Abstract
Bleeding is a common and often challenging complication of malignancy. Etiologies of hemorrhage in this patient population vary, and bleeding may present as an acute, life-threatening emergency or a chronic, low-volume blood loss. For patients with advanced malignancies, interventions to manage bleeding must be balanced by the patient's life expectancy and quality of life. As such, minimally invasive procedures such as transarterial embolization are useful therapeutic options in appropriately selected patients. There is a rich history of palliative transarterial embolization for refractory bleeding in cancer patients. This technique was first applied in the 1970s and has since become an established treatment tool for malignancy-related bleeding throughout the body. While the preponderance of published data comprised case reports and small retrospective studies, the use of embolization continues to expand as experience grows and techniques are refined. In this review, we summarize the literature and provide our perspective on embolization for refractory bleeding in cancer patients.
Keywords: embolization, palliative care, hemorrhage, malignancy, interventional radiology
Objectives : Upon completion of this article, the reader will be able to discuss how embolization plays an important role in the management of bleeding for patients with advanced malignancy.
Accreditation : This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Bleeding is a common and often challenging complication of malignancy, occurring in up to 10% of patients with advanced cancer. 1 Etiologies of hemorrhage in this patient population vary, from local vessel damage and tumoral vascular invasion to systemic processes such as disseminated intravascular coagulopathy and thrombocytopenia. Vascular injury can occur from prior cancer therapies including chemotherapy, surgery, and radiation therapy, as well as from the tumor itself. 2 Bleeding may present as an acute, life-threatening emergency or a chronic, low-volume blood loss.
For patients with advanced malignancies, interventions to manage bleeding must be balanced by the patient's life expectancy and quality of life. Often, the risk profile of invasive approaches such as surgical debulking or vessel ligation outweighs the potential palliative benefit. As such, minimally invasive procedures are useful therapeutic options in appropriately selected patients. Endoscopy, bronchoscopy, and cystoscopy are important considerations in patients with bleeding involving the upper gastrointestinal (GI) tract, 3 lungs, 4 and bladder, 1 respectively. Bleeding vessels can be cauterized, and bleeding tissue can be injected with sclerosants or vasoconstrictors, through an endoscopic approach. External beam radiation has been shown to improve hemoptysis in lung cancer 5 and is also a treatment option for malignant bleeding involving the vagina, rectum, and bladder. 6 However, patients with pelvic malignancies may have received maximal doses of radiotherapy during their treatment and thus may not be candidates for palliative radiation. Additionally, radiation therapy can result in vascular injury, pseudoaneurysms, and vascular-visceral organ fistulae, and is thus the putative cause of, rather than solution to, pelvic bleeding.
There is a rich history of palliative transarterial embolization for refractory bleeding in cancer patients. This technique was first applied in the 1970s and has since become an established treatment tool for malignancy-related bleeding throughout the body. 4 7 8 9 10 11 12 13 14 While the preponderance of published data comprised case reports and small retrospective studies, the use of embolization continues to expand as experience grows and techniques are refined. 1 In this review, we summarize the literature and provide our perspective on embolization for refractory bleeding in cancer patients.
Lungs: Bronchial Artery Embolization
Massive hemoptysis is a significant risk in patients with chronic lung disease from such diseases as sarcoidosis, tuberculosis, and cystic fibrosis, as well as in patients with primary and metastatic lung cancer. 15 Massive hemoptysis carries roughly a 50 to 100% mortality. 16 The bronchial arteries account for the source of hemorrhage in greater than 90% of cases. 17 Previously, surgical intervention was the only therapeutic option for these patients, although these interventions are associated with high morbidity and mortality. In the 1970s, endovascular bronchial artery embolization was introduced and has improved patient outcomes. 18 Classically, hemoptysis due to benign etiologies (such as chronic lung disease, tuberculosis, or bronchiectasis) is secondary to direct invasion, damage, and/or hypertrophy of the bronchial arteries. Alternatively, in cancer patients, hemoptysis is mostly due to the fragility of the neovascularity within the tumor bed and subsequent rupture of these vessels secondary to inflammation and necrosis rather than direct tumor erosion into the bronchial arteries. 19
It is crucial to understand the anatomy of the bronchial arteries for therapeutic planning as well as to avoid procedural complications. The lungs have a dual blood supply, with the pulmonary arteries accounting for approximately 99% of the blood flow, and the bronchial arteries supplying the remaining 1%. The anatomy of the bronchial arteries is variable. The most common anatomic arrangement is two left and one right bronchial arteries. However, this anatomy is found only in less than 50% of the general population. Right-sided bronchial arteries typically arise from an intercostobrachial trunk, while left-sided bronchial arteries typically arise directly from the aorta. 17 Approximately 85% of bronchial arteries arise between the superior endplate of the T5 vertebral body and the inferior endplate of the T6 vertebral body. These are called “orthotopic” bronchial arteries. 20 Under fluoroscopy, the left main stem bronchus serves as a reasonably reliable landmark for the approximate location of these orthotopic bronchial arteries. Roughly 15% of bronchial arteries may have anomalous origins from the subclavian, internal mammary, innominate, pericardiophrenic, superior intercostal, or inferior phrenic arteries. These bronchial arteries are known as “ectopic.” 20 Another key factor when performing bronchial artery embolization is to identify the spinal cord arterial supply. The anterior spinal artery is the dominant arterial supply to the spinal cord and receives its main contributions from the vertebral arteries. However, the anterior spinal artery also receives contributions from the anterior radiculomedullary branches as well as branches from the lumbar and intercostal arteries. The largest anterior medullary branch (artery of Adamkiewicz) typically arises between T8 and L1 but has been reported to arise anywhere between T5 and L4. 21 These anterior medullary branches have a characteristic “hairpin” loop in which the branches have an ascending course that then enters a descending intraspinal segment via a sharp bend ( Fig. 1 ). This classic hairpin configurations aids in identification of these arterial supplies to the anterior spinal artery. 22 23 It is also important to note that numerous anastomoses exist between the pulmonary arteries and bronchial arteries. This leads to a physiologic right-to-left shunt of approximately 5% of the cardiac output. 21 This is important, as a large shunt may predispose the patient to inadvertent embolization.
