Abstract
Painless jaundice is a harbinger of malignant biliary obstruction, with the majority of cases due to pancreatic adenocarcinoma. Despite advances in treatment, including improved surgical techniques and neoadjuvant (preoperative) chemotherapy, long-term survival from pancreatic cancer is rare. This lack of significant improvement in outcomes is believed to be due to multiple reasons, including the advanced stage at diagnosis and lack of an adequate biomarker for screening and early detection, prior to the onset of jaundice or epigastric pain. Close attention is required to select appropriate patients for preoperative biliary decompression, and to prevent morbid complications from biliary drainage procedures, such as pancreatitis and cholangitis. Use of small caliber plastic biliary stents during endoscopic retrograde cholangiopancreatography should be minimized, as metal stents have increased area for improved bile flow and a reduced risk of adverse events during neoadjuvant therapy. Efforts are underway by translational scientists, radiologists, oncologists, surgeons, and gastroenterologists to augment lifespan for our patients and to more readily treat this deadly disease. In this review, the authors discuss the rationale and techniques of endoscopic biliary intervention, mainly focusing on malignant biliary obstruction by pancreatic cancer.
Keywords: malignant biliary obstruction, endoscopic retrograde cholangiopancreatography, endoscopic ultrasound, surgically altered anatomy, interventional radiology
Objectives : Upon completion of this article, the reader will be able to illustrate appropriate selection of patients for endoscopic management of malignant biliary obstruction, advances in the management of pancreatic cancer, and various techniques and their complications involved in endoscopic procedures.
Accreditation : This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit : Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit ™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Obstructive jaundice occurs when the flow of bile, from the liver into the duodenum, is impeded by a blockage or stenosis of the intra- or extrahepatic bile ducts. Biliary obstruction can result from either benign or malignant etiologies. The causes of benign biliary obstruction include primary sclerosing cholangitis, gallstone-induced strictures, chronic pancreatitis, radiation-induced strictures, and autoimmune pancreatitis. 1 On the other hand, the malignant etiologies comprise pancreatic cancer, cholangiocarcinoma, lymphoma, and metastatic cancers. Pancreatic cancer is by far the most common disease responsible for malignant biliary obstruction that is encountered today.
Pancreatic Cancer
Pancreatic cancer is a highly morbid cause of cancer-related death in the United States, with a 5-year survival of a merely 7 to 8%. 2 The most recent estimates predict 53,070 new cases of pancreas cancer in 2016, of which 41,780 patients are expected to die from the disease. In 1985, the Gastrointestinal Tumor Study Group (GITSG) reported a median survival of 20 months for patients with pancreatic cancer who underwent surgical resection followed by adjuvant therapy consisting of radiation and fluorouracil. 3 Thirty-two years later in 2017, The European Study Group for Pancreatic Cancer (ESPAC) reported results of the prospective multicenter trial, comparing the efficacy of combined therapy with gemcitabine plus capecitabine versus gemcitabine monotherapy for resected pancreatic cancer. 4 The median survival for patients in the gemcitabine plus capecitabine group was uninspiring, 28 months (95% confidence interval [CI]: 23.5–31.5) compared with 25.5 months (22.7–27.9) in the gemcitabine-alone group (hazard ratio: 0.82 [95% CI: 0.68–0.98], p = 0.0320. Clearly, the data illustrate a lack of a notable improvement in survival with pancreatic cancer.
Disappointing survival rates are primarily due to a failure in early detection of pancreatic cancer. Nearly 85% of pancreatic cancer patients present with advanced disease stages, where a curative resection is not possible. 5 Therefore, early detection is key to improving survival, and further research is necessary to identify a biomarker which can detect pancreatic cancer prior to the onset of symptoms, such as obstructive jaundice. The ideal tumor biomarker should be accurate, easily obtainable, and economical. Over the past two decades, potential biomarkers, such as mesothelin, glypican-1, circulating microRNAs, and serum thrombospondin-1, were extensively studied; however, none could reliably predict patients with pancreatic cancer. 6 7 8 9 Accordingly, the ideal tumor marker for pancreatic cancer does not exist today. Although it is widely used in monitoring treatment responses and posttreatment surveillance, the serum protein carbohydrate antigen 19–9 (CA 19–9) has poor sensitivity and specificity for pancreatic malignancy. Nevertheless, CA 19–9 has been a key serum biomarker for pancreatic cancer given the lack of viable alternatives.
