Abstract
Objective
Determine the presence and assess the extent of fatty infiltration of the minor salivary glands (SG) of primary SS patients (pSS) as compared to those with non SS sicca (nSS).
Methods
Minor SG biopsy samples from 134 subjects with pSS (n= 72) or nSS (n = 62) were imaged. Total area and fatty replacement area for each glandular cross-section (n=4–6 cross-sections per subject) were measured using Image J (National Institutes of Health, Bethesda, MD, USA). The observer was blinded to subject classification status. The average area of fatty infiltration calculated per subject was evaluated by logistic regression and general linearized models (GLM) to assess relationships between fatty infiltration and clinical exam results, extent of fibrosis and age.
Results
The average area of fatty infiltration for subjects with pSS (median% (range) 4.97 (0.05–30.2)) was not significantly different from that of those with nSS (3.75 (0.087–41.9). Infiltration severity varied widely, and subjects with fatty replacement greater than 6% were equivalently distributed between pSS and nSS participants (χ2 p=0.50). Age accounted for all apparent relationships between fatty infiltration and fibrosis or reduced saliva flow. The all-inclusive GLM for prediction of pSS versus non-SS classification including fibrosis, age, fatty replacement and focus score was not significantly different from any desaturated model. In no iteration of the model did fatty replacement exert a significant effect on the capacity to predict pSS classification.
Conclusions
Fatty infiltration is an age-associated phenomenon and not a selective feature of Sjögren’s syndrome. Sicca patients who do not fulfill pSS criteria have similar rates of fatty infiltration of the minor SG.
Keywords: Sjögren’s Syndrome, aging, fatty replacement, minor salivary gland, pathology
Sjögren’s Syndrome (SS) is a systemic autoimmune disease characterized by chronic, severe dry eyes and mouth [1, 2, 3]. The fundamental mechanisms and causative pathology remain unclear but likely involve environmental [4], genetic [5, 6, 7] and epigenetic [8, 9] factors, dysregulation of innate and adaptive immunity [5, 10] and epithelial cell defects [11]. In addition to sicca symptoms, autoantibodies to Ro/SS-A and La/SS-B [12, 13] and focal lymphocytic infiltration of the salivary and lacrimal glands are cardinal features of SS [14, 15].
Our mechanistic and immunological understanding of SS is incomplete, and the heterogeneity of patient phenotypes confounds the study of SS. It is known that immune cells, predominantly CD4+ T cells [16, 17], congregate into inflammatory foci (>50 cells per 4mm2 tissue) in the salivary gland (SG). A recent study showed that the clonal proliferation of glandular CD4+ T cells associated with decreased saliva flow and fibrotic changes in minor SG tissue [18]. Fibrosis [19], acinar atrophy and other forms of pathological damage are elevated in SS patients [20, 21, 22]. Though loss of salivary and tear flow are cardinal features of SS, the mechanisms linking glandular pathology and loss of function remain cryptic.
Features, or groups of features, which distinguish patient subgroups are an area of interest, both for understanding the disease and subsequently for more effective subject selection in clinical trials. The SG is an attractive tissue for identifying prognostic markers [23, 24], as SG biopsy is already a part of the diagnostic process and part of the gold-standard of SS diagnosis and classification. It is known that risk factors such as hypergammaglobulinemia and recurrent SG enlargement are associated with the development of lymphoma (which occurs in 15% of patients) [6, 25, 26], but strong prognostic indicators of SS alone remain elusive. Sub-categorization of primary SS (pSS) types would aid studies seeking to decipher disease mechanisms and help make inroads into understanding disease pathogenesis, which is necessary for development of effective therapeutic approaches. Fatty infiltration is a fundamental form of SG dysmorphism that has been noted [20, 27, 28] but incompletely assessed.
In this work the hypothesis that fatty infiltration of minor SG is associated with primary SS classification was tested. The corollary hypothesis, that pSS subjects with high fatty infiltration of the minor SG are pathologically distinct from pSS subjects with low/no fatty infiltration, was also evaluated. We employed precise measurements to quantify the average fatty replacement of 72 subjects with pSS and 62 subjects with nSS sicca. We asked whether the extent of minor SG fatty infiltration in primary SS versus nSS controls was different, and whether fatty infiltration associated with other features of disease.
