Abstract
Prehypertension frequently progresses to hypertension, a condition associated with high morbidity and mortality from cardiovascular diseases and stroke. However, the risk factors for developing hypertension from prehypertension remain poorly understood. We conducted a retrospective cohort study using the data from 3,584 prehypertensive Japanese adults (52.1±11.0 years, 2,081 men) found to be prehypertensive in 2004 and reexamined in 2009. We calculated the cumulative incidences of hypertension over five years, examined risk factors and calculated odds ratios (ORs) for developing hypertension after adjustments for age, sex, body mass index, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease, and serum uric acid levels. The additional analysis evaluated whether serum uric acid (hyperuricemia) constituted an independent risk factor for developing hypertension. The cumulative incidence of hypertension from prehypertension over five years was 25.3%. There were no significant differences between women and men (24.4% vs. 26.0%, p=0.28). The cumulative incidence of hypertension in subjects with hyperuricemia (n=726) was significantly higher than those without hyperuricemia (n=2,858) (30.7% vs. 24.0%, p<0.001). After multivariable adjustments, the risk factors for developing hypertension from prehypertension were age (OR:1.023, p<0.001), women (OR:1.595, p<0.001), higher body mass index (OR:1.051, p<0.001), higher baseline systolic (OR:1.072, p<0.001) and diastolic blood pressure (OR:1.085, p<0.001), and higher serum uric acid (OR:1.149, p<0.001). Increased serum uric acid is a strong risk marker for developing hypertension from prehypertension. Further studies are needed to determine if treatment of hyperuricemia in prehypertensive subjects could impede the onset of hypertension.
Keywords: prehypertension, uric acid, hyperuricemia, risk factor, epidemiology
Introduction
Hyperuricemia (elevated serum uric acid) has emerged as one of the strongest risk factors for the development of prehypertension1, primary hypertension2, 3, and resistant hypertension4. Experimentally raising uric acid in rats also causes hypertension.5 Moreover, pilot clinical studies suggest lowering serum uric acid has been reported to lower blood pressure in hypertensive and prehypertensive children and adults in several studies6–12, although this has not been universally observed.13 This, coupled with associations of uric acid with insulin resistance/diabetes mellitus, stroke, heart disease, and chronic kidney disease, has led to interest in uric acid as a potentially modifiable cardiovascular risk factor.14, 15
If hyperuricemia has a contributory role in cardiovascular disease, then it may make sense to reduce serum uric acid as a means to prevent the development of hypertension and cardiovascular disease. In this regard, a trial to lower serum uric acid in normotensive subjects may require a large sample size since the rate of progression to hypertension over a 5 year period is relatively low. However, if hyperuricemia remains a risk factor in prehypertensive subjects for the development of hypertension, then it may be possible to perform a study with sufficient power since the risk for developing hypertension is substantially higher. We therefore performed a study in a large population with prehypertension to determine the risk for developing hypertension after 5 years, with an especial focus on the role of uric acid. Our study suggests that prehypertensive subjects with an elevated serum uric acid are especially at risk for developing hypertension and therefore may be an ideal target population to determine if urate lowering therapy can prevent the development of hypertension.
Methods
Study design and study subjects
This study is a large-scale, retrospective, single-center cohort study in Japan. The database was from the Center for Preventive Medicine, St. Luke’s International Hospital, Tokyo, Japan. The study analyzed 13,201 Japanese subjects who underwent annual medical examinations at the center in 2004 and were reevaluated five years later. When the subjects had more than one annual examination, we only used the first examination of that year to avoid double counts. Every subject had the same work-up, including medical history, routine physical examination, and blood and urine tests collected in this database and detailed in previous publications.16–19
In the present studies, we included subjects between ages 30 and 85 years old in 2004 whose data were available at both 2004 and 2009. Subjects younger than 30 years of age were excluded due to their very modest risk for hypertension and cardiovascular diseases, while subjects aged 85 years old and above have a substantial risk for death over five-year follow-up. Out of the 13,201 subjects, only 121 subjects were less than 30 years old and 10 subjects were 85 years old and above in 2004. 13,070 subjects were enrolled at the first step, 2,599 subjects with hypertension (1,304 subjects on medication for hypertension) at the baseline were excluded. Finally, we analyzed 6,887 subjects with normotensive (blood pressure <120/80 mmHg, age: 47.7±10.3 years old, 37.1% men) and 3,584 prehypertensive subjects (age: 52.1±11.0 years old, 58.1% men) in 2004 separately (Figure 1).
Figure 1. Flow diagram of study enrollment.
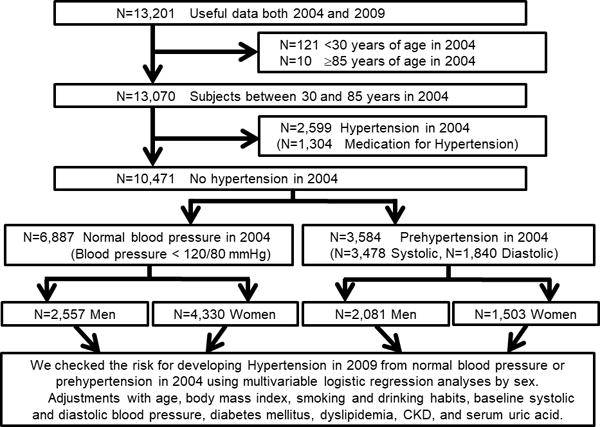
N: number of subjects; BMI: body mass index
We evaluated the cumulative incidence of hypertension from normal blood pressure and prehypertension over five years. Moreover, we evaluated risk factors for developing hypertension from prehypertension over five years and calculated odds ratios (ORs). The distribution of serum uric acid levels differed between men and women, and these analyses were also stratified by sex. We also divided the study population into quartiles according to the serum uric acid levels by sex and compared the risk of developing hypertension from prehypertension between each quartile. Additionally, we compared cumulative incidence of hypertension from prehypertension over 5 years between hyperuricemia and normouricemia after excluding subjects with hypouricemia (serum uric acid levels <3 mg/dL) and/or diabetes mellitus, because hypouricemia sometimes become a risk for hypertension and diabetes mellitus causes decreasing serum uric acid by excretion of urine uric acid. Moreover, to exclude the sampling bias, we also conducted a subanalysis using propensity score matching model to check that hyperuricemia becomes a risk for developing hypertension from prehypertension.
Patient involvement
No patients were involved in setting the research question or outcome measures, nor were they involved in the design and implementation of the study. There are no plans to involve patients in dissemination.
