgery, and colonic or hepatic interposition between the stomach and anterior abdominal wall were once considered absolute contraindications to gastrostomy placement. Gastrostomies can successfully be placed in all these conditions, usually with the assistance of modifications to typical procedural technique, discussed later. 9 13 15 18
Preprocedure Assessment
Patients should stop all oral intake for 8 hours prior to procedure. Threshold laboratory values for coagulation parameters, the management of anticoagulation therapies, and administration of antibiotics vary by institution. Per Society of Interventional Radiology (SIR) guidelines, initial gastrostomy catheter placement confers a moderate risk of bleeding. Recommendations include an INR level less than 1.5 and platelet count greater than 50,000/µL. Clopidogrel should be withheld for 5 days prior to the procedure, a single dose of low-molecular-weight heparin should be held immediately before the procedure, and the cessation of aspirin is not recommended. 19
Transabdominal and Transoral Gastrostomy Placement
Image-guided gastrostomies placed transabdominally are directed into the stomach via direct puncture of the anterior abdominal wall. When gastrostomies are placed via a transoral route, after an incision is made in the anterior abdominal wall and access to the stomach is obtained, with the assistance of a small bore directional catheter, a suture is inserted through the anterior abdominal wall into the stomach up to the oral cavity. The gastrostomy catheter itself is introduced into the oral cavity and the suture used to pull the catheter antegrade into the stomach and through the abdominal wall incision. 20 21 22
Antibiotic Prophylaxis
SIR recommendations for antibiotic prophylaxis vary by technique of placement. The peristomal infection rate associated with transoral gastrostomies is relatively high at 4 to 30% and is attributed to the passage of the catheter through the oral cavity exposing it to oral flora that are then deposited at the stomal site. 23 24 SIR guidelines recommend routine prophylaxis for gastrostomies placed by transoral technique with 1 g of intravenous cefazolin as the antibiotic of choice. 24
There is controversy over the administration of antibiotic prophylaxis for gastrostomies placed by transabdominal technique in the general population, while data suggest that certain subgroups may benefit from antibiotics. In a publication by Cantwell and colleagues, 37 of 57 patients with head and neck cancer received antibiotics before gastrostomy placement via the transabdominal technique. Of the 37 patients who received antibiotic prophylaxis, no patients experienced peristomal infection. Of the 20 patients who did not receive prophylaxis, the rate of peristomal infection was 15%. 25 Accordingly, antibiotic prophylaxis should be considered in patients with head and neck cancer prior to gastrostomy placement.
Technique
Preparation
Prior to the procedure, abdominal computed tomography (CT) or ultrasound images should be reviewed to identify a window from the skin to the stomach with no interposed organs or vasculature. 15 Based on operator preference, 200 mL of dilute barium may be administered approximately 12 hours before the procedure resulting in opacification of the colon under fluoroscopy during the procedure. If a safe window cannot be determined from preprocedure imaging or at the time of the procedure under fluoroscopy, decision may be made to perform the procedure under CT guidance to better delineate patient anatomy. 15 26
Initial Steps
Gastrostomy placement is usually performed under conscious sedation. The procedure can be performed under local sedation alone or general anesthesia, as determined on a case-by-case basis.
Under fluoroscopy, a 4 to 5F nasogastric catheter is passed into the stomach with the use of a guidewire. The catheter is used to insufflate the stomach with air. If it is difficult to keep the stomach distended with air due to peristalsis, 1 mg of glucagon can be used to reduce gastrointestinal motility and promote retention of air in the stomach. 22 A puncture site is chosen approximately two-thirds of the way down the stomach, halfway between the greater and lesser curvature and at least two fingerbreadths below the inferior edge of the ribcage. This allows for access to the stomach away from the vessels at the greater and lesser curvature and below the costal cartilage which can be a source of pain if traversed during placement. 27 The angle of access should be toward the pylorus to facilitate conversion to gastrojejunostomy tube at a later date, if necessary. After sterile preparation and draping of the area, 1 or 2% lidocaine is used to anesthetize the puncture site.
Gastropexy
The use of gastropexy to secure the stomach to the abdominal wall is a source of controversy. To perform gastropexy, one to four T-fasteners (Boston Scientific, Natick, MA) or Cope Suture Wire Anchors (Cook Medical, Bloomington, IN) are deployed through a needle system, into the stomach. After puncture, intraluminal position is confirmed by the aspiration of air and the fasteners are deployed into the stomach by use of an inner stylet or guidewire. After the needle used to place the fasteners is removed, traction is applied to a suture attached to the fastener, approximating the stomach to the abdominal wall. The fastener is cinched into place with the use of a clip. 28 29 30 When gastropexy has been performed, the access site to the stomach is adjacent to or located central to the fasteners.
Advocates of gastropexy report a decreased risk of extraluminal catheter placement, decreased pericatheter leakage, and earlier tract formation from the stomach to anterior abdominal wall. Early maturation of the tract makes replacement of the catheter easier if it is inadvertently removed during the early days after placement. 13 18 27 31 32 33 In addition, some advocate that gastropexy decreases the risk of periprocedural gastric hemorrhage by acting as a tamponade thereby making placement of large bore catheters safe. 18 Others believe there is no justification for the routine use of gastropexy and some associate gastropexy with an increased rate of pericatheter leakage, gastrocutaneous fistulas, and peristomal infection. 18 34 35 Data in the literature are discordant with accounts supporting the successful and safe placement of gastrostomies with the use of no gastropexy and gastropexy with one, two, or three fasteners. 33 36 37 38 39 Accordingly, current use of gastropexy is based on institutional or operator preference.
Transabdominal Gastrostomy Placement
A skin incision is made at the predetermined catheter entrance site and the subcutaneous tissues are gently dissected to prepare for passage of the catheter. An 18-gauge needle with surrounding catheter is inserted through the incision used to puncture the stomach under fluoroscopic guidance. Tenting of the stomach wall indicates proper location of the needle at the outer wall of the stomach. After puncture, intraluminal placement can be confirmed by easy aspiration of air through the needle. The needle is removed while the catheter is advanced into the stomach lumen. A stiff guidewire is advanced through the catheter and coiled within the stomach. Serial dilation of the tract allows for placement of an appropriately sized gastrostomy. The gastrostomy is advanced over the guide wire and maintained in position with the use of a pigtail or balloon, depending on the type of catheter chosen. Injection of contrast through the catheter under fluoroscopic guidance confirms intraluminal position of the catheter.
Transoral Image-Guided Gastrostomy Placement
As described earlier, transoral placement of gastrostomies involves antegrade placement of a gastrostomy catheter, which is introduced through the oral cavity and pulled through to the anterior abdominal wall incision site by the use of a suture. Of note, there have been some cases of tract seeding in patients with head and neck cancers with transoral placement of catheters, suggesting transabdominal placement be used in these cases. 40 Transoral technique traditionally allows for initial placement of larger bore catheters than transabdominal approach; however, placement of larger catheters by transabdominal approach is becoming more common. 39 Most interventional radiology departments predominantly use the transabdominal approach.
