Abstract
Background
Conflicting data exist regarding optimum local therapy for early-stage triple-negative breast cancer (TNBC). We examined outcomes according to local treatment type in a large cohort of node-negative TNBC patients.
Methods
A total of 1,242 consecutive patients with TNBC treated at a single institution from 1999 to 2008 were identified. Of these, 646 with pathologic stage T1-2N0 TNBC underwent breast-conserving therapy (BCT) (N = 448) or total mastectomy (TM) without postmastectomy radiation (N = 198) and comprised the study population. Locoregional recurrence (LRR), distant metastasis (DM), and overall recurrence were investigated with a competing risk analysis using Gray’s test and multivariable Fine and Gray competing risk regression. Overall survival was assessed using standard Kaplan–Meier methods and a Cox proportional hazards analysis.
Results
Median follow-up was 78.3 months (range 1–156). Eight-one percent of patients received adjuvant chemotherapy. TM patients were younger, were more likely to have lymphovascular invasion, and had larger tumors than patients undergoing BCT (all P ≤ 0.05). The 5-year cumulative incidence of LRR was 4.2 and 5.4 % for patients undergoing BCT and TM, respectively. There was no significant difference in LRR, DM, overall recurrence, disease free survival, or overall survival between groups on univariate analysis, or after adjusting for other variables in multivariate models. Lack of chemotherapy and high tumor stage independently predicted for decreased overall survival (both P < 0.001).
Conclusions
A low, 5-year risk of LRR (4.7 %) was achieved in a large group of women with T1-2N0 TNBC treated with multimodality therapy. BCT was as equally effective as TM for local and distant control.
The poor prognosis of triple-negative breast cancer (TNBC) relative to other biologic subtypes of breast cancer has been well established during the past decade.1,2 Several studies, largely consisting of patients treated in an era that predates the use of trastuzumab, have demonstrated the increased risk of LRR of triple-negative and HER2-neupositive breast cancers relative to hormone receptor-positive breast cancers in patients treated with breast-conserving therapy (BCT).3–6 Similarly, an increased risk of locoregional recurrence (LRR) in TNBC patients following total mastectomy (TM) also has been shown, even when postmastectomy radiation is delivered.6,7
Recently, the classic paradigm of equivalent locoregional control with BCT or mastectomy has been questioned in patients with TNBC. Whereas some have reported that BCT provides superior locoregional control compared with mastectomy for early-stage TNBC, others have been unable to validate these findings.8,9 Given the uncertainty regarding optimal local treatment recommendations in this population, we undertook the present study to evaluate the locoregional outcomes of BCT and mastectomy in early-stage, node-negative TNBC patients treated in a contemporaneous era with a modern multimodality therapeutic paradigm.
METHODS
Patient Selection
TNBC patients were identified from clinical pathology reports. Absence of ER or PR staining or HER2 staining classified as 0 or 1+ were considered negative. In the case of tumors with equivocal HER2 status after immunohistochemistry (2+), fluorescence in situ hybridization was performed to assess for amplification (defined as HER2 to probe ratio >2.2). A total of 1,242 consecutive patients with newly diagnosed stage I to III TNBC treated with primary surgery and without neoadjuvant chemotherapy at Memorial Sloan-Kettering Cancer Center (MSKCC) from 1999 to 2008 were identified from an institutional database. The following exclusion criteria were applied: T3/T4 or positive lymph nodes (N = 485), breast-conserving surgery (BCS) without RT (N = 26), or unknown adjuvant chemotherapy or radiation status (N = 84). Patients with isolated tumor cells identified only on immunohistochemical staining (pN0(i+)) were included. There were 646 patients with T1-2N0 TNBC available for analysis. Tumor and lymph node staging was performed according to seventh edition of the American Joint Committee on Cancer.10 Clinical information was abstracted from electronic medical records. Information regarding breast cancer susceptibility gene (BRCA1/BRCA2) mutation status was obtained from the MSKCC clinical genetics service database. This study was approved by the Institutional Review Board.