Definition of terms; hypertension, prehypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, and hyperuricemia
Hypertension is defined as a condition when subjects are on current antihypertensive medication and/or systolic blood pressure of more than or equal to 140 mmHg and/or diastolic blood pressure of more than or equal to 90 mmHg.20, 21 blood pressure readings were obtained using an automatic brachial sphygmomanometer like HEM-9020 (OMRON Corporation, Kyoto, Japan), which was upper arm blood pressure measuring and had passed validation. Two blood pressure examinations were taken after the participants were seated and rested quietly for more than five minutes with their feet on the ground and their back supported. The mean systolic and diastolic blood pressure of each of the subjects were calculated from the recorded measurements. Prehypertension is defined as a condition when subjects have a systolic blood pressure between 120 and 139 mmHg or a diastolic blood pressure between 80 and 89 mmHg.21, 22
Diabetes mellitus is defined as current diabetes mellitus on medication use and/or HbA1c (National Glycohemoglobin Standardization Program) more than or equal to 6.5%, according to International Expert Committee.23 Dyslipidemia is defined as current medication use for dyslipidemia and/or low-density lipoprotein cholesterol more than or equal to 140 mg/dl, high-density lipoprotein cholesterol less than 40 mg/dL, and/or triglyceride more than or equal to 150 mg/dL, according to Japan Atherosclerosis Society guidelines.24
Chronic kidney disease is defined as estimated glomerular filtration rate (eGFR) is less than 60 mL/min/1.73m2. We calculated eGFR using the Japanese GFR equation: eGFR (mL/min/1.73m2) = 194 × serum creatinine−1.094 × age−0.287 (×0.739 if woman).25 Hyperuricemia is defined as serum uric acid level >7.0 mg/dL in men and ≥6.0 mg/dL in women.23, 26–30
Statistical analysis
The statistically significant level was set at probability (p) <0.05 (two sided). Data are expressed as mean ± standard derivation or as percent frequency unless otherwise specified. Comparisons between two groups were performed with student t-tests for normally distributed variables, and χ2 analyses for categorical data. The risk factors for developing hypertension from normal blood pressure or prehypertension in the period of over five years were evaluated both by crude models and by multivariable logistic regression models with adjustments for age, sex, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, body mass index (BMI), diabetes mellitus, dyslipidemia, chronic kidney disease, and serum uric acid (or hyperuricemia), and ORs were analyzed in each group. All analyses were stratified by sex. The cumulative incidences of hypertension from normal blood pressure or prehypertension over five years were compared between the subjects with and without hyperuricemia. When we analyzed the quartile of serum uric acid levels, the lowest quartile served as the reference group. When we conducted a propensity matching analysis, we divided the study subjects into between with and without hyperuricemia at the baseline with adjustments of age, BMI, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease at the baseline by propensity score matching model. We checked the main outcome of the development of hypertension from prehypertension over five year. All the statistical analyses were performed using the SPSS Statistics software (IBM SPSS Statistics version 22 for Windows; IBM, New York, USA).
Ethical considerations
We adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from all subjects by a comprehensive agreement method provided by St. Luke’s International Hospital. All data were collected and compiled in a protected computer database. Individual data were anonymous without identifiable personal information. St. Luke’s International Hospital Ethics Committee approved the protocol for this study.
Results
Demographics of this study subjects
Table 1 shows the demographics for men and women with normal blood pressure and prehypertension. Compared to men with normal blood pressure, women were younger, and had a lower BMI, lower systolic and diastolic blood pressure, faster pulse rate, low prevalence of smoking and drinking habits, diabetes mellitus, dyslipidemia, hyperuricemia, and chronic kidney disease, lower white blood cell counts, hemoglobin, eGFR, C-reactive protein, and serum uric acid levels (Table 1. Normal blood pressure). In contrast, women with prehypertension were older than men, but the others are similar tendency compared to normal blood pressure group (Table 1. Prehypertension).
Table 1.
Demographics of study subjects with prehypertension in 2004
| Normal blood pressure | Total | Women | Men | p |
|---|---|---|---|---|
| Number of subjects | 6,887 | 4,330 | 2,557 | |
|
| ||||
| Age | 47.7±10.3 | 46.8±9.9 | 49.2±10.8 | <0.001 |
| Height (cm) | 162.7±8.2 | 158.1±5.5 | 170.5±5.9 | <0.001 |
| Weight (kg) | 57.0±10.5 | 51.3±6.6 | 69.7±8.5 | <0.001 |
| Body mass index (kg/m2) | 21.4±2.7 | 20.5±2.4 | 22.9±2.5 | <0.001 |
| Systolic BP (mmHg) | 105.6±8.8 | 104.0±9.0 | 108.3±7.6 | <0.001 |
| Diastolic BP (mmHg) | 66.0±6.5 | 64.7±6.6 | 68.3±5.7 | <0.001 |
| Pulse rate (bpm) | 71.9±9.5 | 73.4±9.6 | 69.3±8.9 | <0.001 |
| Smoking | 35.1% | 18.3% | 63.4% | <0.001 |
| Drinking habits | 39.2% | 27.9% | 58.4% | <0.001 |
| Diabetes mellitus | 2.3% | 0.9% | 4.6% | <0.001 |
| Dyslipidemia | 28.9% | 20.7% | 42.7% | <0.001 |
| Hyperuricemia | 11.4% | 4.4% | 23.2% | <0.001 |
| Chronic kidney disease | 2.0% | 1.5% | 2.9% | <0.001 |
| White blood cell (/μL) | 5,062±1,376 | 4,831±1,207 | 5,454±1,666 | <0.001 |
| Hemoglobin (g/dL) | 13.3±1.3 | 12.6±1.0 | 14.5±0.9 | <0.001 |
| Total protein (g/dL) | 7.08±0.35 | 7.09±0.36 | 7.08±0.34 | 0.24 |
| eGFR (mL/min/1.73m2) | 87.6±15.2 | 89.6±15.4 | 84.2±14.3 | <0.001 |
| C-reactive protein (mg/dL) | 0.13±0.21 | 0.12±0.18 | 0.15±0.25 | <0.001 |
| Serum uric acid (mg/dL) | 5.00±1.32 | 4.35±0.88 | 6.10±1.19 | <0.001 |
|
| ||||
| Prehypertension | Total | Women | Men | p |
|
| ||||
| Number of subjects | 3,584 | 1,503 | 2,081 | |
|
| ||||
| Age | 52.1±11.0 | 53.0±10.5 | 51.5±11.3 | <0.001 |
| Height (cm) | 164.4±8.9 | 156.6±5.4 | 170.0±6.2 | <0.001 |
| Weight (kg) | 63.3±12.0 | 54.3±8.3 | 69.8±9.8 | <0.001 |
| Body mass index (kg/m2) | 23.3±3.1 | 22.1±3.1 | 24.1±2.8 | <0.001 |
| Systolic BP (mmHg) | 127.7±5.8 | 127.4±5.7 | 127.9±5.8 | 0.007 |
| Diastolic BP (mmHg) | 79.3±5.2 | 78.4±5.3 | 79.9±5.1 | <0.001 |
| Pulse rate (bpm) | 74.8±10.8 | 78.1±11.1 | 72.4±10.0 | <0.001 |
| Smoking | 40.8% | 13.6% | 60.5% | <0.001 |
| Drinking habits | 45.5% | 23.9% | 61.1% | <0.001 |
| Diabetes mellitus | 4.4% | 2.7% | 5.5% | <0.001 |
| Dyslipidemia | 47.5% | 41.7% | 51.7% | <0.001 |
| Hyperuricemia | 20.3% | 8.7% | 28.6% | <0.001 |
| Chronic kidney disease | 3.5% | 2.6% | 4.2% | 0.008 |
| White blood cell (/μL) | 5,298±1,373 | 4,987±1,244 | 5,522±1,418 | <0.001 |
| Hemoglobin (g/dL) | 13.9±1.3 | 12.9±1.1 | 14.7±1.0 | <0.001 |
| Total protein (g/dL) | 7.21±0.35 | 7.09±0.36 | 7.17±0.34 | <0.001 |
| eGFR (mL/min/1.73m2) | 85.1±15.6 | 86.9±15.6 | 83.8±15.5 | <0.001 |
| C-reactive protein (mg/dL) | 0.16±0.32 | 0.14±0.27 | 0.17±0.35 | 0.019 |
| Serum uric acid (mg/dL) | 5.59±1.39 | 4.63±0.95 | 6.29±1.23 | <0.001 |
BP, blood pressure; bpm, beat per minute; p, probability
Data are presented as mean ± standard deviation.