Fig. 1.

The classic “hairpin” turn of the anterior spinal artery on angiography (arrow).
In cases of massive hemoptysis with diffuse parenchymal disease, the exact culprit vessel or site of hemorrhage may not be identified. In these cases, multiple or all bronchial arteries should be embolized empirically. We prefer to use calibrated microspheres in the 300- to 500-µm range, avoiding smaller microspheres to theoretically reduce the risk of spinal cord infarction due to an unrecognized collateral supply. We also perform digital subtraction angiograms of at least 6 frames per second intermittently during the embolization, as we have occasionally observed that the spinal artery “reveals itself” once the distal bronchial artery is partially embolized. Cone-beam computed tomography (CT) can also be helpful to visualize small segmental medullary branches.
The most common complication following bronchial artery embolization is transient chest pain. 24 Serious complications are rare, occurring in less than 5% of patients. These serious complications involve inadvertent nontarget embolization. Accidental embolization and damage to adjacent structures, such as bronchial wall, esophageal ischemia, and myocardial infarction, are possible given the robust collateralization present in the thorax. Inadvertent embolization of the median anterior spinal artery (artery of Adamkiewicz) is a very serious complication carrying with it significant morbidity for the patient such as Brown-Sequard syndrome and paraplegia, with an estimated incidence of 0.6 to 6.5%. 25 Other complications involve inadvertent distal embolization from reflux of embolization material into the aorta. Examples of these include ischemic stroke, GI infarction, and splenic hematoma/infarction. Distal limb ischemia at the arterial puncture site is also a known complication that is more prevalent in patients with significant atherosclerotic disease. 24 Long-term recurrence rates range from 10 to 60% and are typically due to recanalization of the occluded artery or due to neovascularization.
Lung: Pulmonary Artery Pseudoaneurysms
Pulmonary pseudoaneurysms (PAPs) are a rare entity that may be caused by a variety of etiologies including necrotizing pneumonia, bacterial endocarditis, mucormycosis, tuberculosis, vasculitis (Behcet's disease), trauma, congenital heart disease, pulmonary hypertension, primary or metastatic lung cancer, or iatrogenic causes. Pseudoaneurysms secondary to lung cancer are due to direct tumoral invasion and vascular wall erosion. 26 The most common cancers associated with pulmonary artery pseudoaneurysms are primary squamous cell carcinoma of the lung, primary sarcoma, and metastatic sarcoma. The pulmonary artery pseudoaneurysms associated with cancer typically occur at the site of invasion. 27 The most common presenting symptom is massive hemoptysis. Hemoptysis secondary to PAPs accounts for less than 10% of cases, with the majority of hemoptysis cases arising from a bronchial artery origin. 28 Prompt recognition and therapeutic intervention is crucial for patient's survival due to the risk of asphyxiation. Death is associated with aspiration and asphyxia. Pseudoaneurysm rupture carries an approximate mortality rate of 50%. 26
Bronchoscopy and multidetector CT are the principal means to identify the source of bleeding. PAPs have a predilection for the peripheral pulmonary arterial system with roughly 83% occurring at the segmental or subsegmental levels. The majority are also solitary. Multiple PAPs are typically associated with endocarditis and metastatic disease to the lung. The typical CT findings are a nodular opacity or mass encasing the pulmonary artery. 27
Coil embolization has shown satisfactory results in the management of acute pulmonary hemorrhage secondary to PAPs. Embolization of the feeding pulmonary artery and bronchopulmonary shunt portions can be undertaken. However, these result in infarction of the lung perfused by the respective pulmonary artery. Endovascular stent graft coverage of the PAP can preserve parenchymal perfusion and has shown promising results 24 26 28 29 ( Fig. 2 ).
Fig. 2.

A patient underwent transthoracic needle biopsy of a pulmonary nodule. This was complicated by the development of an iatrogenic pulmonary artery pseudoaneurysm (arrow). This lesion was successfully excluded with a self-expanding stent graft.
Liver
Spontaneous hemorrhage from primary or metastatic liver tumors is a rare but potentially fatal condition. It typically presents as an emergency and requires rapid diagnosis and a multidisciplinary approach involving oncology, hepatobiliary surgery, and interventional radiology. The management of these patients can be complex due to underlying comorbidities including liver dysfunction, either related to chronic cirrhosis or prior hepatotoxic chemotherapy.
Despite the frequency of both primary and metastatic liver tumors in cancer patients, hemorrhage from liver tumors is uncommon, with fewer than 100 cases reported in the United States prior to 1980. 30 However, in areas of high prevalence of hepatocellular carcinoma (HCC), as many as 10% of patients with HCC first present with bleeding. 31 HCC is the most common malignancy associated with spontaneous hepatic hemorrhage, although metastases from sarcoma, squamous cell carcinoma, colon cancer, melanoma, breast cancer, lung cancer, and esophageal cancer have been described 32 ( Fig. 3 ). Tumor size and location, particularly in HCC, do not seem to correlate with the risk of bleeding, as both large, central lesions and small, peripheral lesions have an approximately equal propensity to bleed. 32 The hemorrhage may not even come from the tumor itself; some have suggested that tumoral growth may fracture adjacent liver parenchyma and breach Glisson's capsule from within, thus leading to bleeding. 31 A recent history of blunt trauma may be elicited from patients as the inciting event.
Fig. 3.