While population-based, mass screening for pancreatic cancer is neither practical nor possible, screening asymptomatic high-risk groups was thought to be feasible. As a consequence, a multicenter, prospective trial was performed by Canto et al, focusing mainly on the performance of diagnostic imaging modalities such as magnetic resonance imaging (MRI), computed tomography (CT), and endoscopic ultrasound (EUS). In this study of 225 patients at high risk for pancreas cancer, 92 patients were found by EUS to have at least one pancreatic abnormality (84 cystic lesions, 3 neuroendocrine tumors, and 5 dilated pancreatic duct), although no patients were diagnosed with pancreatic adenocarcinoma. 10 Among the diagnostic tests, EUS was the most sensitive in finding pancreatic abnormalities (42.6%), followed by MRI (33.3%) and CT (11%).
As pancreatic cancer is a systemic disease, the concept of neoadjuvant therapy was conceived from the desire to provide treatment of micrometastatic disease while the primary tumor is intact and relatively well perfused. Neoadjuvant therapy also helps avoid surgery in patients with rapidly progressive disease, thus preventing early unexpected perioperative demise. In a phase II trial with preoperative gemcitabine and radiation therapy, the median survival was 34 months for the patients who underwent pancreaticoduodenectomy, with a median overall survival of 22.7 months. 11 In another phase II trial with preoperative treatment comprising gemcitabine, cisplatin, and radiation therapy, the median survival was 31 months for patients who underwent resection with a median overall survival of 18.7 months. 12
Based on data from these two monumental trials on neoadjuvant treatment, a question could arise whether every patient with either resectable or borderline-resectable pancreatic cancer should undergo neoadjuvant therapy before considering resection. While it is unclear whether patients with a resectable pancreatic cancer would have a better survival if they received neoadjuvant therapy rather than immediate surgical resection, the benefit of neoadjuvant therapy is evident for borderline resectable pancreatic cancer. In borderline resectable pancreatic cancer, it is nearly impossible to accomplish a margin-negative resection without neoadjuvant therapy. As neoadjuvant therapy is widely practiced throughout the world today, management of biliary obstruction has become increasingly important.
Managing Malignant Biliary Obstruction
The benefits of draining an obstructed bile duct include the relief of jaundice and avoidance of hepatotoxicity from chemotherapeutic agents, but the inherent risks in biliary intervention, such as pancreatitis and violation of a sterile environment, cannot be ignored. Therefore, the decision to drain the biliary obstruction should be carefully made, weighing benefits against risks, not out of reflex. In a randomized, multicenter trial of preoperative biliary drainage with plastic stents versus early surgery (within 1 week), serious complications were observed 74% in the biliary drainage group and 39% in the early surgery group. 13 However, the study was limited by a high rate of initial failure rate (25%) of endoscopic retrograde cholangiopancreatography (ERCP) and high ERCP complication rates (46%). Based on this data, it would be reasonable to proceed with surgery without biliary drainage with a plastic stent, if surgery is planned within 2 weeks ( Fig. 1 ). For patients who will receive preoperative therapy, biliary drainage is necessary to initiate and continue chemotherapy.
Fig. 1.
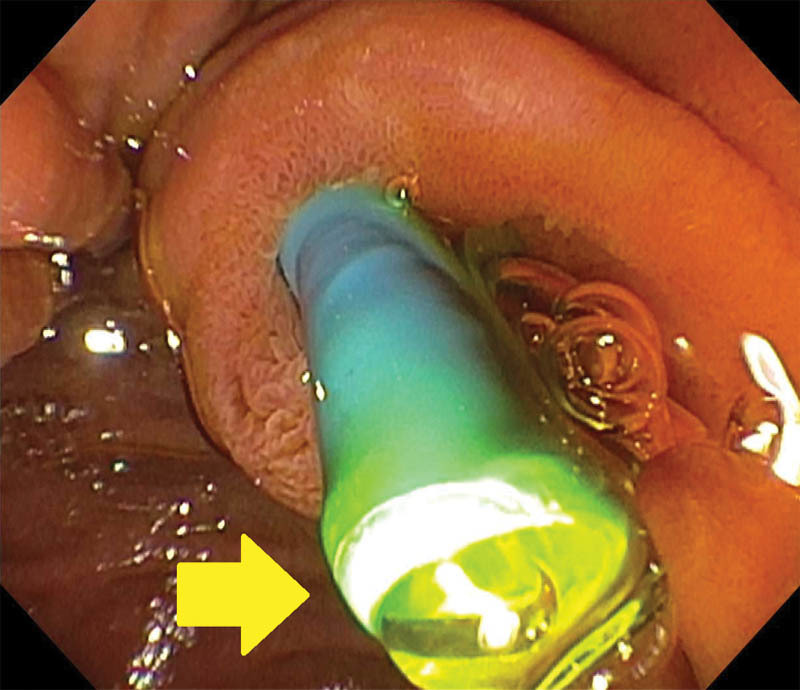
Single photo obtained during endoscopic retrograde cholangiopancreatography for a 58-year-old man with pancreatic cancer demonstrating a 10-Fr, straight plastic biliary stent emanating from the papilla. Arrow demonstrates adequate flow of yellow bile from the tip of the stent. Plastic stents are readily removable and often placed before obtaining a tissue diagnosis of pancreas cancer.
