Abstract
Background
As Sub-Saharan Africa transitions to a new era of universal ART, up-to-date assessments of HIV RNA (viral load, VL) suppression at a population level are needed to understand demographic and geographic sources of ongoing viremia and to inform interventions to optimize ART delivery. We sought to measure population viral load (VL) metrics to assess current viral suppression levels and characterize demographic groups and geographic locations with high-level detectable viremia in East Africa.
Methods
In the SEARCH HIV test-and-treat study (NCT01864683), we conducted baseline HIV testing (89% uptake) and HIV RNA assessments in 32 rural communities in 2013–2014 in Uganda and Kenya (N=303,461). We measured VL in 8,828 HIV+ adults, and defined viral suppression as VL<500 copies/mL. To assess geographic sources of transmission risk, we determined the proportion of all adults (both HIV-positive and HIV-negative) with detectable VL (termed ‘local prevalence of viremia’). Transmission risk ‘hotspots’ were defined as geopolitical subunits within communities with >5.0% local prevalence of viremia. We also assessed sero-discordant couples, measuring the proportion in which the HIV+ partner had detectable viremia.
Findings
Viral suppression was 82% (3,427/4,202) among adults on ART, and 51% (4,490/8,828) among all HIV+ adults. Regional viral suppression among HIV+ adults was 48% (West Uganda), 45% (East Uganda) and 53% (Western Kenya). Transmission risk ‘hotspots’ included 1/21 W.Uganda, 0/18 E.Uganda, and 16/26 Kenya geopolitical subunits. In Uganda, sero-discordancy was 3.1% (492 discordant/16,023 total couples). In 58% of discordant couples, the HIV+ partner was viremic (14% had VL>100,000). In Kenya, sero-discordancy was 10.0% (859/8,616 total couples). In 53%, the HIV+ partner was viremic (15% with VL>100,000).
Interpretation
Prior to the 2013–2014 start of the SEARCH trial, 51% of East African HIV+ adults had viral suppression, reflecting ART scale-up efforts to date. However, geographic ‘hotspots’ of potential HIV transmission risk as well as detectable viremia among sero-discordant couples warrant intensified interventions.
Funding
US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health. President’s Emergency Plan for AIDS Relief (PEPFAR).
Keywords: HIV antiretroviral therapy (ART), population HIV viral load, hotspots, transmission risk, sero-discordant couples
Introduction
As Sub-Saharan Africa transitions to a new era in which ART is offered to all HIV-positive persons regardless of CD4+ cell count,1–3 up-to-date assessments of HIV RNA (viral load, VL) suppression at a population level4–7 can be informative. Viral suppression is the final metric of the UNAIDS 90-90-90 initiative 8 (90% diagnosed/90% on ART/90% virologically suppressed), which aims at maximizing HIV diagnosis, treatment and viral suppression to improve individuals’ health and substantially reduce the potential for HIV transmission.9, 10 As countries begin expanding to universal ART coverage, insights into population-level viral suppression can assist treatment and prevention programs by revealing demographic groups with the highest burden of detectable VL. Population VL analyses can also characterize the geographic distribution of persons with detectable VL and identify ‘hotspots’ of potential HIV transmission risk—areas where persons with HIV or persons with detectable viremia are prevalent.11 Along with behavioral risk factor data, these insights can help programs efficiently target resources to the people and regions most in need of intensified HIV program support.
The scale-up of HIV testing and CD4-guided ART has proceeded at different paces in Sub-Saharan African nations,12 leading to wide-ranging estimates of population-level viral suppression. UNAIDS has estimated that 32% of Sub-Saharan HIV+ adults overall are virally suppressed.13 However, in one recent study from Botswana—one of the first providers of widescale HIV testing and treatment—population viral suppression was estimated to be far higher at 70%.14 In contrast, in two smaller studies in East Africa, viral suppression was in the 40–50% range,15, 16 and in a study from Swaziland, was estimated at 35%.17 Other VL surveys have been limited in size, and several have been derived from persons already in HIV care rather than from comprehensive population samples that include persons unaware of their HIV diagnosis or not yet in HIV care. More broadly, comprehensive measures of VL at a population level have not been frequently conducted due to logistic challenges with population sampling, barriers to VL collection, and cost of VL testing.18
Sero-discordant couples are a high priority population in Sub-Saharan Africa: it is well established that intensive treatment and prevention interventions should be offered to minimize HIV transmission risk. However, data that make the crucial distinction between sero-discordant couples in which the HIV+ partner is virologically suppressed versus viremic are not widely available. Because HIV transmission risk is directly related to levels of viremia,9, 19, 20 understanding the epidemiologic prevalence of viremia within sero-discordant couples is a high priority for developing optimum interventions for this population.
The SEARCH Study (Sustainable East Africa Research in Community Health: NCT01864683) is a cluster randomized trial in Uganda and Kenya of a universal HIV test-and-treat strategy combining universal HIV testing campaigns with efficient universal ART delivery to all HIV-infected persons; SEARCH is assessing the impact of this test-and-treat approach on HIV incidence and secondary individual and community-level health outcomes. During the baseline population assessment in the SEARCH Study, we sought to determine the current state of viral suppression in rural regions of Uganda and Kenya that are rapidly scaling up HIV testing and ART provision. We also sought to assess demographic factors (including sero-discordancy) as well as geographic factors influencing HIV risk related to detectable viremia in these regions.
Methods
Study Design and Population
The Sustainable East Africa Research for Community Health Study (SEARCH:NCT01864603) is a multi-national cluster-randomized trial of a universal HIV ‘test-and-treat’ intervention strategy versus standard country-guided HIV control strategy across 32 communities (approximately N=10,000 each) in rural West Uganda, East Uganda, and Homa Bay and Migori Counties in Kenya. Communities were selected using detailed criteria previously reported,21 including rural setting, approximate population of 10,000 persons, and within the catchment area of a President’s Emergency Plan for AIDS Relief (PEPFAR) supported HIV clinic. Ugandan and Kenyan communities were comprised of geopolitical subunits (‘parishes’ in Uganda; ‘sublocations’ in Kenya).
This study was approved by ethical review boards of Makerere University, Uganda National Council of Science and Technology (Kampala, Uganda), Kenya Medical Research Institute (Nairobi, Kenya), and the University of California, San Francisco (USA). All participants provided verbal informed consent in their preferred language.
Procedures
As previously described, SEARCH performed a household census to enumerate and collect demographic information from all residents of 32 study communities. This survey collected age, gender, marital and occupational status, history of binge alcohol drinking (≥6 drinks at one time), and self-reported estimates of household wealth.21 We performed population-wide HIV testing (using a hybrid approach of community health campaigns [CHCs], followed by home-based testing of persons who did not attend the campaigns) and baseline HIV RNA measurements for adults in the 32 communities.21 We classified participants as on or off ART by their self report. In a random subset of patients, self-reported ART status was confirmed by examining clinic records, which note ART initiation and refill dates.
In HIV-positive participants, plasma was processed by study staff from fingerprick or venous capillary blood as reported previously.6, 22 Plasma HIV RNA levels were determined using commercial real-time PCR assays at accredited laboratories at the University of California, San Francisco, the Joint Clinical Research Centers (JCRC), Uganda, and at the Centers for Disease Control (CDC) HIV-R laboratory in Kisumu, Kenya. Reduced plasma input volumes for capillary sampling (70 uL) increased the lower limit of quantitation (LLOQ) to 500 copies/mL in 29/32 communities, and to 1000 copies/mL in the remaining 3/32 communities.
Analysis
To have a consistent cross-community definition of viral suppression, we excluded the 3/32 SEARCH communities where VL assay LLOQ was 1000 copies/mL, and included the 29/32 communities where VL assay LLOQ was 500 copies/mL. We analyzed adults (≥15 years) who were ‘stable’ community residents, defined by self-report as living in the community for ≥6 months in the preceding year.