Cumulative incidences of hypertension from normal blood pressure over five years
We first sought to confirm multiple prior studies2 that an elevated serum urate is an independent risk factor in the normotensive population. Specifically, we evaluated 6,887 normotensive Japanese adults (age 30 to 85 years) whose records were available and determined the cumulative incidence of hypertension over a 5 year period. Among this population, the cumulative incidence of hypertension was 2.9%, with a higher incidence in men than women (3.8% vs. 2.4%, p=0.002). Subjects with hyperuricemia (n=783) had significantly higher cumulative incidence of hypertension than those without hyperuricemia (n=6,104) (5.6% vs. 2.6%, p<0.001). After stratification by sex, the higher cumulative incidence of hypertension in normotensive subjects with hyperuricemia was observed both in women (6.9% vs. 2.2%, p<0.001) and men (5.2% vs. 3.3%, p=0.036) (Figure 2A). The threshold level (defined as higher incidence of hypertension than the mean) of serum uric acid for developing hypertension from normal blood pressure was 8.0 mg/dL in men and 5.0 mg/dL in women. The overall relative risk for hyperuricemia for developing hypertension was 2.27, which is in alignment with prior studies.
Figure 2. Cumulative incidences of hypertension from prehypertension over five years.
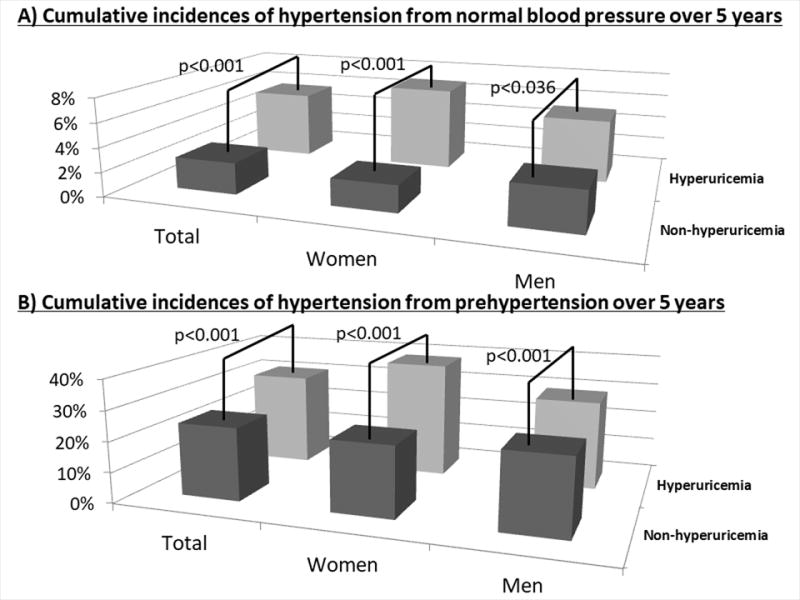
The bars show significant differences in cumulative incidences of hypertension from prehypertension between the subjects with and without hyperuricemia by χ2 analyses.
A) Normal blood pressure
The cumulative incidence of hypertension over five years and the numbers of subjects (n) were 5.6% (n=783) in total with hyperuricemia, 2.6% (n=6,104) in total without hyperuricemia, 6.9% (n=189) in women with hyperuricemia, 2.2% (n=4,141) in women without hyperuricemia, 5.2% (n=594) in men with hyperuricemia, and 3.3% (n=1,963) in men without hyperuricemia.
B) Prehypertension
The cumulative incidence of hypertension over five years and the numbers of subjects (n) were 30.7% (n=726) in total with hyperuricemia, 24.0% (n=2,858) in total without hyperuricemia, 38.2% (n=131) in women with hyperuricemia, 23.1% (n=1,372) in women without hyperuricemia, 29.1% (n=595) in men with hyperuricemia, and 24.8% (n=1,486) in men without hyperuricemia.
Cumulative incidences of hypertension from prehypertension over five years
The cumulative incidence of hypertension from prehypertension over five years was 25.3%. There were no significant differences in the cumulative incidences of hypertension between women and men (24.4% vs. 26.0%, p=0.28). The cumulative incidence of hypertension in subjects with hyperuricemia (n=726) was significantly higher than those without hyperuricemia (n=2,858) (30.7% vs. 24.0%, p<0.001). After stratification by sex, the higher cumulative incidence of hypertension in prehypertensive subjects with hyperuricemia was evident in both women (38.2% vs. 23.1%, p<0.001) and men (29.1% vs. 24.8%, p<0001) (Figure 2B).