Over the past 20 years, among various modalities from multiple specialties, endoscopic drainage has become first-line therapy, mainly due to effective relief of the obstruction, the patient's convenience and acceptance, and rapid recovery postprocedure. Regarding the types of endobiliary stents, multiple studies confirmed endoscopic biliary drainage with metal stents is preferred over endoscopic biliary drainage with plastic stents. 14 15 In a study evaluating rates of perioperative morbidity, mortality, and stent-related complications in 272 consecutive cases of pancreaticoduodenectomy (commonly referred to as the Whipple procedure), no statistically significant difference was found between the group who received metal stents and those who did not receive metal stents, in terms of median estimated blood loss, operating time, and length of hospital stay. However, a considerably higher rate of complications was noted in the plastic stent group (79%) than in the metal stent group (7%) during the preoperative treatment period, mainly secondary to stent occlusion. In the largest study to date comparing the outcomes between covered self-expandable metal stents (CSEMS) and uncovered self-expandable metal stents (USEMS) in malignant biliary obstruction, no significant difference was found in the median overall survival and time to recurrent obstruction between the two groups. 16 Tumor ingrowth with recurrent obstruction was more common in USEMS, but stent migration and acute pancreatitis (6 vs. 1%, p < 0.001) were seen more often in the CSEMS group.
Approaches to Endoscopic Retrograde Cholangiopancreatography
On planning an ERCP for a patient with obstructive jaundice from pancreatic cancer, the first task is to review the available cross-sectional imaging studies. The images are extremely helpful in ascertaining (1) whether there is evidence of gastroduodenal outlet obstruction, (2) the location of biliary obstruction (intra- or extrahepatic), and (3) the location of the tumor(s), which aid to avoid unnecessary contamination of undrainable segments of the liver and prevent hepatic abscess formation. If gastroduodenal outlet obstruction is suspected, then securing the airway by intubation should be completed before ERCP to prevent aspiration of gastric contents. In complex strictures involving both lobes of the liver, the endoscopist should decide whether to drain one or both lobes depending on the location and complexity of the stricture. The goal is to drain at least 50% of the liver volume at the end of the procedure. While ERCP is successful in 90 to 95% of cases, challenges include surgically altered anatomy, obscured and/or friable ampulla from tumor infiltration, and duodenal stenosis from bulky tumors.
Techniques in Endoscopic Retrograde Cholangiopancreatography
Conventional Endoscopic Retrograde Cholangiopancreatography
Usually ERCP begins with attempts to cannulate the bile duct using a cannula or a sphincterotome, and injection of contrast or use of a guidewire. Therefore, conventional techniques include ERCP using a cannula and contrast injection (ERCP-CC), ERCP using a cannula and a guidewire (ERCP-CG), ERCP using a sphincterotome (ERCP-S), and ERCP using a sphincterotome and a guidewire (ERCP-SG). In a systemic review based on 12 randomized controlled trials including 3,450 patients, guidewire-assisted ERCP technique was found to have a significantly reduced rate of post-ERCP pancreatitis (PEP), compared with contrast injection-assisted ERCP technique (relative risk [RR]: 0.51, 95% CI: 0.32–0.82). 17 Moreover, guidewire-assisted ERCP technique was associated with a greater primary cannulation success (RR: 1.07, 95% CI: 1.00–1.15), less use of precut sphincterotomy (RR: 0.75, 95% CI: 0.60–0.95), and no increase in other procedure-related complications.
ERCP Using Double Guidewire Technique
When conventional ERCP technique is unsuccessful in gaining access into the bile duct, a two-wire technique could be used, especially if the guidewire keeps advancing into the pancreatic duct. 18 In this technique, a guidewire is first passed into the pancreatic duct. Then, using a second guidewire, the bile duct is cannulated. The first guidewire placed in the pancreatic duct theoretically “straightens the papillary anatomy” and blocks the second wire from entering the pancreatic duct deflecting it toward the direction of the bile duct. Following double guidewire cannulation, a prophylactic pancreatic duct stent is commonly placed to reduce the risk of PEP ( Fig. 2 ). 19 Interestingly, it is the most widely used technique in Japan after the failure of the conventional approach. 20
Fig. 2.