A patient with metastatic gastrointestinal stromal tumor developed a large intraparenchymal hepatic hemorrhage (left) resulting in pain and hypotension. This mildly hypervascular lesion (middle image, arrow) was successfully embolized with calibrated microspheres. Postprocedure CT (right image) demonstrated successful resolution of hemorrhage.
Conservative management, with active resuscitation and blood product transfusion, is effective in almost 50% of patients who present with spontaneous hepatic hemorrhage. 33 34 For those who fail conservative therapy, transarterial embolization is effective in up to 80% of patients. 35 Relative contraindications to embolization in this setting are similar to those for transarterial chemoembolization and include main portal vein thrombosis and substantial arterial-portal venous shunting. In general, microcoil embolization is avoided to allow for subsequent treatments such as transarterial chemoembolization once the patient has recovered from the acute bleeding episode.
Gastrointestinal Tract
In the first report of transarterial embolization for bleeding in cancer patients, 36 six of the seven patients were treated for GI tract hemorrhage. The most common causes of nonvariceal GI bleeding in cancer patients are the same as those in the noncancer population and include ulcers, arteriovenous malformations, and angiodysplasia. Certainly, cancer patients can be at increased risk for bleeding from these nonmalignant etiologies due to underlying coagulopathy and thrombocytopenia. However, the treatment approach to these lesions is typically the same as for noncancer patients, and a discussion of this topic is beyond the scope of this article. However, we offer the following considerations for cancer patients with chemotherapy-induced thrombocytopenia and refractory, nonmalignant GI bleeding. Thrombocytopenia can occasionally be severe enough that the risk of femoral arterial puncture becomes considerable; we have found that transradial access in such patients is a safe alternative where patent hemostasis can be assured. We will occasionally leave the hemostatic wrist band on the patient for a prolonged period of time, including for 24 hours, while maintaining a pulse oximeter on the ipsilateral thumb to ensure adequate perfusion. Additionally, as chronic, intractable GI bleeding can be an important cause of morbidity as well as treatment delays in cancer patients with thrombocytopenia, an adjunctive intervention to consider that can improve platelet counts and allow for the re-initiation of chemotherapy is partial splenic artery embolization. 37
As with nonmalignant etiologies, endoscopy is generally considered the first line of therapy for patients with malignant GI bleeding. Endoscopy can not only diagnose and localize the cause of the bleed but also provide direct local therapy. However, endoscopy can be unsuccessful for several reasons: the lesion is inaccessible; the area of ulceration/mucosal bleeding is too large to be managed with injections or clips; and the risk of bowel perforation during endoscopy is high due to the use of some chemotherapies such as bevacizumab. Transarterial embolization is a reasonable treatment option when endoscopy is unsuccessful, though there are limited published data on its efficacy.
Tumor invasion or ulceration is an uncommon cause of upper GI bleeding ( Figs. 4 and 5 ). Lee et al described 23 patients who underwent embolization for unresectable gastric cancer. The treatment success rate was 52%, with a median overall survival of 38 days, highlighting the overall poor condition of patients often considered for embolization. 38 Metastases are a rare cause of small bowel bleeding, although autopsy series suggest that the true incidence of small bowel metastases is underappreciated. 39 Metastases from melanoma, lung cancer, cervical cancer, breast cancer, renal cell carcinoma (RCC), and thyroid cancer have all been described, 40 41 with melanoma, lung cancer, and breast cancer the most commonly reported. 39 Several case reports regarding embolization of renal cell metastases have been published. 40
Fig. 4.

Patient presented with a refractory bleeding from a large gastric carcinoma (left image, arrow). Angiography demonstrated an area of active extravasation from the proximal left phrenic artery (right image, arrow); additional tumor supply was seen from the right and left gastric arteries (not shown). All three arteries were embolized to stasis with gelatin sponge.
Fig. 5.

A patient with renal cell carcinoma was found to have active bleeding on endoscopy ( a ) from a metastasis involving the duodenum noted on a CT scan ( b , arrow). Angiography revealed the metastasis with arterial blood supply (arrowheads) from branches of the middle colic artery shared with loops of small bowel. Endovascular coils in the gastroduodenal artery from a previously unsuccessful attempt at tumor embolization are also present (arrow) ( c ). Cone-beam CT ( D ) was used to isolate arterial branches that supplied the tumor alone and could therefore be embolized safely with gelatin sponge. Postembolization angiography demonstrated significant devascularization of the tumor following embolization ( e ).
As opposed to the upper GI tract, tumor invasion can account for up to 26% of lower GI bleeds. 42 Malignancies commonly associated with GI bleeding include lymphoma, gastric carcinoma, small or large bowel adenocarcinoma, GI stromal tumors, and metastases. Colon carcinoma accounts for 2 to 9% of hematochezia and is the most common cause of chronic lower GI bleeding. 43 Embolization is best tolerated in the upper GI tract given the rich collateral network. However, multiple series show that lower GI tract embolization can be performed safely in appropriately selected patients by taking the necessary precautions. 43 44
In our experience, outcomes following embolization of malignant causes of GI bleeding are varied. Complete cessation of bleeding is rare, for several reasons. First, bleeding may be venous in nature, and so the best one can hope for, with arterial embolization, is to reduce to blood flow to allow for hemostasis. Second, arterial supply to the tumor independent of the bowel mucosa is rarely seen, and so the risk of bowel ischemia must be balanced with the extent of embolization. That being said, it is certainly possible to palliate patients with embolization by reducing transfusion requirements and potentially allowing for the re-initiation of chemotherapy. Occasionally, patients can be bridged to definitive surgery, which is where the best overall survival is identified. 45 As with nonmalignant causes of GI bleeding, coils and gelatin sponge are the most common embolic agents used for malignant GI bleeding, although there is also an important role for n-butyl cyanoacrylate and calibrated particles as well.
