Abstract
Accurate risk stratification of smooth muscle tumors is essential for appropriate patient management. Yet, the rarity of smooth muscle tumors of the vagina and vulva makes development of a prognostically meaningful classification system challenging. While 2 classification methods for vulvar smooth muscle tumors and 1 for vaginal smooth muscle tumors have been proposed, it is our experience that many pathologists tend to apply criteria for uterine smooth muscle tumors when evaluating vulvovaginal tumors.
We retrospectively reviewed a large cohort of vulvovaginal smooth muscle tumors with clinical follow up and evaluated which method most accurately classified tumors according to patient outcome. A total of 71 tumors, 53 vaginal (75%) and 18 vulvar (25%), from 71 patients were identified. All tumors were centrally examined for degree of cytologic atypia, morphology (spindled, epithelioid, myxoid), mitotic index per 10 high power fields, atypical mitotic figures, tumor cell necrosis, ischemic necrosis, tumor interface (circumscribed or infiltrative) and margin status. Clinical features were recorded for each patient. Follow up was available for 63 patients (89%), and ranged from 1 to 234 months (median 64 months). While site-specific and uterine criteria showed equally excellent sensitivity in classifying smooth muscle neoplasms as leiomyosarcoma according to patient outcome, uterine criteria showed improved specificity relatively to site-specific methods in classifying non-sarcoma tumors according to patient outcome. We recommend that uterine smooth muscle tumor criteria and nomenclature be adopted for evaluation and classification of vulvovaginal smooth muscle tumors.
Keywords: smooth muscle tumor, leiomyosarcoma, leiomyoma, vulva, vagina
Introduction
Smooth muscle tumors (SMTs) are the most common mesenchymal neoplasms of the vulva and vagina(1), yet their infrequency is demonstrated by the prevalence of published reports as individual cases or small series. The rarity of these tumors makes development of a prognostic classification system challenging. However, accurate risk stratification of tumors according to pathologic findings is critical for appropriate management of patients.
To date, 3 site-specific classification methods have been proposed: 2 for vulvar SMTs and 1 for vaginal SMTs (Table 1). The first sets of criteria were published in 1979 by Tavassoli and Norris, 1 for vulvar SMTs(2) and 1 for vaginal SMTs(3). A review of 32 vulvar SMTs led to identification of 3 main risk determinants based on tumor recurrence: gross size ≥ 5 cm, mitotic index ≥ 5 figures/10 high power fields (HPFs) and infiltrative tumor interface. Finding 2 of 3 features qualifies a tumor as low grade leiomyosarcoma and the presence of all 3 features warrant a diagnosis of leiomyosarcoma. A similar review of 60 vaginal SMTs established varying combinations of risk determinants including degree of cytologic atypia, mitotic index and infiltrative tumor interface. Tumors with moderate or severe cytologic atypia and mitotic activity of ≥ 5 figures/10 HPFs are designated leiomyosarcoma. Further, of the 5 recurrent tumors in their series, the 1 tumor that metastasized had infiltrative margins in contrast to the 4 other locally recurrent tumors that were circumscribed. It was suggested by the authors that the presence of infiltration alone warranted classification as leiomyosarcoma in vaginal SMTs until proven otherwise due to infiltration as a general indicator of more aggressive behavior in mesenchymal tumors.
Table 1.
Proposed Criteria for Classification of Vaginal or Vulvar Smooth Muscle Tumors as Leiomyosarcoma.
| 1979 Vaginal and Vulvar Criteria | 1996 Vulvar Criteria | Uterine SMT Criteria |
|---|---|---|
|
| ||
| Vagina: | Any 3 of 4 warrant diagnosis of leiomyosarcoma
|
Any 2 of 3 warrant diagnosis of leiomyosarcoma
|
2 of 2 warrant diagnosis of leiomyosarcoma
| ||
| OR | ||
1 of 1 warrant diagnosis of leiomyosarcoma
| ||
| Vulva: | ||
Any 2 of 3 warrant diagnosis of low grade leiomyosarcoma and 3 of 3 warrant diagnosis of leiomyosarcoma
| ||
HPFs (high power fields)
Prognostic criteria were expanded in 1996 by Nielsen and colleagues(4) following review of 25 SMTs of the vulva. A set of 4 criteria incorporating gross and microscopic findings were proposed: gross size (≥ 5 cm), presence of infiltrative margin, mitotic index (≥ 5 mitoses/10 HPFs) and degree of cytologic atypia (moderate to severe). If 0 or 1 criterion was met, the tumor should be interpreted as leiomyoma; if 2 criteria were satisfied, the tumor should be interpreted as atypical leiomyoma; and if 3 or 4 criteria were fulfilled, the tumor should be interpreted as leiomyosarcoma.
Despite these proposed site-specific classification systems, it is our experience that many pathologists tend to apply criteria for SMTs of the uterus(5) when evaluating vulvovaginal SMTs. The 2014 World Health Organization (WHO) Classification of Tumors of Female Reproductive Organs(1) outlines 3 morphologic components to assess in uterine SMTs: degree of cytologic atypia, mitotic index and the presence of tumor cell necrosis. Most practitioners label a tumor as leiomyosarcoma when at least 2 of these 3 components are identified, requiring that the degree of cytologic atypia be at least moderate and the mitotic index be a minimum of 10 figures/10 HPFs. Other combinations of these 3 components, whether due to a lesser degree of cytologic atypia, lower mitotic index or presence/absence of tumor cell necrosis, fall within a spectrum of SMTs such as smooth muscle tumor of uncertain malignant potential (STUMP), leiomyoma with bizarre nuclei and mitotically active leiomyoma that have been correlated with potential for aggressive behavior(6–9).
Additionally, prior to 2004, morphologic criteria for risk stratification of ovarian SMTs were non-existent. A review of 54 SMTs of the ovary by Lerwill and investigators(10) led to validation of diagnostic categories and prognostic criteria of uterine SMTs. The authors concluded that, as in the uterus, ovarian leiomyosarcoma may be diagnosed when at least 2 of 3 criteria are satisfied: moderate to severe cytologic atypia, mitotic index in excess of 10 figures/10 HPFs and the presence of tumor cell necrosis.
The aim of our study was to retrospectively review a large cohort of vulvovaginal SMTs with clinical follow up and statistically evaluate which of the proposed site-specific criteria or uterine SMT criteria most accurately classified tumors according to patient outcome.
Materials and Methods
Case Selection
The archives of 7 institutions were searched for SMTs of the vulva and vagina using keywords “smooth muscle tumor/neoplasm,” “leiomyoma,” “smooth muscle tumor of uncertain malignant potential” and “leiomyosarcoma.” Cases were limited to internal patients of each institution to ensure access to all original slides, relevant clinical information and follow up. A total of 71 tumors from 71 patients were identified. Clinical and pathologic data were obtained from electronic medical records and pathology reports. These data included the patient’s age at diagnosis, tumor site, clinical presentation, reported pathologic diagnosis, gross measurement of tumor size, clinical management (due to inter-institutional differences in descriptions of surgical procedures, excision specimens were consolidated to simple excision if the tumor was conservatively excised or enucleated [Fig. 1A] and wide local excision if excision margins were attempted), presence of and site(s) of recurrence(s), additional therapy (if applicable) and clinical status at last follow up.