We calculated viral suppression (defined as VL<500 copies/mL) among all HIV+ persons self-reporting ART use, reflecting one aspect of ART program effectiveness. We further characterized VL metrics among all HIV+ persons (regardless of ART use) as an overall assessment of the success of ART penetration in Uganda/Kenya under national guidelines, which at that time made ART available to persons with CD4<350/uL. VL measurements were categorized into five strata: suppressed (VL<500 copies/mL), 500–1,000, 1,000–10,000, 10,000–100,000, and >100,000. Median and mean log10(VL) were calculated within each community and in each region (W. Uganda/E. Uganda/ Kenya).
To assess risk factors for potential HIV transmission among all HIV+ adults regardless of ART use, we evaluated individual-level predictors of having a detectable VL. We used logistic regression accounting for household-level clustering to assess predictors including geographic region (W. Uganda/E. Uganda/ Kenya), age stratum, gender, marital status (married vs. not married), occupation (farmer/agricultural vs. other), binge alcohol drinking (yes vs. no), household wealth quintile (from principal component analysis derived from a household assets questionnaire), and HIV testing location (community health campaign vs. at home). Adjusted odds ratios for each predictor were calculated using a model containing all variables.
To characterize transmission risk existing in any community for an encounter occurring in that community, we estimated the ‘local prevalence of viremia’ in three steps: (1) measuring the fraction of HIV-positive stable adult community residents with a measured VL who had detectable viremia (≥500 copies/mL), (2) multiplying this fraction by the total number of stable adult residents who tested HIV-positive in the community, and (3) dividing this estimate of viremic adults by the total adult stable resident population with a measured HIV status (both HIV-positive and HIV-negative). This method considered adults with missing VL values and HIV serostatus values as missing completely at random. We estimated local prevalence of viremia in this manner for geopolitical subunits within communities, and for communities overall. We compared median local prevalence of viremia of communities within each region using Wilcoxon rank-sum tests.
HIV transmission risk ‘hotspots’ were assessed as present or absent based on whether any geopolitical subunit within a community had a local prevalence of viremia of ≥5.0%.
HIV sero-discordant couples with a viremic HIV-positive partner paired with an HIV-negative partner are at high risk for potential HIV transmission,23 and warrant urgent treatment and prevention interventions. To study the magnitude and characteristics of this population in Uganda and Kenya, we assessed self-identified male-female household couples. Couples were either sero-concordant (two HIV-negative persons, or two HIV-positive persons), or sero-discordant (HIV-positive person with HIV-negative person). In discordant couples, furthermore, an HIV-positive partner could have detectable viremia or be virally suppressed. In each region, we tabulated couples of each type resulting from combinations of male and female gender with HIV status. For discordant couples with a viremic HIV+ partner, we assessed how frequently the viremic partner had a VL>100,000—a level denoting the highest potential for transmission.20, 23
Role of the Funding Source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
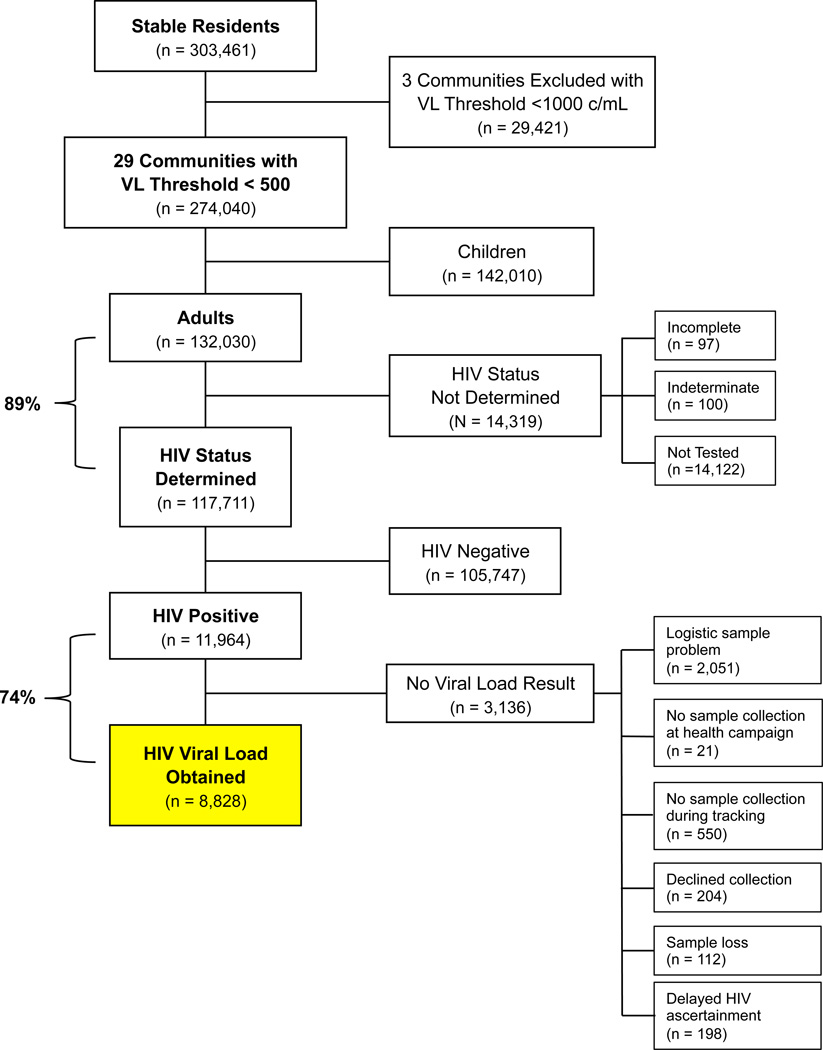
Overall, 274,040 stable residents were enumerated in 29 study communities from April 2, 2013, to June 8, 2014, including 132,030 adults (Fig. 1; Uganda N=79,682, Kenya N=52,348) and 142,010 children. Among adults, we determined HIV status in 117,711/132,030 (89%). Overall, 11,964 adults were HIV-positive (6.3% prevalence [2,096/33,424] in West Uganda, 3.2% [1,238/38,446] in East Uganda, and 18.6% [8,510/45,646] in Kenya), 66% [7,848/11,964] were female, 54% [6,413/11,964] worked in agriculture, and 70% [8,315/11,964] were married. Overall, 76% [9,090/11,964] of individuals were diagnosed with HIV at community health campaigns, and 24% via home-based tracking (Table 1). Demographic data for individuals within each individual community are given in the appendix (Page 1).
Figure 1. CONSORT Diagram.
Selection of 8,828 adults for analysis who had stable residence in study communities, underwent HIV testing, and had HIV RNA level (viral load) determined. Three of 32 communities in the SEARCH Study were excluded because viral load testing utilized a higher lower limit of quantitation (<1,000 copies/mL) than was used study-wide (<500 c/mL). Children were excluded. HIV status was not determined in some individuals. Of HIV sero-positive individuals, viral load results were unavailable in a subset of participants.
Table 1.