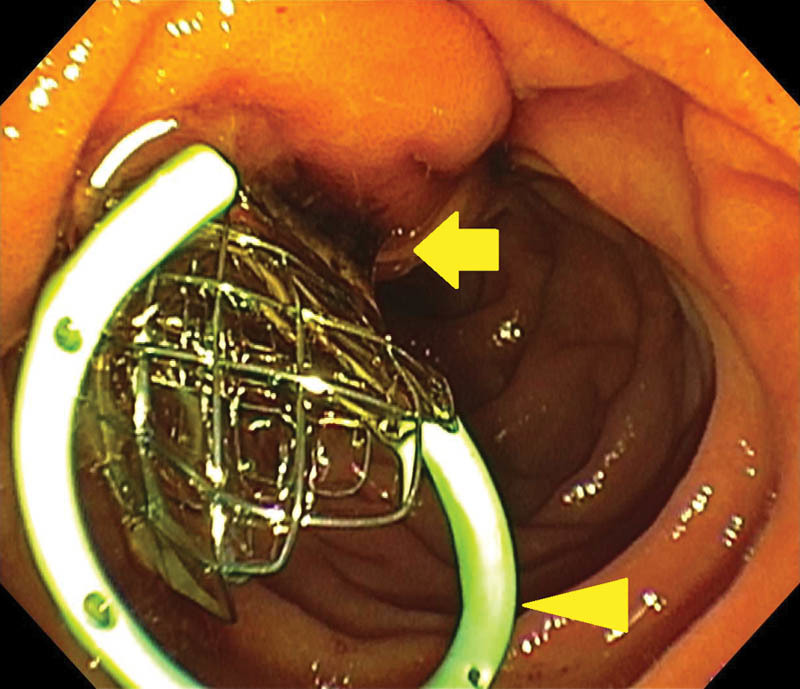
Endoscopic photo obtained following endoscopic retrograde cholangiopancreatography in a 61-year-old woman with locally advanced pancreatic cancer and high-grade biliary obstruction which required use of the double guidewire technique. To prevent pancreatitis and augment flow of pancreatic secretions, a 5-Fr, single pigtail pancreatic duct stent (arrowhead) is placed, followed by deployment of the uncovered metal biliary stent. Dark bile is seen draining through the braided wire of the metal biliary stent (arrow). When velocity is constant, a 10-mm metal stent allows roughly 10 times more bile flow, in comparison to a 10-Fr plastic stent of the same length.
ERCP Using a Precut Biliary Access Technique
When both the conventional and double guidewire techniques are proven to be unsuccessful, a precut technique is a useful maneuver to gain access into the bile duct. Precut techniques are divided into three classes based on the anatomy incised to facilitate biliary cannulation. 21 The most common methods involve the use of a needle knife and electrosurgical cautery. In the first technique, a sphincterotomy is made from inside the papillary os , superiorly in direction of the bile duct, while using the needle knife with pulses of cutting cautery. Often a small plastic pancreatic stent (5 Fr or less in diameter) is placed into the pancreatic duct before the precut sphincterotomy is made, to prevent pancreatitis secondary to cautery effect at the pancreatic ductal opening. In the second technique, called “precut fistulotomy,” the incision is made on the mound of the intraduodenal segment of the papilla from north to south stopping well before the papillary os. Therefore, the papillary os is completely untouched minimizing the risk of PEP. This technique, however, is somewhat more difficult to perform than the first and requires proficiency in handling the needle knife in ERCP. The third precut technique makes use of a guidewire that is passed into the pancreatic duct, where in the sphincterotome is used to cut the “septum” between the pancreatic duct and the bile duct—the transpancreatic precut sphincterotomy. This technique requires no additional instruments, but carries a theoretically higher risk of pancreatitis. 22
Endoscopic Ultrasound–Assisted ERCP
EUS-assisted technique comprises rendezvous technique, EUS-guided intrahepatic duct access, and EUS-guided extrahepatic duct access.