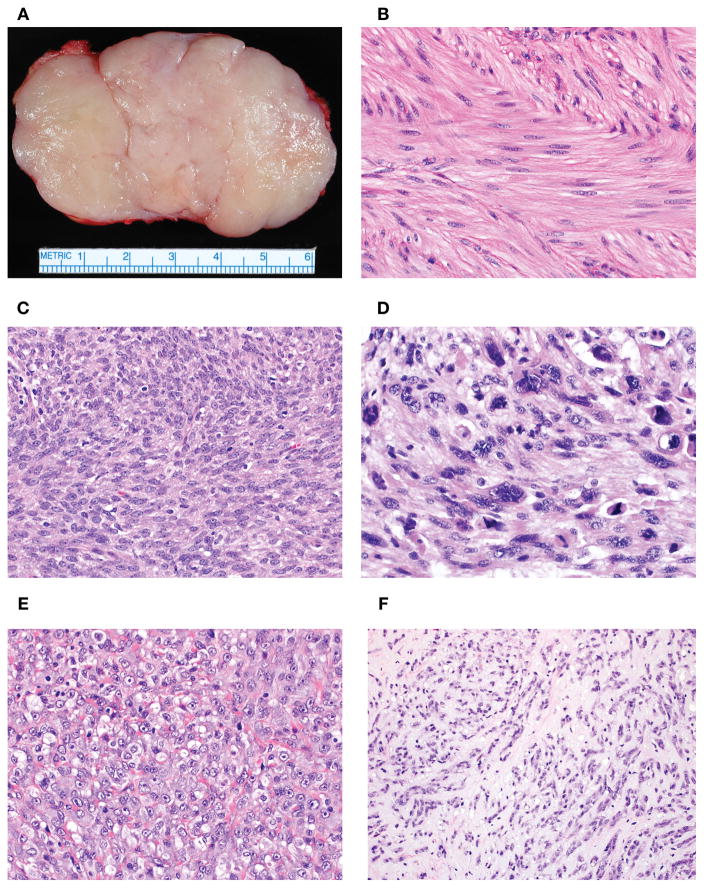
Figure 1.
Vulvovaginal SMTs are often conservatively excised or enucleated in an absence of clinically concerning features for malignancy given their proximity to sensitive anatomic structures (A). This surgical procedure often results in problematic microscopic assessment of tumor interface (infiltrative or circumscribed). SMTs with mild cytologic atypia exhibited minimal variation in nuclear size and shape, stippled and evenly dispersed chromatin and small to inconspicuous nucleoli (B). Tumors with moderate cytologic atypia had larger nuclei with irregular nuclear membrane contours, uneven chromatin and more prominent nucleoli (C). Tumors with severe atypia demonstrated significant nuclear enlargement and pleomorphism with coarse chromatin and large nucleoli (D). Epithelioid morphology showed polygonal to rounded cells with a nested or sheet-like growth (E). Myxoid morphology featured prominent quantities of myxoid acid-mucin stroma that often resulted in dyscohesion or separation of individual cells (F).
Morphologic Analysis
Complete sets of hematoxylin and eosin slides were centrally reviewed by 2 of the authors (SS and JKS). Each tumor was confirmed to be an SMT and its original diagnosis was recorded from the pathology report. Tumors were examined for degree of cytologic atypia (mild, moderate, severe), morphology (spindled, epithelioid, myxoid), mitotic index per 10 HPFs (a minimum of 3 sets of 10 HPFs were counted by 40× objective and 10× ocular eyepiece and the highest number was recorded), presence of atypical mitotic figures, presence of tumor cell necrosis, presence of ischemic necrosis, tumor interface (circumscribed or infiltrative) and margin status.
Cytologic atypia was defined by the extent of nuclear pleomorphism and enlargement, hyperchromasia, coarse chromatin and size of nucleoli. Tumors with mild atypia had minimal variation in nuclear size and shape, stippled and evenly dispersed chromatin and small to inconspicuous nucleoli (Fig. 1B). Tumors with moderate atypia exhibited larger nuclei, with irregular nuclear membrane contours, uneven chromatin and more prominent nucleoli (Fig. 1C). Tumors with severe atypia exhibited frank nuclear enlargement and pleomorphism, coarse chromatin and large nucleoli (Fig. 1D). If a tumor showed a gradient of atypia, it was recorded as a range. For instance, if a tumor had significant quantities of moderate and severe atypia, it was interpreted as moderate to severe.
For morphology, a tumor was considered spindled (typical/conventional) when it had a fascicular growth of cells with eosinophilic, fibrillary cytoplasm and elongated nuclei. A tumor was categorized as epithelioid when its cells were polygonal to rounded, resembling an epithelial neoplasm (Fig. 1E). A designation of myxoid was given when a tumor had prominent quantities of myxoid acid-mucin stroma that often resulted in dyscohesion or separation of individual cells (Fig. 1F). If the tumor had a mixture of morphologies, it was recorded as a spectrum with the predominant morphologic pattern first followed by the secondary morphologic pattern. For example, if a tumor was 70% spindled and 30% myxoid, it was designated as spindled to myxoid. If a morphologic pattern comprised 10% or less of the tumor, it was given a designation of focal; e.g., spindled to focally myxoid.
Mitotic figures were considered atypical when there was deviation from standard metaphase patterns, variations of polarity or other non-physiological division forms. Tumor cell necrosis exhibited an abrupt shift between viable to non-viable tumor without evidence of intervening tissue (Fig. 2A) contrasted by ischemic necrosis which showed a transition of granulation tissue and/or hyalinization between viable and non-viable tumor (Fig. 2B). Tumor interface was interpreted as circumscribed when it was well-delineated relative to surrounding non-neoplastic tissue or had an expansile growth (Fig. 2C), and as infiltrative when permeative growth or destructive invasion of surrounding tissue was seen (Fig. 2D). Lastly, margin status of the specimens was noted as negative, focally positive (< 3 mm of tumor involving tissue margin) or diffusely positive (≥ 3 mm of tumor involving tissue margin). If a specimen’s margins were extensively involved by tumor to an extent that prevented assessment of the tumor being circumscribed or infiltrative, tumor interface was designated as unknown.