Subjects with higher baseline serum uric acid level had higher cumulative incidences of hypertension over five years both in women and men except for hypouricemic women (serum uric acid levels <2.0 mg/dL) (Figure S1B). For women, the risk for hypertension rose rapidly with serum uric acid levels over 5 mg/dl, with 50 percent of women with a serum uric acid of 7 mg/dl developing hypertension and nearly 65 percent of women with a serum uric acid of 8 mg/dl or greater. In contrast, the risk of developing hypertension with increasing serum uric acid levels was less in men, and a level of ≥7 mg/dl carried a risk of about 30% that did not increase significantly at higher levels of serum uric acid. The threshold level of serum uric acid for developing hypertension from prehypertension was 7.0 mg/dL in men and 5.0 mg/dL in women.
Risk factors for developing hypertension from prehypertension over five years
We conducted a multivariable logistic regression analyses and calculated ORs for developing hypertension from prehypertension in each group with adjustments for age, sex, BMI, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease, and serum uric acid (or hyperuricemia). The risk factors for developing hypertension from prehypertension over five years were age (OR per 1 year increased: 1.023; 95% CI, 1.015–1.032), women (OR versus men: 1.595; 95% CI, 1.269–2.005), higher BMI (OR per 1 kg/m2 increased: 1.051; 95% CI 1.021–1.081), higher baseline systolic blood pressure (OR per 1 mmHg increased: 1.072; 95% CI, 1.055–1.089) and diastolic blood pressure (OR per 1 mmHg increased: 1.085; 95% CI, 1.065–1.106), lower pulse rate (OR pre 1 bpm increased: 0.991; 95% CI, 0.983–1.855), and higher serum uric acid (OR pre 1 mg/dL increased: 1.149; 95% CI, 1.066–1.238) (Table 2 Total). When analyses were separated by sex, the results were similar, except that BMI in women and pulse rate in both women and men did not become risks for developing hypertension from prehypertension (Table 2. Men, Women). Moreover, we conducted subanalyses using eGFR, fasting blood glucose and HbA1c instead of chronic kidney disease and diabetes mellitus (Table S1). The results gave similar results as our primary analyses (Table 2. Total).
Table 2.
Risk factors for developing hypertension from prehypertension over five years
| Total | Crude
|
Adjusted*
|
|||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | ||
| Age | per 1 year increased | 1.024 | 1.017–1.031 | <0.001 | 1.023 | 1.015–1.032 | <0.001 |
| Female gender | versus male gender | 0.920 | 0.789–1.072 | 0.283 | 1.595 | 1.269–2.005 | <0.001 |
| Body mass index | per 1 kg/m2 increased | 1.066 | 1.041–1.092 | <0.001 | 1.051 | 1.051–1.081 | <0.001 |
| Smoking | positive versus negative | 1.200 | 1.031–1.397 | 0.019 | 1.103 | 0.914–1.330 | 0.31 |
| Drinking habits | positive versus negative | 1.100 | 0.946–1.279 | 0.216 | 1.075 | 0.897–1.289 | 0.43 |
| Baseline systolic BP | per 1 mmHg increased | 1.115 | 1.100–1.131 | <0.001 | 1.072 | 1.055–1.089 | <0.001 |
| Baseline diastolic BP | per 1 mmHg increased | 1.128 | 1.109–1.147 | <0.001 | 1.085 | 1.065–1.106 | <0.001 |
| Pulse rate | per 1 bpm increased | 0.995 | 0.998–1.002 | 0.15 | 0.991 | 0.983–0.999 | 0.030 |
| Diabetes | positive versus negative | 1.502 | 1.067–2.114 | 0.020 | 1.277 | 0.878–1.855 | 0.20 |
| Dyslipidemia | positive versus negative | 1.098 | 0.944–1.276 | 0.22 | 0.857 | 0.724–1.014 | 0.073 |
| Chronic kidney disease | positive versus negative | 1.047 | 0.699–1.570 | 0.82 | 0.700 | 0.449–1.089 | 0.114 |
| Serum uric acid | per 1 mg/dL increased | 1.125 | 1.066–1.188 | <0.001 | 1.149 | 1.066–1.238 | <0.001 |
|
| |||||||
| Hyperuricemia | positive versus negative | 1.406 | 1.175–1.683 | <0.001 | 1.348 | 1.101–1.650 | 0.004 |
| Women |
Crude
|
Adjusted†
|
|||||
| OR | 95% CI | p | OR | 95% CI | p | ||
|
| |||||||
| Age | per 1 year increased | 1.030 | 1.018–1.042 | <0.001 | 1.028 | 1.014–1.043 | <0.001 |
| Body mass index | per 1 kg/m2 increased | 1.075 | 1.037–1.115 | <0.001 | 1.041 | 0.998–1.086 | 0.064 |
| Smoking | positive versus negative | 1.101 | 0.785–1.543 | 0.58 | 1.039 | 0.718–1.503 | 0.84 |
| Drinking habits | positive versus negative | 1.089 | 0.829–1.431 | 0.541 | 1.256 | 0.918–1.718 | 0.15 |
| Baseline systolic BP | per 1 mmHg increased | 1.125 | 1.100–1.149 | <0.001 | 1.080 | 1.053–1.107 | <0.001 |
| Baseline diastolic BP | per 1 mmHg increased | 1.130 | 1.101–1.160 | <0.001 | 1.085 | 1.054–1.118 | <0.001 |
| Pulse rate | per 1 bpm increased | 0.998 | 0.987–1.008 | 0.66 | 0.993 | 0.981–1.005 | 0.26 |
| Diabetes | positive versus negative | 2.026 | 1.069–3.837 | 0.030 | 1.593 | 0.799–3.176 | 0.186 |
| Dyslipidemia | positive versus negative | 1.227 | 0.968–1.556 | 0.091 | 0.796 | 0.602–1.053 | 0.109 |
| Chronic kidney disease | positive versus negative | 0.671 | 0.294–1.533 | 0.344 | 0.443 | 0.180–1.089 | 0.076 |
| Serum uric acid | per 1 mg/dL increased | 1.386 | 1.224–1.569 | <0.001 | 1.242 | 1.080–1.429 | 0.002 |
|
| |||||||
| Hyperuricemia (≥6.0 mg/dL) | positive versus negative | 2.054 | 1.413–2.987 | <0.001 | 1.513 | 1.003–2.282 | 0.048 |
| Men | Crude | Adjusted† | |||||
| OR | 95% CI | p | OR | 95% CI | P | ||
| Age | per 1 year increased | 1.021 | 1.012–1.030 | <0.001 | 1.02 | 1.010–1.030 | <0.001 |
| Body mass index | per 1 kg/m2 increased | 1.061 | 1.025–1.098 | <0.001 | 1.056 | 1.015–1.099 | 0.007 |
| Smoking | positive versus negative | 1.242 | 1.013–1.521 | 0.037 | 1.133 | 0.910–1.411 | 0.27 |
| Drinking habits | positive versus negative | 1.072 | 0.876–1.311 | 0.50 | 1.002 | 0.804–1.250 | 0.98 |
| Baseline systolic BP | per 1 mmHg increased | 1.109 | 1.089–1.129 | <0.001 | 1.068 | 1.046–1.090 | <0.001 |
| Baseline diastolic BP | per 1 mmHg increased | 1.129 | 1.104–1.154 | <0.001 | 1.085 | 1.058–1.112 | <0.001 |
| Pulse rate | per 1 bpm increased | 0.994 | 0.984–1.004 | 0.23 | 0.990 | 0.979–1.000 | 0.061 |
| Diabetes | positive versus negative | 1.318 | 0.878–1.980 | 0.18 | 1.158 | 0.740–1.812 | 0.52 |
| Dyslipidemia | positive versus negative | 1.005 | 0.826–1.223 | 0.96 | 0.886 | 0.714–1.099 | 0.27 |
| Chronic kidney disease | positive versus negative | 1.224 | 0.765–1.958 | 0.40 | 0.836 | 0.499–1.401 | 0.50 |
| Serum uric acid | per 1 mg/dL increased | 1.085 | 1.002–1.176 | 0.046 | 1.101 | 1.006–1.204 | 0.037 |
|
| |||||||
| Hyperuricemia (>7.0 mg/dL) | positive versus negative | 1.245 | 1.007–1.540 | 0.043 | 1.268 | 1.003–1.602 | 0.047 |
BP, blood pressure; bpm, beat per minute; OR, odds ratio; p, probability
Data adjusted for age, sex, body mass index, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease, and serum uric acid (or hyperuricemia).