EUS-Guided Rendezvous Technique
In this technique, the bile duct is visualized via EUS and accessed using a 19-guage EUS needle under sonographic and fluoroscopic guidance. The preferable location of the bile duct entry is the common bile duct (CBD) surrounded by the pancreas, rather than the common hepatic duct (CHD) which is free-standing without any surrounding organs. However, accessing CBD is not always possible due to the tight strictures incurred by the pancreatic tumor process constricting the duct. Once the bile duct is accessed, the stylet is removed and bile is aspirated confirming that the needle tip is in the bile duct. Contrast is injected cross-checking the location of the needle tip. At this point, an angled 0.035 inch long, guidewire is advanced into the bile duct through the EUS needle and torqued to direct toward the ampulla. The guidewire is then pushed through the ampulla enough to create loops in the duodenum. Subsequently, the EUS scope is exchanged with ERCP scope and the wire is brought out through the therapeutic channel using a biopsy forceps or a snare. Now, typical ERCP can be performed, eventually placing a metal biliary stent. This technique has an overall success rate of 81% with a complication rate of 10%. 23 When this technique was compared with precut needle-knife technique in 206 patients (58 in the EUS-guided rendezvous technique [EUS-RV] group and 144 in the precut needle-knife group), the technical success rate was significantly higher for the EUS-RV group at 98.3% in contrast to 90.3% in the precut needle-knife group, p = 0.03, with no significant difference in the complication rate. 24
EUS-Guided Intrahepatic Duct Access
The concept of accessing the bile duct through the intrahepatic duct is analogous to percutaneous transabdominal biliary drainage that has been perfected by our colleagues in interventional radiology. In this technique, a branch of the left hepatic duct is accessed through the stomach wall using a 19-gauge EUS needle under EUS guidance. A 0.035-inch guidewire is then advanced through the EUS needle after confirming the position of the needle by bile aspiration, EUS, and fluoroscopic view. 25 26 The guidewire should be advanced to and through the ampulla and then EUS scope is exchanged with an ERCP scope. The wire is grabbed by a snare using an ERCP scope, and traditional ERCP is followed. In cases with severe gastric outlet obstruction, sending the wire through the ampulla is of no use, as the ERCP scope cannot be advanced to the second portion of the duodenum. In such cases, the tract from the stomach to the left hepatic duct is dilated and a metal stent is deployed over the guidewire across the biliary stricture. As expected, this procedure can result a leak at the puncture site in the stomach and clipping of the site should be considered after dilation and stent placement. 26 Additionally, securing access into a branch of the left hepatic duct is often challenging due to respiratory movement of patients. 27
EUS-Guided Extrahepatic Duct Access
When the scope cannot be advanced to the second portion of the duodenum due to a severe stricture at the juncture of the first and second portion of the duodenum, the bile duct can be directly accessed using a 19-gauge needle and a guidewire as in the rendezvous technique with an EUS scope placed in the duodenal bulb ( Fig. 3a–f ). 25 26 In contrast to the rendezvous technique, however, no attempt is made to advance the wire toward the ampulla. Instead, the wire is advanced in retrograde fashion to the intrahepatic duct. Over the guidewire, a metal stent is directly placed through the choledochoduodenostomy just created via EUS-guided needle advancement. Regarding the need for dilation over the guidewire before placing a stent, it is usually not necessary as metal stents are stiff and can be pushed through the duodenal wall, provided that the guidewire is advanced deep enough into the intrahepatic duct. Moreover, dilation of the tract is discouraged if the biliary access is through CHD where the bile duct is free-standing without a surrounding structure. With high pressure from a long-standing obstruction, a considerable risk of bile leak exists upon dilation and exchange of dilator and metal stent. Although EUS-guided ERCP has a high technical success rate with an acceptably low complication rate for the cases where conventional ERCP attempts failed to gain access into the bile duct, we must reflect that the published data are from endoscopists in centers with experience and proficiency performing both procedures. Furthermore, the complication rates are, though acceptable, still significant and EUS-guided ERCP does not appear to fare better than the alternative, percutaneous transabdominal biliary drainage by our colleagues in interventional radiology.
Fig. 3.