Participants and methods
Participants
All patient exams and sample collections were conducted at one of two Sjögren’s research clinic sites (Oklahoma Medical Research Foundation or University of Minnesota – Twin Cities) [29]. The respective Institutional Review Boards approved all research methods and exams and the study participants provided written informed consent in compliance with the Declaration of Helsinki. Clinic participants were self- or physician-referred and underwent a phone-based screening interview using validated dryness questions [2]; full clinical evaluation with specimen collection as previously described [29] was limited to those with at least two qualifying ocular and oral dryness complaints. After verifying that no participant in this study had AECG exclusion criteria or features of secondary or overlap SS (concurrent rheumatoid arthritis, systemic lupus erythematosus or systemic sclerosis), subjects were classified as primary SS or non-SS sicca via the 2002 revised American European Consensus Group Sjögren’s Syndrome criteria [30]. A total of 134 subjects (pSS = 72, nSS = 62) were included in this study. Imaging and scoring of participant biopsy tissue was performed independently. The study was performed in two phases. In the first phase, total tissue area and adipocyte area were assessed with blinding of classification status, and in consultation with an oral pathologist. In the second phase, after unblinding of samples, a retrospective analysis of pattern of adipocyte replacement was assessed in response to manuscript review. Demographic and clinical data of participants are shown in Table 1.
Table 1.
Subject Demographics
| pSS | nSS | ||
|---|---|---|---|
|
|
|||
| Total participants (n) | 72 | 62 | |
|
|
|||
| p-value1 | |||
| Age mean (SD) | 55 (12.1) | 48 (14) | 0.078 |
| p-value2 | |||
| Female (%) | 86 | 87.1 | 1 |
| Anti-Ro/SSA positive (%) | 63.8 | 1.6 | <0.0001 |
| Anti-La/SSB positive (%) | 60.8 | 4.8 | <0.0001 |
| WUSF Positive3 (%) | 63.8 | 37.1 | 0.0031 |
| Schirmer’s Positive4 (%) | 51 | 34.8 | 0.0544 |
| VanBijsterveld Positive5 (%) | 57 | 27.4 | 0.0008 |
| FS ≥16 (n) | 62.5 | 14.5 | <0.0001 |
unpaired two-tailed t test
Fisher’s exact test
Whole unstimulated saliva flow; positive test = ≤1.5mL/15 minutes
Schirmer’s positive test = ≤5mm/min
Van Bijsterveld Positive = ≥4
FS = Focus Score
Salivary gland (SG) biopsy and imaging
Four to six minor labial SGs per participant were formalin-fixed, embedded in paraffin, sectioned (4μM) and stained with hematoxylin and eosin (H&E) in a hospital pathology laboratory. The H&E slide assessed for focus scoring was imaged using a Zeiss 710 confocal microscope equipped with a motorized stage. Each glandular cross-section was imaged at 200x magnification in overlapping sections. These sections were then digitally stitched together using the Zeiss ZenBlue software package (Zeiss, Thornwood, NY, USA) to yield reconstructions of entire glandular cross-sections (Figure 1, left and third columns) (Supplementary Figure 1). All reconstructed cross-sections were de-identified and assigned random identity numbers. Scorers did not have access to the identification key. Re-identification was done only after all images (n= 839) were scored. Total section area was measured using the ‘pen’ tool in Image J (National Institutes of Health, Bethesda, MD, USA) on a touch-enabled tablet. Areas of adipocyte replacement (Figure 1, second and far right columns) were measured, and the total area of adipocyte replacement for each cross section calculated. The average percent area of fatty replacement (of 4–6 sections per subject) was calculated for all subjects. Fatty replacement is reported as a percentage of total section area.
Figure 1.
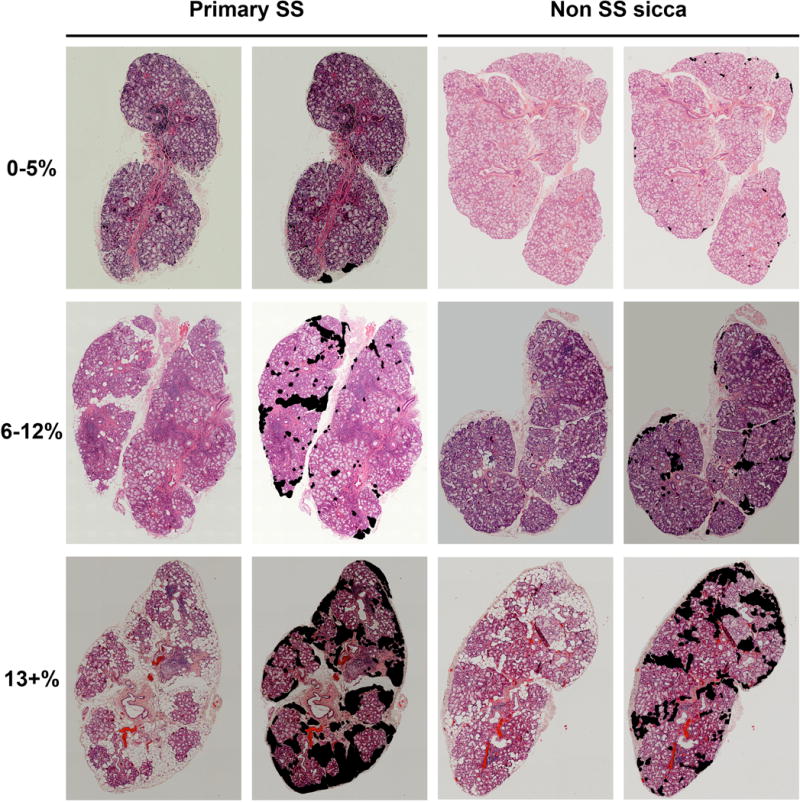
Digital salivary gland sections are scored for fatty replacement area. H&E stained sections are shown unscored (left) and with the adipocytes quantified (right) for both pSS and nSS subjects, covering the full range of observed replacement.