Renal Embolization for Hematuria
Renal cell carcinoma presents a challenge for clinicians and treatments due to its poor response to chemotherapeutic agents. 46 Definitive treatment for localized disease is radical nephrectomy, but advanced disease may preclude the patient from undergoing curative treatments. As the disease progresses, patients may start to experience significant symptoms such as hematuria and flank pain from their underlying malignancy. In these patients, palliative renal artery embolization may remain as the only possible treatment for symptom control ( Figs. 6 and 7 ). 47 48
Fig. 6.

A patient with renal cell carcinoma presented with refractory hematuria. CT ( a ) demonstrated a right lower pole renal mass (asterisk) with blood clots (arrow) within the collecting system. Embolization of the lesion was performed with absolute ethanol [preembolization ( b ) and postembolization ( c ) angiograms are noted]. Subsequent CT imaging demonstrated complete devascularization of the lesion (arrow) ( d ).
Fig. 7.
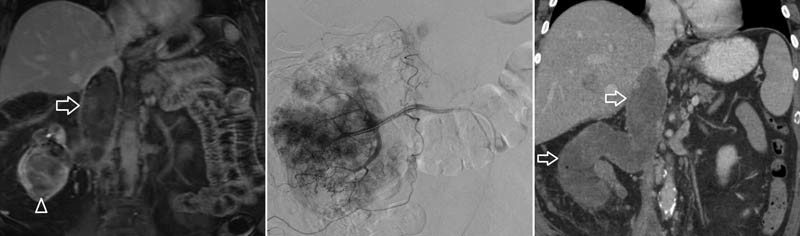
A patient with advanced renal cell carcinoma, with tumor noted on CT (arrowhead, left image) extending into the inferior vena cava (arrow) presented with persistent hematuria. A hypervascular lesion was noted on angiography essentially replacing the entire renal arterial vascular supply; embolization of the kidney was performed with absolute ethanol (middle image). Postprocedure CT imaging demonstrated complete devascularization of the renal lesion and its extension into the inferior vena cava (arrows, right image).
Multiple studies have shown successful control of local symptoms such as hematuria. Munro et al 48 and Nurmi et al 49 demonstrated successful palliation of symptoms following embolization. Further evidence suggests that embolization may actually provide a prolonged survival benefit to patients with advanced RCC as well as successful control of local symptoms. 50 The goals for palliative embolization in patients with metastatic RCC are to achieve control of symptoms attributable to the primary tumor. Embolic agents vary considerably between institutions, but common agents include absolute alcohol, polyvinyl alcohol particles, and n-butyl-2-cyanoacrylate glue.
Bladder Embolization for Hematuria
Bladder hemorrhage can occur secondary to radiation cystitis, bladder carcinoma, cyclophosphamide-induced cystitis, and infection. 51 Incidence of late radiation cystitis following radiation therapy is approximately 5 to 10%. Hematuria is the most common presenting symptom and varies in severity from mild hematuria to life-threatening hemorrhage. Initial treatment usually involves intravenous fluid replacement, blood transfusions, correction of any coagulopathies/thrombocytopenia, and transurethral catheterization with bladder irrigation and washout. 52 Alternative options include intravesical instillation of numerous agents (such as aluminum, formalin, prostaglandins, and placental extract) to achieve hemostasis. Other methods such as cystoscopy with fulguration (i.e., electrocoagulation, diathermy, argon laser) are an option. 52 However, in some patients, these initial methods fail to achieve satisfactory hemostasis. Radical cystectomy still remains an option but carries with it a high operative risk. Bilateral nephrostomy catheter placement to arrest the flow of urine and its thrombolytic components such as urokinase can also reduce bleeding in the setting of cystitis.
Minimally invasive transarterial embolization serves as a therapeutic option in which these other treatment options have failed and surgery is not an option ( Fig. 8 ). A success rate of 90% has been reported. 51 The bladder is supplied by the inferior vesical arteries, arising from the internal iliac artery. The inferior vesical artery may also have a common trunk with the superior gluteal and internal pudendal artery or branch directly from the internal pudendal. It is important to understand the different anatomic variants in males and females. In females, the inferior vesical artery may be a branch off the vaginal artery. In males, the inferior vesical artery also supplies the prostate, seminal vesicles, and ductus deferens. The superior vesical artery also typically branches from the internal iliac artery and gives off numerous branches that supply the upper portion of the bladder as well as portions of the ureter. 51 53
Fig. 8.

A patient with a history of myelodysplastic syndrome developed thrombocytopenia and BK virus cystitis. This was initially managed with urinary diversion with bilateral nephrostomy tubes. Due to persistent gross hematuria, selective embolization of the bilateral vesical arteries was performed. Intraprocedural CT was performed to delineate the arterial anatomy.