Special Considerations in Gastrostomy Placement
Peritoneal Carcinomatosis
Peritoneal carcinomatosis presents with tumor implants and omental caking that may manifest as a hard, tense abdomen. Once considered a contraindication to percutaneous gastrostomy, it is no longer regarded as such and modifications in technique can assist in any difficulty this condition causes during placement. 8 A safe window to the stomach may be difficult to determine, as implants may obscure the underlying peritoneal anatomy and in some cases, imaging by CT may be helpful. 10 Operators should be aware that the sensation of passing through hard tissue may occur and longer needles may be needed to pass through the tumor ( Fig. 1 ).
Fig. 1.
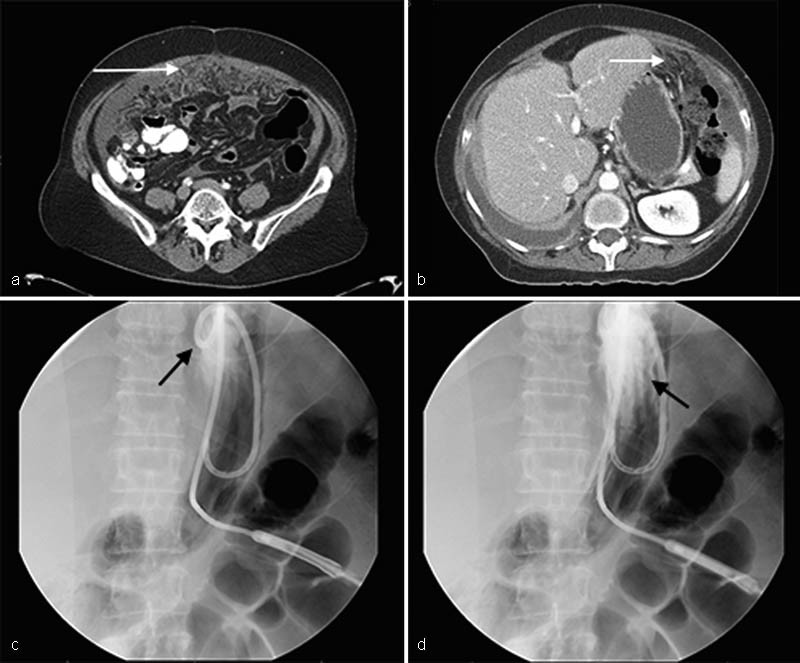
Decompressive gastrostomy placement in a 55-year-old woman with ovarian carcinoma, bowel obstruction, and peritoneal carcinomatosis. Axial contrast-enhanced computed tomographic images show ( a ) omental carcinomatosis inferoanteriorly in the peritoneal cavity (white arrow) and ( b ) omental carcinomatosis extending superiorly, anterior to the stomach, along the projected path of gastrostomy access (white arrow). ( c ) Single fluoroscopic image shows gastrostomy successfully placed into the stomach with the locking loop of the catheter overlying the gastric fundus (black arrow). ( d ) Contrast injection under fluoroscopy opacifies gastric rugae (black arrow) confirming intraluminal position of the gastrostomy.
Ascites
Ascites was considered a contraindication to gastrostomy, as it confers an increased risk of leakage and peritonitis. Many studies have shown that gastrostomy can be placed safely in the setting of ascites, provided that paracentesis is performed. 8 10 41 42 In a recent study published in the Journal of Palliative Medicine , O'Connor and colleagues reviewed gastrostomy and gastrojejunostomy catheter placement in 69 patients with ascites and concluded that effective gastrostomy catheter placement is possible in patients with large volume ascites as long as the ascites is drained before procedure and a gastrocutaneous fistula can successfully be formed. 43 If ascites is recurrent, serial postprocedure imaging may be used to evaluate if ascitic fluid has reaccumulated and repeat paracentesis may be performed to ensure catheter tract maturation in the setting of large volume ascitic fluid. 10 Although there is controversy over the use of gastropexy, it is general consensus that use of gastropexy in the setting of ascites is advantageous as it assists in securing the catheter to the abdominal wall in the setting of excess fluid. 10 18 44 ( Fig. 2 )
Fig. 2.
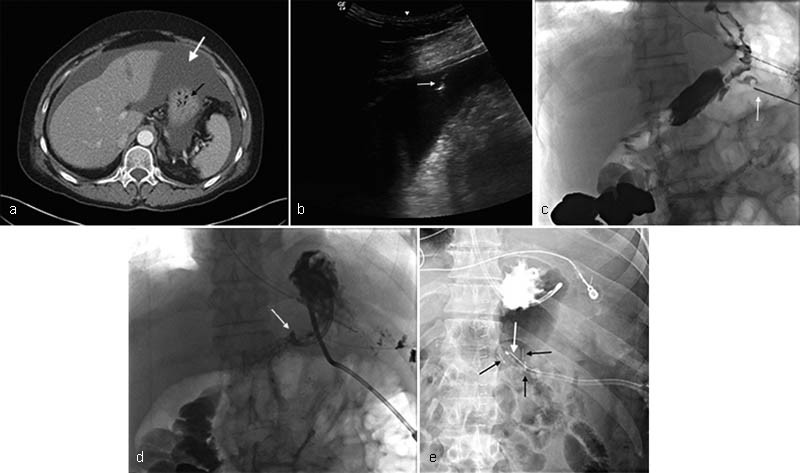
Decompressive gastrostomy placement in a 57-year-old woman with ovarian carcinoma, bowel obstruction, and recurrent ascites. ( a ) Axial contrast-enhanced computed tomographic image demonstrates the presence of ascites (white arrow). The fluid surrounds the stomach (black arrow). ( b ) Gray-scale sonographic image immediately prior to gastrostomy placement shows the tip of an 18-cauge needle (white arrow) entering anechoic ascitic fluid. Large volume paracentesis was performed with removal of 3,500 mL clear yellow fluid. The patient was well known to the IR department and she did not require paracenteses more frequently than every 4 weeks; the decision was made to not use gastropexy during placement. ( c ) A single fluoroscopic image shows access being obtained to the stomach (white arrow) without gastropexy, two-thirds of the way down the gastric body, between the greater and lesser curvature with some distance between the 12th anterior rib and the access site, suggesting placement below the costal cartilage. ( d ) Single fluoroscopic image shows the tip of the newly placed gastrostomy catheter tenting the inner wall of the now decompressed stomach. (white arrow). ( e ). In a different patient with recurrent ascites, a single fluoroscopic image shows three gastropexy fasteners (black arrows) apposing the stomach to the abdominal wall. Access to the stomach was obtained in the center of a triangle made by the three fasteners and the balloon-type gastrostomy catheter can be seen entering the stomach in this area.