Treatment and Follow-up
Surgical treatment consisted of breast-conserving surgery or TM. Eighty-seven percent of patients underwent sentinel lymph node biopsy alone and 13 % of patients had axillary dissections. Negative margins, defined as tumor cells not touching the ink, were obtained in 99 % of patients. All patients undergoing BCS received postoperative whole breast radiation. Separate supraclavicular or axillary boost fields were not utilized. At the discretion of the treating radiation oncologist, 92.6 % of BCT patients received a boost to the lumpectomy cavity. The total median radiation dose was 60.4 Gy (range 42.4–69.0). No patient undergoing mastectomy received postmastectomy radiation.
Endpoints and Statistics
All endpoints were defined in accordance with the STEEP criteria.11 LRR were biopsy-proven and defined as tumor recurring in the breast, chest wall, or the ipsilateral regional lymph nodes (axillary, supraclavicular, internal mammary) either as a first breast cancer event or simultaneously with distant recurrence.
Time to local and distant relapse was calculated from the date of core biopsy, as was survival time. Baseline clinical characteristics were compared using Fisher’s exact test. For LRR, distant metastasis (DM), and overall recurrence, competing risk analysis was used to estimate the cumulative incidence rate (CIR). Gray’s test was used to compare the CIRs. For LRR and DM, given a limited number of events, variables with univariate P value <0.15 were examined in a Fine and Gray multivariable model along with the surgery type, chemotherapy, and tumor stage. For overall recurrence, all variables were included in the multivariate model.
Analysis for disease-free survival and overall survival was similar to overall recurrence, except that the standard Kaplan-Meier method was used to estimate actuarial survival probabilities and a Cox proportional hazards model was used to estimate univariate and multivariate hazard ratios and 95 % confidence intervals. All statistical analyses were performed in software packages SAS 9.2 (SAS Institute Inc., Cary, NC) and R version 2.13 (The R Foundation for Statistical Computing, Vienna, Austria). A test with the P value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics for patients by surgery type are described in Table 1. Overall, 448 patients (69 %) received BCT and 198 (31 %) underwent TM. The median age at diagnosis was 54 years (range 20–88). Eighty-one percent of patients received adjuvant chemotherapy. For those receiving chemotherapy, systemic regimens were anthracycline and taxane-based in 46 %, anthracycline-based without taxanes in 13 %, cyclophosphamide, methotrexate, and fluorouracil (CMF) based in 37 %, and another regimen in 4 %. Compared with patients undergoing TM, BCT patients were older (P < 0.001), more likely to identify as non-black race (P = 0.040), less likely to have lymphovascular invasion (P = 0.042), and had a lower tumor stage (P = 0.023). Additionally, patients receiving BCT were significantly less likely to undergo axillary dissection (P < 0.001) and to have BRCA mutations (P < 0.001). No significant difference in tumor grade or chemotherapy use was noted between the groups.
TABLE 1.
Clinical characteristics
| BCT | Mastectomy | P value | |
|---|---|---|---|
| No. of patients (N) | 448 | 198 | |
| Median follow-up (months) | 79.7 | 77.1 | |