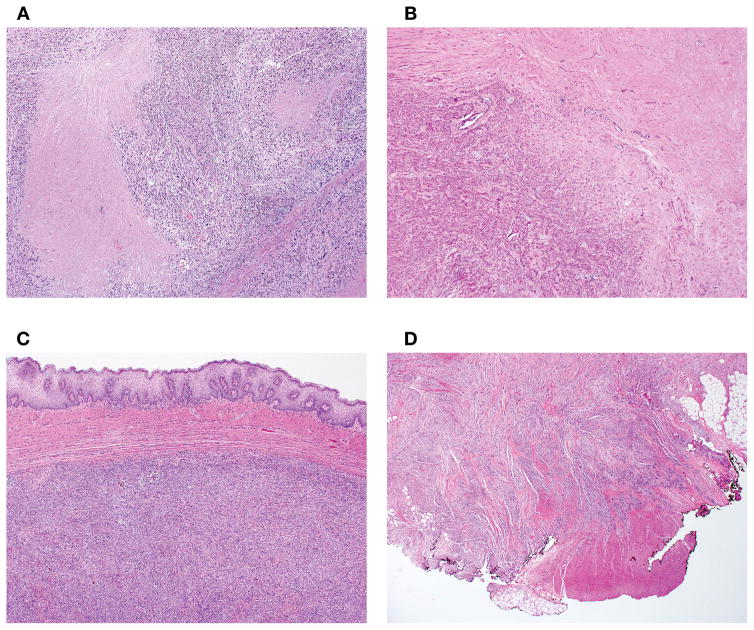
Figure 2.
Tumor cell necrosis exhibited an abrupt transition between viable to non-viable tumor without evidence of intervening tissue (A). In contrast, ischemic necrosis showed a zone of granulation tissue and/or hyalinization between viable and non-viable tumor (B). Tumor interface was classified as circumscribed when it was well-demarcated relative to surrounding non-neoplastic tissue or had an expansile growth (C) and as infiltrative when permeative growth or destructive invasion of surrounding tissue was present (D).
Statistical Analysis
For statistical analysis, SMTs were grouped as vulvar or vaginal and leiomyosarcoma or non-sarcoma. Clinical and pathologic variables were summarized with means and medians or ranges for continuous data, and with frequencies and percentages for categorical data. Sensitivity in detecting leiomyosarcoma and specificity in detecting non-sarcoma cases were calculated for each proposed classification method according to clinical outcome. Analysis of time to recurrence was summarized with 2-year risk estimates using the Kaplan-Meier method, and was compared between groups with likelihood ratio tests from Cox proportional hazards regression models. p-values less than 0.05 were considered statistically significant. All analyses were performed by SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Selected clinicopathologic features of all vulvovaginal SMTs are shown in Table 2. Features of recurrent tumors are outlined in Table 3. Comprehensive clinical and pathologic findings of all tumors are presented in Supplementary Digital Content Tables 1 and 2. A total of 71 SMTs, 53 vaginal (75%) and 18 vulvar (25%), from 71 patients were identified. Median patient age was 52 years (range 15 to 87 years). Clinical presentation was variable and included patient complaint of a vaginal or vulvar mass/nodule/cyst, incidental finding during physical examination or radiologic imaging for evaluation of an unrelated issue or incidental finding during intraoperative management for an unrelated indication. Of the 53 vaginal tumors, 39 were originally diagnosed as spindled leiomyoma, 1 as mitotically active leiomyoma, 1 as leiomyoma with bizarre nuclei, 11 as spindled leiomyosarcoma and 1 as myxoid leiomyosarcoma. Of the 18 vulvar tumors, 12 were originally diagnosed as spindled leiomyoma, 1 as myxoid leiomyoma, 1 as spindled STUMP, 1 as myxoid STUMP and 3 as spindled leiomyosarcoma. Follow up from institutional medical records was available for 63 patients (89%), and ranged from 1 to 234 months with a median of 64 months.
Table 2.
Selected Clinicopathologic Features of Vaginal and Vulvar Smooth Muscle Tumors.
| Vaginal Spindled Leiomyoma (N=39) | Vaginal Mitotically Active Leiomyoma (N=1) | Vaginal Leiomyoma with Bizarre Nuclei (N=1) | Vaginal Spindled Leiomyosarcoma (N=11) | Vaginal Myxoid Leiomyosarcoma (N=1) | Vulvar Spindled Leiomyoma (N=13) | Vulvar STUMP (N=2) | Vulvar Spindled Leiomyosarcoma (N=3) | |
|---|---|---|---|---|---|---|---|---|
| Age | ||||||||
| Mean (SD) | 50 (13) | 71 | 36 | 53.5 (21.8) | 67 | 51.8 (16.1) | 45, 50 | 56, 68, 72 |
| Range | (22–87) | (15–78) | (28–77) | |||||
| Tumor Size (cm, greatest dimension) | ||||||||
| N | 39 | 1 | 1 | 11 | 1 | 12 | 2 | 3 |
| Mean (SD) | 2.6 (1.5) | 0.5 | 4.5 | 5.9 (1.6) | 4 | 4 (2.9) | 6, 8.5 | 5.5, 11, 13.5 |
| Range | (0.6–7) | (3.5–9) | (0.5–11.2) | |||||
| Degree of Atypia* | ||||||||
| Mild to moderate | 39 (100%) | 1 (100%) | 0 (0%) | 1 (9.1%) | 0 (0%) | 13 (100%) | 0 (0%) | 0 (0%) |
| Moderate to severe | 0 (0%) | 0 (0%) | 1 (100%) | 10 (90.9%) | 1 (100%) | 0 (0%) | 2 (100%) | 3 (100%) |
| Mitotic Index (per 10 HPFs)* | ||||||||
| Median | 0 | 10 | 0 | 15 | 6 | 0 | 1, 1 | 8, 34, 23 |
| Q1, Q3 | 0, 0 | 11, 23 | 0, 1 | |||||
| Range | (0–7) | (5–32) | (0–1) | |||||
| Atypical Mitotic Figures | ||||||||
| Mitotic index of zero | 32 (82.1%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 7 (53.8%) | 0 (0%) | 0 (0%) |
| N | 7 (17.9%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (46.2%) | 2 (100%) | 1 (33.3%) |
| Y | 0 (0%) | 0 (0%) | 0 (0%) | 11 (100%) | 1 (100%) | 0 (0%) | 0 (0%) | 2 (66.7%) |
| Tumor Cell Necrosis* | ||||||||
| N | 39 (100%) | 1 (100%) | 1 (100%) | 4 (36.4%) | 0 (0%) | 13 (100%) | 2 (100%) | 0 (0%) |
| Y | 0 (0%) | 0 (0%) | 0 (0%) | 7 (63.6%) | 1 (100%) | 0 (0%) | 0 (0%) | 3 (100%) |
| Ischemic Necrosis | ||||||||
| N | 35 (89.7%) | 1 (100%) | 1 (100%) | 5 (45.5%) | 0 (0%) | 11 (84.6%) | 2 (100%) | 0 (0%) |
| Y | 4 (10.3%) | 0 (0%) | 0 (0%) | 6 (54.5%) | 1 (100%) | 2 (15.4%) | 0 (0%) | 3 (100%) |
| Tumor Interface | ||||||||
| Circumscribed | 22 (56.4%) | 0 (0%) | 0 (0%) | 4 (36.4%) | 0 (0%) | 8 (61.5%) | 1 (50%) | 0 (0%) |
| Infiltrative | 4 (10.3%) | 0 (0%) | 0 (0%) | 5 (45.5%) | 1 (100%) | 3 (23.1%) | 1 (50%) | 3 (100%) |
| UNK | 13 (33.3%) | 1 (100%) | 1 (100%) | 2 (18.2%) | 0 (0%) | 2 (15.4%) | 0 (0%) | 0 (0%) |
| Margin Status | ||||||||
| Negative | 7 (17.9%) | 0 (0%) | 0 (0%) | 6 (54.5%) | 1 (100%) | 3 (30.8%) | 2 (100%) | 1 (33.3%) |
| Positive | 32 (82.1%) | 1 (100%) | 1 (100%) | 3 (45.5%) | 0 (0%) | 9 (69.2%) | 0 (0%) | 2 (66.7%) |
| Follow Up (months) | ||||||||
| N | 35 | 1 | 1 | 11 | 1 | 9 | 2 | 3 |
| Median | 85 | 15 | 180 | 64 | 150 | 12 | 9, 19 | 4, 36, 56 |
| Range | (1–226) | (2–234) | (1–137) | |||||
| Any Recurrence | 0 | 0 | 0 | 8 | 1 | 0 | 0 | 3 |
Includes only feature assessment of pre-neoadjuvant therapy tumors
STUMP (smooth muscle tumor of uncertain malignant potential); HPFs (high power fields); UNK (unknown)
Table 3.