Colonic or Hepatic Interposition
Patients with interposition of the colon or liver between the stomach and the anterior abdominal wall may have gastrostomy catheters placed via an infracolic route. 45 In a small study by Cantwell and colleagues, five patients with anatomy prohibitive of typical supracolic gastrostomy catheter placement safely underwent placement of infracolic percutaneous gastrostomy. 46 Of note, gastropexy is not recommended when taking an infracolic route, as there is a risk of resultant mechanical bowel obstruction. 47 Patients with neurologic disorders may suffer from superior displacement of the stomach. An intercostal or subcostal angled route via the lowest anterior intercostal space has been described by Thornton and colleagues. 48 In the minimal number of cases where the colon cannot be displaced either superiorly or inferiorly from a position between the stomach and the anterior abdominal wall, surgical gastrostomy placement may be necessary. 15
Prior Gastric Surgery
Previous gastric surgery can make percutaneous gastrostomy catheter placement challenging. In some cases, gastrojejunal or other postsurgical anastomoses make insufflation of the stomach unsafe. In other cases, altered anatomy and location make percutaneous access to the stomach seem impossible. Previously, gastric surgery was considered a contraindication to gastrostomy, but it is no longer regarded as such. Most of the time, challenges can be overcome and access obtained through modifications in technique including CT guidance, use of rotational angiography, use of longer needles to access the desirable puncture location and even access via a transhepatic route 18 ( Fig. 3 ).
Fig. 3.
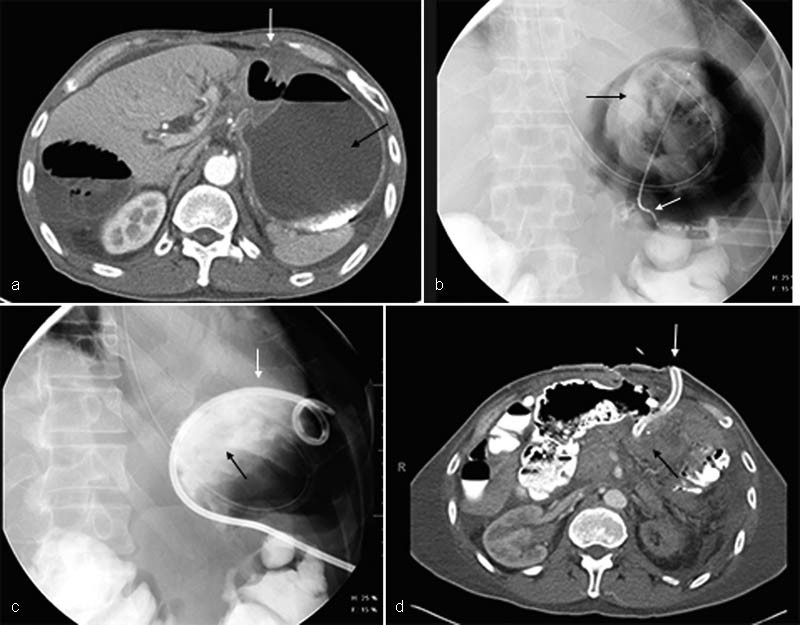
Decompressive gastrostomy placement in a 57-year-old man with a history of gastric cancer post–Billroth II procedure 1-year previously. The patient presented to the emergency room with symptoms and imaging findings consistent with gastric outlet obstruction. ( a ) Axial contrast-enhanced computed tomographic image shows a dilated fluid-filled stomach (black arrow) with a small safe window anteriorly. ( b ) Fluoroscopic image shows access has been obtained to the stomach, despite altered anatomy from prior surgery. A small catheter is in place (white arrow) and injected contrast confirms intraluminal position (black arrow). ( c ) Fluoroscopic image confirms gastrostomy (white arrow) placement into the stomach, as newly injected contrast (black arrow) is visualized in the gastric fundus. ( d ) Axial contrast-enhanced image of the gastrostomy entrance site demonstrates successful placement of the catheter into the stomach despite its altered anatomy (white arrow). There has been successful decompression of the previously dilated, fluid-filled stomach (black arrow).
Complications of Gastrostomy Placement
Minor complications of gastrostomy placement include superficial stomal bleeding, pericatheter stomal infection, excessive granulation tissue formation at the stomal site, catheter occlusion, catheter dislodgement, and pericatheter leakage. Major complications include severe hemorrhage, bowel perforation, peritonitis, abscess formation, and deep skin infections. 49 In a meta-analysis of publications on gastrostomy catheter placement, Wollman and colleagues reported a major complication rate of image-guided gastrostomies of up to 5.9% and a minor complication rate of 7.8%. 50
Outcomes
While a substantial amount of literature exists on placement of percutaneous gastrostomies, there is a relative paucity of research specifically addressing the use gastrostomies as a palliative treatment for bowel obstruction. In the current literature, for purposes of discussion and comparison, data are usually derived from subsets of patients in a larger cohort who received percutaneous gastrostomies for a variety of reasons or appropriated from publications on percutaneous endoscopic gastrostomy placement for decompression.
Decompressive gastrostomy catheters, placed either endoscopically or by image guidance, relieve nausea and vomiting in approximately 84 to 100% of patients with bowel obstruction. 8 11 12 In a meta-analysis of 14 studies, DeEulis and Yennurajalingam determined that whether gastrostomies were placed surgically ( n = 69), endoscopically, or by image guidance ( n = 700), patients experienced a successful rate of symptom resolution. 51 While many of the studies concentrated on decompression of malignant bowel obstruction, decompression of nonmalignant disease has also proven successful. In a study by Daigle and colleagues, 72 out of 72 patients who received gastrostomy catheters for nonmalignant gastrointestinal disease were successfully decompressed and discharged home with gastrostomies in place. 14
Decompressive gastrostomy catheters not only relieve obstruction and obstructive symptoms but also can allow for resumption of per oral nutritional intake. Out of 72 patients who received gastrostomies in the study by Daigle and colleagues, 36 patients (50%) who were discharged on TPN experienced eventual complete resolution of mechanical obstruction and return to oral intake, after a median of 51 days postdischarge. The re-admittance rate after gastrostomy placement is remarkably low. In the study by Daigle and colleagues, only 9 of 72 patients were readmitted within 30 days after discharge, 6 of who were admitted for issues not related to gastrostomy placement. 14 In a study by Issaka and colleagues, 30 of 32 patients (93%) who responded well to nasogastric decompression and ultimately underwent gastrostomy placement had no new hospitalizations after discharge. 8
The positive clinical response to nasogastric tube placement in this and other studies suggests a correlation between symptomatic improvement with use of a nasogastric and success of gastrostomy in palliating symptoms. 8 44 Accordingly, in some departments, the patient's response to nasogastric tube placement is a highly weighted factor in the decision to place a gastrostomy. 44
Although not much data have been gathered on the subject of catheter size and efficacy of decompression, it seems intuitive that larger catheter sizes would allow for quicker decompression with a lower rate of catheter obstruction. Transoral placement of catheters has historically been considered more stable than transabdominal placement, as the lower risk of malposition allows for the safe placement of larger bore catheters than those placed by transabdominal approach. In a subset of studies reviewed by DeEulis and Yennurajalingam where catheter size was recorded, 280 patients received a large bore catheter of 20F or above by endoscopic placement, while 311 patients received a catheter under 20F by image-guided placement. There was a similar rate of symptomatic control regardless of catheter size or technique of placement. 51 In a study by Mohan and colleagues, initial placement of 485 image-guided gastrostomies or gastrojejunostomies ranging from 10 to 18F with minimal major and minor complications (0.2 and 0.6%, retrospectively) demonstrates that placement of relatively large bore catheters by image guidance is safe and effective. 39 The connection between catheter size and successful decompression needs further evaluation.