Statistical Analysis
Data normality was tested using the Shapiro-Wilks test, and, where necessary, non-parametric tests were applied. Comparisons between groups and correlations among variables were calculated in Prism 6 (GraphPad, La Jolla, CA, USA) using the Kolmogorov-Smirnov test and Spearman correlation or diagnostic test evaluation statistics, as appropriate. All tests were 2-tailed. Logistic regression and disease prediction were performed using generalized linear models (GLMs) via the ‘stats’ package in R.
Results
Salivary gland (SG) fatty replacement is not specific to SS
The average area of minor SG fatty infiltration for pSS patients (median % (range) 4.97 (0.05–30.2)) was not significantly different from that of nSS patients (3.75 (0.087–41.9) (Figure 2A). We then assessed if subjects with fatty infiltration greater than the average median (5%) were overrepresented in either patient group. The extent of infiltration varied widely, and those subjects with fatty replacement greater than 6% were equivalently distributed between pSS and nSS classifications (χ2 p = 0.50). Although patients were not selected to be age-matched, patient age was not significantly different between pSS and nSS groups (Figure 2B).
Figure 2.
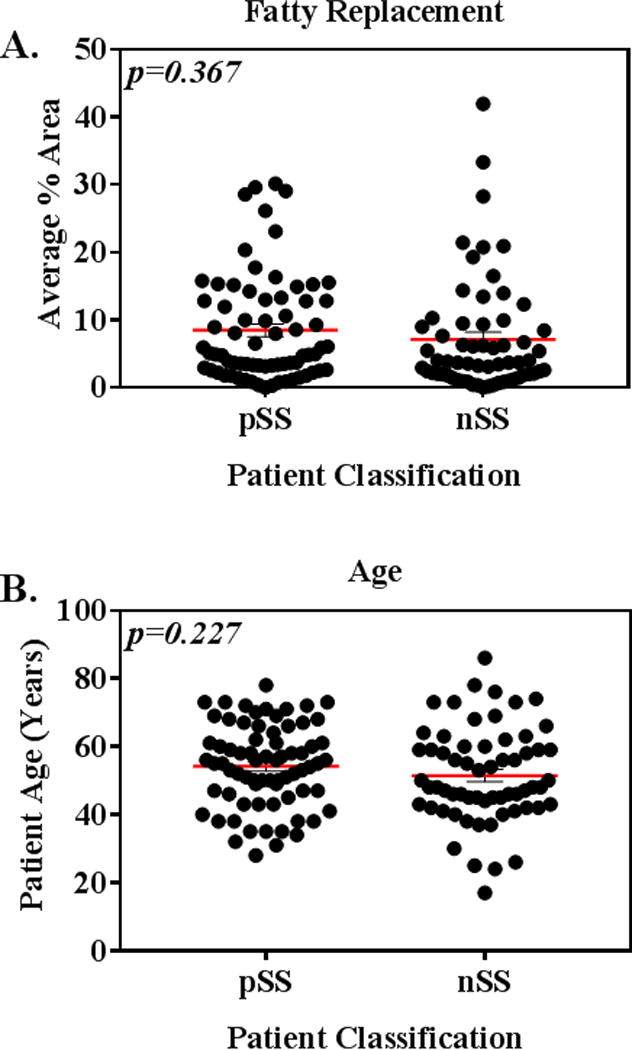
(A) Salivary gland fatty replacement is not significantly different (p= 0.367) in pSS versus nSS patients. 2-tailed Kolmogorov-Smirnov test. (B) Patient age in pSS and nSS groups is not significantly different. Unpaired 2-tailed t-test.