Bilateral embolization is usually preferred due to the rich collateralization present within the pelvis. Numerous embolic agents have been used, but current preference is to use permanent particulate embolic agents, such as calibrated microspheres. 54
Post embolization syndrome of nausea and pain can occur as well as buttock and perineal pain. Also, as with any embolization procedure, bladder embolization carries with it the risk of nontarget embolization. Potential complications that have been reported include bladder necrosis and Brown-Sequard syndrome due to the anastomoses present with the sacral arteries that may supply the spinal cord. 51 55 56
Prostate
Hematuria secondary to prostate cancer may be related to direct tumor invasion of the bladder, tumor invasion into the prostatic urethra, or mucosal edema and ischemia that is associated with radiation therapy. 57 58 Prostate radiation causes robust, progressive obliterative endarteritis which leads to cellular hypoxia and subsequent hemorrhage. 59 Initial therapy is conservative in nature with restrictions to physical activity, Foley catheter placement, bladder irrigation, and fluid resuscitation. However, when conventional methods fail to achieve hemostasis, transarterial embolization of the prostate gland is a reliable next step in treatment. 57 Success rates of up to 88% have been reported. 60
The anatomy of the prostatic arteries is highly variable. The number of independent prostatic arteries arising from each pelvic sidewall is variable as well. Bilhim et al showed that 57% of internal iliac arteries have one prostatic artery, while the other 43% have two prostatic arteries. 53 The most common origin of the prostatic artery is the internal pudendal, but it may arise from the superior vesical, anterior gluteal/pudendal trunk, obturator, inferior gluteal, accessory pudendal, or superior gluteal arteries. The prostatic arteries then bifurcate before penetrating the prostate gland into an anterior-lateral prostatic branch and a posterior-lateral prostatic branch. The anterior-lateral prostatic branch supplies the area of prostate gland responsible for benign prostatic hypertrophy (BPH) and can be enlarged relative to the posterior-lateral branch due to increased demand if BPH occurs in concert with prostate cancer. Most common anastomoses identified within the pelvis are to the internal pudendal, contralateral and ipsilateral prostatic branches, rectal and vesical arteries. 51 53 61
As prostate cancer progresses, the neovascularity that is associated with these higher grade tumors increases, and multiple collaterals within the pelvis develop. It is important to identify these collaterals with preprocedure imaging to avoid nontarget embolization. Superselective angiography, as well as cone-beam CT, can help identify these collateral vessels. 58 Selective technique is preferable to avoid nontarget embolization. However, superselective embolization may miss some feeding arteries and therefore fail to achieve full hemostasis. Chen et al propose a delicate balance between selective and nonselective techniques when performing prostate artery embolization. 58 Bilateral embolization is the standard practice to achieve adequate hemostasis due to the robust collateralization present within the pelvis and prostate regions. 57 Polyvinyl alcohol and calibrated microspheres are most commonly used. 59
Complications are usually mild and consist of pain, hematospermia, urethral burning, rectal bleeding, urinary tract infection, and acute urinary retention. More severe complications are related to nontarget embolization such as bladder ischemia/necrosis and gluteal ischemia. 62
Gynecologic Malignancies
While vaginal bleeding from gynecologic malignancies may present as a chronic, slow bleed, it can also result in acute, massive hemorrhage. The initial management for patients in the latter setting includes vaginal packing and transfusion of blood products. Emergency radiation therapy can be considered, but this treatment approach has several limitations in the acute setting. First, patients may have already received maximal radiation doses previously and would therefore not be candidates for palliative radiation. Second, even short-course palliative radiation therapy schedule requires sessions spread over multiple days and is thus not feasible in patients with acute bleeding. 63 Moreover, complications of prior radiation therapy are occasionally implicated as the cause of vaginal bleeding, thus rendering further radiation therapy somewhat counterintuitive.
Transarterial embolization is therefore often the intervention of choice in the emergent management of refractory vaginal bleeding from gynecologic tumors ( Fig. 9 ). Indeed, one of the first roles of transarterial embolization was for pelvic hemorrhage, 64 65 as the technique was introduced as an alternative to open surgical ligation of the internal iliac arteries in the 1970s. The reported success rate for embolization in this clinical setting is high. In one of the first and largest studies, Pisco et al 66 performed gelatin sponge and polyvinyl alcohol embolization in 108 patients with intractable bleeding due to pelvic malignancies. The anterior division of the internal iliac arteries was selected when possible; otherwise, embolization was performed from the main trunk of the internal iliac arteries. This study found complete cessation of hemorrhage in 74 of 108 patients and partial control in 23 of 108 patients. Additional smaller series have similarly shown high success rates. 10 67 68 69
Fig. 9.

A patient with cervical cancer underwent chemoradiation therapy, complicated by vesicovaginal fistula formation. The patient presented to the emergency department with massive vaginal bleeding resulting in hemodynamic instability. CT angiography demonstrated active extravasation immediately superior to the vaginal packing material, seen as dense material on the sagittal CT reconstructions (arrow). Emergent angiography demonstrated a large right uterine artery pseudoaneurysm (arrow) that was successfully embolized with cyanoacrylate/Ethiodol.
Angiography may not always reveal a discrete site of bleeding, in which case empiric embolization can be performed similar to the paradigm of pelvic trauma. Given the vast array of contemporary endovascular tools, selective catheterization of the anterior division of the internal iliac artery should be feasible in almost every patient. In the authors' opinion, further superselective catheterization of the “usual suspect” arteries, such as the uterine artery and cervicovaginal trunk, should be pursued. Unless a focal vascular defect such as a pseudoaneurysm is seen, in which microcoils are appropriate, embolization is most commonly performed with gelatin sponge or microspheres. Embolization should always be performed bilaterally given the rich collateral network in the pelvis. As in other clinical settings for which internal iliac embolization is performed, complications of this procedure include skin necrosis, neurologic dysfunction, buttock claudication, impotence, and bladder ischemia.
Transarterial embolization also has a role in chronic, low-volume bleeding from gynecologic malignancies. The typical clinical scenario is a patient with a necrotic, friable mass who has already received chemoradiation therapy and who requires intermittent transfusions but otherwise has a high performance status. Both selective (i.e., anterior division of internal iliac artery) and superselective embolization can be helpful for these patients. 68 Unlike patients with benign uterine leiomyomas undergoing uterine fibroid embolization, the relevant arteries in patients with chronic malignant bleeding may not demonstrate substantial hypertrophy. Moreover, prior radiation therapy can cause vascular sclerosis, degrading the arterial supply into a spider's web of tiny nourishing branches. We have found that intraprocedural cross-sectional imaging tools such as cone-beam CT can be invaluable in helping navigate the pelvic vascular tree to identify the tumoral arterial supply.
Conclusion
Transarterial embolization plays an important role in the management of bleeding for patients with advanced malignancy. Patients in this clinical setting often have a poor prognosis, but this alone should not dissuade interventionalists from considering embolization. Bleeding results in a wide range of debilitating symptoms, and interventions to improve a patient's quality of life by reducing or arresting bleeding should be considered. By approaching embolization from a palliative rather than curative perspective, the role of embolization can be greatly broadened across the spectrum of patients with advanced malignancy.