The use of decompressive gastrostomies for patients with bowel obstruction in need of palliative treatment is increasing and recommendations for decompressive percutaneous gastrostomy placement are now included in some institutional guidelines for enteral access and treatment of bowel obstruction. 3 52 Despite this growth, decompressive gastrostomies remain underutilized, possibly affected by a wider underutilization of palliative services. 2 3 In a retrospective cohort study of elderly Medicare patients with malignant bowel obstruction, Lilley and colleagues discovered that after initial admission for malignant bowel obstruction, these patients had a median survival of 3 months but fewer than 5% had a palliative care consultation. Of patients admitted multiple times for malignant bowel obstruction, only 20% underwent placement of a decompressive gastrostomy. Lilley and colleagues discuss other ways in which decompressive gastrostomies assist in delivering quality palliative care. A discussion with patient and family about the appropriateness of gastrostomy placement can serve as an introduction to further discussions on end-of-life care goals. Patients with decompressive gastrostomies do not require long-term use of nasogastric catheters and are more likely able to tolerate further palliative treatment, as they are no longer symptomatic. Patients with gastrostomies go for hospice care more often, spend less time in the intensive care unit, and have fewer in-hospital deaths, which are all quality metrics for end-of-life care of cancer patients, as they have been shown to result in a more favorable end-of-life experience for both patients and their families. 2
Decompressive Cecostomy
Cecostomies offer artificial access to the cecum by transabdominal catheter insertion. 15 Crass and colleagues published the first report of image-guided placement of a catheter into the cecum, which was used to treat Ogilvie's syndrome (idiopathic colonic pseudoobstruction) in 1985. 5 The standard treatment for Ogilvie's syndrome is supportive care followed by colonoscopic decompression and, if treatment is unsuccessful, surgical decompression. Crass and colleagues successfully placed a cecostomy in a patient who had failed colonoscopic decompression but was a poor surgical candidate. The cecostomy decompressed the patients' tense, distended abdomen immediately, colonic perforation did not occur, and a small amount of persistent abdominal distention resolved slowly over the next few days. 5
Indications for Placement of Decompressive Cecostomy
Obstruction is highly prevalent in the cancer population (3–15%) with 33% of these cases involving the large bowel. 2 Nonmalignant causes of colonic obstruction include Ogilvie's syndrome, degenerative neurologic conditions, cecal volvulus, and pseudomembranous colitis. 4 13 53 54 The main treatment goal of cecostomy is to prevent unwanted bowel perforation by decompression of the colon. Cecostomies also relieve pain, bloating, and discomfort associated with colonic obstruction. 10 55
Contraindications to Cecostomy
Contraindications to cecostomy catheter placement are similar to those for other enteral access to the abdomen and include bowel ischemia, active peritonitis, and uncorrectable coagulopathy. Relative contraindications include hemodynamic instability and recent gastrointestinal hemorrhage with persistent risk of serious hemorrhage as in the presence of diffuse varices. 15
Preprocedure Assessment
Patients should cease all oral intake 8 hours prior to the procedure. 56 Threshold laboratory values for coagulation parameters, management of anticoagulation therapies, and antibiotic administration vary per institution. No societal recommendations specific to cecostomy placement have been published. Some literature suggests the intravenous administration of a second-generation cephalosporin alone for antibiotic prophylaxis, while other reports suggest a triple antibiotic regimen consisting of intravenous ampicillin, metronidazole, and gentamicin. 55 56 57
Patients are maintained on a clear liquid diet for 24 to 48 hours prior to placement. 55 56 A bowel prep is then administered, consisting of bisacodyl (Dulcolax; Boehringer Ingelheim Pharmaceuticals, Inc, Ridgefield, CT) and Miralax (Bayer Corporation, Pittsburgh, PA). Severely constipated patients may be admitted to the hospital for administration of bowel preparation consisting of bisacodyl and a polyethylene glycol solution (GoLYTELY; Braintree Laboratories, Inc., Braintree, MA). If a patient is unable to have a bowel movement within 12 hours of the procedure, this preparation can be administered again with the addition of enemas. 56 If patients are suffering from colonic pseudoobstruction, endoscopically guided lavage may be performed instead of oral bowel preparation. 10
Prior to cecostomy placement, abdominal CT should be performed and reviewed to determine the location of the cecum. 56 In patients who have anatomy not well delineated on CT, a barium enema may be useful. 58
Technique
Cecostomies are placed with the patient under monitored moderate sedation. A 22F silicone catheter is placed into the rectum and a retention balloon on the catheter is inflated. An access path to the cecum is chosen via fluoroscopic imaging and the area is prepped and draped under sterile conditions. Air is administered through the 22F silicone catheter under fluoroscopic guidance, until sufficient filling of the cecum is visualized. 56 Care should be taken to avoid overdistention of the cecum, as patients with obstruction and/or malignant processes are likely at increased risk of perforation. 10 After administration of lidocaine for local anesthesia at the proposed entry site, the cecum is accessed under fluoroscopic guidance. Based on operator preference, from zero to three T-fasteners (Boston Scientific) or Cope Suture Wire Anchors (Cook Medical) are deployed within the cecum and used to approximate the cecal wall to the abdominal wall. 55 56 After fastener placement, the cecum accessed with an 18-gauge needle. 56 Contrast injected through the needle under fluoroscopy is used to confirm intracecal positioning of the needle. The needle is removed and its outer catheter advanced into the cecum. A 0.035-inch guidewire is advanced through the catheter and coiled within the cecum. The needle catheter is exchanged out for a drainage catheter. The catheter is locked and intracecal position is confirmed with contrast injection under fluoroscopy. 55 56 The catheter is attached to a bag, which allows for gravity drainage. Small punctures may be made in the drainage bag to facilitate exiting of air from the bag, preventing overfilling of the bag with colonic gas which may cause the bag to rupture or prevent effective drainage due to backpressure on the catheter 55 ( Fig. 4 ).
Fig. 4.