Pathologic Features of Recurrent Vaginal and Vulvar Smooth Muscle Tumors.
| Case | Age | Site | Reported Diagnosis |
Tumor Size (greatest dimension) |
Degree of Atypia |
Morphology | Mitotic Index (per 10 HPFs) |
Atypical Mitotic Figures |
Tumor Cell Necrosis |
Ischemic Necrosis |
Tumor Interface |
Margin Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 42 | 67 | Vagina | Myxoid leiomyosarcoma | 4 cm | Moderate | Myxoid | 6 | Y | Y | Y | Infiltrative | Negative |
| 43 | 44 | Vagina | Leiomyosarcoma | 6 cm | Moderate (pre-neoadjuvant biopsy and resection) | Epithelioid and focally spindled | 18 (biopsy); 2 (resection) | Y | Y (pre-neoadjuvant biopsy and resection) | Y | Infiltrative | Focally positive |
| 44 | 75 | Vagina | Leiomyosarcoma | 3.5 cm | Mild to moderate | Spindled | 24 | Y | Y | N | Circumscribed | Negative |
| 45 | 64 | Vagina | Leiomyosarcoma | 9 cm | Moderate to severe | Spindled | 14 | Y | N | Y | Infiltrative | Negative |
| 47 | 57 | Vagina | Leiomyosarcoma | 5.5 cm | Moderate to focally severe (pre-neoadjuvant biopsy); moderate to severe (resection) | Spindled | 11 (pre-neoadjuvant biopsy); 4 (resection) | Y | Y | Y | Circumscribed | Negative |
| 50 | 26 | Vagina | Leiomyosarcoma | 7 cm | Moderate to severe (pre-neoadjuvant biopsy and resection) | Spindled and myxoid | 15 (pre-neoadjuvant biopsy); 2 (resection) | Y | Y (pre-neoadjuvant biopsy); N (resection) | Y | Infiltrative | Negative |
| 51 | 62 | Vagina | Leiomyosarcoma | 6 cm | Severe | Spindled and focally myxoid | 5 | Y | Y | Y | Infiltrative | Diffusely positive |
| 52 | 78 | Vagina | Leiomyosarcoma | 4.2 cm | Moderate to severe | Spindled | 23 | Y | Y | N | UNK | Diffusely positive |
| 53 | 28 | Vagina | Leiomyosarcoma | 5 cm | Severe | Spindled | 17 | Y | N | N | UNK | Diffusely positive |
| 69 | 72 | Vulva | Leiomyosarcoma | 11 cm | Moderate to severe | Epithelioid and spindled | 8 | N | Y | Y | Infiltrative | Focally positive |
| 70 | 56 | Vulva | Leiomyosarcoma | 13.5 cm | Moderate to severe | Spindled and focally myxoid | 34 | Y | Y | Y | Infiltrative | Focally positive |
| 71 | 68 | Vulva | Leiomyosarcoma | 5.5 cm | Moderate to severe | Spindled and focally myxoid | 23 | Y | Y | Y | Infiltrative | Negative |
UNK (unknown); HPFs (high power fields)
Vaginal SMTs Reported as Spindled Leiomyoma (n=39)
Patient age ranged from 22 to 87 years (mean 50 years). Tumor size was 0.6 cm to 7 cm in greatest dimension (mean 2.6 cm). Simple excision was performed in all 39 cases.
Each tumor had uniformly spindled cells with no more than mild cytologic atypia. Mitotic index ranged from 0 to 7 figures per 10 HPFs and no atypical mitotic figures were seen. Tumor cell necrosis was consistently absent. Ischemic necrosis was noted in 4 tumors (10%). Infiltrative tumor interface was present in 4 tumors (10%), of which 3 had diffusely positive margins and 1 had negative margins. Circumscribed tumor interface was present in 22 cases (56%). Tumor interface could not be assessed in 13 cases (33%).
Tumor recurrence was not identified in any patient, including patients with positive margins (31 cases diffusely positive, 1 case focally positive) and/or infiltration. Follow up was available for 35 patients and ranged from 1 month to 226 months (median 85 months).
Vaginal SMT Reported as Mitotically Active Leiomyoma (n=1)
The patient was a 71 year old woman with a history of cutaneous melanoma whom had a 0.5 cm in greatest dimension polypoid vaginal mass discovered during physical examination. The patient underwent simple excision of the mass.
The spindled cells exhibited no more than mild cytologic atypia, up to 10 mitotic figures per 10 HPFs without atypical figures and no evidence of tumor cell necrosis or ischemic necrosis. Due to diffuse margin involvement, tumor interface could not be assessed.
No evidence of recurrence was identified despite diffusely positive margins. The patient died of complications of melanoma 15 months later.
Vaginal SMT Reported as Leiomyoma with Bizarre Nuclei (n=1)
A 36 year old woman underwent simple excision of a 4.5 cm in greatest dimension vaginal mass.
The spindled cells had moderate to focally severe cytologic atypia without mitotic activity (0 figures) and tumor cell necrosis. Ischemic necrosis was absent. Secondary to diffuse margin involvement, tumor interface could not be assessed.
After 180 months of follow up, recurrence was not reported.