Characteristics of Ugandan and Kenyan HIV-Positive Adults with and without Measured HIV RNA Levels (n=11,964)
| Characteristic | Total (n = 11,964) |
With VL Result (n = 8,828) |
No VL Result (n = 3,136) |
|---|---|---|---|
| Region | |||
| West Uganda | 2,156 (18.0) | 1,827 (20.7) | 329 (10.5) |
| East Uganda | 1,278 (10.7) | 1,147 (13.0) | 131 (2.4) |
| Kenya | 8,530 (71.3) | 5,854 (66.3) | 2,676 (85.3) |
| Gender | |||
| Female | 7,848 (65.6) | 5,843 (66.2) | 2,005 (63.9) |
| Male | 4,116 (34.3) | 2,985 (33.8) | 1131 (36.1) |
| Occupation | |||
| Farmer/agricultural work | 6,413 (53.6) | 5,133 (58.1) | 1,280 (40.8) |
| Non-agricultural workA | 5,551 (46.4) | 3,695 (41.9) | 1,856 (59.2) |
| Age | |||
| 15 to 20 years | 534 (4.5) | 400 (4.5) | 134 (4.3) |
| 21 to 30 years | 3,458 (28.9) | 2,427 (27.5) | 1,031 (32.9) |
| 31 to 40 years | 3,830 (32.0) | 2,839 (32.2) | 991 (31.6) |
| 41 to 50 years | 2,391 (20.0) | 1,821 (20.6) | 570 (18.2) |
| 51 to 60 years | 1,201 (10.0) | 935 (10.6) | 266 (8.5) |
| > 60 years | 550 (4.6) | 406 (4.6) | 144 (4.6) |
| Marital status | |||
| Married | 8,315 (69.6)B | 6,075 (68.9)C | 2,240 (71.6)D |
| Single or divorced | 3,633 (30.4) | 2,743 (31.1) | 890 (28.4) |
| Household wealth | |||
| 1st quintile (lowest wealth) | 1,949 (16.6)E | 1,461 (16.7) | 488 (16.2) G |
| 2nd quintile | 1,898 (16.1) | 1,502 (17.2) | 396 (13.1) |
| 3rd quintile | 2,244 (19.1) | 1,670 (19.1) | 574 (19.1) |
| 4th quintile | 2,609 (22.2) | 1,933 (22.1) | 676 (22.4) |
| 5th quintile (highest wealth) | 3,057 (26.0) | 2,179 (24.9) F | 878 (29.2) |
| Mode of HIV testing | |||
| At community health campaign | 9,090 (76.1)H | 6,822 (77.3) I | 2,268 (72.5)J |
| At home, via home-based testing | 2,863 (23.9) | 2,003 (22.7) | 860 (27.5) |
NOTE.
Fisher (n=1,555 [28.0%]), shopkeeper/vendor (n=1,274 [22.9%]), teacher (n=225 [4.1%]), transportation (n=157 [2.8%]), other (n=1,636 [29.5%]), not provided (n=13 [0.2%]), no job (n=691 [12.5%]).
Frequencies: B: 11,948; C : 8,818; D: 3,130; E: 11,757; F: 8,745; G: 3,012; H: 11,953; I: 8,825; J: 3,128.
Among HIV+ adults, we determined VL in 8,828/11,964 (74%). We were unable to determine VL in 3,136/11,964 (26%) of tested HIV+ persons (Fig. 1). Top reasons were (1) logistic sample problems (n=2,051/3,136 [65% of missing VLs]) including barriers related to staffing, transportation, reagents/materials, and sporadic sample processing problems, (2) no sample collected during health campaign (n=21), (3) no sample collected during home-based tracking (n=550), (4) participants declined sample collection (n=204/3,136 [6.5%]), (5) sample loss (n=112/3,136 [3.6%]), and (6) delayed HIV ascertainment preventing VL collection (n=198/3,136 [6.3%]). Demographic characteristics were similar between persons with (n=8,828) vs. without a VL (n=3,136, Table 1).
Among HIV+ adults on ART by self-report, VL was suppressed in 82% (3,427/4,202; Table 2). Overall, among HIV+ adults with a measured VL, viral suppression was 51% (4,490/8,828; Table 2). Across study regions, VL was low (500–10,000 c/mL), moderate (10,001–100,000 c/mL) and high (>100,000 c/mL) in 14.9% [1,318/8,828], 21.7% [1,914/8,828], and 12.5% [1,106/8,828] of HIV+ individuals, respectively. In West Uganda, 48% [881/1,827] of HIV+ adults overall and 84% [568/675] of adults on ART had suppressed VL. In East Uganda, 45% [516/1,147] of HIV+ adults overall and 75% [345/458] of those on ART were suppressed. In Kenya, 53% [3,093/5,854] of HIV+ adults overall and 82% [2,514/3,069] of those on ART were suppressed (Table 2). Viral suppression among HIV+ persons ranged in geopolitical subunits of communities from 27.2%-61.1% in West Uganda, 26.0%-61.5% in East Uganda, and 41.9%-63.9% in Kenya (Table 3). HIV+ adults with VL>100,000 copies/mL—at highest risk for potential HIV transmission attributable to VL—were 11% [198/1,827] in West Uganda, 16% [180/1,147] in East Uganda, and 12% [728/5,854] in Kenya (Table 2).
Table 2.
Viral Suppression by Region and ART Status
| WEST UGANDA | EAST UGANDA | KENYA | ||||
|---|---|---|---|---|---|---|
| VL Metric | Region Total (n=1,827) |
On ART (n=675) |
Region Total (n=1,147) |
On ART (n=458) |
Region Total (n=5,854) |
On ART (n=3,069) |
| < 500 (copies/mL) | 881 (48.2%) |
568 (84.2%) |
516 (45.0%) |
345 (75.3%) |
3,093 (52.8%) |
2,514 (81.9%) |
| 500–1,000 | 38 (2.1%) |
6 (0.9%) |
28 (2.4%) |
10 (2.2%) |
113 (1.9%) |
43 (1.4%) |
| 1,000–10,000 | 284 (15.5%) |
37 (5.5%) |
133 (11.6%) |
36 (7.9%) |
722 (12.3%) |
168 (5.5%) |
| 10,001–100,000 | 426 (23.3%) |
42 (6.2%) |
290 (25.3%) |
49 (10.7%) |
1,198 (20.5%) |
215 (7.0%) |
| > 100,000 | 198 (10.8%) |
22 (3.3%) |
180 (15.7%) |
18 (3.9%) |
728 (12.4%) |
129 (4.2%) |
| Median VL, IQR | 874 (<500—24,983) |
<500 (<500—<500) |
2,124 (<500—45,404) |
<500 (<500—<500) |
<500 (<40—24,268) |
<500 (<40—<500) |
|
Mean log10(VL) 95% CI |
3.47 (3.41–3.52) |
2.79 (2.73–2.86) |
3.66 (3.59–3.73) |
3.00 (2.92–3.08) |
3.20 (3.17–3.24) |
2.50 (2.46–2.54) |
NOTE. VL, viral load; ART, antiretroviral therapy; IQR, interquartile range; CI, confidence interval
Table 3.