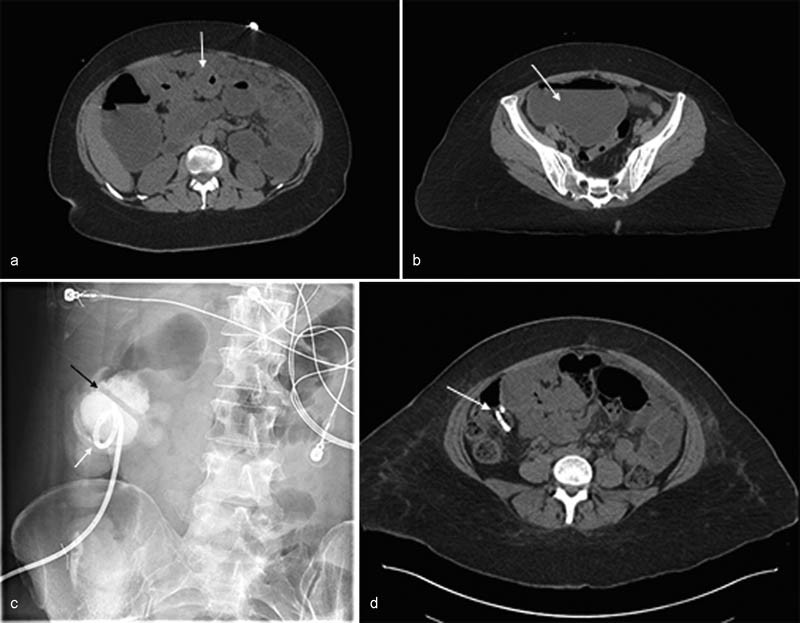
Decompressive cecostomy placement in a 39-year-old woman with adenocarcinoma of the colon and bowel obstruction. ( a ) Axial non–contrast-enhanced computed tomographic (CT) image demonstrates an eccentric mass within the transverse colon consistent with patients' known history of colonic adenocarcinoma (white arrow). ( b ) Axial non–contrast-enhanced CT image from the pelvic region shows a dilated fluid-filled cecum (white arrow) secondary to the distal transverse colonic obstruction. The cecum measures up to 12.7 cm. ( c ) Fluoroscopic image shows the locking loop of the newly placed pigtail catheter (white arrow) within the cecum. Contrast outlines haustral markings (black arrow), confirming intracolonic location of the catheter. ( d ) Axial non–contrast-enhanced CT image taken 5 days after the procedure shows the presence of the cecostomy within a decompressed cecum that no longer contains fluid and is of normal caliber (white arrow).
The indwelling catheter can be exchanged for a Chait trapdoor cecostomy catheter 6 weeks after the placement. 58 59
Complications of Cecostomy Placement
Minor complications include superficial hemorrhage, pericatheter leakage, and excessive granulation tissue formation. Major complications include severe hemorrhage and peritonitis. 60 A concern for intraperitoneal spill of fecal contents with resulting peritonitis has been a subject of discussion in the literature. 2 5 It is believed that cecopexy with the use of T-fasteners, as described earlier, may decrease the incidence of peristomal leakage of fecal contents. While the use of cecopexy is based on operator preference in some institutions, many sources describing cecostomy placement include cecopexy as a routine step of the procedure or overtly recommend use of cecopexy to specifically prevent inadvertent fecal spillage. 4 10 15 54 55 58 59 61
Outcomes
Much of the early data on cecostomy catheter placement focuses on treating symptoms of chronic constipation and/or fecal incontinence in the pediatric population, with some mention of these conditions in the adult population. 57 58 59 61 62 The research on percutaneous cecostomy used for decompression of bowel obstruction is relatively sparse, but promising.
Most literature on decompressive cecostomies discusses the use of the catheter in preventing impending colonic perforation in a dilated obstructed colon. 4 5 53 54 63 64 More recent data have focused on the palliative role of cecostomies in providing symptomatic relief of abdominal bloating and pain associated with bowel obstruction. 10 55 56 Cecostomies may also be used as a temporizing measure to palliate symptoms while awaiting a more decisive treatment for the underlying bowel obstruction. 55
The end-stage cancer population experiences a 3 to 15% rate of bowel obstruction. The small bowel is commonly involved (61%), but colonic obstruction remains significant at 33%. 12 The cause of obstruction are many, with not only malignant causes but also benign causes as a result of the presence of malignancy, including adhesions, hernia, and post–radiation therapy bowel damage. Tewari and colleagues published on the use of cecostomy catheters to treat 27 patients with malignant large bowel obstruction. The rationale behind cecostomy placement was decompression of the colon to prevent perforation; however, in this study, specific attention is also given to the subject of palliation of bowel obstruction in this high-risk population. Of the 27 patients, no cecal perforation occurred postcecostomy. In 24 of the 27 patients (89%), medical records documented relief of pain. Sixteen of the 24 patients died with the catheter in place over an average of 123 days after the procedure. In eight patients, the catheter was removed after a mean of 29 days and three patients had the catheter removed during colostomy, which was performed for reasons other than technical failure of the indwelling cecostomy catheter. 55 Though the study group is small, these data support the use of cecostomy catheters as a safe and effective treatment to prevent colonic perforation, treat obstructive symptoms for both longer and shorter terms at end of life, and bridge patients to more definitive treatment.
Marker and colleagues studied safety and effect on quality of life when cecostomy catheters were placed in adults for chronic constipation and fecal incontinence. The results showed a statistically significant increase in satisfaction scores and mean quality-of-life scores. 56 A comparison is difficult, as these catheters were placed to prevent soiling events and offer patients more independence rather than to resolve discomfort and pain. Although not placed to relieve obstructive symptoms, the results demonstrate a favorable experience for patients who underwent placement of a cecostomy and lived with a cecostomy in place. This suggests that having a cecostomy in place does not necessarily result in dissatisfaction or discomfort and those benefits conferred by cecostomy catheter placement may outweigh risks. Studies are needed to determine quality-of-life outcomes and pain relief in patients with bowel obstruction treated with decompressive cecostomies.