Retrospective, subjective evaluation of patterns of adipocyte replacement using unblinded samples revealed no obvious differences in fatty distribution patterns between pSS and nSS groups. Glandular cross-sections from individual subjects tended to display very similar patterns of adipocyte distribution. Adipocytes most commonly occurred at borders of lobes, radiating inward, while patterns of diffuse infiltration (12% of cases in both groups) and lobe replacement (8–9% of cases in both groups) were less common. Notably, proximity of adipocytes to lymphocytic foci was rare.
Age accounts for association between fatty replacement and pathologic features of SS
Although the extent of fatty replacement did not associate with disease classification, we asked whether fatty replacement associated with clinical, demographic or behavioral features of the participants. Patient heterogeneity is one of the confounding factors in SS research, and opportunities to subset patients must be explored. Simple Spearman correlations showed that fatty replacement positively associated with both the extent of fibrotic changes (r = 0.27, p = 0.002) and patient age (r = 0.55, p<0.0001). Whole unstimulated salivary flow (WUSF) inversely correlated with the extent of fatty replacement (r = −0.300, p = 0.0005). Historical patient smoking behavior data (never smoked and have ever smoked) had no age-independent association with disease classification or fatty replacement (data not shown).
The strong correlation between fatty replacement and age mandated an age-corrected analysis, specifically to address whether the extent of fatty replacement was solely attributable to advanced subject age. Linear models using two (Fatty replacement and WUSF) or three (Fatty replacement, age and WUSF) parameters were constructed and compared (Table 2). Age accounted for the entirety of the association between fatty replacement and WUSF (β2 = −0.065, p = 0.44) and fatty replacement and fibrosis (β2 = 0.012, p = 0.90).
Table 2.
Fatty replacement does not associate with clinical SS features
| Uncorrected | Age-corrected | |||
|---|---|---|---|---|
|
|
||||
| Variable | β-value | p-value | β-value | p-value |
| Fibrosis1 | 0.166 | 0.056 | 0.013 | 0.876 |
| VanBijsterveld | 0.042 | 0.638 | <0.00001 | 0.999 |
| Schirmer’s | −0.054 | 0.537 | 0.099 | 0.224 |
| WUSF2 | −0.233 | 0.001 | −0.065 | 0.435 |
| Biopsy Focus score | −0.006 | 0.942 | −0.028 | 0.725 |
Average percent area fibrotic change in minor salivary glands of SS patients
WUSF = whole unstimulated saliva flow
Fatty Replacement adds no predictive value to SS classification models
Our final approach was to assess if fatty replacement improved the accuracy of a predictive model of SS classification status. Our previous work demonstrated that, along with focus score, the area of morphologically-detected glandular fibrosis was predictive of primary SS classification [19]. The known confounding effect of age was also considered in that study, and we found that age could not explain elevated fibrosis in pSS subjects. This result, and our further analyses, indicated that fibrosis in pSS patients is a part of SS pathology, not simply a feature of age. Thus, we constructed generalized linear models to explore the relative contributions of age, fibrotic replacement, and focus score to assess if the inclusion of fatty replacement had any effect on the model (Table 3A). The all-inclusive model (Table 3, upper) of fibrosis, age, fatty replacement and focus score was not significantly different from any other analysis. In no iteration of the model did fatty replacement exert a significant effect on the model’s outcome (Table 3). The addition or absence of either age or fatty replacement to a predictive model containing focus score and fibrosis had no significant positive or negative effect on the predicted outcome. Thus, the extent of minor SG fatty replacement is not a selective feature of Sjögren’s syndrome.
Table 3.