Data adjusted for age, body mass index, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease, and serum uric acid (or hyperuricemia).
Serum uric acid as a risk factor for developing hypertension from prehypertension
We calculated each serum uric acid quartile by sex to account for gender differences in serum uric acid levels. In men, subjects in the highest quartile (serum uric acid of ≥7.2 mg/dL) had 1.35-fold (95% CI, 1.023–1.768) higher OR of developing hypertension than those in the lowest quartile (serum uric acid of <5.6 mg/dL). Women in the 3rd quartile (serum uric acid from 4.6 to 5.2 mg/dL) and the highest quartile (serum uric acid of ≥5.2 mg/dL) had 1,77-fold (95% CI, 1.231–2.556) and 2.40-fold (95% CI, 1.693–3.400) higher ORs of developing hypertension than those in the lowest quartile (serum uric acid of <4.0 mg/dL) (Table 3. Crude). After adjustment for age, BMI, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, and chronic kidney disease, these ORs were 1.37 (95% CI: 1.008–1.859) in men, 1.66 (95% CI: 1.120–2.462) in the 3rd quartile and 1.97 (95% CI: 1.338–2.901) in the highest quartile in women compared to the lowest quartile (Table 3. Adjusted).
Table 3.
Relative odds of developing hypertension from prehypertension over five years by serum uric acid quartile
| Crude
|
Adjusted
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Women | N | Cumulative incidence | OR | 95% C.I. | p | OR | 95% C.I. | p |
| 1st quartile (<4.0 mg/dL) | 354 | 16.7% | Reference | Reference | ||||
| 2nd quartile (4.0≤ <4.6 mg/dL) | 386 | 21.5% | 1.370 | 0.946–1.983 | 0.096 | 1.342 | 0.907–1.998 | 0.14 |
| 3rd quartile (4.6≤ <5.2 mg/dL) | 359 | 26.2% | 1.774 | 1.231–2.556 | 0.002 | 1.661 | 1.120–2.462 | 0.012 |
| 4th quartile (5.2≤ mg/dL) | 404 | 32.4% | 2.399 | 1.693–3.400 | <0.001 | 1.970 | 1.338–2.901 | <0.001 |
|
| ||||||||
| Men | ||||||||
| 1st quartile (<5.6 mg/dL) | 550 | 24.4% | Reference | Reference | ||||
| 2nd quartile (5.6≤ <6.4 mg/dL) | 534 | 25.3% | 1.050 | 0.797–1.384 | 0.73 | 1.086 | 0.811–1.454 | 0.85 |
| 3rd quartile (6.4≤ <7.2 mg/dL) | 504 | 24.4% | 1.002 | 0.756–1.328 | 0.99 | 0.970 | 0.715–1.316 | 0.85 |
| 4th quartile (7.2≤ mg/dL) | 493 | 30.2% | 1.345 | 1.023–1.768 | 0.034 | 1.369 | 1.008–1.859 | 0.044 |
N, number of subjects; OR, odds ratio; p, probability
Data adjusted for age, body mass index, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease, and serum uric acid quartile.
Additionally, we also performed an analysis in which we excluded subjects with severe hypouricemia (who largely have a genetic cause31–33 and may have increased risk for hypertension34–36) and diabetes mellitus subjects (who often have low uric acid due to the effects of glycosuria37), as both groups may therefore alter the normal relationship of uric acid with hypertension. This analysis therefore excluded 55 hypouricemic subjects (11 men and 44 women, defined as serum uric acid <3.0 mg/dL) and 156 diabetes mellitus subjects (115 men and 41 women, 1 man and 1 woman had both hyperuricemia and diabetes mellitus) from 3,584 prehypertensive subjects, leaving 3,375 subjects (1,956 men). The cumulative incidence of hypertension from prehypertension over 5 years in subjects with hyperuricemia was higher than those without hyperuricemia (30.4% vs. 23.7%, p<0.001). The difference of cumulative incidence of hypertension between hyperuricemia and normouricemia in women (38.4% vs 22.8%, p<0.001) is much larger than in men (28.7% vs. 24.5%, p=0.054).
Propensity score matching model
We conducted a subanalysis using propensity score matching model. We divided the study subjects into between with and without hyperuricemia at the baseline with adjustments of age, BMI, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease by propensity score matching model (Table 4). The results showed subjects with hyperuricemia had more than 20% higher incidence of hypertension from prehypertension over five years compared to those without hyperuricemia (30.1% vs. 25.0%, p=0.040).
Table 4.