Footnotes
Conflict of Interest No authors declare any conflicts of interest.
References
- 1.Pereira J, Phan T. Management of bleeding in patients with advanced cancer. Oncologist. 2004;9(05):561–570. doi: 10.1634/theoncologist.9-5-561. [DOI] [PubMed] [Google Scholar]
- 2.Dutcher J P. Hematologic abnormalities in patients with nonhematologic malignancies. Hematol Oncol Clin North Am. 1987;1(02):281–299. [PubMed] [Google Scholar]
- 3.Akhtar K, Byrne J P, Bancewicz J, Attwood S E. Argon beam plasma coagulation in the management of cancers of the esophagus and stomach. Surg Endosc. 2000;14(12):1127–1130. doi: 10.1007/s004640000266. [DOI] [PubMed] [Google Scholar]
- 4.Patel U, Pattison C W, Raphael M.Management of massive haemoptysis Br J Hosp Med 199452(2-3):74–76., 76–78 [PubMed] [Google Scholar]
- 5.Brundage M D, Bezjak A, Dixon P et al. The role of palliative thoracic radiotherapy in non-small cell lung cancer. Can J Oncol. 1996;6 01:25–32. [PubMed] [Google Scholar]
- 6.Ferris F D, Bezjak A, Rosenthal S G. The palliative uses of radiation therapy in surgical oncology patients. Surg Oncol Clin N Am. 2001;10(01):185–201. [PubMed] [Google Scholar]
- 7.Knott-Craig C J, Oostuizen J G, Rossouw G, Joubert J R, Barnard P M. Management and prognosis of massive hemoptysis. Recent experience with 120 patients. J Thorac Cardiovasc Surg. 1993;105(03):394–397. [PubMed] [Google Scholar]
- 8.Wells I. Internal iliac artery embolization in the management of pelvic bleeding. Clin Radiol. 1996;51(12):825–827. doi: 10.1016/s0009-9260(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 9.Recordare A, Bonariol L, Caratozzolo E et al. Management of spontaneous bleeding due to hepatocellular carcinoma. Minerva Chir. 2002;57(03):347–356. [PubMed] [Google Scholar]
- 10.Nabi G, Sheikh N, Greene D, Marsh R. Therapeutic transcatheter arterial embolization in the management of intractable haemorrhage from pelvic urological malignancies: preliminary experience and long-term follow-up. BJU Int. 2003;92(03):245–247. doi: 10.1046/j.1464-410x.2003.04328.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita Y, Harada M, Yamamoto H et al. Transcatheter arterial embolization of obstetric and gynaecological bleeding: efficacy and clinical outcome. Br J Radiol. 1994;67(798):530–534. doi: 10.1259/0007-1285-67-798-530. [DOI] [PubMed] [Google Scholar]
- 12.Hayes M C, Wilson N M, Page A, Harrison G SM. Selective embolization of bladder tumours. Br J Urol. 1996;78(02):311–312. [PubMed] [Google Scholar]
- 13.Jenkins C N, McIvor J. Survival after embolization of the internal iliac arteries in ten patients with severe haematuria due to recurrent pelvic carcinoma. Clin Radiol. 1996;51(12):865–868. doi: 10.1016/s0009-9260(96)80084-5. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava D N, Gandhi D, Julka P K, Tandon R K. Gastrointestinal hemorrhage in hepatocellular carcinoma: management with transheptic arterioembolization. Abdom Imaging. 2000;25(04):380–384. doi: 10.1007/s002610000056. [DOI] [PubMed] [Google Scholar]
- 15.Wholey M H, Chamorro H A, Rao G, Ford W B, Miller W H. Bronchial artery embolization for massive hemoptysis. JAMA. 1976;236(22):2501–2504. [PubMed] [Google Scholar]
- 16.Najarian K E, Morris C S. Arterial embolization in the chest. J Thorac Imaging. 1998;13(02):93–104. doi: 10.1097/00005382-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz J, Sheth D, Patel J. Bronchial artery embolization. Semin Intervent Radiol. 2012;29(03):155–160. doi: 10.1055/s-0032-1326923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remy J, Voisin C, Ribet Met al. Treatment, by embolization, of severe or repeated hemoptysis associated with systemic hypervascularization[in French].Nouv Presse Med 19732312060. [PubMed] [Google Scholar]
- 19.Garcia-Olivé I, Sanz-Santos J, Centeno C et al. Results of bronchial artery embolization for the treatment of hemoptysis caused by neoplasm. J Vasc Interv Radiol. 2014;25(02):221–228. doi: 10.1016/j.jvir.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Walker C M, Rosado-de-Christenson M L, Martínez-Jiménez S, Kunin J R, Wible B C. Bronchial arteries: anatomy, function, hypertrophy, and anomalies. Radiographics. 2015;35(01):32–49. doi: 10.1148/rg.351140089. [DOI] [PubMed] [Google Scholar]
- 21.Ittrich H, Klose H, Adam G. Radiologic management of haemoptysis: diagnostic and interventional bronchial arterial embolisation. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2015;187(04):248–259. doi: 10.1055/s-0034-1385457. [DOI] [PubMed] [Google Scholar]
- 22.Burke C T, Mauro M A. Bronchial artery embolization. Semin Intervent Radiol. 2004;21(01):43–48. doi: 10.1055/s-2004-831404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPherson S, Routh W D, Nath H, Keller F S. Anomalous origin of bronchial arteries: potential pitfall of embolotherapy for hemoptysis. J Vasc Interv Radiol. 1990;1(01):86–88. doi: 10.1016/s1051-0443(90)72509-2. [DOI] [PubMed] [Google Scholar]
- 24.Khalil A, Fedida B, Parrot A, Haddad S, Fartoukh M, Carette M-F.Severe hemoptysis: from diagnosis to embolization Diagn Interv Imaging 201596(7-8):775–788. [DOI] [PubMed] [Google Scholar]