Local Prevalence of Viremia by Community (n = 114,449 stable adult residents)
| West Uganda |
Community | Parish | HIV Pos./ Total Adult |
HIV Prevalence |
Viremic/ HIV Pos. |
Viremia in HIV Pos. Adults |
Viremia in Total AdultsA |
Local Prevalence of ViremiaA |
|---|---|---|---|---|---|---|---|---|
| Bugamba | Community Total | 273/5222 | 5.2% | 141/268 | 52.6% | 144/5222 | 2.8% | |
| Kabarama | 121/2811 | 4.3% | 69/118 | 58.5% | 71/2811 | 2.5% | ||
| Rweibogo | 152/2411 | 6.3% | 72/150 | 48.0% | 73/2411 | 3.0% | ||
| Kitwe | Community Total | 228/3974 | 5.7% | 105/211 | 49.8% | 113/3974 | 2.9% | |
| Kitwe | 66/718 | 9.2% | 30/58 | 51.7% | 34/718 | 4.8% | ||
| Nshenyi | 162/3256 | 5.0% | 75/153 | 49.0% | 79/3256 | 2.4% | ||
| Mitooma | Community Total | 313/4286 | 7.3% | 124/284 | 43.7% | 137/4286 | 3.2% | |
| Nkinga | 104/1539 | 6.8% | 35/90 | 38.9% | 40/1539 | 2.6% | ||
| Nyakishojwa | 125/1590 | 7.9% | 56/114 | 49.1% | 61/1590 | 3.9% | ||
| Ward I | 51/680 | 7.5% | 19/48 | 39.6% | 20/680 | 3.0% | ||
| Ward II | 33/477 | 6.9% | 14/32 | 43.8% | 14/477 | 3.0% | ||
| Nsiika | Community Total | 164/4608 | 3.6% | 95/158 | 60.1% | 99/4608 | 2.1% | |
| Kyeyare | 74/1734 | 4.3% | 46/73 | 63.0% | 47/1734 | 2.7% | ||
| Nsiika Town | 33/1102 | 3.0% | 17/31 | 54.8% | 18/1102 | 1.6% | ||
| Nyakishojwa | 37/872 | 4.2% | 20/34 | 58.8% | 22/872 | 2.5% | ||
| Rwengwe | 20/900 | 2.2% | 12/20 | 60.0% | 12/900 | 1.3% | ||
| Rubaare | Community Total | 164/4051 | 4.0% | 98/153 | 64.1% | 105/4051 | 2.6% | |
| Nyarwanya | 87/2088 | 4.2% | 59/81 | 72.8% | 63/2088 | 3.0% | ||
| Rukiri | 77/1963 | 3.9% | 39/72 | 54.2% | 42/1963 | 2.1% | ||
| Rugazi | Community Total | 369/3888 | 9.5% | 172/336 | 51.2% | 189/3888 | 4.9% | |
| Buzenga | 81/1077 | 7.5% | 28/66 | 42.4% | 34/1077 | 3.2% | ||
| Magambo | 152/1400 | 10.9% | 73/141 | 51.8% | 79/1400 | 5.6% | ||
| Rugazi | 136/1411 | 9.6% | 71/129 | 55.0% | 75/1411 | 5.3% | ||
| Ruhoko | Community Total | 311/3722 | 8.4% | 90/165 | 54.5% | 170/3722 | 4.6% | |
| Kanyansheko | 126/1332 | 9.5% | 41/76 | 53.9% | 68/1332 | 5.1% | ||
| Kayenje | 185/2390 | 7.7% | 49/89 | 55.1% | 102/2390 | 4.3% | ||
| Rwashamire | Community Total | 274/3673 | 7.5% | 93/204 | 45.6% | 125/3673 | 3.4% | |
| Nkongoro | 142/2262 | 6.3% | 52/107 | 48.6% | 69/2262 | 3.1% | ||
| Rwashamaire | 132/1411 | 9.4% | 41/97 | 42.3% | 56/1411 | 4.0% | ||
| Region | Total | 2096/33424 | 6.3% | 918/1779 | 51.6% | 1082/33424 | 3.2% | |
|
NOTE. A: Estimates based on proportional accounting for viral loads missing at random | ||||||||
|
East Uganda |
Community | Parish |
HIV Pos./ Total Adult |
HIV Prevalence |
Viremic/ HIV Pos. |
Viremia in HIV Pos Adults |
Viremia in Total AdultsA |
Local Prevalence of ViremiaA |
| Bugono | Community Total | 120/3767 | 3.2% | 58/109 | 53.2% | 64/3767 | 1.7% | |
| Bugono | 50/1351 | 3.7% | 27/46 | 58.7% | 29/1351 | 2.2% | ||
| Itanda | 70/2416 | 2.9% | 31/63 | 49.2% | 34/2416 | 1.4% | ||
| Kadama | Community Total | 113/3728 | 3.0% | 72/104 | 69.2% | 78/3728 | 2.1% | |
| Nabunyere | 30/1007 | 3.0% | 15/27 | 55.6% | 17/1007 | 1.7% | ||
| Nandere | 83/2721 | 3.1% | 57/77 | 74.0% | 61/2721 | 2.3% | ||
| Kameke | Community Total | 58/3991 | 1.5% | 27/58 | 46.6% | 27/3991 | 0.7% | |
| Nyakoi | 32/2027 | 1.6% | 17/32 | 53.1% | 17/2027 | 0.8% | ||
| Oboliso | 26/1964 | 1.3% | 10/26 | 38.5% | 10/1964 | 0.5% | ||
| Kamuge | Community Total | 101/4643 | 2.2% | 47/96 | 49.0% | 49/4643 | 1.1% | |
| Kagoli | 34/2029 | 1.7% | 17/32 | 53.1% | 18/2029 | 0.9% | ||
| Kalapata | 67/2614 | 2.6% | 30/64 | 46.9% | 31/2614 | 1.2% | ||
| Kiyeyi | Community Total | 180/4637 | 3.9% | 80/176 | 45.5% | 82/4637 | 1.8% | |
| Nabuyoga | 57/1498 | 3.8% | 28/56 | 50.0% | 29/1498 | 1.9% | ||
| Pawanga | 123/3139 | 3.9% | 52/120 | 43.3% | 53/3139 | 1.7% | ||
| Kiyunga | Community Total | 179/4412 | 4.1% | 90/155 | 58.1% | 104/4412 | 2.4% | |
| Bugonyoka | 40/1500 | 2.7% | 20/39 | 51.3% | 21/1500 | 1.4% | ||
| Nakabugo | 139/2912 | 4.8% | 70/116 | 60.3% | 84/2912 | 2.9% | ||
| Merikit | Community Total | 198/4624 | 4.3% | 117/178 | 65.7% | 130/4624 | 2.8% | |
| Kachinga | 86/2260 | 3.8% | 54/83 | 65.1% | 56/2260 | 2.5% | ||
| Merikit | 112/2364 | 4.7% | 63/95 | 66.3% | 74/2364 | 3.1% | ||
| Nankoma | Community Total | 146/4549 | 3.2% | 73/139 | 52.5% | 77/4549 | 1.7% | |
| Matovu | 83/2110 | 3.9% | 38/78 | 48.7% | 40/2110 | 1.9% | ||
| Namakoko | 63/2439 | 2.6% | 35/61 | 57.4% | 36/2439 | 1.5% | ||
| Nsiinze | Community Total | 143/4095 | 3.5% | 67/132 | 50.8% | 73/4095 | 1.8% | |
| Nawaikona | 46/1879 | 2.4% | 28/46 | 60.9% | 28/1879 | 1.5% | ||
| Nsinze | 97/2216 | 4.4% | 39/86 | 45.3% | 44/2216 | 2.0% | ||
| Region | Total | 1238/38446 | 3.2% | 631/1147 | 55.0% | 681/38446 | 1.8% | |
| Kenya | Community | Sublocation |
HIV Pos./ Total Adult |
HIV Prevalence |
Viremic/ HIV Pos. |
Viremia in HIV Pos Adults |
Viremia in Total AdultsA |
Local Prevalence of ViremiaA |
| Bware | Community Total | 197/3301 | 6.0% | 88/174 | 50.6% | 100/3301 | 3.0% | |
| Bware | 197/3301 | 6.0% | 88/174 | 50.6% | 100/3301 | 3.0% | ||
| Kisegi | Community Total | 798/4103 | 19.4% | 135/271 | 49.8% | 398/4103 | 9.7% | |
| Gwasi North | 188/1215 | 15.5% | 23/53 | 43.4% | 82/1215 | 6.7% | ||
| Kitawa | 295/1594 | 18.5% | 62/132 | 47.0% | 139/1594 | 8.7% | ||
| Uterere | 315/1294 | 24.3% | 50/86 | 58.1% | 183/1294 | 14.2% | ||
| Kitare | Community Total | 801/3746 | 21.4% | 114/282 | 40.4% | 324/3746 | 8.6% | |