| Age (years) | <0.001 | ||
| ≤50 | 155 (35 %) | 104 (53 %) | |
| >50 | 293 (65 %) | 94 (47 %) | |
| Race | 0.040 | ||
| Non-black | 391 (87 %) | 184 (93 %) | |
| Black | 57 (13 %) | 14 (7 %) | |
| T-stage | 0.023 | ||
| Tmic/T1a/T1b | 124 (28 %) | 65 (33 %) | |
| T1c | 210 (47 %) | 70 (35 %) | |
| T2 | 114 (25 %) | 63 (32 %) | |
| LVI | 0.042 | ||
| No | 389 (87 %) | 156 (79 %) | |
| Yes | 59 (13 %) | 38 (19 %) | |
| Unknowna | 0 (0 %) | 4 (2 %) | |
| Grade | 0.586 | ||
| 1 and 2 | 47 (10 %) | 24 (12 %) | |
| 3 | 401 (90 %) | 174 (88 %) | |
| BRCA | <0.001 | ||
| Not tested | 380 (85%) | 149 (76%) | |
| Tested negative | 48 (11%) | 22 (11%) | |
| Polymorphism | 2 (0.4%) | 0 (0%) | |
| BRCA1 mutation | 13 (3%) | 23 (12%) | |
| BRCA2 mutation | 3 (0.7%) | 6 (3%) | |
| Axillary surgery | <0.001 | ||
| Sentinel lymph node biopsy | 414 (92 %) | 151 (76 %) | |
| Axillary dissection | 34 (8 %) | 47 (24 %) | |
| Chemotherapy | 0.444 | ||
| No | 88 (20 %) | 33 (17 %) | |
| Yes | 360 (80 %) | 165 (83 %) | |
| Chemotherapy regimen | 0.586 | ||
| Anthracycline/taxane-based | 148 (33 %) | 91 (46 %) | |
| Anthracycline-based (no taxane) | 56 (13 %) | 14 (7 %) | |
| CMF | 142 (32 %) | 51 (26 %) | |
| Other | 14 (3 %) | 9 (5 %) | |
| Radiation | <0.001 | ||
| No | 0 (0 %) | 198 (100 %) | |
| Yes | 448 (100 %) | 0 (0 %) |
BCT breast-conserving therapy, LVI lymphovascular invasion, CMF cyclophosphamide methotrexate fluorouracil
Unknowns were removed from the test
In total, 117 patients were tested for BRCA mutations, including 51 undergoing mastectomy and 66 undergoing BCT. Of these, there were 45 deleterious BRCA mutations detected, including 29 in mastectomy patients and 16 in BCT patients. Median follow-up was 76.4 months (range 1–156) for surviving patients, and was similar for patients undergoing BCT (79.7 months) or mastectomy (77.1 months).
There were 33 LRR (crude LRR rate = 5.3%) in our cohort. Twenty of 448 BCT patients (4.5 %) experienced LRR, including 10 local recurrences, 6 regional recurrences, and 4 simultaneous local and regional recurrences. A similar pattern was seen after TM with a total LRR rate of 6.6 %, which included 8 local recurrences, 4 regional recurrences, and 1 patient with both. The 5-year cumulative incidence of LRR for patients undergoing BCT was 4.2 % compared with 5.4 % for patients receiving mastectomy (Fig. 1a). There was no significant difference in LRR when comparing BCT and mastectomy in a competing risk univariate analysis (Table 2, hazard ratio (HR) = 1.5, 95 % confidence interval (CI) 0.75–3.01, P = 0.25). There were trends toward increased LRR for patients with lymphovascular invasion (HR = 2.12, 95 % CI 1–4.53, P = 0.051) and those not receiving chemotherapy (HR = 3.62, 95 % CI 0.85–15.36, P = 0.081). After multivariate adjustment, there continued to be no difference in locoregional outcomes between BCT and mastectomy (Table 3, odds ratio (OR) = 1.44, 95 % CI 0.71–2.92, P = 0.31). No independent predictors for LRR were identified in the final multivariate model.
FIG. 1.
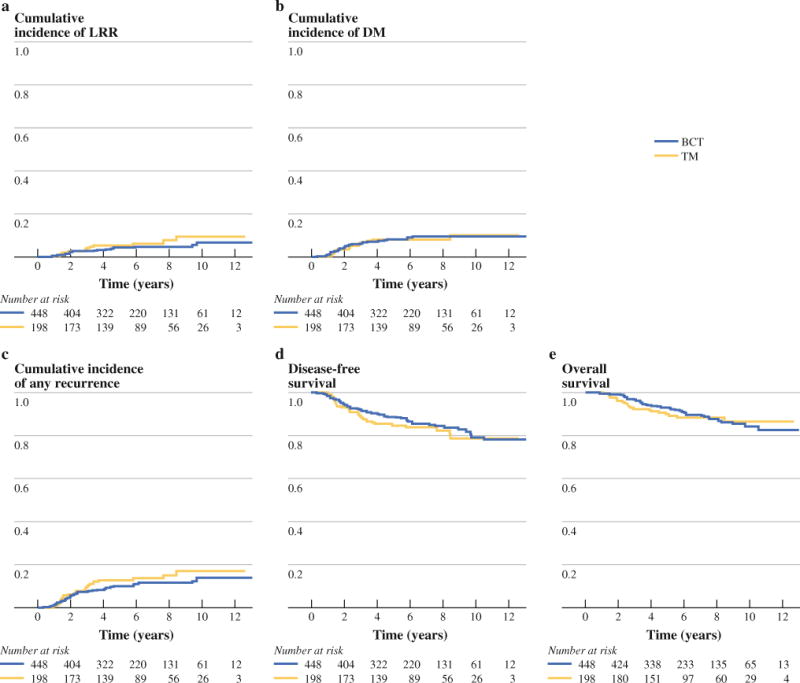
Cumulative incidence of locoregional recurrence (a), cumulative incidence of distant metastasis (b), cumulative incidence of overall recurrence (c), disease-free survival (d), and overall survival (e) for patients undergoing breast-conserving therapy and mastectomy
TABLE 2.