Propensity score matching model
| Normouricemia | Hyperuricemia | p | |
|---|---|---|---|
| Number of subjects | 688 | 688 | |
|
| |||
| Age | 51.3±11.0 | 51.6±11.1 | 0.57 |
| Male gender | 80.7% | 81.1% | 0.84 |
| Height (cm) | 167.7±8.3 | 167.7±8.3 | 0.95 |
| Weight (kg) | 69.2±11.4 | 69.2±11.2 | 0.97 |
| Body mass index (kg/m2) | 24.5±3.0 | 24.5±2.9 | 0.96 |
| Systolic BP (mmHg) | 128.1±5.9 | 128.1±5.9 | 0.96 |
| Diastolic BP (mmHg) | 79.8±5.2 | 79.7±5.2 | 0.94 |
| Pulse rate (bpm) | 73.5±10.6 | 73.6±10.5 | 0.90 |
| Smoking | 54.7% | 55.8% | 0.66 |
| Drinking habits | 55.5% | 56.4% | 0.74 |
| Diabetes mellitus | 2.5% | 3.1% | 0.51 |
| Dyslipidemia | 61.5% | 61.6% | 0.96 |
| Chronic kidney disease | 6.1% | 5.4% | 0.56 |
BP, blood pressure; bpm, beats per minute; p, probability
Data are presented as mean ± standard deviation.
The subjects with hyperuricemia had significantly higher cumulative incidence of hypertension from prehypertension over five years compared to those without hyperuricemia by d χ2 analyses (30.1% vs. 25.0%, p=0.040).
Discussion
The primary goal was to identify the major risk factors for developing hypertension in a large healthy Japanese prehypertensive population. Higher baseline systolic blood pressure, diastolic blood pressure, increasing BMI, female gender, and age emerged as risk factors, as well as hyperuricemia. Over the five-year period, approximately 25 percent of the prehypertensive subjects developed hypertension. However, the striking finding was that, among prehypertensive subjects, a high serum uric acid further increased the risk for hypertension. While in men the presence of hyperuricemia increased the risk to approximately 35 percent, in women the increased risk for hypertension was much higher, with hypertension developing in 1.7-fold in the 3rd serum uric acid quartile and 2.0-fold in the highest quartile compared with the lowest quartile, respectively. These results provide a framework of reference for the design and size of clinical trials aimed to evaluate whether urate lowering therapies can prevent hypertension in prehypertensive subjects.
There is a report that uric acid is an independent predictor for developing prehypertension.1 However, our study is the first to evaluate whether the progression from prehypertension to hypertension is influenced by serum urate levels. Our primary finding was that, while hyperuricemic normotensive subjects had a greater than two-fold increased risk for developing hypertension over 5 years (5.6 percent versus 2.6 percent, respectively), that the risk for developing hypertension in hyperuricemic prehypertensive subjects was much greater, even when compared to normouricemic prehypertensive subjects (30.7 vs 24.0 percent, respectively). Thus, our study suggests that targeting the prehypertensive hyperuricemic individual may be the ideal group to evaluate the role of urate lowering to prevent hypertension.
After multiple adjustments, the risk factors for developing hypertension from prehypertension continued to include age, female gender, higher BMI, higher baseline systolic and diastolic blood pressure, lower pulse rate, and higher serum uric acid, but not smoking and drinking habits, diabetes mellitus, dyslipidemia, or chronic kidney disease. However, after stratification by sex, BMI in women and pulse rate in both women and men did not become risks for developing hypertension from prehypertension. These results suggest the importance of focusing on obesity, serum uric acid levels, as well as blood pressure as modifiable risk factors to delay or impede the development of hypertension. Unexpectedly, smoking and drinking habits, diabetes mellitus, dyslipidemia, and chronic kidney disease were not independent risk factors for developing hypertension in prehypertensive subjects. These factors are known to carry increased risks of cardiovascular disease, but perhaps play an indirect role in the pathogenesis of hypertension in the Japanese population.
Another interesting finding is the greater risk for the development of hypertension carried by hyperuricemia in prehypertensive women than in prehypertensive men. It has been known for some time that for equivalent levels of serum uric acid, women tend to carry a greater risk for hypertension and kidney disease.29, 38, 39 This may relate to differences in expression of urate transporters in nonrenal tissues40, as SLC2A9, for example, is known to be differentially regulated in male and female mice41. Estrogen also decreases serum uric acid levels, and after menopause serum uric acid levels increase in women.34 The other hypothesis is that hyperuricemic women have large urate production with increased xanthine oxidative activity, which generates reactive oxygen products because molecular oxygen is used as an electron acceptor.42 These reactive oxygen products combines nitric oxide and prevent vasodilation.43 Moreover, uric acid itself stimulates proliferation, angiotensin II production, and oxidative stress through the tissue renin-angiotensin system.44 In the results, hyperuricemic women had more risk for hypertension than hyperuricemic men.
While early clinical trials of lowering uric acid in subjects has been promising,6–8, 10, 12 there have been negative studies13, 45, 46, and Mendelian genetic studies have also not been able to show a relationship between serum uric acid and hypertension.47, 48 However, the negative studies have generally been observed in subjects whose blood pressure is already under treatment and the genetic studies are often limited by not considering other influencing conditions, such as diet. Moreover, the EURIKA study showed serum uric acid is a risk factor for resistant hypertension.4 Our study in pre-hypertensive (untreated) subjects underlines the need to design a definitive study to evaluate the role of lowering uric acid in the prevention of hypertension.
Our study has several limitations. First, this study is a retrospective single center study, which may have introduced selection bias. On the other hand, single center studies offer the advantage of the similarity of methodology. Second, this longitudinal study lacks time-to-event data which precluded survival analysis. Third, our study does not rule out that the effect of uric acid might be confounded by subtle changes in kidney function. While we could not show chronic kidney disease or eGFR to be an independent risk factor in this study, this may be limited by the few patients with chronic kidney disease or because including uric acid in the analysis may make kidney function not an independent risk factor due to the potential that they could both be working via the same mechanism. Thus, it remains possible that an elevation of serum uric acid may increase the risk for hypertension in part because a higher serum uric acid is associated with subtle renal dysfunction. Fourth, this study did not account for presence of metabolic syndrome, although we did include various components such as prehypertension, dyslipidemia, and diabetes. While we recognized metabolic syndrome could be an important risk factor for hypertension, including these other variables could act as confounders and hence we avoided performing such an analysis. Fifth, some hypertensive subjects might have white-coat hypertension and some non-hypertensive subjects might have home hypertension because we measured blood pressure only at the center. Ambulatory blood pressure monitoring is the best method for blood pressure evaluation, but it is difficult in practice in the setting of an annual medical examination. Finally, this is an observation study, and intervention studies are needed to clarify whether the treatments for hyperuricemia in prehypertensive subjects are useful to prevent the development of hypertension.
Supplementary Material
Perspectives.
Increased serum uric acid is a strong risk marker for developing hypertension from prehypertension. Therefore, serum uric acid may be an important modifiable risk factor in managing patients with prehypertension. Further research and especially clinical trials are needed to evaluate whether uric acid lowering can impede the development of hypertension from pre-hypertension.