| Gembe East | 372/1688 | 22.0% | 63/141 | 44.7% | 166/1688 | 9.8% | ||
| Ngodhe | 160/763 | 21.0% | 25/69 | 36.2% | 58/763 | 7.6% | ||
| Waondo | 269/1295 | 20.8% | 26/72 | 36.1% | 97/1295 | 7.5% | ||
| Magunga | Community Total | 701/4041 | 17.3% | 168/411 | 40.9% | 287/4041 | 7.1% | |
| Kiabuya | 275/1488 | 18.5% | 62/161 | 38.5% | 106/1488 | 7.1% | ||
| Magunga | 150/902 | 16.6% | 45/104 | 43.3% | 65/902 | 7.2% | ||
| Nyancha | 276/1651 | 16.7% | 61/146 | 41.8% | 115/1651 | 7.0% | ||
| Nyamrisra | Community Total | 780/3411 | 22.9% | 308/642 | 48.0% | 374/3411 | 11.0% | |
| Nyamrisra | 485/1947 | 24.9% | 184/385 | 47.8% | 232/1947 | 11.9% | ||
| Rangwa West | 295/1464 | 20.2% | 124/257 | 48.2% | 142/1464 | 9.7% | ||
| Nyatoto | Community Total | 660/4431 | 14.9% | 230/502 | 45.8% | 302/4431 | 6.8% | |
| Nyatoto | 214/1412 | 15.2% | 82/183 | 44.8% | 96/1412 | 6.8% | ||
| Sumba East | 158/1280 | 12.3% | 40/106 | 37.7% | 60/1280 | 4.7% | ||
| Sumba West | 288/1739 | 16.6% | 108/213 | 50.7% | 146/1739 | 8.4% | ||
| Ogongo | Community Total | 795/4491 | 17.7% | 325/653 | 49.8% | 396/4491 | 8.8% | |
| Ogongo | 293/1685 | 17.4% | 132/248 | 53.2% | 156/1685 | 9.3% | ||
| Ruri East | 280/1481 | 18.9% | 107/219 | 48.9% | 137/1481 | 9.2% | ||
| Ruri West | 222/1325 | 16.8% | 86/186 | 46.2% | 103/1325 | 7.7% | ||
| Ongo | Community Total | 611/3912 | 15.6% | 191/381 | 50.1% | 306/3912 | 7.8% | |
| Kanyimach | 351/2272 | 15.4% | 113/227 | 49.8% | 175/2272 | 7.7% | ||
| South Kanya | 260/1640 | 15.9% | 78/154 | 50.6% | 132/1640 | 8.0% | ||
| Othoro | Community Total | 892/3629 | 24.6% | 403/869 | 46.4% | 414/3629 | 11.4% | |
| Ka Atieno 2 | 892/3629 | 24.6% | 403/869 | 46.4% | 414/3629 | 11.4% | ||
| Sena | Community Total | 741/3176 | 23.3% | 165/406 | 40.6% | 301/3176 | 9.5% | |
| Wakinga | 364/1549 | 23.5% | 80/189 | 42.3% | 154/1549 | 9.9% | ||
| Waware | 377/1627 | 23.2% | 85/217 | 39.2% | 148/1627 | 9.1% | ||
| Sibuoche | Community Total | 795/4219 | 18.8% | 409/750 | 54.5% | 434/4219 | 10.3% | |
| Kajulu 2 | 795/4219 | 18.8% | 409/750 | 54.5% | 434/4219 | 10.3% | ||
| Tom Mboya | Community Total | 739/3186 | 23.2% | 225/510 | 44.1% | 326/3186 | 10.2% | |
| Kamasengre | 555/2328 | 23.8% | 171/369 | 46.3% | 257/2328 | 11.0% | ||
| Kaswanga | 184/858 | 21.4% | 54/141 | 38.3% | 70/858 | 8.2% | ||
| Region | Total | 8510/45646 | 18.6% | 2761/5851 | 47.2% | 4016/45646 | 8.8% | |
We examined individual-level predictors of detectable viremia among HIV+ adults to assess factors that could raise HIV transmission risk. In multivariable analysis, we found that region (E. Uganda: odds ratio (OR) 1.49 [95% CI, 1.29–1.72] vs. Kenya), younger age (OR 4.07 [3.21–5.17] for age 21–30 vs. >60), male gender (OR 1.48 [1.34–1.63]), married status (OR 1.19 [1.08–1.32] vs. single/divorced), binge alcohol drinking (OR 1.62 [1.28–2.05]) and lower wealth (OR 1.52 [1.32–1.77] for lowest vs. highest quintile) independently predicted detectable viremia among HIV+ adults (Table 4). Individuals diagnosed via home-testing were more frequently viremic than persons tested at community health campaigns (OR 1.41 [1.26–1.57]; Table 4).
Table 4.
Predictors of Detectable HIV RNA among HIV-Positive Adults (n=8,828)
| Characteristic | aOR (95% CI) | p-value |
|---|---|---|
| Region | ||
| West Uganda | 1.09 (0.97–1.23) | 0.16 |
| East Uganda | 1.46 (1.26–1.69) | < 0.0001 |
| Kenya | Ref. | — |
| Age (years) | ||
| 15 to 20 | 7.05 (5.11–9.73) | < 0.0001 |
| 21 to 30 | 4.04 (3.18–5.12) | < 0.0001 |
| 31 to 40 | 2.14 (1.70–2.70) | < 0.0001 |
| 41 to 50 | 1.44 (1.14–1.83) | 0.002 |
| 51 to 60 | 1.13 (0.87–1.45) | 0.36 |
| >60 | Ref. | — |
| Gender | ||
| Female | Ref. | — |
| Male | 1.42 (1.29–1.57) | < 0.0001 |
| Marital Status | ||
| Married | 1.20 (1.08–1.33) | 0.0006 |
| Single or divorced | Ref. | — |
| Occupation | ||
| Farmer | Ref. | — |
| Non-farmer | 0.94 (0.86–1.03) | 0.18 |
| Wealth | ||
| 1st quintile (lowest wealth) | 1.52 (1.31–1.76) | < 0.0001 |
| 2nd quintile | 1.27 (1.10–1.47) | 0.0013 |
| 3rd quintile | 1.10 (0.95–1.26) | 0.19 |
| 4th quintile | 1.11 (0.97–1.27) | 0.13 |
| 5th quintile (highest wealth) | Ref. | — |
| Binge alcohol drinking | ||
| No | Ref. | — |
| Yes | 1.62 (1.28–2.05) | < 0.0001 |
| SEARCH testing mode | ||
| At community health campaign | Ref. | — |
| At home-based testing | 1.62 (1.28–2.05) | < 0.0001 |
NOTE. aOR, adjusted odds ratio
Local prevalence of viremia (percentage of all adults [regardless of HIV status] estimated to have detectable VL) was higher in Kenya communities (median 8.3%, range 3.0%-14.2%) vs. West Uganda (median 3.0%, range 1.3%-5.6%; p=0.0007; Table 3). Local prevalence of viremia was also higher in Kenya vs. East Uganda (median 1.7%, range 0.5%-3.1%, p=0.0001), and higher in West Uganda vs. East Uganda (median 3.0% vs. 1.7%, p=0.002).
There was substantial geographic variation in community VL metrics: in West Uganda, estimates of local prevalence of viremia varied >4-fold across the geopolitical subunits comprising communities (range 1.3% [Rwengwe parish, Nsiika] to 5.6% [Magambo parish, Rugazi]; Table 3). In East Uganda, although local prevalence of viremia was lower overall, it still varied >6-fold (range 0.5% [Oboliso parish, Kameke] to 3.1% [Merikit perish, Merikit]). Finally, in Kenya, local prevalence of viremia also ranged >4-fold (range 3.0% [Bware] to 14.2% [Uterere sublocation, Kisegi; Table 3).