Fatty Replacement adds no predictive value to SS classification models
| Number of variables | Input | Point Estimate | p-value | OR | Accuracy (95% CI) | Sensitivity | Specificity | ANOVA p value | |
|---|---|---|---|---|---|---|---|---|---|
| A | 4 | Focus Score | 0.936 | 0.002 | 2.549 | 0.75 (0.667, 0.821) | 0.82 | 0.69 | N/A |
| Fibrosis | 0.097 | 0.001 | 1.102 | ||||||
| Age | −0.012 | 0.529 | 0.988 | ||||||
| Fatty Replacement | 0.003 | 0.910 | 1.003 | ||||||
|
| |||||||||
| B | 3 | Focus Score | 0.941 | 0.001 | 2.562 | 0.75 (0.667, 0.821) | 0.82 | 0.69 | 0.91 |
| Fibrosis | 0.097 | 0.001 | 1.102 | ||||||
| Age | −0.011 | 0.517 | 0.989 | ||||||
|
| |||||||||
| C | 3 | Focus Score | 0.945 | 0.001 | 2.573 | 0.758 (0.675, 0.828) | 0.836 | 0.69 | 0.53 |
| Fibrosis | 0.091 | 0.001 | 1.096 | ||||||
| Fatty Replacement | −0.005 | 0.849 | 0.995 | ||||||
|
| |||||||||
| D | 2 | Focus Score | 0.938 | 0.001 | 2.554 | 0.765 (0.684, 0.835) | 0.836 | 0.704 | 0.8 |
| Fibrosis | 0.091 | 0.001 | 1.095 | ||||||
Discussion
Fatty infiltration is a well-noted but poorly understood aspect of SG dysmorphism in SS. Our study examined fatty infiltration in subjects with pSS or nSS sicca and found no significant difference between the groups in the area of replacement. Using a precise, manual method of measurement, we quantified the total gland area replaced by adipocytes. We eliminated observer bias by measuring all fat in the tissue section and by blinding the observer to classification status. As a percentage of total available gland area, no statistical difference in fatty replacement was found between those with pSS or nSS. A positive biopsy focus score is the cardinal histopathologic feature of SS [31, 32], but focus score-negative primary SS patients exist. There are numerous validated features of SS used in classifying patients, all with differing specificity and sensitivity for SS. Surprisingly, many of the features are weakly or not significantly associated with each other. The cardinal indicators of SS are positive biopsy focus score and presence of anti-Ro autoantibodies. Associations between other clinical signs and SS are weak, as are associations between signs (i.e. vanBijsterveld score and saliva flow) [29]. This heterogeneity encourages the exploration of novel, potentially useful categorizing features of disease.
Glandular pathologies such as focal lymphocytic infiltration [1, 20], fibrosis [19] and acinar cell atrophy [20, 21] associate with SS classification and other clinical features [29]. However, in the present study the extent of fatty infiltration was driven exclusively by the age of the individual and did not associate with SS classification, the extent of SG fibrosis as assessed by morphologic criteria, ocular damage, saliva flow (WUSF), tear flow (Schirmer’s eye test) or biopsy focus score. Previously, we demonstrated that significant association of fibrosis with both classification and signs (corneal damage and focus score) of SS were not explained by patient age [19]. In contrast, our current findings show that associations between fatty replacement and salivary flow or morphologically detected fibrotic changes are driven solely by patient age. After age-correction of the linear models, there is no association between fatty infiltration and other SS disease features, and a retrospective analysis showed that adipocytes are rarely in direct contact or close proximity to focal infiltrates. Overall, our data demonstrate that unlike other glandular pathologies, fatty infiltration of the SG is not associated with Sjögren’s syndrome per se.
Normal aging processes may explain fatty replacement in SS SG. Fatty infiltration and fatty replacement are terms that describe processes by which tissue, generally exocrine tissue, is replaced in whole or in part by adipocytes [33, 34, 35]. Fatty infiltration of tissue is a normal part of the aging process, though pathological and accelerated forms can occur after tissue injury [36, 37]. Thymic involution, for example, is a systematic replacement of thymic tissue with adipocytes which occurs as an organism ages [38]. The initial accumulation of leptin in metabolically inactive thymic tissue allows for orderly apoptosis and an expansion of adipocytes in the organ volume.
Fatty replacement due to soft tissue injury has been described in soft tissues (especially that of the rotator cuff [37]), traumatic bone injury [39] and in pancreatic injury due to primary duct stenosis or calcification [33, 34, 35]. In well-characterized rodent models of pancreatic duct ligation, the pancreatic tissue undergoes a series of predictable alterations. First, the tissue becomes edematous, exocrine acini swell, then shrink and atrophy. This is followed by rapid parenchymal swelling, recession, and ultimately complete adipocyte replacement of the organ [34]. Additionally, human case studies have shown that calculus blockage of the pancreatic duct leads to total fatty replacement of the acinar pancreatic tissue, with preservation of the islets [33]. Ductal dilation, basal laminar dysfunction and epithelial irregularities are notable features in SS glands [40, 41, 42, 43], though no decisive model of the role of each in disease yet exists. Fatty infiltration has been characterized as a normal biological response to injury in exocrine tissue, and its presence in the SG of both SS and nSS sicca patients may be a shared mechanism for resolution or maintenance of damaged SG tissue.