Vaginal SMTs Reported as Spindled Leiomyosarcoma (n=11)
Patient age ranged from 15 to 78 years (mean 53 years). Tumor size varied from 3.5 to 9 cm in greatest dimension (mean 5.9 cm).
All tumors exhibited at least moderate cytologic atypia. One tumor had mild to moderate cytologic atypia, another had purely moderate cytologic atypia and the 9 tumors had moderate to severe or severe cytologic atypia. Spindled morphology was seen in 7 tumors (64%), spindled to myxoid morphology in 3 tumors (27%) and epithelioid to spindled morphology in 1 tumor (9%). The mitotic index ranged from 5 to 32 figures per 10 HPFs with a median of 15 figures per 10 HPFs (pre-neoadjuvant assessment only). Atypical mitotic figures were identified in each tumor. Tumor cell necrosis was present in 7 tumors (64%). Tumor interface was infiltrative in 5 cases (45%), circumscribed in 4 (36%) and unknown in 2 (18%). Six tumors had negative margins and 4 had diffusely positive margins.
Seven patients underwent wide local excision (4 patients received neoadjuvant therapy before excision based on prior biopsy diagnosis of leiomyosarcoma), 3 patients underwent simple excision and 1 had a posterior exenteration. One patient presented with liver metastasis at time of primary surgical intervention. Patients were managed with assorted combinations of adjuvant therapy and surgical resection following recurrence(s). Follow up ranged from 2 to 234 months (median 64 months). A total of 8 patients recurred, either locally (2 patients) or distant sites (6 patients). At last follow up, 4 patients had died of disease, 2 were alive with disease and 5 had no evidence of disease. Additionally, 2 patients with no evidence of disease at last follow up were deceased from an unknown cause.
Vaginal SMT Reported as Myxoid Leiomyosarcoma (n=1)
A 67 year old woman presented with a vaginal mass and underwent simple excision of the lesion. Grossly, the tumor measured 4 cm in greatest dimension.
Microscopically, the tumor cells exhibited moderate cytologic atypia with purely myxoid morphology. The mitotic index was 6 figures per 10 HPFs and atypical figures were seen. Tumor cell necrosis was present. The tumor had an infiltrative interface and excision margins were negative.
The patient experienced multiple pelvic recurrences, the first occurring at 48 months, and eventually developed chest wall metastases. Recurrences were treated by surgical resection, chemotherapy and radiotherapy. The patient died of disease at 150 months.
Vulvar SMTs Reported as Spindled Leiomyoma (n=13)
Patient age ranged from 28 to 77 years (mean 52 years). Tumor size was known in all but 1 tumor and ranged from 0.5 cm to 11.2 cm in greatest dimension (mean 4 cm). Simple excision was performed in 11 cases and wide local excision was performed in 2 cases.
Each tumor uniformly had spindled cells with no more than mild cytologic atypia. Focal myxoid morphology was noted in 3 cases (23%). Mitotic indices varied from 0 to 1 figure per 10 HPFs and none had atypical mitotic figures. Tumor cell necrosis was consistently absent. Ischemic necrosis was found in 2 tumors (15%). Infiltrative tumor interface was present in 3 tumors (23%), of which 2 had diffusely positive margins and 1 had negative margins. Circumscribed tumor interface was present in 8 cases (61%). Tumor interface could not be assessed in 2 cases (17%).
Tumor recurrence was not identified in any patient, including patients with positive margins (8 cases diffusely positive, 1 case focally positive) and/or infiltration. Follow up was available for 9 patients and ranged from 1 month to 137 months (median 12 months).
Vulvar SMTs Reported as STUMP (n=2)
One tumor was reported as spindled STUMP and another was reported as myxoid STUMP.
The spindled STUMP occurred in a 50 year old patient. She presented with a painful right-sided vulvar mass and underwent wide local excision. Grossly, the tumor measured 8.5 cm in greatest dimension.
Microscopically, the tumor consisted of a pure population of spindled cells with moderate to severe cytologic atypia, 1 mitotic figure per 10 HPFs and no tumor cell necrosis. Tumor interface was circumscribed and excision margins were negative. At last follow up 9 months post-op, the patient had no evidence of disease.
A 45 year old patient presented with a vulvar mass and was diagnosed with myxoid STUMP. The tumor was excised by simple excision and measured 6 cm in greatest dimension.
The cells were predominantly myxoid with focal areas of spindled morphology. The degree of cytologic atypia was moderate to severe and 1 mitotic figure per 10 HPFs was identified. Tumor cell necrosis was absent. The tumor was infiltrative, but margins were negative. The patient had no evidence of recurrence at last follow up (19 months).
Vulvar SMTs Reported as Spindled Leiomyosarcoma (n=3)
Patients’ age at diagnosis were 56, 68 and 72 years. All 3 presented with a vulvar mass, 1 of which was a suspected Bartholin cyst. Each patient underwent wide local excision. Gross sizes of the tumors were 5.5 cm, 11 cm and 13.5 cm.
Each tumor demonstrated moderate to severe cytologic atypia. The morphology of the tumors included 1 tumor with a mixture of epithelioid and spindled features while 2 others had spindled to focally myxoid features. The mitotic index was 8, 23 or 34 figures per 10 HPFs for the 3 tumors and atypical mitotic figures were identified in 2 tumors. Tumor cell necrosis was present in all 3 tumors. Each tumor had an infiltrative interface. Two of 3 tumors had focally positive margins and the remaining had negative margins.
Recurrence in each patient occurred at differing intervals: metastasis to lung at 1 month in 1 patient, metastasis to lung at 36 months in another patient and extensive local recurrence of the pelvis at 41 months in the remaining patient. One patient was treated with adjuvant radiotherapy and 2 patients were treated with chemotherapy after recurrence. All 3 patients died of disease (4 months, 36 months and 56 months).
Statistical Analysis of SMTs According to Site-specific Criteria and Uterine SMT Criteria
Results of sensitivity and specificity calculations are summarized in Tables 4 and 5. When vaginal leiomyosarcomas were classified by patient outcome, the sensitivity of site-specific 1979 vaginal criteria was 88.9% (8/9) and uterine criteria was 100%. Likewise, for vulvar leiomyosarcomas classified by patient outcome, the sensitivity of site-specific 1979 vulvar criteria, 1996 vulvar criteria and uterine criteria was 100% for each (3/3).
Table 4.
Sensitivity of Proposed Criteria for Vulvovaginal Leiomyosarcomas According to Clinical Outcome.
| Proposed Criteria | Vagina (n=9) | Vulva (n=3) |
|---|---|---|
| 1979 Vaginal Criteria | Criteria not satisfied for LMS = 1 (11.1%) Criteria satisfied for LMS = 8 (88.9%) |
N/A |
| 1979 Vulvar Criteria | N/A | Criteria satisfied for LMS = 3 (100%) |
| 1996 Vulvar Criteria | N/A | Criteria satisfied for LMS = 3 (100%) |
| Uterine Criteria | Criteria satisfied for LMS = 9 (100%) | Criteria satisfied for LMS = 3 (100%) |
LMS (leiomyosarcoma)
Table 5.