In the spectrum of treatment for colonic obstruction, percutaneous cecostomy placement is still new. In patients with Ogilvie's syndrome, when cessation of oral intake, nasogastric catheter placement, and pharmacologic therapies fail, colonoscopic decompression is usually the next step with or without endoscopic placement of a cecostomy catheter. Unfortunately, the reported rate of symptomatic recurrence after colonoscopic decompression has been 15 to 40%. 4 53 The patient can undergo repeat colonoscopic decompression but after failure of endoscopic treatment(s) and/or if the cecum remains dilated to 10 to 12 cm, the patient may be referred for surgical decompression which, while generally successful (90% success rate), also confers high morbidity and mortality rates of 30 and 6%, respectively. 4 In quite a few published series of patients with Ogilvie's syndrome or other causes of significant colonic dilation, percutaneous cecostomy placement was performed and successfully prevented colonic perforation in patients with severe colonic dilation. 4 5 60 63
Endoscopically placed colonic stents may be used to treat malignant bowel obstruction refractory to supportive and pharmacologic measures. In the study on cecostomies for malignant bowel obstruction by Tewari and colleagues, 3 of 27 patients failed pharmacologic therapy, 24 had surgical consultations but were considered poor candidates, and 26 patients had gastroenterology consultations. Six of the 26 patients evaluated by gastroenterologists underwent lower endoscopy, of which 3 had obstructions that were not amenable to stent placement and 3 had stents placed without resolution of symptoms. After these attempts, percutaneous cecostomy placement was performed in all 27 patients with a 100% technical success rate and relief of symptoms in 89% of patients. 55
Currently, recommendations for cecostomy catheter placement are sparse, but the high recurrence rate after endoscopic decompression, the significant morbidity and mortality associated with surgical interventions, the presence of likely concomitant comorbidities in this population that may prevent surgical intervention, and apparent limitations of endoscopic stent placement indicate that there is a gap in the current treatment paradigm. Decompressive cecostomy placement offers a minimally invasive, safe, and effective way to prevent bowel perforation, palliate symptoms, and temporize the patient while awaiting more definitive therapy. As more data are acquired, recommendations on the use of decompressive cecostomy catheter placement should be better delineated in the literature.
Conclusion
Both gastrostomy and cecostomy catheters developed into decompressive treatments for bowel obstruction over just the past 30 years. There remains a paucity of data on the use of gastrostomy and cecostomy tube placement as a decompressive and palliative treatment for bowel obstruction. The available data support the use of these procedures in the treatment of malignant and nonmalignant bowel obstruction.
The use of gastrostomies and cecostomies as a palliative treatment is of particular interest, especially since malignant bowel obstruction is common and is a strong predictor of prognosis. In these and other nonmalignant cases with long-term obstructive symptoms, gastrostomies and cecostomies may be able to have a great effect, by improving patient-centered outcomes, quality of life, and end-of-life care. Current Interventional Radiology and palliative care literature supports the use of decompressive catheters placed by image guidance, as more invasive surgical and even endoscopic procedures may be contraindicated or result in further distress to the already compromised patient. Although more data are needed, it has already been shown that gastrostomies and cecostomies are safe and effective in decompressing the bowel and relieving the associated symptoms, thus improving the end-of-life quality measures.
Footnotes
Conflict of Interest The authors report no financial or nonfinancial conflicts.
References
- 1.Zerey M, Sechrist C W, Kercher K W, Sing R F, Matthews B D, Heniford B T.The laparoscopic management of small-bowel obstruction Am J Surg 200719406882–887., discussion 887–888 [DOI] [PubMed] [Google Scholar]
- 2.Lilley E J, Scott J W, Goldberg J Eet al. Survival, healthcare utilization, and end-of-life care among older adults with malignancy-associated bowel obstruction: comparative study of surgery, venting gastrostomy, or medical management Ann Surg 2017 10.1097/SLA.0000000000002164. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laval G, Marcelin-Benazech B, Guirimand F et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48(01):75–91. doi: 10.1016/j.jpainsymman.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Chevallier P, Marcy P Y, Francois E et al. Controlled transperitoneal percutaneous cecostomy as a therapeutic alternative to the endoscopic decompression for Ogilvie's syndrome. Am J Gastroenterol. 2002;97(02):471–474. doi: 10.1111/j.1572-0241.2002.05457.x. [DOI] [PubMed] [Google Scholar]
- 5.Crass J R, Simmons R L, Frick M P, Maile C W. Percutaneous decompression of the colon using CT guidance in Ogilvie syndrome. AJR Am J Roentgenol. 1985;144(03):475–476. doi: 10.2214/ajr.144.3.475. [DOI] [PubMed] [Google Scholar]
- 6.Wills J S, Oglesby J T. Percutaneous gastrostomy. Radiology. 1983;149(02):449–453. doi: 10.1148/radiology.149.2.6414043. [DOI] [PubMed] [Google Scholar]
- 7.Tao H H, Gillies R R. Percutaneous feeding gastrostomy. AJR Am J Roentgenol. 1983;141(04):793–794. doi: 10.2214/ajr.141.4.793. [DOI] [PubMed] [Google Scholar]
- 8.Issaka R B, Shapiro D M, Parikh N D et al. Palliative venting percutaneous endoscopic gastrostomy tube is safe and effective in patients with malignant obstruction. Surg Endosc. 2014;28(05):1668–1673. doi: 10.1007/s00464-013-3368-7. [DOI] [PubMed] [Google Scholar]
- 9.Zucchi E, Fornasarig M, Martella L et al. Decompressive percutaneous endoscopic gastrostomy in advanced cancer patients with small-bowel obstruction is feasible and effective: a large prospective study. Support Care Cancer. 2016;24(07):2877–2882. doi: 10.1007/s00520-016-3102-9. [DOI] [PubMed] [Google Scholar]
- 10.Holm A N, Baron T H. Palliative use of percutaneous endoscopic gastrostomy and percutaneous endoscopic cecostomy tubes. Gastrointest Endosc Clin N Am. 2007;17(04):795–803. doi: 10.1016/j.giec.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Kawata N, Kakushima N, Tanaka M et al. Percutaneous endoscopic gastrostomy for decompression of malignant bowel obstruction. Dig Endosc. 2014;26(02):208–213. doi: 10.1111/den.12139. [DOI] [PubMed] [Google Scholar]