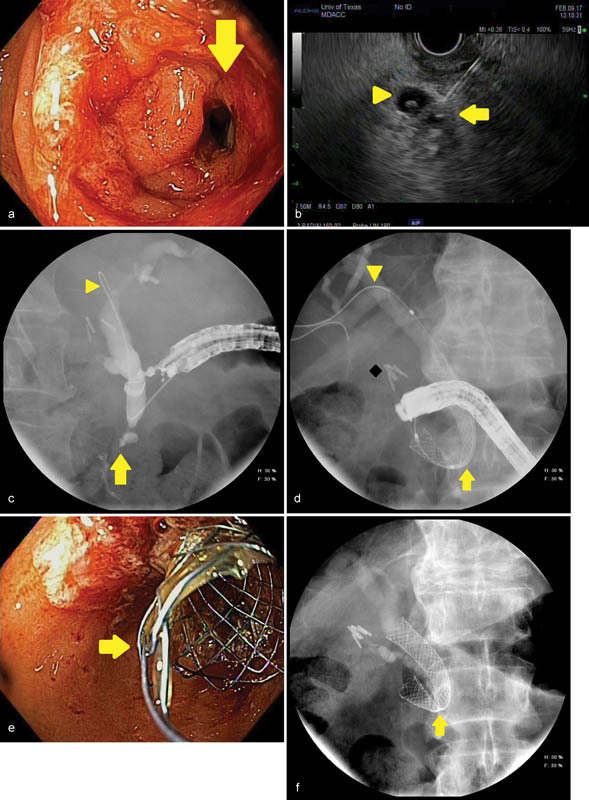
An image series is presented to illustrate endoscopic ultrasound (EUS)-guided biliary access in a 73-year-old man with an unresectable, infiltrating pancreatic cancer complicated by combined biliary obstruction and gastroduodenal outlet obstruction. ( a ) A high-grade duodenal stenosis (arrow) is identified obstructing the area of the papilla, which prevents endoscope passage for conventional endoscopic retrograde cholangiopancreatography (ERCP). ( b ) An endosonographic image obtained shows EUS being used to pass a 19-gauge needle through the scope into the bile duct (arrow). A periampullary diverticulum is noted with internal debris (arrowhead). ( c ) Fluoroscopy confirms a distal biliary stricture (arrow) following passage of an angled-tip 0.035 inch by 460-cm guidewire (arrowhead) through the needle; the needle and EUS scope are subsequently withdrawn leaving the guidewire in place. ( d ) The ERCP scope is passed over the guidewire and a covered metal biliary stent is deployed (arrow), creating a therapeutic choledochoduodenal fistula for biliary drainage. The guidewire (arrowhead) and deployment catheter are then removed. Surgical clips after cholecystectomy are noted (diamond). ( e ) Endoscopic image obtained with the ERCP scope demonstrating the covered metal biliary stent (arrow) emanating from the medial wall of the duodenal bulb. ( f ) The final fluoroscopic image after completion of the procedure confirms appropriate position and expansion of the metal biliary stent. A narrowing or “waist” is seen in the stent as it passes through the duodenal wall (arrow).
ERCP in Surgically Altered Anatomy
Recurrent biliary obstruction may develop within months of pancreaticoduodenectomy or Roux-en-Y hepaticojejunostomy performed for curative intent of pancreaticobiliary cancers. The etiologies of recurrent biliary obstruction include metastatic spread via hematologic or lymphatic routes, or due to growth of micro-metastases present at the surgical margin (so-called R1 resection, with histologic evidence of cancer at the cut margin in the surgical specimen). 28 Such cases of biliary obstruction in patients with surgically altered anatomy present a unique challenge for the interventional gastroenterologist owing to the length of bowel needing to be traversed, difficulty in identifying the neo-biliary orifice, and technical limitations of the instruments (such as endoscopes and accessory devices; Fig. 4 ).
Fig. 4.
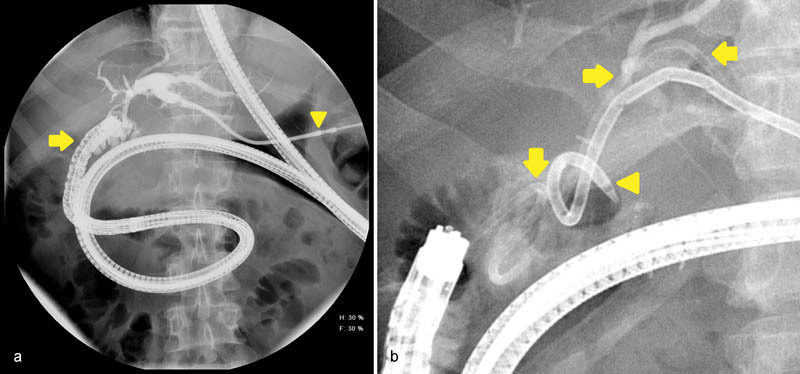
( a ) Fluoroscopic images during endoscopic retrograde cholangiopancreatography (ERCP) in a 58-year-old man following Roux-en-Y hepaticojejunostomy demonstrates markedly redundant pediatric colonoscope (arrow) is passed deep into the jejunum to reach the choledochostomy. Contrast is injected through the percutaneous biliary drain (arrowhead) prior to biliary cannulation which allows the endoscopist to estimate the amount of liver being drained. ( b ) A 7-Fr plastic biliary stent (arrows) is deployed into a segment of the left liver lobe. The percutaneous biliary drain (arrowhead) is capped for 2 weeks as a trial prior to drain removal. Performing ERCP in patients with surgically altered anatomy is technically demanding due to the increased length of bowel that must be traversed and the use of nonstandard or individually modified equipment.