Novelty and Significance.
What is New
This is the first study to identify the major risk factors for developing hypertension in subjects with prehypertension by a large-scale longitudinal design. Moreover, all the analyses were adjusted for age, body mass index, smoking and drinking habits, baseline systolic and diastolic blood pressure, pulse rate, diabetes mellitus, dyslipidemia, chronic kidney disease, and serum uric acid (or hyperuricemia), and these analyses were also stratified by sex.
What is Relevant
The cumulative incidence of hypertension in prehypertensive subjects with hyperuricemia was more than 25% higher than those without hyperuricemia. Moreover, after multivariable adjustments, higher serum uric acid became a strong risk marker for developing hypertension from prehypertension, as well as age, higher body mass index, and higher baseline systolic and diastolic blood pressure.
Summary.
This study compared the relative risk for serum uric acid in predicting the development of hypertension in both the normotensive and prehypertensive population, and showed that the relative risk increased from 5 percent to 30 percent. Moreover, increased serum uric acid became a strong risk marker for developing hypertension from prehypertension both in men and women after multiple adjustments. Thus we identify the hyperuricemic, prehypertensive population as an ideal group for future prevention trials.
Acknowledgments
All the authors of this paper fulfill the criteria of authorship. The authors thank the patients and all staff in Center for Preventive Medicine, St. Luke’s International Hospital, for assistance with data collection. Dr. Kuwabara reports the grant for studying abroad from Federation of National Public Service Personnel Mutual Aid Association in Japan.
Sources of Funding
There is no source of funding for this study
Dr. Johnson has equity with XORT Therapeutics that is developing novel xanthine oxidase inhibitors and with Colorado Research Partners LLC that is developing inhibitors of fructose metabolism. In addition, Dr. Johnson is an inventor on several patents licensed to XORT Therapeutics. (US Patent No 7,799,794, US Patent No. 8,557,831).
Footnotes
Conflict of interest
The remaining authors have nothing to disclose.
References
- 1.Liu L, Gu Y, Li C, et al. Serum uric acid is an independent predictor for developing prehypertension: A population-based prospective cohort study. J Hum Hypertens. 2017;31:116–120. doi: 10.1038/jhh.2016.48. [DOI] [PubMed] [Google Scholar]
- 2.Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2011;63:102–110. doi: 10.1002/acr.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feig DI, Madero M, Jalal DI, Sanchez-Lozada LG, Johnson RJ. Uric acid and the origins of hypertension. J Pediatr. 2013;162:896–902. doi: 10.1016/j.jpeds.2012.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghi C, Tubach F, De Backer G, Dallongeville J, Guallar E, Medina J, Perk J, Roy C, Banegas JR, Rodriguez-Artalejo F, Halcox JP. Lack of control of hypertension in primary cardiovascular disease prevention in europe: Results from the eurika study. Int J Cardiol. 2016;218:83–88. doi: 10.1016/j.ijcard.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 5.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 6.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soletsky B, Feig DI. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension. 2012;60:1148–1156. doi: 10.1161/HYPERTENSIONAHA.112.196980. [DOI] [PubMed] [Google Scholar]
- 8.Higgins P, Walters MR, Murray HM, McArthur K, McConnachie A, Lees KR, Dawson J. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: A randomised controlled trial. Heart. 2014;100:1085–1092. doi: 10.1136/heartjnl-2014-305683. [DOI] [PubMed] [Google Scholar]
- 9.Beattie CJ, Fulton RL, Higgins P, Padmanabhan S, McCallum L, Walters MR, Dominiczak AF, Touyz RM, Dawson J. Allopurinol initiation and change in blood pressure in older adults with hypertension. Hypertension. 2014;64:1102–1107. doi: 10.1161/HYPERTENSIONAHA.114.03953. [DOI] [PubMed] [Google Scholar]
- 10.Madero M, Rodriguez Castellanos FE, Jalal D, Villalobos-Martin M, Salazar J, Vazquez-Rangel A, Johnson RJ, Sanchez-Lozada LG. A pilot study on the impact of a low fructose diet and allopurinol on clinic blood pressure among overweight and prehypertensive subjects: A randomized placebo controlled trial. J Am Soc Hypertens. 2015;9:837–844. doi: 10.1016/j.jash.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, Uz E, Akcay A, Yigitoglu R, Covic A. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–1233. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 12.Kanbay M, Huddam B, Azak A, Solak Y, Kadioglu GK, Kirbas I, Duranay M, Covic A, Johnson RJ. A randomized study of allopurinol on endothelial function and estimated glomerular filtration rate in asymptomatic hyperuricemic subjects with normal renal function. Clin J Am Soc Nephrol. 2011;6:1887–1894. doi: 10.2215/CJN.11451210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borgi L, McMullan C, Wohlhueter A, Curhan GC, Fisher ND, Forman JP. Effect of uric acid-lowering agents on endothelial function: A randomized, double-blind, placebo-controlled trial. Hypertension. 2017;69:243–248. doi: 10.1161/HYPERTENSIONAHA.116.08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borghi C, Rosei EA, Bardin T, Dawson J, Dominiczak A, Kielstein JT, Manolis AJ, Perez-Ruiz F, Mancia G. Serum uric acid and the risk of cardiovascular and renal disease. J Hypertens. 2015;33:1729–1741. doi: 10.1097/HJH.0000000000000701. discussion 1741. [DOI] [PubMed] [Google Scholar]
- 15.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwabara M, Motoki Y, Sato H, Fujii M, Ichiura K, Kuwabara K, Nakamura Y. Low frequency of toothbrushing practices is an independent risk factor for diabetes mellitus in male and dyslipidemia in female: A large-scale, 5-year cohort study in japan. J Cardiol. 2017;70:107–112. doi: 10.1016/j.jjcc.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Kuwabara M, Niwa K, Hisatome I, Nakagawa T, Roncal-Jimenez CA, Andres-Hernando A, Bjornstad P, Jensen T, Sato Y, Milagres T, Garcia G, Ohno M, Lanaspa MA, Johnson RJ. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: Five-year japanese cohort study. Hypertension. 2017;69:1036–1044. doi: 10.1161/HYPERTENSIONAHA.116.08998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwabara M, Bjornstad P, Hisatome I, Niwa K, Roncal-Jimenez CA, Andres-Hernando A, Jensen T, Milagres T, Sato Y, Garcia G, Ohno M, Lanaspa MA, Johnson RJ. Elevated serum uric acid level predicts rapid decline in kidney function. Am J Nephrol. 