Univariate analysis of outcomes in women with T1-2N0 triple-negative breast cancer
| Variable | LRR
|
DM
|
Overall recurrence
|
Disease-free survival
|
Overall survival
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | HR (95 % CI) | P value | HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Surgery type | ||||||||||
| Mastectomy versus BCT | 1.5 (0.75–3.01) | 0.25 | 0.97 (0.54–1.75) | 0.92 | 1.32 (0.83–2.12) | 0.24 | 1.21 (0.79–1.84) | 0.379 | 1.07 (0.64–1.79) | 0.801 |
| Age (year) | ||||||||||
| >50 versus ≤50 | 1.26 (0.64–2.5) | 0.51 | 1.15 (0.67–1.98) | 0.61 | 1.22 (0.77–1.93) | 0.39 | 0.99 (0.66–1.49) | 0.965 | 1.01 (0.62–1.65) | 0.966 |
| Race | ||||||||||
| Black versus non-black | 1.88 (0.77–4.6) | 0.16 | 1.97 (0.99–3.9) | 0.054 | 2.03 (1.13–3.64) | 0.017 | 1.75 (1.02–2.99) | 0.041 | 1.71 (0.89–3.26) | 0.106 |
| T stage | ||||||||||
| Tmic/T1a/T1b | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| T1c | 1.59 (0.66–3.84) | 0.3 | 1.5 (0.74–3.04) | 0.26 | 1.38 (0.76–2.51) | 0.29 | 1.57 (0.92–2.66) | 0.095 | 2.77 (1.33–5.78) | 0.007 |
| T2 | 1.55 (0.59–4.03) | 0.37 | 1.78 (0.85–3.75) | 0.13 | 1.79 (0.97–3.33) | 0.064 | 1.89 (1.09–3.3) | 0.025 | 3.07 (1.43–6.61) | 0.004 |
| LVI | ||||||||||
| Yes versus no | 2.12 (1–4.53) | 0.051 | 0.83 (0.38–1.82) | 0.64 | 1.17 (0.65–2.11) | 0.6 | 1.28 (0.77–2.14) | 0.344 | 1 (0.51–1.96) | 0.996 |
| Grade | ||||||||||
| 3 versus 1 and 2 | 4.06 (0.56–29.37) | 0.16 | 1.16 (0.46–2.89) | 0.75 | 1.41 (0.61–3.22) | 0.42 | 1.96 (0.86–4.47) | 0.112 | 1.97 (0.72–5.42) | 0.188 |
| Chemotherapy | ||||||||||
| Yes versus no | 3.62 (0.85–15.36) | 0.081 | 0.75 (0.39–1.42) | 0.37 | 1.05 (0.57–1.92) | 0.88 | 0.76 (0.47–1.23) | 0.264 | 0.6 (0.35–1.04) | 0.068 |
LRR locoregional recurrence, DM distant metastasis, LVI lymphovascular invasion, BCT breast-conserving therapy, HR hazard ratio. CI confidence interval
TABLE 3.