Initial attempts at biliary cannulation in such patients were met with high complication rates; however, with refinement in techniques and devices, a significant proportion of patients may be safely managed with internal biliary stents. Single-balloon enteroscopy (SBE) has emerged as a leading method to reach the biliary anastomosis and facilitate ERCP. 29 30 Using this technique, the endoscope is fitted with an over-the-scope balloon. The endoscope is then advanced as deep as possible into the small bowel, followed by insufflation of the balloon, which acts as an anchor to allow the endoscope to again be advanced deeper into the small bowel. In a meta-analysis of 461 patients with surgically altered anatomy who underwent SBE-assisted ERCP, the procedural success rate was 61.7% (95% CI; 52.9–70.5%). 30 These data suggest, for nonurgent indications, enteroscopy-assisted ERCP is a reasonable first-line approach. Caution should be advised in patients with ascending cholangitis, as delaying biliary drainage due to suboptimal success rates of ERCP in this patient population may lead to adverse outcomes and mortality. 31
Conclusion
Although managing pancreatic cancer is seemingly difficulty and efforts to improve survival have met numerous obstacles over the past three decades, we have seen unceasing effort made in translational research, identifying improved tumor biomarkers, advances in imaging modalities, and endoscopic approaches. ERCP techniques have evolved from conventional cannulation to EUS-assisted ERCP with a higher success rate and declining complication rates. Managing pancreatic cancer undoubtedly calls for a multidisciplinary team approach, including radiologists, cytologists, researchers, medical oncologists, radiation oncologists, gastroenterologists, and surgeons. With ongoing collaborative translational research, clinical trials, and clinical management, we are making incremental advances toward better outcomes for our patients.
Acknowledgments
No financial support was received relevant to the publication of this article. We would like to acknowledge contributions by Dr. Aman Deep, Dr. Faisal S. Ali, Dr. Samreen Khuwaja, and Ms. Laura G. Romero in editing images and organizing references related to this publication.
Footnotes
Disclosure The authors declare no potential conflicts of interest relevant to the publication of this article.
References
- 1.Baron T H, Sr, Davee T. Endoscopic management of benign bile duct strictures. Gastrointest Endosc Clin N Am. 2013;23(02):295–311. doi: 10.1016/j.giec.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Cancer of the Pancreas - Cancer Stat Facts. Available at:https://seer.cancer.gov/statfacts/html/pancreas.html. Accessed March 1, 2017
- 3.Kalser M H, Ellenberg S S. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120(08):899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos J P, Palmer D H, Ghaneh Pet al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial Lancet 2017389(10073):1011–1024. [DOI] [PubMed] [Google Scholar]
- 5.Rosewicz S, Wiedenmann B.Pancreatic carcinoma Lancet (London, England) 1997349(9050):485–489. [DOI] [PubMed] [Google Scholar]
- 6.Argani P, Iacobuzio-Donahue C, Ryu B et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7(12):3862–3868. [PubMed] [Google Scholar]
- 7.Jenkinson C, Elliott V L, Evans A et al. Decreased serum thrombospondin-1 levels in pancreatic cancer patients up to 24 months prior to clinical diagnosis: association with diabetes mellitus. Clin Cancer Res. 2016;22(07):1734–1743. doi: 10.1158/1078-0432.CCR-15-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J-Y, Sun Y-W, Liu D-J, Zhang J-F, Li J, Hua R. MicroRNAs in stool samples as potential screening biomarkers for pancreatic ductal adenocarcinoma cancer. Am J Cancer Res. 2014;4(06):663–673. [PMC free article] [PubMed] [Google Scholar]
- 9.Herreros-Villanueva M, Bujanda L. Glypican-1 in exosomes as biomarker for early detection of pancreatic cancer. Ann Transl Med. 2016;4(04):64. doi: 10.3978/j.issn.2305-5839.2015.10.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Canto M I, Hruban R H, Fishman E Ket al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals Gastroenterology 201214204796–804., quiz e14–e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D B, Varadhachary G R, Crane C H et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 12.Varadhachary G R, Wolff R A, Crane C H et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 13.van der Gaag N A, Rauws E A, van Eijck C H et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362(02):129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 14.Wasan S M, Ross W A, Staerkel G A, Lee J H. Use of expandable metallic biliary stents in resectable pancreatic cancer. Am J Gastroenterol. 2005;100(09):2056–2061. doi: 10.1111/j.1572-0241.2005.42031.x. [DOI] [PubMed] [Google Scholar]