- 25.Mal H, Rullon I, Mellot F et al. Immediate and long-term results of bronchial artery embolization for life-threatening hemoptysis. Chest. 1999;115(04):996–1001. doi: 10.1378/chest.115.4.996. [DOI] [PubMed] [Google Scholar]
- 26.Pelage J P, El Hajjam M, Lagrange C et al. Pulmonary artery interventions: an overview. Radiographics. 2005;25(06):1653–1667. doi: 10.1148/rg.256055516. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Gilman M D, Humphrey K L et al. Pulmonary artery pseudoaneurysms: clinical features and CT findings. AJR Am J Roentgenol. 2017;208(01):84–91. doi: 10.2214/AJR.16.16312. [DOI] [PubMed] [Google Scholar]
- 28.Shin S, Shin T-B, Choi H et al. Peripheral pulmonary arterial pseudoaneurysms: therapeutic implications of endovascular treatment and angiographic classifications. Radiology. 2010;256(02):656–664. doi: 10.1148/radiol.10091416. [DOI] [PubMed] [Google Scholar]
- 29.Dallaudière B, Hummel V, Pierre Laissy J. The use of covered stents for treatment of pulmonary artery pseudoaneurysms. J Vasc Interv Radiol. 2013;24(02):296–298. doi: 10.1016/j.jvir.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Van Landingham S B, Hendricks J C, Roberts J W. Spontaneous rupture of hepatocellular carcinoma. J Surg Oncol. 1985;29(02):129–131. doi: 10.1002/jso.2930290212. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L X, Wang G S, Fan S T. Spontaneous rupture of hepatocellular carcinoma. Br J Surg. 1996;83(05):602–607. doi: 10.1002/bjs.1800830507. [DOI] [PubMed] [Google Scholar]
- 32.Srinivasa S, Lee W G, Aldameh A, Koea J B. Spontaneous hepatic haemorrhage: a review of pathogenesis, aetiology and treatment. HPB. 2015;17(10):872–880. doi: 10.1111/hpb.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung K L, Lau W Y, Lai P B, Yiu R Y, Meng W C, Leow C K. Spontaneous rupture of hepatocellular carcinoma: conservative management and selective intervention. Arch Surg. 1999;134(10):1103–1107. doi: 10.1001/archsurg.134.10.1103. [DOI] [PubMed] [Google Scholar]
- 34.Rosales A, Que F G. Spontaneous hepatic hemorrhage: a single institution's 16-year experience. Am Surg. 2016;82(11):1117–1120. [PubMed] [Google Scholar]
- 35.Kirikoshi H, Saito S, Yoneda M et al. Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol. 2009;9(01):29. doi: 10.1186/1471-230X-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein H M, Medellin H, Ben-Menachem Y, Wallace S. Transcatheter arterial embolization in the management of bleeding in the cancer patient. Radiology. 1975;115(03):603–608. doi: 10.1148/15.3.603. [DOI] [PubMed] [Google Scholar]
- 37.Kauffman C R, Mahvash A, Kopetz S, Wolff R A, Ensor J, Wallace M J. Partial splenic embolization for cancer patients with thrombocytopenia requiring systemic chemotherapy. Cancer. 2008;112(10):2283–2288. doi: 10.1002/cncr.23432. [DOI] [PubMed] [Google Scholar]
- 38.Lee H J, Shin J H, Yoon H-K et al. Transcatheter arterial embolization in gastric cancer patients with acute bleeding. Eur Radiol. 2009;19(04):960–965. doi: 10.1007/s00330-008-1216-2. [DOI] [PubMed] [Google Scholar]
- 39.Rosato F E, Jr, Rosato E L. Current surgical management of intestinal metastases. Semin Oncol. 2008;35(02):177–182. doi: 10.1053/j.seminoncol.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Gordon B, Lossef S V, Jelinger E, Barth K H. Embolotherapy for small bowel hemorrhage from metastatic renal cell carcinoma: case report. Cardiovasc Intervent Radiol. 1991;14(05):311–313. doi: 10.1007/BF02578457. [DOI] [PubMed] [Google Scholar]
- 41.Rustagi T, Rangasamy P, Versland M. Duodenal bleeding from metastatic renal cell carcinoma. Case Rep Gastroenterol. 2011;5(01):249–257. doi: 10.1159/000327996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thacker P G, Friese J L, Loe M, Biegler P, Larson M, Andrews J. Embolization of nonliver visceral tumors. Semin Intervent Radiol. 2009;26(03):262–269. doi: 10.1055/s-0029-1225667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2009;6(11):637–646. doi: 10.1038/nrgastro.2009.167. [DOI] [PubMed] [Google Scholar]
- 44.Busch O RC, van Delden O M, Gouma D J. Therapeutic options for endoscopic haemostatic failures: the place of the surgeon and radiologist in gastrointestinal tract bleeding. Best Pract Res Clin Gastroenterol. 2008;22(02):341–354. doi: 10.1016/j.bpg.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Meehan T, Stecker M S, Kalva S P, Oklu R, Walker T G, Ganguli S. Outcomes of transcatheter arterial embolization for acute hemorrhage originating from gastric adenocarcinoma. J Vasc Interv Radiol. 2014;25(06):847–851. doi: 10.1016/j.jvir.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Almgård L E, Fernström I, Haverling M, Ljungqvist A. Treatment of renal adenocarcinoma by embolic occlusion of the renal circulation. Br J Urol. 1973;45(05):474–479. doi: 10.1111/j.1464-410x.1973.tb06806.x. [DOI] [PubMed] [Google Scholar]
- 47.Mukund A, Gamanagatti S. Ethanol ablation of renal cell carcinoma for palliation of symptoms in advanced disease. J Palliat Med. 2010;13(02):117–120. doi: 10.1089/jpm.2009.0243. [DOI] [PubMed] [Google Scholar]