Specificity of Proposed Criteria for Non-malignant Vulvovaginal Smooth Muscle Tumors According to Clinical Outcome.
| Proposed Criteria | Vagina (n=41) | Vulva (n=15) |
|---|---|---|
| 1979 Vaginal Criteria | Criteria not satisfied for LMS = 37 (90.2%) Criteria satisfied for LMS = 4 (9.8%) |
N/A |
| 1979 Vulvar Criteria | N/A | Criteria not satisfied for LMS = 13 (86.7%) Criteria satisfied for at least low grade LMS = 2 (13.3%) |
| 1996 Vulvar Criteria | N/A | Criteria not satisfied for LMS = 14 (93.3%) Criteria satisfied for LMS = 1 (6.7%) |
| Uterine Criteria | Criteria not satisfied for LMS = 41 (100%) | Criteria not satisfied for LMS = 15 (100%) |
LMS (leiomyosarcoma)
Specificity for non-recurrent vulvovaginal SMTs showed greater variability. For vaginal SMTs, site-specific 1979 criteria had a specificity of 90.2% (37/41) and uterine criteria had a specificity of 100% (41/41). For vulvar SMTs, site-specific 1979 criteria had a specificity of 86.7% (13/15), 1996 criteria had a septicity of 93.3% (14/15) and uterine criteria had a specificity of 100% (15/15).
Additionally, a range of gross and morphologic variables were significantly (p<0.05) associated with recurrence among SMTs. Analysis of these features and their relationship to 2 year recurrence-free survival are summarized in Table 6.
Table 6.
Statistical Analysis of Recurrent Smooth Muscle Tumors Among Patients with Clinical Follow Up.
| Variable | Tumors | Any Recurrence | 2 Year Recurrence-free Survival % (95% CI) | p-value |
|---|---|---|---|---|
| Site | 0.4883 | |||
| Vagina | 49 | 9 | 81.9% (69.6%, 94.2%) | |
| Vulva | 14 | 3 | 92.9% (79.4%, 100%) | |
| Tumor Size ≥ 5 cm | 0002 | |||
| No | 45 | 3 | 94.2% (86%, 100%) | |
| Yes | 18 | 9 | 60.3% (34.7%, 86%) | |
| Degree of Atypia | <0001 | |||
| Mild to Moderate | 46 | 1 | 96.4% (89.6%, 100%) | |
| Moderate to Severe | 17 | 11 | 53.4% (27.1%, 79.8%) | |
| Mitotic Index ≥ 10/10 HPFs | <0001 | |||
| No | 51 | 3 | 97.4% (92.5%, 100%) | |
| Yes | 12 | 9 | 26.7% (0%, 57.2%) | |
| Atypical Mitotic Figures | <0001 | |||
| Mitotic Index of Zero | 36 | 0 | 100%* | |
| No | 13 | 1 | 100%* | |
| Yes | 14 | 11 | 36.3% (8.8%, 63.8%) | |
| Tumor Cell Necrosis | <0001 | |||
| No | 52 | 2 | 97.5% (92.7%, 100%) | |
| Yes | 11 | 10 | 31.2% (2.2%, 60.1%) | |
| Ischemic Necrosis | <0001 | |||
| No | 49 | 3 | 94.5% (86.7%, 100%) | |
| Yes | 14 | 9 | 52.6% (24.6%, 80.6%) | |
| Pattern of Infiltration | 0053 | |||
| Circumscribed | 29 | 2 | 85.7% (67.4%, 100%) | |
| Infiltrative | 16 | 8 | 66.1% (41.7%, 90.5%) | |
| Unknown | 18 | 2 | 94.4% (83.9%, 100%) | |
| Pattern of Infiltration (Unknown Excluded) | 0042 | |||
| Circumscribed | 29 | 2 | 85.7% (67.4%, 100%) | |
| Infiltrative | 16 | 8 | 66.1% (41.7%, 90.5%) |
Confidence interval non-estimable due to sparse data
HPFs (high power fields)
Discussion
Our study has some advantages to prior series of vulvovaginal SMTs. First, our cohort of patients was comprised solely of institutional cases which provided us with comprehensive pathologic data for each patient and access to all original H&E slides. Second, we assessed the value of tumor cell necrosis as a feature of malignant potential given its significance in uterine and ovarian SMTs. Third, we had access to detailed clinical history and follow up from institutional medical records and for the majority of patients (follow up median of 5.3 years, 89% of patients). Lastly, our study reviewed, to the best of our knowledge, the largest series of clinically aggressive vulvovaginal SMTs to date.
Similar to the 1979 studies of vulvovaginal SMTs(2, 3), we did not retrospectively reclassify tumors, opting for clinical outcome (local or distant recurrence) to be the standard by which tumors are considered sarcoma. Whatever methodology was used to classify and report the 71 tumors included our study, we did not notice a discordance in diagnosis when linked to patient outcome. All 54 tumors that were labeled as leiomyoma behaved as expected, and even simple excision with diffusely positive margins was sufficient treatment since none recurred. This result supports the continued practice of conservative excision of lesions of low clinical suspicion and/or pose complicated or debilitating removal due to their anatomic location. The 2 tumors in our study classified as STUMP have yet to show evidence of recurrence. Due to the small size of this group and limited follow up for these patients (9 and 19 months), it remains to be seen whether classifying vaginal and vulvar SMTs as STUMP using uterine criteria is associated with a similarly low risk of recurrence as has been reported in their uterine counterparts.
Likewise, whatever threshold was utilized to diagnose a tumor as leiomyosarcoma, 12 of 15 cases diagnosed as leiomyosarcoma recurred, either distantly or locally (Table 3). Distant metastasis occurred in 10 of 12 patients, 8 of whom are dead of disease, 1 of whom is alive with disease and 1 of whom has no evidence of disease. Two patients diagnosed with leiomyosarcoma experienced only local recurrence (cases 43 and 52). Case 43 was a 6 cm vaginal tumor with moderate cytologic atypia and predominantly epithelioid morphology, 18 mitotic figures per 10 HPFs, tumor cell necrosis and infiltration with persistent/recurrent disease in the vagina 2 months after excision. The patient is without disease at last follow up 234 months post-op. Case 52 was a 4.2 cm vaginal tumor with moderate to severe cytologic atypia, spindled morphology, 23 mitoses per 10 HPFs with tumor cell necrosis and unknown tumor interface due to diffusely positive tumor margins. This case is more recent, and has only limited follow up of 2 months. Nevertheless, persistent/recurrent disease at the site of excision was clinically identified 1 month after excision. Both cases were included as recurrent tumors for statistical analysis. Tumors of either mode of recurrence satisfied both site-specific criteria and uterine criteria for designation as leiomyosarcoma with 1 exception. Case 44 was a 3.5 cm tumor with mild to moderate atypia, 24 mitoses per 10 HPFs, tumor cell necrosis and circumscribed tumor interface. Since the tumor lacked significant cytologic atypia and/or infiltration, it did not qualify as leiomyosarcoma according to 1979 vaginal criteria, yet met uterine criteria for malignancy. This patient experienced spread of tumor to the pelvic sidewall 23 months after diagnosis and underwent surgical resection and radiation therapy. She died of an unknown cause at 76 months.