- 15.Mullen J T, Lee J H, Gomez H Fet al. Pancreaticoduodenectomy after placement of endobiliary metal stents J Gastrointest Surg 20059081094–1104., discussion 1104–1105 [DOI] [PubMed] [Google Scholar]
- 16.Lee J H, Krishna S G, Singh A et al. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest Endosc. 2013;78(02):312–324. doi: 10.1016/j.gie.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 17.Tse F, Yuan Y, Moayyedi P, Leontiadis G I. Guidewire-assisted cannulation of the common bile duct for the prevention of post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. Cochrane Database Syst Rev. 2012;12:CD009662. doi: 10.1002/14651858.CD009662.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao W-C, Angsuwatcharakon P, Isayama H et al. International consensus recommendations for difficult biliary access. Gastrointest Endosc. 2017;85(02):295–304. doi: 10.1016/j.gie.2016.09.037. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Fujita N, Noda Y et al. Can pancreatic duct stenting prevent post-ERCP pancreatitis in patients who undergo pancreatic duct guidewire placement for achieving selective biliary cannulation? A prospective randomized controlled trial. J Gastroenterol. 2010;45(11):1183–1191. doi: 10.1007/s00535-010-0268-7. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda I, Isayama H, Bhatia V. Current situation of endoscopic biliary cannulation and salvage techniques for difficult cases: current strategies in Japan. Dig Endosc. 2016;28 01:62–69. doi: 10.1111/den.12591. [DOI] [PubMed] [Google Scholar]
- 21.Davee T, Garcia J A, Baron T H. Precut sphincterotomy for selective biliary duct cannulation during endoscopic retrograde cholangiopancreatography. Ann Gastroenterol. 2012;25(04):291–302. [PMC free article] [PubMed] [Google Scholar]
- 22.Buscaglia J M, Kalloo A N. Pancreatic sphincterotomy: technique, indications, and complications. World J Gastroenterol. 2007;13(30):4064–4071. doi: 10.3748/wjg.v13.i30.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol. 2014;7(02):94–102. doi: 10.1007/s12328-014-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos) Gastrointest Endosc. 2012;75(02):354–359. doi: 10.1016/j.gie.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 25.Chavalitdhamrong D, Draganov P V. Endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2012;18(06):491–497. doi: 10.3748/wjg.v18.i6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy M J. Therapeutic endoscopic ultrasound for biliary and pancreatic disorders. Curr Gastroenterol Rep. 2010;12(02):141–149. doi: 10.1007/s11894-010-0090-7. [DOI] [PubMed] [Google Scholar]
- 27.Dhir V, Bhandari S, Bapat M, Joshi N, Vivekanandarajah S, Maydeo A. Comparison of transhepatic and extrahepatic routes for EUS-guided rendezvous procedure for distal CBD obstruction. United European Gastroenterol J. 2013;1(02):103–108. doi: 10.1177/2050640613480145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Wagensveld B A, Coene P P, van Gulik T M, Rauws E A, Obertop H, Gouma D J. Outcome of palliative biliary and gastric bypass surgery for pancreatic head carcinoma in 126 patients. Br J Surg. 1997;84(10):1402–1406. [PubMed] [Google Scholar]
- 29.De Koning M, Moreels T G. Comparison of double-balloon and single-balloon enteroscope for therapeutic endoscopic retrograde cholangiography after Roux-en-Y small bowel surgery. BMC Gastroenterol. 2016;16(01):98. doi: 10.1186/s12876-016-0512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inamdar S, Slattery E, Sejpal D V et al. Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc. 2015;82(01):9–19. doi: 10.1016/j.gie.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Khashab M A, Tariq A, Tariq U et al. Delayed and unsuccessful endoscopic retrograde cholangiopancreatography are associated with worse outcomes in patients with acute cholangitis. Clin Gastroenterol Hepatol. 2012;10(10):1157–1161. doi: 10.1016/j.cgh.2012.03.029. [DOI] [PubMed] [Google Scholar]