Notable geographic juxtaposition of communities with higher and lower local prevalence of viremia was evident. Figure 2 shows community locations and estimates of local prevalence of viremia. The Kenyan community with the highest viremia prevalence (Othoro, 11.2%) closely borders one of the lowest viremia prevalence communities (Bware, 2.7%; Fig. 2C).
Figure 2. Local community prevalence of viremia by region.
(A) Geographic maps showing locations of West Uganda study communities [map + inset map]. For each community, the local prevalence of viremia is indicated (% of adults [regardless of HIV status] who had HIV viremia). (B) Maps showing East Uganda community locations and local prevalence of viremia. (C) Maps showing Kenya community locations and local prevalence of viremia.
HIV transmission risk ‘hotspots’ (i.e., geographic regions with higher local prevalence of viremia) denote higher HIV transmission risk, because random contact in these regions signifies likelier exposure to a person with detectable virus. Overall, 3/21 parishes in Western Uganda (e.g., Magambo, Rugazi; Table 3), 0/18 parishes in East Uganda, and 23/26 sublocations in Kenya were transmission risk hotspots (Table 3). In Kenya, the highest local prevalence of viremia was 14.2%, seen in Uterere (Kisegi; Table 3).
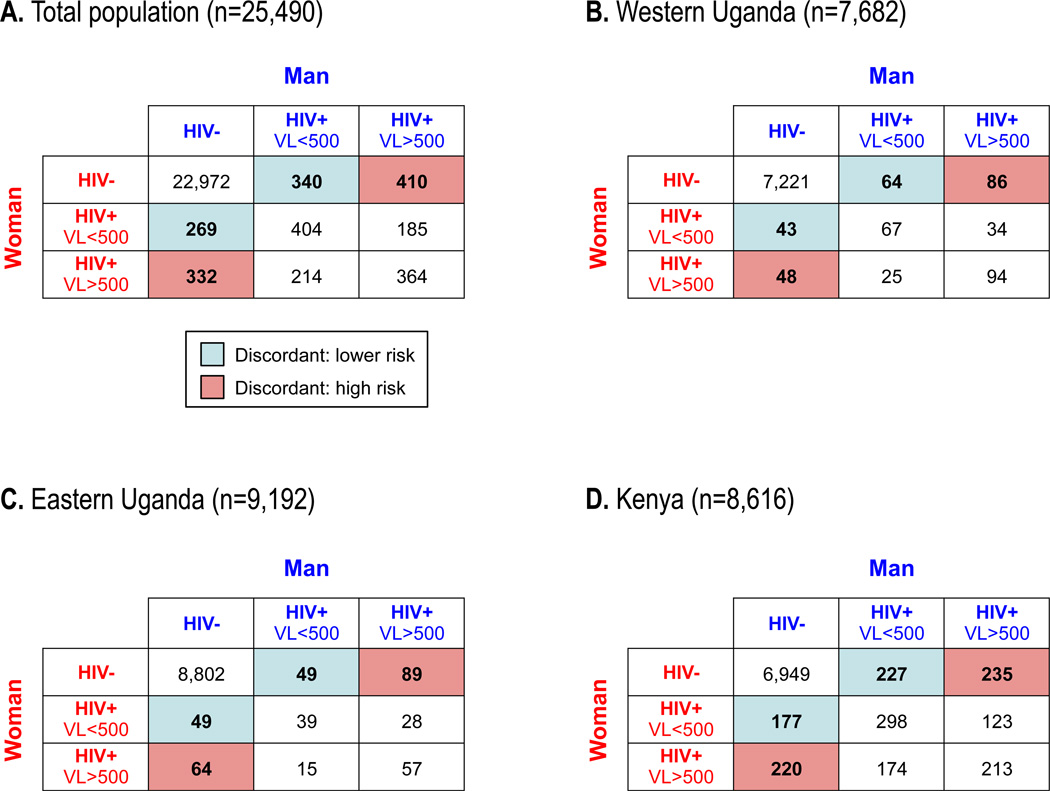
To assess detectable viremia and HIV transmission risk within sero-discordant couples, we enumerated 25,490 couples (Fig. 3A). Of these, 1,351 (5.3%) were sero-discordant (i.e., composed of one HIV-positive and one HIV-negative partner). In 742/1,351 sero-discordant couples (55%), the HIV+ partner had a detectable VL, indicating immediate transmission risk (Fig. 3A). In Uganda, 492/16,023 (3.1%) of couples were sero-discordant. In 58% of these sero-discordant couples, the HIV+ partner was viremic, and in 15% the viremic partner had VL>100,000 c/mL, indicating marked transmission risk (Fig 3B–C). In Kenya, sero-discordancy was >3-fold more common than in Uganda, occurring in 859/8,616 (10.0%) of couples (Fig. 3D). In 53% of the sero-discordant couples, the HIV+ partner was viremic, and 13% had a viremic partner with VL>100,000 c/mL.
Figure 3. Viral suppression by sero-discordancy characteristics of male-female couples.
(A) Overall numbers of male-female couples [n=25,490] defined by the male and female partner being either HIV-negative, HIV-positive with VL>500 c/mL, or HIV-positive with VL>500 c/mL. Sero-discordant couples with lower risk (light blue boxes) and higher risk (red boxes) are indicated. Analogous tables of sero-discordant couples in (B) West Uganda [n=7,682 couples], (C) East Uganda [n=9,192 couples], and (D) Kenya [8,616 couples] are shown.
Discussion
We conducted a cross-sectional analysis of population-level HIV RNA levels during the baseline assessment for a test-and-treat study (SEARCH) in rural Uganda and Kenya. We found 51% of HIV-infected adults had viral suppression, reflecting the impact of CD4-guided ART scale-up. Conversely, nearly half of all HIV-infected adults had detectable viremia, creating risk for potential HIV transmission. We also identified geopolitical units where 5–10% of the entire adult population had detectable VL, representing ‘hotspots’ of potential HIV transmission risk. Finally, we found that in over half of sero-discordant couples, the HIV+ partner had detectable VL. These data—the largest ever population-sampled viral load survey in East Africa—provide an up-to-date view of the HIV epidemic as a new era of universal ART eligibility begins.
Our finding that 51% of HIV+ persons have viral suppression contrasts the 32% aggregate Sub-Saharan estimate by UNAIDS.13 Some data comprising the UNAIDS study predated 2013, and may not fully reflect current progress in ART scale-up. A recent report from Botswana reported even higher viral suppression among 3,596 HIV+ adults out of 12,610 sampled. They found 70% of HIV+ individuals had viral suppression (VL<400 c/mL).14 Botswana began ART scale-up earlier than many other countries using CD4-restricted guidelines. Because of its successful HIV testing program, and successful ART programs achieving >95% viral suppression, Botswana has reached this impressive population viral suppression threshold.
Two smaller East African studies also estimated viral suppression. In a 2012 household survey in Homa Bay County, Kenya, (N=1,397; HIV prevalence, 24%), viral suppression (VL<1000 c/mL) was 39.7%.15 A study from Rwanda using electronic medical record data (N=3,066 patients on ART) found 82.1% virologically suppressed (VL<40 c/mL). Modeling the HIV care cascade, authors estimated that 106,371/204,889 (52%) of HIV+ Rwandans were virologically suppressed. Our data span 2013–2014, offering recent estimates from East Africa, and relied on comprehensive population sampling rather than being restricted to selected households or patients already in HIV care. Together, these studies are consistent with an emerging consensus that viral suppression in East Africa is most likely in the 40–50% range currently.