A recent study [28] found that SS patients with positive focus scores had a higher incidence of “moderate” and “significant” fatty infiltration, in contrast to largely “negative” nSS. The authors did not define “negative” as the complete absence of adipocytes, but rather as no “significant” presence. The subjects in our study had a lower focus score, on average, as compared to their patients (1.5 versus 3.25), which may indicate different disease stages, and could explain the differences in our findings. Of interest, they also reported that adipocytes in pSS tissue produce IL-6, a pro-inflammatory cytokine [28], located away from areas with lymphocytic infiltrates. Our approach examined fatty infiltration as a function of area, allowing increased precision, in addition to a slightly larger study cohort. While we found no difference in the extent of fatty infiltration between pSS and nSS sicca, the metabolic nature of the adipocytes between these groups could differ. In this study, we observed no association between SG focus score, an indicator of disease severity [23, 44, 45], and degree of SG fatty infiltration. Moreover, proximity of adipocytes to lymphocytic infiltrates was rare, and patterns of fatty replacement between pSS and nSS groups did not obviously differ. We cannot formally exclude, however, the possibility that extent of fatty replacement may vary with the composition of the infiltrates. Our results are in agreement with those of Katona et al [46]. We concur that SG fatty replacement is not a distinguishing feature of SS. Further, our study demonstrates that in addition to no association with SS classification, fatty infiltration has no significant association with any other evaluated clinical sign or symptom within our cohort.
Fatty tissue in inflammatory disease is known to produce pro-inflammatory agents [47, 48], and SG epithelium has been shown to produce adiponectin in SS [49] which may support the process of fatty replacement. Although we detected no difference in the volume of fatty infiltration between those with sicca or SS, our findings do not preclude the existence of as-yet cryptic differences between these two groups. The driving mechanisms, chronology, and metabolic nature of fatty infiltration and other aspects of SS remain to be explored. The fundamental heterogeneity of SS is a confounding aspect both in researching and in treating the disease. We have herein demonstrated that minor SG fatty replacement is primarily a feature of aging and does not associate with SS signs or disease classification. This work refines our understanding of SS pathogenesis and may help shape future inquiries into the mechanisms and true pathology of the disease.
Supplementary Material
Acknowledgments
We would like to acknowledge the help and assistance of the OMRF Imaging Core for generating the assembled images, Farris Tedder for her summer of input on this project, Rachel Smith for assistance with figures and Louise Williamson for clerical assistance.
Footnotes
Declaration of interest statement
The authors report no conflicts of interest.
Supplementary file available online
Additional magnified sections of adipocyte-containing MSG are shown in Supplementary Figure 1.
References
- 1.Greenspan JS, Daniels TE, Talal N, et al. The histopathology of Sjögren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37(2):217–229. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 2.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjogren’s syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 3.Tapinos NI, Polihronis M, Tzioufas AG. Immunopathology of Sjogren’s syndrome. Ann Med Interne (Paris) 1998 Feb;149(1):17–24. [PubMed] [Google Scholar]
- 4.Kivity S, Arango MT, Ehrenfeld M, et al. Infection and autoimmunity in Sjogren’s syndrome: a clinical study and comprehensive review. J Autoimmun. 2014 Jun;51:17–22. doi: 10.1016/j.jaut.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Lessard CJ, Li H, Adrianto I, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjogren’s syndrome. Nat Genet. 2013;45(11):1284–92. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjogren’s syndrome. Nat Rev Rheumatol. 2013;9(9):544–56. doi: 10.1038/nrrheum.2013.110. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Zhang K, Chen H, et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjogren’s syndrome at 7q11.23. Nat Genet. 2013;45(11):1361–5. doi: 10.1038/ng.2779. [DOI] [PubMed] [Google Scholar]