Of the 3 non-recurrent tumors diagnosed as leiomyosarcoma (Nos. 46, 48 and 49), 2 satisfied criteria for leiomyosarcoma by site-specific and uterine criteria and both of these patients had limited follow up (Nos. 46 and 49). The 7 cm tumor from case 46 had significant cytologic atypia, 32 mitoses per 10 HPFs, tumor cell necrosis and infiltration. The patient died of an unknown cause 8 months after diagnosis. Case 49 had a follow up period of 10 months. This patient had a 4.8 cm tumor with moderate to severe atypia, 12 mitoses per 10 HPFs and no evidence of tumor cell necrosis. The remaining non-recurrent tumor classified as leiomyosarcoma (No. 48) would be classified as STUMP according to uterine criteria due to its moderate to severe cytologic atypia, 5 mitotic figures per 10 HPFs and lack of tumor cell necrosis. This last patient was without disease as of 151 months post-op.
Many of the gross and morphologic features that were evaluated demonstrated statistical significance for recurrence risk. The variables of tumor dimension greater than 5 cm, significant cytologic atypia, mitotic index > 10 figures per 10 HPFs, presence of atypical mitotic figures, presence of tumor cell necrosis and pattern of tumor interface each had a p < .05. In the context of these variables, while all versions of site-specific criteria and uterine criteria demonstrated excellent sensitivity for classifying biologic leiomyosarcoma, differences in specificity were evident. Site-specific 1979 criteria for the 41 vaginal SMTs that did not exhibit aggressive behavior classified 4 tumors as leiomyosarcoma (specificity of 90.2%) whereas uterine criteria classified all 41 vaginal tumors as non-sarcoma (specificity of 100%). Of the 15 vulvar SMTs that did not exhibit aggressive behavior, 2 were classified as at least low grade leiomyosarcoma by 1979 vulvar criteria (specificity of 86.7%) and 1 was classified as leiomyosarcoma by site-specific 1996 vulvar criteria (specificity of 93.3%). Uterine criteria classified all 15 tumors as non-sarcoma (specificity of 100%). For both sensitivity and specificity, uterine criteria outperformed site-specific criteria in classification of vulvovaginal SMTs according to patient outcome.
An issue we encountered in proposed site-specific criteria was assessment of tumor interface. Whether due to low clinical concern for malignancy, incidental discovery or need for conservative excision given the relative location of tumor to complex and sensitive anatomic structures, vulvovaginal SMTs were often simply excised (80% of all tumors). Frequently, this resulted in positive margins, and hindered or prevented evaluation of a tumor’s interface as circumscribed or infiltrative. Accordingly, a designation of “unknown” for tumor interface was necessary in 27% of cases. Furthermore, neither circumscription nor infiltration seemed to be a reliable indicator of malignant potential since 4 leiomyosarcomas were circumscribed but metastasized and 6 infiltrative tumors without other concerning features and did not recur. While tumor interface is statistically significantly associated with recurrence in our analysis (p=.0053), it is a difficult feature to reliably evaluate and incorporate into classification of vulvovaginal SMTs. Additionally, our data do not support infiltration as a feature pathognomonic of malignancy in vulvovaginal SMTs.
Comparing the overall incidence of vaginal and vulvar SMTs in our study to the separate cohorts reported by Tavassoli and Norris(2, 3), vaginal SMTs occur more frequently than vulvar SMTs. Our study identified 53 vaginal tumors and 18 vulvar tumors in contrast to their 60 vaginal tumors and 32 vulvar tumors. In general, distinction of vaginal versus vulvar primary tumor can be difficult when pelvic floor musculature is sufficiently distorted. While each patient’s anatomical composition varies, it is our experience that tumors exceeding 10 cm in size become problematic for site assignment. Only 3 of 71 tumors (4%) in our series were larger than 10 cm, and we recorded them as vulvar origin based on clinical interpretation.
It is important to consider other entities in the differential diagnosis that can be confused with vulvovaginal SMTs such as cellular angiofibroma, angiomyofibroblastoma, deep angiomyxoma, malignant melanoma and dermatofibrosarcoma protuberans. Cellular angiofibroma can be confused with cellular SMTs since both show a fascicular growth of spindled cells with interspersed thick-walled blood vessels. However, cellular angiofibroma tends to form shorter fascicles of fibroblastic cells and has a more enriched vasculature of small to medium sized vessels with hyalinized walls(11). Additionally, contrary to SMTs, desmin is usually negative and CD34 is strongly positive in cellular angiofibroma(12). Another benign genital stromal tumor to consider is angiomyofibroblastoma. Angiomyofibroblastoma exhibits characteristic alternating zones of cellularity of cytologically bland spindled to plasmacytoid cells in an myxoedematous to fibrocollagenous extracellular matrix(13). Unlike SMTs, the vessels of angiomyofibroblastoma are small and thin-walled, and myofibroblastic tumor cells have a distinctive perivascular organization. Further, while angiomyofibroblastoma consistently expresses desmin, smooth muscle actins are usually negative(14). When vulvovaginal SMTs become large and markedly myxoid, deep angiomyxoma should be excluded. Deep angiomyxoma is an infiltrative and uniformly hypocellular neoplasm composed of mildly atypical spindled to stellate myofibroblastic cells set in a prominently myxoedematous matrix with medium to large, thick-walled vessels, some of which are hyalinized(15). Although deep angiomyxoma can have collections of smooth muscle cells or myoid bundles adjacent to its vasculature, it does not form fascicles to the extent of SMTs(16). Furthermore, even when myxoid, SMTs are typically more cellular than deep angiomyxoma. Both desmin and smooth muscle actins are frequently expressed by deep angiomyxoma(16). Cutaneous or mucosal melanoma can morphologically mimic a spindled or epithelioid SMT, but identification of a junctional component or expression of S-100 protein, melan-A, tyrosinase or SOX10 is helpful. However, there is immunohistochemical overlap between melanoma and SMTs. Melanoma can express desmin(17) and SMTs can express HMB45(18), but SMTs are not usually positive for other melanocytic markers (at least to the extent expected in melanoma). Dermatofibrosarcoma protuberans, including variants with fibrosarcomatous transformation, can resemble SMTs. Classic dermatofibrosarcoma protuberans has an infiltrative or nodular architecture of mildly atypical spindled fibroblastic cells with a characteristic storiform growth, thin-walled vasculature and collagenous stroma that can become variably myxoid(19). Tumors that have progressed to fibrosarcomatous transformation show a greater degree of cytologic atypia and mitotic activity as well as herringbone growth pattern(20). In diagnostically difficult cases, a panel of CD34, desmin and smooth muscle actins are helpful since dermatofibrosarcoma protuberans consistently expresses CD34, even in fibrosarcomatous transformed examples (although overall expression is decreased in the transformed component), and is negative for desmin and smooth muscle actins (except in unusual variants with focal myoid bundles)(21, 22). Additionally, if molecular genetic or cytogenetic testing is pursued, t(17;22)(q22;q13) which results in COL1A1-PDGFB fusion, is diagnostic of dermatofibrosarcoma protuberans and is not found in SMTs(23).