We found detectable VL (i.e., viremia) in nearly half of the HIV+ adults we studied in Uganda and Kenya. Predictors of detectable viremia (and therefore HIV transmission) included younger age, male gender, married status, and lower household wealth. Being HIV-tested at home rather than at a community health fair also predicted viremia. This may be a proxy characteristic for non-health-seeking behavior. Binge alcohol drinking was also predictive of detectable viremia, consistent with an emerging literature linking this practice to both ART non-adherence24 as well as virologic non-suppression in Sub-Saharan Africa.25 Our analysis, which did not adjust for self-reported ART use, suggests that somewhere along the HIV care cascade, these populations are less likely to engage in care, receive ART, and adhere to therapy. These risk groups are well-documented in the literature to face both structural and behavioral barriers to linkage and retention in care.26
In addition to demographic risk factors for detectable viremia, we also assessed the geographic distribution of viremic individuals. There is no universally accepted definition of a ‘hotspot,’ and HIV transmission risk may be predominantly influenced by sexual or injection drug use patterns. However, hotspot analysis has helped design both prevention and treatment strategies for HIV27, 28 and other diseases like tuberculosis.29
In this study, by assessing VL values in geographic units within a large population, ‘hotspots’ of higher prevalence of viremia were seen in regions with HIV prevalence ranging from 6.3% (West Uganda) to 18.6% (Kenya).21 Thus, regional and community-specific data can reveal important features of local epidemics within areas of generalized epidemics. Relying solely on aggregated administrative data, especially in lower-prevalence regions, may fail to reveal small locations of substantially higher potential transmission risk embedded within larger areas, underscoring the value of geographic analysis to identify intervention targets.
Reasons for ‘hotspots’ likely differ between communities, and will require local study. In prior studies, ‘hotspots’ have tracked closely with transportation routes,11, 30 and have clustered within occupations such as fisherfolk and sex workers.28 It is also likely that markets, bars, and other locations influence geographic risk. Detailed analyses of our communities with high viremia prevalence are underway, to characterize factors influencing ‘hotspot’ regions, including geography, transport routes, locations of HIV care provision, and ART coverage. Approaches like ours that combine a census with community-based HIV testing, along with behavioral and demographic data, provide a powerful approach to identifying risk locations, allowing for targeted interventions. These could include enhanced HIV testing services, for example, at locations with high viremia prevalence, or enhanced ART care services such as community ART groups or community ART delivery points in communities with high local prevalence of viremia.
Alongside geographic risks, we also demonstrate marked risk within HIV sero-discordant Ugandan and Kenyan couples. Over half of sero-discordant couples had a partner with detectable viremia, and 15% of these had VL>100,000, signifying immediate high risk for potential HIV transmission.31 Although behavioral interventions can mitigate this risk, the risk of transmission when VL>100,000 c/mL may be 8-fold higher than when VL=1,000 c/mL.32 Notably, the number of viremic male partners was similar to viremic female partners, indicating that transmission risk is driven by both genders. Although guidelines for management of discordant couples have recommended interventions including regular HIV testing and counseling, condom use, and screening for sexually-transmitted infections, these have been challenging to implement. Newer interventions including pre-exposure prophylaxis (PrEP) for HIV-negative individuals, and immediate ART for HIV-positive individuals in sero-discordant couples, could have substantial impact given these challenges.3
Our study had certain limitations. First, we did not ascertain HIV status on all individuals; however, we did achieve 89% overall adult testing coverage. Further, community health campaigns one year after baseline successfully tested 50% of those who did not test in the baseline year; these adults had a similar HIV prevalence (9.7%) to baseline testers.33 Second, among HIV-positive individuals, our population sample did not measure VL on all HIV+ individuals, missing 26% mainly due to sporadic logistic problems during field conditions. While informative missingness is a possible source of bias, demographic characteristics were similar between persons with and without measured VLs. Third, our geographic analysis was done at parish and community levels—we thus could not assess person-to-person relationships and more granular ‘hotspots’ centered around locations of employment, schools, or social activities. Fourth, our sero-discordant couple analyses only assessed self-reported household relationships. We thus could not evaluate undeclared or secondary partnerships that also influence transmission risk.
In summary, in a large population-based study in East Africa, we found evidence that ART scale-up has achieved measurable impact on levels of viral suppression. Nevertheless, nearly half of HIV+ adults are viremic, geographic ‘hotspots’ of transmission risk exist, and sero-discordant couples harbor substantial levels of potential HIV transmission risk. Our data advance a comprehensive up-to-date view of the East African HIV epidemic as the era of universal ART begins.
Supplementary Material
Research in Context.
Evidence before this study
Evidence has accumulated that HIV antiretroviral therapy improves the health of all HIV-infected persons regardless of CD4+ T-cell count, and dramatically lowers the potential for HIV transmission. Global guidelines now endorse universal ART, and Sub-Saharan Africa is transitioning to a new era in which countries are scaling up ART to all HIV-positive persons. In tandem, UNAIDS conceptualized the ‘90-90–90’ initiative (aiming for 90% of HIV-positive persons to be diagnosed, 90% of diagnosed persons to be on ART, and >90% of persons on ART to achieve viral suppression), translating to a population viral suppression of 73%. However, very few population level assessments of viral load metrics have been performed, particularly in East Africa, due to difficulties sampling a broad population, logistics of phlebotomy and the current costs of viral load testing. This type of information from East Africa is crucial for understanding where we stand as we initiate the new era of universal ART. This information is needed to set HIV program priorities, match funding to areas of greatest need, and understand ongoing drivers of circulating viremia.
We searched PubMed, and public documents and reports from UNAIDS, WHO and the CDC using combinations of search terms including ―population viral load,‖ ―East Africa,‖ ―antiretroviral therapy,‖ and ―universal ART‖ in English language sources up to October 1, 2016. Few available estimates of viral suppression in East Africa exist; current reports are derived from ART program data that only account for patients already in HIV care, rather than patients who are HIV-positive but not yet diagnosed or in care. Other available estimates include population-level data but are from prior years, have limitations in sample size and breadth of the population assessed, and do not enumerate specific groups or types of geographic locations at risk for having elevated levels of detectable HIV RNA. Limited data are available about the current state of the HIV epidemic in East Africa as the era of universal ART begins.
Added value of this study
Within a large-scale East African population-based cluster-randomized clinical trial of a universal HIV test-and-treat intervention (the SEARCH Study) that is ongoing across 32 communities in Uganda and Kenya (approximately 10,000 persons per community), we performed what is to our knowledge the largest ever assessment of HIV viral loads of general populations in Uganda and Kenya, two East African countries rapidly scaling up ART and adopting universal therapy programs. We found that 51% of the East African population assessed had viral suppression—higher than previous UNAIDS estimates of 30–35%, but lower than a recent estimate of 70% from Botswana in Southern Africa. We also found specific geographic areas with elevated levels of viremia: ‘hotspots’ that are likely to have higher risk of ongoing HIV transmission. Additionally, we found that East African discordant couples (male-female pairs in which one person is HIV-positive, and one person is HIV-negative) have very high rates of detectable viral load, and thus are harboring substantial risk for HIV transmission.
Implications of all the available evidence
In this largest ever cross-sectional description of the East African HIV epidemic, we find that over half of adults are virally suppressed—evidence that ART scale-up is continuing successfully. However, we also find ‘hotspots’ of risk: geographic locations and large numbers of discordant couples with elevated levels of viremia. These locations and populations at risk will require dedicated interventions.
Acknowledgments
Financial Support. This work was supported by National Institutes of Health U01 AI069502 to DH, P30 AI027763 to Paul Volberding, UCSF, and UCSF-CTSI KL2TR000143 to VJ, and the President’s Emergency Plan for AIDS Relief (PEPFAR).