- 48.Munro N P, Woodhams S, Nawrocki J D, Fletcher M S, Thomas P J. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003;92(03):240–244. doi: 10.1046/j.1464-410x.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- 49.Nurmi M, Satokari K, Puntala P. Renal artery embolization in the palliative treatment of renal adenocarcinoma. Scand J Urol Nephrol. 1987;21(02):93–96. doi: 10.3109/00365598709180300. [DOI] [PubMed] [Google Scholar]
- 50.Park J H, Kim S H, Han J K, Chung J W, Han M C. Transcatheter arterial embolization of unresectable renal cell carcinoma with a mixture of ethanol and iodized oil. Cardiovasc Intervent Radiol. 1994;17(06):323–327. doi: 10.1007/BF00203951. [DOI] [PubMed] [Google Scholar]
- 51.Loffroy R, Pottecher P, Cherblanc V et al. Current role of transcatheter arterial embolization for bladder and prostate hemorrhage. Diagn Interv Imaging. 2014;95(11):1027–1034. doi: 10.1016/j.diii.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Smit S G, Heyns C F. Management of radiation cystitis. Nat Rev Urol. 2010;7(04):206–214. doi: 10.1038/nrurol.2010.23. [DOI] [PubMed] [Google Scholar]
- 53.Bilhim T, Pisco J M, Rio Tinto H et al. Prostatic arterial supply: anatomic and imaging findings relevant for selective arterial embolization. J Vasc Interv Radiol. 2012;23(11):1403–1415. doi: 10.1016/j.jvir.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 54.Loffroy R, Guiu B, Cercueil J-P, Krausé D. Endovascular therapeutic embolisation: an overview of occluding agents and their effects on embolised tissues. Curr Vasc Pharmacol. 2009;7(02):250–263. doi: 10.2174/157016109787455617. [DOI] [PubMed] [Google Scholar]
- 55.Liguori G, Amodeo A, Mucelli F P et al. Intractable haematuria: long-term results after selective embolization of the internal iliac arteries. BJU Int. 2010;106(04):500–503. doi: 10.1111/j.1464-410X.2009.09192.x. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi T, Kusano S, Matsubayashi T, Uchida T. Selective embolization of the vesical artery in the management of massive bladder hemorrhage. Radiology. 1980;136(02):345–348. doi: 10.1148/radiology.136.2.7403507. [DOI] [PubMed] [Google Scholar]
- 57.Pereira K, Halpern J A, McClure T D et al. Role of prostate artery embolization in the management of refractory haematuria of prostatic origin. BJU Int. 2016;118(03):359–365. doi: 10.1111/bju.13524. [DOI] [PubMed] [Google Scholar]
- 58.Chen J-W, Shin J H, Tsao T-F et al. Prostatic arterial embolization for control of hematuria in patients with advanced prostate cancer. J Vasc Interv Radiol. 2017;28(02):295–301. doi: 10.1016/j.jvir.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Rastinehad A R, Caplin D M, Ost M C et al. Selective arterial prostatic embolization (SAPE) for refractory hematuria of prostatic origin. Urology. 2008;71(02):181–184. doi: 10.1016/j.urology.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Korkmaz M, Şanal B, Aras B et al. The short- and long-term effectiveness of transcatheter arterial embolization in patients with intractable hematuria. Diagn Interv Imaging. 2016;97(02):197–201. doi: 10.1016/j.diii.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Bilhim T, Tinto H R, Fernandes L, Martins Pisco J. Radiological anatomy of prostatic arteries. Tech Vasc Interv Radiol. 2012;15(04):276–285. doi: 10.1053/j.tvir.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Cizman Z, Isaacson A, Burke C. Short- to midterm safety and efficacy of prostatic artery embolization: a systematic review. J Vasc Interv Radiol. 2016;27(10):1487–14930. doi: 10.1016/j.jvir.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 63.Kim D H, Lee J H, Ki Y K et al. Short-course palliative radiotherapy for uterine cervical cancer. Radiat Oncol J. 2013;31(04):216–221. doi: 10.3857/roj.2013.31.4.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Katzen B T, Rossi P, Passairello R, Simonetti G. Transcatheter therapeutic arterial embolization. Radiology. 1976;120(03):523–531. doi: 10.1148/120.3.523. [DOI] [PubMed] [Google Scholar]
- 65.Riley J. The role of embolization in palliative care. Palliat Med. 1996;10(02):168. doi: 10.1177/026921639601000216. [DOI] [PubMed] [Google Scholar]
- 66.Pisco J M, Martins J M, Correia M G. Internal iliac artery: embolization to control hemorrhage from pelvic neoplasms. Radiology. 1989;172(02):337–339. doi: 10.1148/radiology.172.2.2748811. [DOI] [PubMed] [Google Scholar]
- 67.Field K, Ryan M J, Saadeh F A, Kamran W. Selective arterial embolisation for intractable vaginal haemorrhage in genital tract malignancies. Eur J Gynaecol Oncol. 2016;37(03):736–740. [PubMed] [Google Scholar]
- 68.Mann W J, Jr, Jander H P, Partridge E E et al. Selective arterial embolization for control of bleeding in gynecologic malignancy. Gynecol Oncol. 1980;10(03):279–289. doi: 10.1016/0090-8258(80)90095-5. [DOI] [PubMed] [Google Scholar]
- 69.Mihmanli I, Cantasdemir M, Kantarci F, Halit Yilmaz M, Numan F, Mihmanli V. Percutaneous embolization in the management of intractable vaginal bleeding. Arch Gynecol Obstet. 2001;264(04):211–214. doi: 10.1007/s004040000119. [DOI] [PubMed] [Google Scholar]