- 8.Altorok N, Coit P, Hughes T, et al. Genome-wide DNA methylation patterns in naïve CD4+ T cells from patients with primary Sjögren’s Syndrome. Arthritis Rheum. 2014;66(3):731–9. doi: 10.1002/art.38264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konsta OD, Thabet Y, Le Dantec C, et al. The contribution of epigenetics in Sjogren’s Syndrome. Front Genet. 2014;5:71. doi: 10.3389/fgene.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbelo PD, Ambatipudi K, Alevizos I. Genome-wide association studies in Sjogren’s syndrome: What do the genes tell us about disease pathogenesis? Autoimmun Rev. 2014;13(7):756–61. doi: 10.1016/j.autrev.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teos LY, Zhang Y, Cotrim AP, et al. IP3R deficit underlies loss of salivary fluid secretion in Sjogren’s Syndrome. Sci Rep. 2015;5:13953. doi: 10.1038/srep13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mariette X. Pathophysiology of Sjogren’s syndrome. Ann Med Interne (Paris) 2003;154(3):157–68. [PubMed] [Google Scholar]
- 13.Garcia-Carrasco M, Fuentes-Alexandro S, Escarcega RO, et al. Pathophysiology of Sjogren’s syndrome. Arch Med Res. 2006;37(8):921–32. doi: 10.1016/j.arcmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Jonsson R, Vogelsang P, Volchenkov R, et al. The complexity of Sjogren’s syndrome: novel aspects on pathogenesis. Immunol Lett. 2011 Dec 30;141(1):1–9. doi: 10.1016/j.imlet.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Mavragani CP, Moutsopoulos HM. Sjogren’s syndrome. Annu Rev Pathol. 2014;9:273–85. doi: 10.1146/annurev-pathol-012513-104728. [DOI] [PubMed] [Google Scholar]
- 16.Brookes SM, Cohen SB, Price EJ, et al. T cell clones from a Sjogren’s syndrome salivary gland biopsy produce high levels of IL-10. Clin Exp Immunol. 1996;103(2):268–72. doi: 10.1046/j.1365-2249.1996.d01-623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler S, Korner M, Forger F, et al. Evaluation of histological, serological and clinical changes in response to abatacept treatment of primary Sjogren’s syndrome: A pilot study. Arthritis Care Res (Hoboken) 2013;65(11):1862–8. doi: 10.1002/acr.22052. [DOI] [PubMed] [Google Scholar]
- 18.Joachims ML, Leehan KM, Lawrence C, et al. Single-cell analysis of glandular T cell receptors in Sjogren’s syndrome. JCI Insight. 2016 Jun 2;1(8) doi: 10.1172/jci.insight.85609. pii: e85609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leehan KM, Pezant N, Rasmussen A, et al. Minor salivary gland fibrosis in Sjögren’s syndrome is elevated, associated with focus score and not solely a consequence of aging. Clin Exp Rheumatol. 2017 Forthcoming. [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda Y. Histopathological studies of the labial salivary glands in patients with Sjogren’s syndrome. Part I: light microscopic study. Bull Tokyo Med Dent Univ. 1980 Mar;27(1):9–25. [PubMed] [Google Scholar]
- 21.Bookman AA, Shen H, Cook RJ, et al. Whole stimulated salivary flow: correlation with the pathology of inflammation and damage in minor salivary gland biopsy specimens from patients with primary Sjogren’s syndrome but not patients with sicca. Arthritis Rheum. 2011;63(7):2014–20. doi: 10.1002/art.30295. [DOI] [PubMed] [Google Scholar]
- 22.Llamas-Gutierrez FJ, Reyes E, Martinez B, et al. Histopathological environment besides the focus score in Sjogren’s syndrome. Int J Rheum Dis. 2014;17(8):898–903. doi: 10.1111/1756-185X.12502. [DOI] [PubMed] [Google Scholar]
- 23.Carubbi F, Alunno A, Cipriani P, et al. Is minor salivary gland biopsy more than a diagnostic tool in primary Sjogrens syndrome? Association between clinical, histopathological, and molecular features: a retrospective study. Semin Arthritis Rheum. 2014;44(3):314–24. doi: 10.1016/j.semarthrit.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Carubbi F, Alunno A, Cipriani P, et al. A retrospective, multicenter study evaluating the prognostic value of minor salivary gland histology in a large cohort of patients with primary Sjogren’s syndrome. Lupus. 2015;24(3):315–20. doi: 10.1177/0961203314554251. [DOI] [PubMed] [Google Scholar]
- 25.Skopouli FN, Dafni U, Ioannidis JP, et al. Clinical evolution, and morbidity and mortality of primary Sjogren’s syndrome. Semin Arthritis Rheum. 2000;29(5):296–304. doi: 10.1016/s0049-0172(00)80016-5. [DOI] [PubMed] [Google Scholar]
- 26.Quartuccio L, Isola M, Baldini C, et al. Biomarkers of lymphoma in Sjogren’s syndrome and evaluation of the lymphoma risk in prelymphomatous conditions: results of a multicenter study. J Autoimmun. 2014 Jun;51:75–80. doi: 10.1016/j.jaut.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Madrigal F, Micheau C. Histology of the Major Salivary Glands. Am J Surg Pathol. 1989;13(10):879–899. doi: 10.1097/00000478-198910000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Skarstein K, Aqrawi LA, Øijordsbakken G, et al. Adipose tissue is prominent in salivary glands of Sjögren’s syndrome patients and appears to influence the microenvironment in these organs. Autoimmunity. 2016;49(5):338–346. doi: 10.1080/08916934.2016.1183656. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen A, Ice JA, Li H, et al. Comparison of the American-European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised SICCA cohort. Ann Rheum Dis. 2014;22(73):31–38. doi: 10.1136/annrheumdis-2013-203845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniels TE, Silverman S, Jr, Michalski JP, et al. The oral component of Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol. 1975;39(6):875–885. doi: 10.1016/0030-4220(75)90108-5. [DOI] [PubMed] [Google Scholar]