Multivariate analysis of outcomes in women with T1-2N0 triple-negative breast cancer
| Variable | LRR
|
DM
|
Overall recurrence
|
Disease-free survival
|
Overall survival
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | HR (95 % CI) | P value | HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Surgery type | ||||||||||
| Mastectomy vs. BCT | 1.44 (0.71–2.92) | 0.31 | 1.09 (0.58–2.07) | 0.79 | 1.42 (0.84–2.38) | 0.19 | 1.25 (0.8–1.93) | 0.325 | 1.09 (0.63–1.87) | 0.762 |
| Age (year) | ||||||||||
| >50 versus ≤50 | — | — | — | — | 1.16 (0.71–1.9) | 0.56 | 0.99 (0.65–1.51) | 0.967 | 0.95 (0.57–1.57) | 0.832 |
| Race | ||||||||||
| Black versus non-black | — | — | 1.91 (0.92–3.95) | 0.083 | 2.09 (1.12–3.9) | 0.021 | 1.76 (1.02–3.05) | 0.043 | 1.68 (0.87–3.25) | 0.125 |
| T stage | ||||||||||
| Tmic/T1a/T1b | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref | 1 | Ref |
| T1c | 1.22 (0.53–2.85) | 0.64 | 1.81 (0.84–3.92) | 0.13 | 1.4 (0.74–2.67) | 0.3 | 2.09 (1.13–3.89) | 0.019 | 4.21 (1.89–9.35) | <0.001 |
| T2 | 1.1 (0.44–2.77) | 0.84 | 2.24 (0.99–5.07) | 0.053 | 1.78 (0.9–3.5) | 0.097 | 1.82 (1.02–3.25) | 0.044 | 4.71 (2.00–11.06) | <0.001 |
| LVI | ||||||||||
| Yes versus no | 1.86 (0.88–3.95) | 0.11 | 0.81 (0.36–1.82) | 0.61 | 1.07 (0.58–1.95) | 0.83 | 1.21 (0.72–2.04) | 0.474 | 0.93 (0.47–1.85) | 0.841 |
| Grade | ||||||||||
| 3 versus 1 and 2 | — | — | — | — | 1.19 (0.52–2.75) | 0.68 | 1.67 (0.72–3.86) | 0.231 | 1.51 (0.54–4.22) | 0.427 |
| Chemotherapy | ||||||||||
| Yes versus no | 3.06 (0.75–12.43) | 0.12 | 0.54 (0.26–1.13) | 0.1 | 0.77 (0.38–1.54) | 0.45 | 0.54 (0.31–0.93) | 0.026 | 0.32 (0.17–0.59) | <0.001 |
LRR locoregional recurrence, DM distant metastasis, LVI lymphovascular invasion, BCT breast-conserving therapy, HR hazard ratio. CI confidence interval
Fifty-two patients (8 %) developed distant metastasis, including 36 (8 %) BCT patients and 16 (8.1 %) TM patients. The 5-year cumulative incidence of DM was 8.2 % for patients receiving BCT compared with 8.1 % for those receiving TM (Fig. 1b). On univariate analysis, the rate of DM did not differ among patients having BCT or TM (Table 2, HR = 0.97, 95 % CI 0.54–1.75, P = 0.92). Multivariate analysis (Table 3) failed to identify any independent predictors for distant metastasis, although there was a trend for an increased rate of distant metastasis with T2 tumors (HR = 2.24, 95 % CI 0.99–5.07, P = 0.053).
Finally, there was no difference in overall survival with either BCT or TM in univariate (Table 2, HR = 1.07, 95 % CI 0.64–1.79, P = 0.801) or multivariate analysis (Table 3, HR = 1.09, 95 % CI 0.63–1.87, P = 0.762). Chemotherapy use (HR = 0.32, 95 % CI 0.17–0.59, P < 0.001) was associated with reduced risk of mortality in multivariate analysis. Additionally, patients with pathologic tumor stage T1c (HR = 4.21, 95 % CI 1.89–9.35, P < 0.001) and T2 tumors (HR = 4.71, 95 % CI 2–11.06, P < 0.001) had significantly decreased overall survival compared with patients with stage T1a–T1b tumors.
DISCUSSION
In this study of 646 patients with T1-2N0 TNBC treated at our institution over nearly a decade, we found no advantage for either BCT or mastectomy in terms of locoregional control, distant relapse, overall recurrence, or overall survival. The 5-year cumulative incidence rate of LRR was 4.2 % with BCT versus 5.4 % with mastectomy, underscoring the favorable locoregional control rates that are achievable in this population utilizing a modern multimodality therapeutic approach. Given the low number of locoregional events, we were unable to identify any independent predictors for an increased risk of LRR in our multivariate model.