2017;45:330–337. doi: 10.1159/000464260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwabara M, Hisatome I, Roncal-Jimenez CA, Niwa K, Andres-Hernando A, Jensen T, Bjornstad P, Milagres T, Cicerchi C, Song Z, Garcia G, Sanchez-Lozada LG, Ohno M, Lanaspa MA, Johnson RJ. Increased serum sodium and serum osmolarity are independent risk factors for developing chronic kidney disease; 5 year cohort study. PLoS One. 2017;12:e0169137. doi: 10.1371/journal.pone.0169137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimamoto K, Ando K, Fujita T, et al. The japanese society of hypertension guidelines for the management of hypertension (jsh 2014) Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 21.Black HR, Sica D, Ferdinand K, White WB, American Heart Association E, Arrhythmias Committee of Council on Clinical Cardiology CoCDiYCoC, Stroke Nursing CoFG, Translational B, American College of C. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task force 6: Hypertension: A scientific statement from the american heart association and the american college of cardiology. Circulation. 2015;132:e298–302. doi: 10.1161/CIR.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 22.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart L, Blood Institute Joint National Committee on Prevention DE, Treatment of High Blood P, National High Blood Pressure Education Program Coordinating C Blood Institute Joint National Committee on Prevention DE, Treatment of High Blood P, National High Blood Pressure Education Program Coordinating C. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 23.International Expert C. International expert committee report on the role of the a1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teramoto T, Sasaki J, Ishibashi S, et al. Executive summary of the japan atherosclerosis society (jas) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in japan -2012 version. J Atheroscler Thromb. 2013;20:517–523. doi: 10.5551/jat.15792. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated gfr from serum creatinine in japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Yamanaka H, Japanese Society of G, Nucleic Acid M. Japanese guideline for the management of hyperuricemia and gout: Second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30:1018–1029. doi: 10.1080/15257770.2011.596496. [DOI] [PubMed] [Google Scholar]
- 27.Desai RV, Ahmed MI, Fonarow GC, Filippatos GS, White M, Aban IB, Aronow WS, Ahmed A. Effect of serum insulin on the association between hyperuricemia and incident heart failure. Am J Cardiol. 2010;106:1134–1138. doi: 10.1016/j.amjcard.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin KC, Lin HY, Chou P. Community based epidemiological study on hyperuricemia and gout in kin-hu, kinmen. J Rheumatol. 2000;27:1045–1050. [PubMed] [Google Scholar]
- 29.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing esrd in a screened cohort. Am J Kidney Dis. 2004;44:642–650. [PubMed] [Google Scholar]
- 30.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the us general population: The national health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 31.Sugihara S, Hisatome I, Kuwabara M, Niwa K, Maharani N, Kato M, Ogino K, Hamada T, Ninomiya H, Higashi Y, Ichida K, Yamamoto K. Depletion of uric acid due to slc22a12 (urat1) loss-of-function mutation causes endothelial dysfunction in hypouricemia. Circ J. 2015;79:1125–1132. doi: 10.1253/circj.CJ-14-1267. [DOI] [PubMed] [Google Scholar]
- 32.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 33.Kuwabara M, Niwa K, Ohtahara A, Hamada T, Miyazaki S, Mizuta E, Ogino K, Hisatome I. Prevalence and complications of hypouricemia in a general population: A large-scale cross-sectional study in japan. PLoS One. 2017;12:e0176055. doi: 10.1371/journal.pone.0176055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwabara M. Hyperuricemia, cardiovascular disease, and hypertension. Pulse (Basel) 2016;3:242–252. doi: 10.1159/000443769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The piuma study. Hypertension. 2000;36:1072–1078. doi: 10.1161/01.hyp.36.6.1072. [DOI] [PubMed] [Google Scholar]
- 36.De Leeuw PW, Thijs L, Birkenhager WH, Voyaki SM, Efstratopoulos AD, Fagard RH, Leonetti G, Nachev C, Petrie JC, Rodicio JL, Rosenfeld JJ, Sarti C, Staessen JA, Systolic Hypertension in Europe Trial I Prognostic significance of renal function in elderly patients with isolated systolic hypertension: Results from the syst-eur trial. J Am Soc Nephrol. 2002;13:2213–2222. doi: 10.1097/01.asn.0000027871.86296.92. [DOI] [PubMed] [Google Scholar]
- 37.Shichiri M, Iwamoto H, Shiigai T. Diabetic renal hypouricemia. Arch Intern Med. 1987;147:225–228. [PubMed] [Google Scholar]
- 38.Lee JJ, Ahn J, Hwang J, Han SW, Lee KN, Kim JB, Lee S, Na JO, Lim HE, Kim JW, Rha SW, Park CG, Seo HS, Oh DJ, Kim EJ. Relationship between uric acid and blood pressure in different age groups. Clin Hypertens. 2015;21:14. doi: 10.1186/s40885-015-0022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S. Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res. 2001;24:691–697. doi: 10.1291/hypres.24.691. [DOI] [PubMed] [Google Scholar]
- 40.Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, Endou H, Johnson RJ. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–1795. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- 41.Doring A, Gieger C, Mehta D, et al. Slc2a9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008;40:430–436. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- 42.Borghi C, Cicero AFG. Serum uric acid and cardiometabolic disease: Another brick in the wall? Hypertension. 2017;69:1011–1013. doi: 10.1161/HYPERTENSIONAHA.117.09081. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe E. Uric acid and atrial fibrillation - cause or other association? Circ J. 2012;76:584–585. doi: 10.1253/circj.cj-12-0057. [DOI] [PubMed] [Google Scholar]
- 44.Kuwabara M, Sato Y, Kanbay M, Johnson RJ. Uric acid and left ventricular hypertrophy: A potentially new modifiable target? Am J Hypertens. 2017;30:229–231. doi: 10.1093/ajh/hpw195. [DOI] [PubMed] [Google Scholar]
- 45.McMullan CJ, Borgi L, Fisher N, Curhan G, Forman J. Effect of uric acid lowering on renin-angiotensin-system activation and ambulatory bp: A randomized controlled trial. Clin J Am Soc Nephrol. 2017;12:807–816. doi: 10.2215/CJN.10771016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal MS, Srinivas TR, Mohandas R, Shuster JJ, Wen X, Whidden E, Tantravahi J, Johnson RJ. The effect of the addition of allopurinol on blood pressure control in african americans treated with a thiazide-like diuretic. J Am Soc Hypertens. 2015;9:610–619. e611. doi: 10.1016/j.jash.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottgen A, Albrecht E, Teumer A, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45:145–154. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Q, Kottgen A, Dehghan A, et al. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3:523–530. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