- 32.Daniels TE. Labial salivary gland biopsy in Sjögren’s syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984;27(2):147–56. doi: 10.1002/art.1780270205. [DOI] [PubMed] [Google Scholar]
- 33.Anand R, Narula MK, Chaudhary V, et al. Total pancreatic lipomatosis with malabsorption syndrome. Indian J Endocrinol Metab. 2011;15(1):51–3. doi: 10.4103/2230-8210.77587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walters MN. Adipose atrophy of the exocrine pancreas. J Pathol Bacteriol. 1966;92(2):547–57. doi: 10.1002/path.1700920232. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe S, Abe K, Anbo Y, et al. Changes in the mouse exocrine pancreas after pancreatic duct ligation: a qualitative and quantitative histological study. Arch Histol Cytol. 1995;58(3):365–74. doi: 10.1679/aohc.58.365. [DOI] [PubMed] [Google Scholar]
- 36.Davies MR, Liu X, Lee L, et al. TGF-beta Small Molecule Inhibitor SB431542 Reduces Rotator Cuff Muscle Fibrosis and Fatty Infiltration By Promoting Fibro/Adipogenic Progenitor Apoptosis. PLoS ONE. 2016;11(5):e0155486. doi: 10.1371/journal.pone.0155486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Ning AY, Chang NC, et al. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J. 2016;6(1):6–15. doi: 10.11138/mltj/2016.6.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamas A, Lopez E, Carrio R, et al. Adipocyte and leptin accumulation in tumor-induced thymic involution. Int J Mol Med. 2016;37(1):133–8. doi: 10.3892/ijmm.2015.2392. [DOI] [PubMed] [Google Scholar]
- 39.Rosenthal DI, Hayes CW, Rosen B, et al. Fatty replacement of spinal bone marrow due to radiation: demonstration by dual energy quantitative CT and MR imaging. J Comput Assist Tomogr. 1989;13(3):463–5. doi: 10.1097/00004728-198905000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Goicovich E, Molina C, Perez P, et al. Enhanced degradation of proteins of the basal lamina and stroma by matrix metalloproteinases from the salivary glands of Sjogren’s syndrome patients: correlation with reduced structural integrity of acini and ducts. Arthritis Rheum. 2003;48(9):2573–84. doi: 10.1002/art.11178. [DOI] [PubMed] [Google Scholar]
- 41.Molina C, Alliende C, Aguilera S, et al. Basal lamina disorganisation of the acini and ducts of labial salivary glands from patients with Sjogren’s syndrome: association with mononuclear cell infiltration. Ann Rheum Dis. 2006;65(2):178–83. doi: 10.1136/ard.2004.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker JL, Menko AS, Khalil S, et al. Diverse roles of E-cadherin in the morphogenesis of the submandibular gland: insights into the formation of acinar and ductal structures. Dev Dyn. 2008;237(11):3128–41. doi: 10.1002/dvdy.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramer JM. Early events in Sjogren’s Syndrome pathogenesis: the importance of innate immunity in disease initiation. Cytokine. 2014;67(2):92–101. doi: 10.1016/j.cyto.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 44.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren’s syndrome. J Autoimmun. 2010;34(4):400–7. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Risselada AP, Kruize AA, Goldschmeding R, et al. The prognostic value of routinely performed minor salivary gland assessments in primary Sjogren’s syndrome. Ann Rheum Dis. 2014;73(8):1537–40. doi: 10.1136/annrheumdis-2013-204634. [DOI] [PubMed] [Google Scholar]
- 46.Katona K, Farkas N, Suto G, et al. Adipose tissue infiltration in minor salivary glands of patients with Sjogren’s syndrome: Lack of significant correlation with the disease. An image analysis of 174 cases. Autoimmunity. 2017;50(4):199–201. doi: 10.1080/08916934.2017.1316381. [DOI] [PubMed] [Google Scholar]
- 47.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7(12):885–96. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 48.Henry SL, Bensley JG, Wood-Bradley RJ, et al. White adipocytes: More than just fat depots. Int J Biochem Cell Biol. 2012;44(3):435–440. doi: 10.1016/j.biocel.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, et al. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006;54(7):2295–9. doi: 10.1002/art.21944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