These findings corroborate those of a recently published study of 1,325 patients with TNBC treated at the MD Anderson Cancer Center, in which surgery type was not found to be a predictor of LRR after multivariate adjustment for known prognostic factors (HR = 1.07, 95 % CI 0.86–1.34, P = 0.55).9 Despite the relatively large sample size, there were several limitations of this study, including a heterogeneous population including all clinical stages and significant imbalances between the BCT and mastectomy groups in terms of tumor stage, nodal stage, and lymphovascular invasion, all favoring patients receiving BCT. In addition, more than half of the patients were treated before 2000 and 58 % of the entire cohort had node-positive disease. Both of these factors in part may explain the 27 % 5-year LRR rate observed in these patients. In aggregate, the heterogeneous patient population and the time period during which the majority of patients were accrued may limit the applicability of these results to current populations of early-stage TNBC patients.
In contrast, a retrospective subset analysis from the University of Alberta demonstrated that in 468 women with T1-2N0 TNBC, patients treated with BCT had significantly better locoregional control compared with women treated with mastectomy.8 With a median follow-up of 7.2 years, BCT patients had a 5-year actuarial LRR rate of 4 % compared with 10 % in patients who underwent mastectomy (P = 0.022). The authors attempted to correct for potential imbalances in clinical and pathological characteristics in the two surgical groups by performing multivariate analyses for both the entire T1-2N0 subset and in 195 pairs of patients from either treatment arm matched for tumor stage. In both of these analyses, the significantly improved locoregional outcomes for patients undergoing BCT persisted.
Several features may explain the discrepant results between our study and the University of Alberta series. In keeping with modern standards, the majority (81 %) of patients in our study received adjuvant chemotherapy compared with only 35 % of those with T1-2N0 breast cancer in the University of Alberta study. Given the beneficial impact of systemic therapy on locoregional control, it is possible that the underutilization of chemotherapy may account partially for the higher 5-year LRR of 10 % observed after mastectomy in the University of Alberta study compared with 5.4 % in our study.12 Additionally, details regarding the comparison for prognostic factors, such as margin status, number of nodes removed, BRCA status, chemotherapy type, and length of follow-up between the treatment groups were not reported in the University of Alberta study and may have influenced the results. Finally, the statistical methods employed were inherently different. The University of Alberta study employed Kaplan–Meier and Cox regression analysis to calculate actuarial LRR rates, which examines only first events and ignores the dependent relationship between locoregional and distant events in breast cancer. In our study, we utilized competing risk analyses that minimized potential bias by taking into account the effect of multiple locoregional and distant events, a statistical practice that is particularly germane to studies of TNBC, in which there is a higher propensity for early events compared with other biologic subtypes of breast cancer.13
Our series represents the largest population of TNBC patients with exclusively T1-2N0 tumors to date and expands upon the results of a previous study from our center reporting locoregional and distant outcomes in 194 patients with subcentimeter, node-negative TNBC.14 Even with the addition of 458 patients with larger TNBC tumors, locoregional outcomes in the current study were comparable to the 5-year, LRR-free survival of 96 % observed in the T1a/T1bN0 TNBC patients from the prior study, of whom only 58 % received chemotherapy. These data challenge conventional beliefs regarding the effect of increasing tumor size on locoregional outcomes. It is possible that classic tumor size-prognosis relationships are not relevant to node-negative TNBC, analogous to the observation that number of positive nodes is unrelated to prognosis in node-positive TNBC.15
Another notable aspect of our study was the availability of genetics information for a proportion of the study population. Given the modest proportion (18 %) of the study cohort tested, it is unlikely that genetic testing results significantly biased the choice of surgical treatment. The 38 % prevalence of BRCA mutations amongst the 117 patients in our cohort undergoing BRCA testing is somewhat higher than the 20–29 % incidence rate of BRCA mutations reported in other TNBC series, but most likely represents more rigorous selection process for genetic testing based on family history, ethnicity, and age.16–18 In any event, in our study BRCA mutation status had no detectable impact on study outcomes (data not shown), consistent with other studies reporting similar overall prognoses of BRCA mutation carriers and noncarriers, although the low number of events amongst the 45 patients testing positive for BRCA mutations limits the ability to draw robust statistical conclusions.19