In summary, we reviewed the clinical and pathologic features of a large cohort of vulvovaginal SMTs with long term follow up and correlated our findings with patient outcome. Although previously proposed site-specific criteria have high sensitivity for identifying SMTs with aggressive behavior, our analysis revealed that uterine criteria were equally as sensitive and more specific than site-specific criteria. Further study of vulvovaginal SMTs classified as STUMP or other leiomyoma variants, such as leiomyoma with bizarre nuclei and mitotically active leiomyoma, is necessary to determine whether the same morphologic thresholds for uterine and ovarian SMTs are valid in tumors of the vulvovaginal region. We recommend use of uterine SMT criteria and nomenclature for evaluation and diagnosis of vulvovaginal SMTs.
Supplementary Material
References
- 1.Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO classification of tumours of female reproductive organs. Lyon, France: IARC Press; 2014. [Google Scholar]
- 2.Tavassoli FA, Norris HJ. Smooth muscle tumors of the vulva. Obstet Gynecol. 1979;53:213–217. [PubMed] [Google Scholar]
- 3.Tavassoli FA, Norris HJ. Smooth muscle tumors of the vagina. Obstet Gynecol. 1979;53:689–693. [PubMed] [Google Scholar]
- 4.Nielsen GP, Rosenberg AE, Koerner FC, et al. Smooth-muscle tumors of the vulva. A clinicopathological study of 25 cases and review of the literature. Am J Surg Pathol. 1996;20:779–793. doi: 10.1097/00000478-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- 6.Croce S, Young RH, Oliva E. Uterine leiomyomas with bizarre nuclei: a clinicopathologic study of 59 cases. Am J Surg Pathol. 2014;38:1330–1339. doi: 10.1097/PAS.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 7.Ip PP, Cheung AN, Clement PB. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009;33:992–1005. doi: 10.1097/PAS.0b013e3181a02d1c. [DOI] [PubMed] [Google Scholar]
- 8.Ly A, Mills AM, McKenney JK, et al. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol. 2013;37:643–649. doi: 10.1097/PAS.0b013e3182893f36. [DOI] [PubMed] [Google Scholar]
- 9.Mills AM, Ly A, Balzer BL, et al. Cell cycle regulatory markers in uterine atypical leiomyoma and leiomyosarcoma: immunohistochemical study of 68 cases with clinical follow-up. Am J Surg Pathol. 2013;37:634–642. doi: 10.1097/PAS.0b013e318287779c. [DOI] [PubMed] [Google Scholar]
- 10.Lerwill MF, Sung R, Oliva E, et al. Smooth muscle tumors of the ovary: a clinicopathologic study of 54 cases emphasizing prognostic criteria, histologic variants, and differential diagnosis. Am J Surg Pathol. 2004;28:1436–1451. doi: 10.1097/01.pas.0000141393.99300.d0. [DOI] [PubMed] [Google Scholar]
- 11.Nucci MR, Granter SR, Fletcher CD. Cellular angiofibroma: a benign neoplasm distinct from angiomyofibroblastoma and spindle cell lipoma. Am J Surg Pathol. 1997;21:636–644. doi: 10.1097/00000478-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Iwasa Y, Fletcher CD. Cellular angiofibroma: clinicopathologic and immunohistochemical analysis of 51 cases. Am J Surg Pathol. 2004;28:1426–1435. doi: 10.1097/01.pas.0000138002.46650.95. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CD, Tsang WY, Fisher C, et al. Angiomyofibroblastoma of the vulva. A benign neoplasm distinct from aggressive angiomyxoma. Am J Surg Pathol. 1992;16:373–382. doi: 10.1097/00000478-199204000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Laskin WB, Fetsch JF, Tavassoli FA. Angiomyofibroblastoma of the female genital tract: analysis of 17 cases including a lipomatous variant. Hum Pathol. 1997;28:1046–1055. doi: 10.1016/s0046-8177(97)90058-7. [DOI] [PubMed] [Google Scholar]
- 15.Steeper TA, Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am J Surg Pathol. 1983;7:463–475. doi: 10.1097/00000478-198307000-00009. [DOI] [PubMed] [Google Scholar]
- 16.van Roggen JF, van Unnik JA, Briaire-de Bruijn IH, et al. Aggressive angiomyxoma: a clinicopathological and immunohistochemical study of 11 cases with long-term follow-up. Virchows Arch. 2005;446:157–163. doi: 10.1007/s00428-004-1135-9. [DOI] [PubMed] [Google Scholar]
- 17.Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033–1042. doi: 10.1038/modpathol.2015.62. [DOI] [PubMed] [Google Scholar]
- 18.Vang R, Kempson RL. Perivascular epithelioid cell tumor (‘PEComa’) of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumors. Am J Surg Pathol. 2002;26:1–13. doi: 10.1097/00000478-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher CD, Evans BJ, MacArtney JC, et al. Dermatofibrosarcoma protuberans: a clinicopathological and immunohistochemical study with a review of the literature. Histopathology. 1985;9:921–938. doi: 10.1111/j.1365-2559.1985.tb02878.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghorbani RP, Malpica A, Ayala AG. Dermatofibrosarcoma protuberans of the vulva: clinicopathologic and immunohistochemical analysis of four cases, one with fibrosarcomatous change, and review of the literature. Int J Gynecol Pathol. 1999;18:366–373. [PubMed] [Google Scholar]
- 21.Calonje E, Fletcher CD. Myoid differentiation in dermatofibrosarcoma protuberans and its fibrosarcomatous variant: clinicopathologic analysis of 5 cases. J Cutan Pathol. 1996;23:30–36. doi: 10.1111/j.1600-0560.1996.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 22.Edelweiss M, Malpica A. Dermatofibrosarcoma protuberans of the vulva: a clinicopathologic and immunohistochemical study of 13 cases. Am J Surg Pathol. 2010;34:393–400. doi: 10.1097/PAS.0b013e3181cf7fc1. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher CDM, Bridge JA, Hogendoorn P, et al. WHO classification of tumours of soft tissue and bone. Lyon, France: IARC Press; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.