- 12.Ripamonti C, Twycross R, Baines M et al. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Support Care Cancer. 2001;9(04):223–233. doi: 10.1007/s005200000198. [DOI] [PubMed] [Google Scholar]
- 13.Scheidbach H, Horbach T, Groitl H, Hohenberger W. Percutaneous endoscopic gastrostomy/jejunostomy (PEG/PEJ) for decompression in the upper gastrointestinal tract. Initial experience with palliative treatment of gastrointestinal obstruction in terminally ill patients with advanced carcinomas. Surg Endosc. 1999;13(11):1103–1105. doi: 10.1007/s004649901182. [DOI] [PubMed] [Google Scholar]
- 14.Daigle C R, Boules M, Corcelles R et al. Percutaneous endoscopic gastrostomy for decompression of nonmalignant gastrointestinal disease. J Laparoendosc Adv Surg Tech A. 2015;25(10):804–807. doi: 10.1089/lap.2014.0619. [DOI] [PubMed] [Google Scholar]
- 15.Itkin M, DeLegge M H, Fang J C et al. Multidisciplinary practical guidelines for gastrointestinal access for enteral nutrition and decompression from the Society of Interventional Radiology and American Gastroenterological Association (AGA) Institute, with endorsement by Canadian Interventional Radiological Association (CIRA) and Cardiovascular and Interventional Radiological Society of Europe (CIRSE) Gastroenterology. 2011;141(02):742–765. doi: 10.1053/j.gastro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Gassas A, Kennedy J, Green G et al. Risk of ventriculoperitoneal shunt infections due to gastrostomy feeding tube insertion in pediatric patients with brain tumors. Pediatr Neurosurg. 2006;42(02):95–99. doi: 10.1159/000090462. [DOI] [PubMed] [Google Scholar]
- 17.Sane S S, Towbin A, Bergey E A et al. Percutaneous gastrostomy tube placement in patients with ventriculoperitoneal shunts. Pediatr Radiol. 1998;28(07):521–523. doi: 10.1007/s002470050401. [DOI] [PubMed] [Google Scholar]
- 18.Lyon S M, Pascoe D M. Percutaneous gastrostomy and gastrojejunostomy. Semin Intervent Radiol. 2004;21(03):181–189. doi: 10.1055/s-2004-860876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel I J, Davidson J C, Nikolic B et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23(06):727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Clark J A, Pugash R A, Pantalone R R. Radiologic peroral gastrostomy. J Vasc Interv Radiol. 1999;10(07):927–932. doi: 10.1016/s1051-0443(99)70140-5. [DOI] [PubMed] [Google Scholar]
- 21.Laasch H U, Wilbraham L, Bullen K et al. Gastrostomy insertion: comparing the options--PEG, RIG or PIG? Clin Radiol. 2003;58(05):398–405. doi: 10.1016/s0009-9260(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 22.Szymski G X, Albazzaz A N, Funaki B et al. Radiologically guided placement of pull-type gastrostomy tubes. Radiology. 1997;205(03):669–673. doi: 10.1148/radiology.205.3.9393519. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein O A, Campbell J, Rajan D Ket al. Randomized trial comparing radiologic pigtail gastrostomy and peroral image-guided gastrostomy: intra- and postprocedural pain, radiation exposure, complications, and quality of life J Vasc Interv Radiol 201526111680–1686., quiz 1686 [DOI] [PubMed] [Google Scholar]
- 24.Venkatesan A M, Kundu S, Sacks Det al. Practice guidelines for adult antibiotic prophylaxis during vascular and interventional radiology procedures[corrected]J Vasc Interv Radiol 201021111611–1630., quiz 1631 [DOI] [PubMed] [Google Scholar]
- 25.Cantwell C P, Perumpillichira J J, Maher M M et al. Antibiotic prophylaxis for percutaneous radiologic gastrostomy and gastrojejunostomy insertion in outpatients with head and neck cancer. J Vasc Interv Radiol. 2008;19(04):571–575. doi: 10.1016/j.jvir.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Möhlenbruch M, Nelles M, Thomas D et al. Cone-beam computed tomography-guided percutaneous radiologic gastrostomy. Cardiovasc Intervent Radiol. 2010;33(02):315–320. doi: 10.1007/s00270-009-9641-4. [DOI] [PubMed] [Google Scholar]
- 27.de Baere T, Chapot R, Kuoch V et al. Percutaneous gastrostomy with fluoroscopic guidance: single-center experience in 500 consecutive cancer patients. Radiology. 1999;210(03):651–654. doi: 10.1148/radiology.210.3.r99mr40651. [DOI] [PubMed] [Google Scholar]
- 28.Brown A S, Mueller P R, Ferrucci J T., Jr Controlled percutaneous gastrostomy: nylon T-fastener for fixation of the anterior gastric wall. Radiology. 1986;158(02):543–545. doi: 10.1148/radiology.158.2.2934763. [DOI] [PubMed] [Google Scholar]
- 29.Coleman C C, Coons H G, Cope Cet al. Percutaneous enterostomy with the Cope suture anchor Radiology 1990174(3, Pt 1):889–891. [DOI] [PubMed] [Google Scholar]
- 30.Cope C. Suture anchor for visceral drainage. AJR Am J Roentgenol. 1986;146(01):160–162. doi: 10.2214/ajr.146.1.160. [DOI] [PubMed] [Google Scholar]
- 31.Dewald C L, Hiette P O, Sewall L E, Fredenberg P G, Palestrant A M. Percutaneous gastrostomy and gastrojejunostomy with gastropexy: experience in 701 procedures. Radiology. 1999;211(03):651–656. doi: 10.1148/radiology.211.3.r99ma04651. [DOI] [PubMed] [Google Scholar]
- 32.Given M F, Lyon S M, Lee M J. The role of the interventional radiologist in enteral alimentation. Eur Radiol. 2004;14(01):38–47. doi: 10.1007/s00330-003-1911-y. [DOI] [PubMed] [Google Scholar]
- 33.Thornton F J, Fotheringham T, Haslam P J, McGrath F P, Keeling F, Lee M J. Percutaneous radiologic gastrostomy with and without T-fastener gastropexy: a randomized comparison study. Cardiovasc Intervent Radiol. 2002;25(06):467–471. doi: 10.1007/s00270-001-0089-4. [DOI] [PubMed] [Google Scholar]
- 34.Funaki B, Zaleski G X, Lorenz J et al. Radiologic gastrostomy placement: pigtail- versus mushroom-retained catheters. AJR Am J Roentgenol. 2000;175(02):375–379. doi: 10.2214/ajr.175.2.1750375. [DOI] [PubMed] [Google Scholar]
- 35.Kavin H, Messersmith R. Radiologic percutaneous gastrostomy and gastrojejunostomy with T-fastener gastropexy: aspects of importance to the endoscopist. Am J Gastroenterol. 2006;101(09):2155–2159. doi: 10.1111/j.1572-0241.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 36.Akinci D, Ciftci T T, Kaya D, Ozmen M N, Akhan O. Long-term results of percutaneous radiologic gastrostomy and gastrojejunostomy in children with emphasis on technique: single or double gastropexy? AJR Am J Roentgenol. 2010;195(05):1231–1237. doi: 10.2214/AJR.09.4042. [DOI] [PubMed] [Google Scholar]
- 37.Deutsch L S, Kannegieter L, Vanson D T, Miller D P, Brandon J C. Simplified percutaneous gastrostomy. Radiology. 1992;184(01):181–183. doi: 10.1148/radiology.184.1.1609078. [DOI] [PubMed] [Google Scholar]