We acknowledge several limitations of this study; the most significant is that it is a single institution, retrospective, nonrandomized study. Not surprisingly, there were differences in the clinical and pathologic characteristics of patients receiving BCT compared with those undergoing mastectomy. Although we attempted to correct for these imbalances using multivariate analyses, unmeasured confounding factors may have influenced the outcome. Furthermore, despite the large size of our patient cohort, there were relatively few locoregional events, limiting the statistical power of the study to identify predictors of LRR or to detect a small, but statistically significant, difference in LRR. However, given that the absolute cumulative incidence of LRR at 5 years was 4.2 and 5.4 % for BCT and mastectomy, respectively, and that four LRRs must be prevented at 5 years to prevent one death from breast cancer at 15 years, it is highly unlikely that increased patient numbers would produce a clinically meaningful change in our results.20
Given that it is unlikely that a randomized trial comparing BCT to mastectomy for TNBC will be conducted in the near future, we believe that the results of this retrospective study contribute to the growing body of evidence showing that early-stage TNBC patients treated with a modern multimodality paradigm employing contemporary surgery and radiation techniques in combination with chemotherapy can achieve acceptable locoregional outcomes regardless of the extent of surgery. The major implication of these data is that once tumor burden is reduced to subclinical disease following adequate surgical treatment, tumor biology and the quality of systemic therapy, not the extent of surgery, are the main determinants of prognosis in TNBC. To this end, more aggressive local therapy is unlikely to improve outcomes significantly in these patients. Rather, improving upon the results observed to date will require refining our understanding of the biologic heterogeneity of TNBC, defining clinically applicable molecular signatures that predict treatment response and patterns of recurrence, and developing systemic agents that are selectively lethal to TNBC cells.21–25
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 3.Arvold ND, Taghian AG, Niemierko A, Abi Raad RF, Sreedhara M, Nguyen PL, et al. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29(29):3885–91. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solin LJ, Hwang WT, Vapiwala N. Outcome after breast conservation treatment with radiation for women with triple-negative early-stage invasive breast carcinoma. Clin Breast Cancer. 2009;9(2):96–100. doi: 10.3816/CBC.2009.n.018. [DOI] [PubMed] [Google Scholar]
- 5.Millar EK, Graham PH, O’Toole SA, McNeil CM, Browne L, Morey AL, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27(28):4701–8. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 6.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 7.Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(9):1419–26. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 8.Abdulkarim BS, Cuartero J, Hanson J, Deschenes J, Lesniak D, Sabri S. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29(21):2852–8. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adkins FC, Gonzalez-Angulo AM, Lei X, Hernandez-Aya LF, Mittendorf EA, Litton JK, et al. Triple-negative breast cancer is not a contraindication for breast conservation. Ann Surg Oncol. 2011;18(11):3164–73. doi: 10.1245/s10434-011-1920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th. New York: Springer; 2010. [Google Scholar]
- 11.Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25(15):2127–32. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 12.Buchholz TA, Tucker SL, Erwin J, Mathur D, Strom EA, McNeese MD, et al. Impact of systemic treatment on local control for patients with lymph node-negative breast cancer treated with breast-conservation therapy. J Clin Oncol. 2001;19(8):2240–6. doi: 10.1200/JCO.2001.19.8.2240. [DOI] [PubMed] [Google Scholar]
- 13.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 14.Ho AY, Gupta G, King TA, Perez CA, Patil SM, Rogers KH, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer. 2012;118(20):4944–52. doi: 10.1002/cncr.27480. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Angulo AM, Timms KM, Liu S, Chen H, Litton JK, Potter J, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17(5):1082–9. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulkes WD, Stefansson IM, Chappuis PO, Begin LR, Goffin JR, Wong N, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95(19):1482–5. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 17.Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24(36):5652–7. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Aya LF, Chavez-Macgregor M, Lei X, Meric-Bern-stam F, Buchholz TA, Hsu L, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29(19):2628–34. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayraktar S, Gutierrez-Barrera AM, Liu D, Tasbas T, Akar U, Litton JK, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat. 2011;130(1):145–53. doi: 10.1007/s10549-011-1711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 21.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–9. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metzger-Filho O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30(15):1879–87. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364(3):205–14. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 25.Perez EA, Patel T, Moreno-Aspitia A. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res Treat. 2010;121(2):261–71. doi: 10.1007/s10549-010-0824-0. [DOI] [PubMed] [Google Scholar]