VJ, MLP, DK, CRC, EDC, MRK and DVH have received grants from the National Institutes of Health (NIH). VJ has received research grant support from Gilead Sciences. DVH has received non-financial support (donation of study drug Truvada) from Gilead Sciences. CRC has received personal fees from expert witness service, and from Symbiomix Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Prior Presentation. This work was presented at the 2016 Conference on Retroviruses and Opportunistic Infections (Boston, MA, February 25, 2016; Abstract #1026).
Declarations of Interests. All other authors declare no interests.
Author Contributions. VJ, MLP, MRK and DVH contributed to the study design, data analysis and interpretation, writing of the manuscript and preparation of figures and tables. TL, DK, GC, EAB, CRC, and EDC contributed to study design, data interpretation, and writing of the manuscript. DMB, NS, DB, TDC, AL, AP, JK, and ES contributed to data collection and interpretation.
Additional contributions. We are grateful to the participants of the Sustainable East Africa Research in Community Health (SEARCH) Study and to staff of the Makerere University Joint AIDS Program (MJAP), the Makerere University – University of California San Francisco (MU-UCSF) Collaboration, the Infectious Disease Research Collaboration (IDRC, Kampala), and the SEARCH Collaboration.
References
- 1.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015:1–78. [PubMed] [Google Scholar]
- 4.Das M, Chu PL, Santos GM, Scheer S, Vittinghoff E, McFarland W, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLOS ONE. 2010;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castel AD, Befus M, Willis S, Griffin A, West T, Hader S, et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS. 2012;26(3):345–353. doi: 10.1097/QAD.0b013e32834de5fe. [DOI] [PubMed] [Google Scholar]
- 6.Jain V, Liegler T, Kabami J, Chamie G, Clark TD, Black D, et al. Assessment of population-based HIV RNA levels in a rural east African setting using a fingerprick-based blood collection method. Clin Infect Dis. 2013;56(4):598–605. doi: 10.1093/cid/cis881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller WC, Powers KA, Smith MK, Cohen MS. Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect Dis. 2013;13(5):459–464. doi: 10.1016/S1473-3099(12)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014. pp. 1–40. [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanser F, Barnighausen T, Cooke GS, Newell ML. Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int J Epidemiol. 2009;38(4):1008–1016. doi: 10.1093/ije/dyp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vella S, Schwartlander B, Sow SP, Eholie SP, Murphy RL. The history of antiretroviral therapy and of its implementation in resource-limited areas of the world. AIDS. 2012;26(10):1231–1241. doi: 10.1097/QAD.0b013e32835521a3. [DOI] [PubMed] [Google Scholar]
- 13.Joint United Nations Programme on HIV/AIDS. How AIDS changed everything: MDG 6 executive summary. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2015. pp. 1–140. [Google Scholar]
- 14.Gaolathe T, Wirth KE, Holme MP, Makhema J, Moyo S, Chakalisa U, et al. Botswana’s progress toward achieving the 2020 UNAIDS 90-90-90 antiretroviral therapy and virological suppression goals: a population-based survey. Lancet HIV. 2016;3(4) doi: 10.1016/S2352-3018(16)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maman D, Zeh C, Mukui I, Kirubi B, Masson S, Opolo V, et al. Cascade of HIV care and population viral suppression in a high-burden region of Kenya. AIDS. 2015;29(12):1557–1565. doi: 10.1097/QAD.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nsanzimana S, Kanters S, Remera E, Forrest JI, Binagwaho A, Condo J, et al. HIV care continuum in Rwanda: a cross-sectional analysis of the national programme. Lancet HIV. 2015;2(5):e208–e215. doi: 10.1016/S2352-3018(15)00024-7. [DOI] [PubMed] [Google Scholar]
- 17.Justman J, Ellman T, Donnell D, Duong Y, Reed J, Bicego G, et al. Population HIV Viral Load Estimate in Swaziland: Assessing ART Program Effectiveness and Transmission Potential. Program and abstracts of the 20th Conference on Retroviruses and Opportunistic Infections; March 5, 2013; Atlanta, GA. Abstract 96. 2013. [Google Scholar]
- 18.Roberts T, Cohn J, Bonner K, Hargreaves S. Scale-up of Routine Viral Load Testing in Resource-Poor Settings: Current and Future Implementation Challenges. Clin Infect Dis. 2016;62(8):1043–1048. doi: 10.1093/cid/ciw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual Activity Without Condoms and Risk of HIV Transmission in Serodifferent Couples When the HIV-Positive Partner Is Using Suppressive Antiretroviral Therapy. JAMA. 2016;316(2):171–181. doi: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 20.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 21.Chamie G, Clark TD, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, et al. A hybrid mobile approach for population-wide HIV testing in rural east Africa: an observational study. Lancet HIV. 2016;3(3):e111–e119. doi: 10.1016/S2352-3018(15)00251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. Leveraging Rapid Community-Based HIV Testing Campaigns for Non-Communicable Diseases in Rural Uganda. PLOS ONE. 2012;7(8):e43400. doi: 10.1371/journal.pone.0043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 24.Braithwaite RS, Bryant KJ. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Health. 2010;33(3):280–287. [PMC free article] [PubMed] [Google Scholar]
- 25.Fatch R, Emenyonu NI, Muyindike W, Kekibiina A, Woolf-King S, Hahn JA. Alcohol Interactive Toxicity Beliefs and ART Non-adherence Among HIV-Infected Current Drinkers in Mbarara, Uganda. AIDS Behav. 2016 doi: 10.1007/s10461-016-1429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware NC, Wyatt MA, Geng EH, Kaaya SF, Agbaji OO, Muyindike WR, et al. Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Med. 2013;10(1):e1001369–e1001369. doi: 10.1371/journal.pmed.1001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwer KC, Rusch ML, Weeks JR, Lozada R, Vera A, Magis-Rodriguez C, et al. Spatial Epidemiology of HIV among Injection Drug Users in Tijuana, Mexico. Ann Assoc Am Geogr. 2012;102(5):1190–1199. doi: 10.1080/00045608.2012.674896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshi T, Fuji Y, Nzou SM, Tanigawa C, Kiche I, Mwau M, et al. Spatial Distributions of HIV Infection in an Endemic Area of Western Kenya: Guiding Information for Localized HIV Control and Prevention. PLOS ONE. 2016;11(2):e0148636. doi: 10.1371/journal.pone.0148636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izumi K, Ohkado A, Uchimura K, Murase Y, Tatsumi Y, Kayebeta A, et al. Detection of Tuberculosis Infection Hotspots Using Activity Spaces Based Spatial Approach in an Urban Tokyo, from 2003 to 2011. PLOS ONE. 2015;10(9):e0138831. doi: 10.1371/journal.pone.0138831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris CN, Ferguson AG. Estimation of the sexual transmission of HIV in Kenya and Uganda on the trans-Africa highway: the continuing role for prevention in high risk groups. Sex Transm Infect. 2006;82(5):368–371. doi: 10.1136/sti.2006.020933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murnane PM, Celum C, Mugo N, Campbell JD, Donnell D, Bukusi E, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27(13):2155–2160. doi: 10.1097/QAD.0b013e3283629037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hughes JP, Baeten JM, Lingappa JR, Magaret AS, Wald A, de Bruyn G, et al. Determinants of Per-Coital-Act HIV-1 Infectivity Among African HIV-1-Serodiscordant Couples. J Infect Dis. 2012;205(3):358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamie G, Kabami J, Ssemmondo E, Clark TD, Bukusi E, Petersen M, et al. 94% Population HIV Testing Coverage With Repeat Hybrid Mobile Testing in East Africa. Program and Abstracts of the 2016 Conference on Retroviruses and Opportunistic Infections; February 22–25, 2016; Boston, MA. 2016. Abstract 979. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.