- 38.Jeong E J, Song H Y, Park J H et al. Preliminary results of percutaneous radiologic gastrostomy in a pediatric population: a modified Chiba-needle puncture technique with single gastropexy. AJR Am J Roentgenol. 2015;205(01):W133-7. doi: 10.2214/AJR.14.12543. [DOI] [PubMed] [Google Scholar]
- 39.Mohan P, Dupaix R, Tewari S et al. Abstract no. 225 - Safety and technical success of percutaneous radiologic gastrostomy/gastrojejunostomy placement without gastropexy. J Vasc Interv Radiol. 2017;28(02):S99. [Google Scholar]
- 40.Tucker A T, Gourin C G, Ghegan M D, Porubsky E S, Martindale R G, Terris D J. ‘Push’ versus ‘pull’ percutaneous endoscopic gastrostomy tube placement in patients with advanced head and neck cancer. Laryngoscope. 2003;113(11):1898–1902. doi: 10.1097/00005537-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Lee M J, Saini S, Brink J A, Morrison M C, Hahn P F, Mueller P R. Malignant small bowel obstruction and ascites: not a contraindication to percutaneous gastrostomy. Clin Radiol. 1991;44(05):332–334. doi: 10.1016/s0009-9260(05)81270-x. [DOI] [PubMed] [Google Scholar]
- 42.Ryan J M, Hahn P F, Mueller P R. Performing radiologic gastrostomy or gastrojejunostomy in patients with malignant ascites. AJR Am J Roentgenol. 1998;171(04):1003–1006. doi: 10.2214/ajr.171.4.9762985. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor O J, Diver E, McDermott S et al. Palliative gastrostomy in the setting of voluminous ascites. J Palliat Med. 2014;17(07):811–821. doi: 10.1089/jpm.2013.0397. [DOI] [PubMed] [Google Scholar]
- 44.Shaw C, Bassett R L, Fox P S et al. Palliative venting gastrostomy in patients with malignant bowel obstruction and ascites. Ann Surg Oncol. 2013;20(02):497–505. doi: 10.1245/s10434-012-2643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirich D R, Gray R R. Infracolic percutaneous gastrojejunostomy: technical note. Cardiovasc Intervent Radiol. 1989;12(06):340–341. doi: 10.1007/BF02575435. [DOI] [PubMed] [Google Scholar]
- 46.Cantwell C P, Gervais D A, Hahn P F, Mueller P R. Feasibility and safety of infracolic fluoroscopically guided percutaneous radiologic gastrostomy. J Vasc Interv Radiol. 2008;19(01):129–132. doi: 10.1016/j.jvir.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 47.Hennigan T W, Forbes A. Colonic obstruction caused by endoscopic percutaneous gastrostomy. Eur J Surg. 1992;158(08):435. [PubMed] [Google Scholar]
- 48.Thornton F J, Varghese J C, Haslam P J, McGrath F P, Keeling F, Lee M J. Percutaneous gastrostomy in patients who fail or are unsuitable for endoscopic gastrostomy. Cardiovasc Intervent Radiol. 2000;23(04):279–284. doi: 10.1007/s002700010069. [DOI] [PubMed] [Google Scholar]
- 49.Covarrubias D A, O'Connor O J, McDermott S, Arellano R S. Radiologic percutaneous gastrostomy: review of potential complications and approach to managing the unexpected outcome. AJR Am J Roentgenol. 2013;200(04):921–931. doi: 10.2214/AJR.11.7804. [DOI] [PubMed] [Google Scholar]
- 50.Wollman B, D'Agostino H B, Walus-Wigle J R, Easter D W, Beale A. Radiologic, endoscopic, and surgical gastrostomy: an institutional evaluation and meta-analysis of the literature. Radiology. 1995;197(03):699–704. doi: 10.1148/radiology.197.3.7480742. [DOI] [PubMed] [Google Scholar]
- 51.DeEulis T G, Yennurajalingam S. Venting gastrostomy at home for symptomatic management of bowel obstruction in advanced/recurrent ovarian malignancy: a case series. J Palliat Med. 2015;18(08):722–728. doi: 10.1089/jpm.2014.0355. [DOI] [PubMed] [Google Scholar]
- 52.Sutcliffe J, Wigham A, Mceniff N, Dvorak P, Crocetti L, Uberoi R. CIRSE standards of practice guidelines on gastrostomy. Cardiovasc Intervent Radiol. 2016;39(07):973–987. doi: 10.1007/s00270-016-1344-z. [DOI] [PubMed] [Google Scholar]
- 53.Jain A, Vargas H D. Advances and challenges in the management of acute colonic pseudo-obstruction (Ogilvie syndrome) Clin Colon Rectal Surg. 2012;25(01):37–45. doi: 10.1055/s-0032-1301758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison M C, Lee M J, Stafford S A, Saini S, Mueller P R. Percutaneous cecostomy: controlled transperitoneal approach. Radiology. 1990;176(02):574–576. doi: 10.1148/radiology.176.2.2367677. [DOI] [PubMed] [Google Scholar]
- 55.Tewari S O, Getrajdman G I, Petre E N et al. Safety and efficacy of percutaneous cecostomy/colostomy for treatment of large bowel obstruction in adults with cancer. J Vasc Interv Radiol. 2015;26(02):182–188. doi: 10.1016/j.jvir.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marker D R, Perosi N, Ul Haq F, Morefield W, Mitchell S. Percutaneous cecostomy in adult patients: safety and quality-of-life results. J Vasc Interv Radiol. 2015;26(10):1526–15320. doi: 10.1016/j.jvir.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 57.Khan W U, Satkunasingham J, Moineddin R et al. The percutaneous cecostomy tube in the management of fecal incontinence in children. J Vasc Interv Radiol. 2015;26(02):189–195. doi: 10.1016/j.jvir.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Chait P G, Shlomovitz E, Connolly B L et al. Percutaneous cecostomy: updates in technique and patient care. Radiology. 2003;227(01):246–250. doi: 10.1148/radiol.2271020574. [DOI] [PubMed] [Google Scholar]
- 59.Donkol R H, Al-Nammi A. Percutaneous cecostomy in the management of organic fecal incontinence in children. World J Radiol. 2010;2(12):463–467. doi: 10.4329/wjr.v2.i12.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.vanSonnenberg E, Varney R R, Casola G et al. Percutaneous cecostomy for Ogilvie syndrome: laboratory observations and clinical experience. Radiology. 1990;175(03):679–682. doi: 10.1148/radiology.175.3.2343112. [DOI] [PubMed] [Google Scholar]
- 61.Shandling B, Chait P G, Richards H F. Percutaneous cecostomy: a new technique in the management of fecal incontinence. J Pediatr Surg. 1996;31(04):534–537. doi: 10.1016/s0022-3468(96)90490-x. [DOI] [PubMed] [Google Scholar]
- 62.Chait P G, Shandling B, Richards H M, Connolly B L. Fecal incontinence in children: treatment with percutaneous cecostomy tube placement--a prospective study. Radiology. 1997;203(03):621–624. doi: 10.1148/radiology.203.3.9169678. [DOI] [PubMed] [Google Scholar]
- 63.Casola G, Withers C, vanSonnenberg E, Herba M J, Saba R M, Brown R A. Percutaneous cecostomy for decompression of the massively distended cecum. Radiology. 1986;158(03):793–794. doi: 10.1148/radiology.158.3.3945754. [DOI] [PubMed] [Google Scholar]
- 64.Haaga J R, Bick R J, Zollinger R M., Jr CT-guided percutaneous catheter cecostomy. Gastrointest Radiol. 1987;12(02):166–168. doi: 10.1007/BF01885131. [DOI] [PubMed] [Google Scholar]


