SUMMARY
Commensal intestinal microbes are collectively beneficial in preventing local tissue injury and augmenting systemic antimicrobial immunity. However, given the near-exclusive focus on bacterial species in establishing these protective benefits, the contributions of other types of commensal microbes remain poorly defined. Here we show that commensal fungi can functionally replace intestinal bacteria, by conferring protection against injury to mucosal tissues and positively calibrating the responsiveness of circulating immune cells. Susceptibility to colitis and influenza A virus infection that occur upon commensal bacteria eradication are efficiently overturned by monocolonization with either Candida albicans or Saccharomyces cerevisiae. The protective benefits of commensal fungi are mediated by mannans, a highly conserved component of fungal cell walls, since intestinal stimulation with this moiety alone overrides disease susceptibility in mice depleted of commensal bacteria. Thus, commensal enteric fungi safeguard local and systemic immunity by providing tonic microbial stimulation that can functionally replace intestinal bacteria.
eTOC BLURB
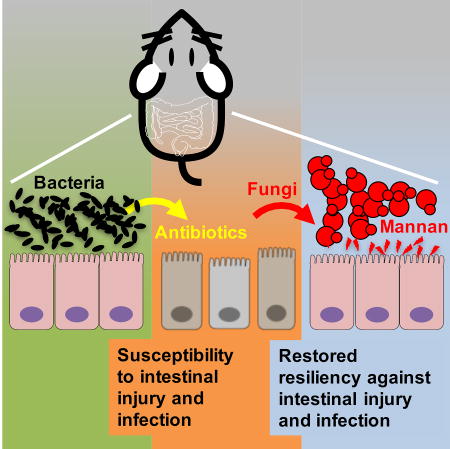
Intestinal colonization is not restricted to bacteria alone, but includes fungi whose symbiotic interactions with the mammalian host remain incompletely defined. Jiang et al. demonstrate that diverse species of fungi can functionally replace enteric bacteria by conferring protection against infectious and inflammatory disorders.
INTRODUCTION
Commensal intestinal microbes collectively play beneficial roles in calibrating immunological responsiveness to improve the outcomes of inflammatory disorders and infections (Chung and Kasper, 2010; Hooper et al., 2012; Round and Mazmanian, 2009). This symbiotic relationship between enteric commensal microbes and the mammalian host has primarily been probed by analyzing intestinal bacteria. For example, oral administration of broad-spectrum antibiotics that deplete enteric bacteria impairs resiliency and survival against dextran sodium sulfate (DSS)-induced colitis (Rakoff-Nahoum et al., 2004). Interestingly, these protective benefits are not confined to local intestinal tissues, but extend distally to enhance responsiveness of immune cells that protect against pathogens that disseminate or replicate in extra-intestinal tissues (Abt et al., 2012; Ichinohe et al., 2011). These collective benefits of commensal bacteria are reproduced by intestinal stimulation using conserved bacterial structural components such as peptidoglycan or LPS (Abt et al., 2012; Clarke et al., 2010; Ichinohe et al., 2011; Rakoff-Nahoum et al., 2004). Thus, commensal bacteria, through their principal molecular components, play important immune-modulatory roles in protecting against disease locally in the intestine and systemically in extra-intestinal tissues.
Importantly however, microbial commensalism is not restricted only to bacteria, but includes viral and fungal species each capable of colonizing mammalian hosts. In this regard, mono-colonization with individual strains of murine norovirus has been recently shown to replace commensal bacteria in promoting resistance against DSS induced colitis and maintaining intestinal homeostasis (Kernbauer et al., 2014). This functional overlap between commensal bacteria and viruses gives rise to fundamental questions regarding whether other classes of intestinal microbes play similar beneficial roles in positively calibrating local and systemic immunity.
Fungi are a ubiquitous component of the mammalian microbiome. Despite being estimated to comprise <1% of commensal microbial species by genomic equivalence (Arumugam et al., 2011; Qin et al., 2010), individual fungi are also of >100-fold increased size compared with bacteria (Underhill and Lliev, 2014). Host sensing of intestinal fungi through the microbial pattern recognition receptor, dectin-1, in mice harboring commensal enteric bacteria has been associated with protective immune responses during DSS induced tissue injury (Iliev et al., 2012). Nonetheless, since intestinal bacteria can independently provide immune modulatory signals, it remains unclear if these beneficial properties attributed to intestinal fungi reflect direct interactions with the mammalian host, or indirect consequences from shifts in the composition of bacterial communities. For example, dectin-1 deficient mice have increased proportions of lactobacilli that can independently ameliorate DSS induced colitis by enhancing colonic accumulation of protective regulatory T cells (Tang et al., 2015). Thus, whether fungal-host interactions are autonomously beneficial for influencing disease outcomes remains undefined.
RESULTS
C. albicans intestinal mono-colonization overrides the protective necessity of commensal bacteria
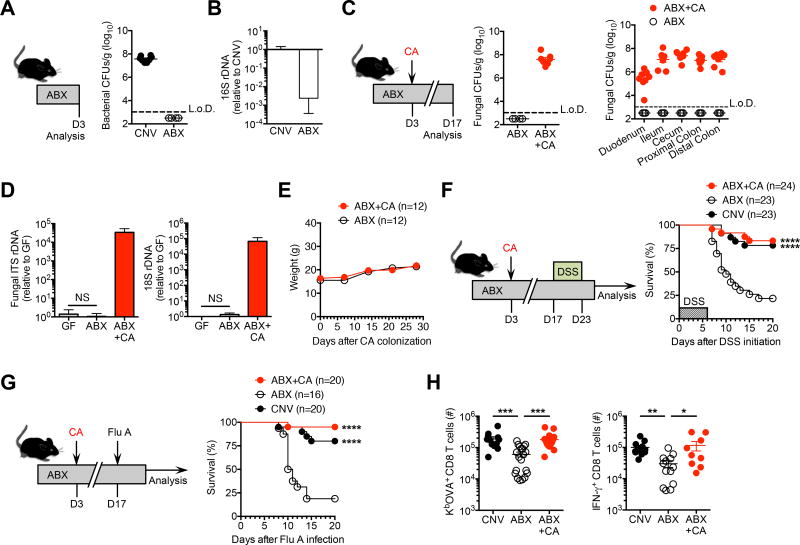
Fungi are naturally impervious to antibiotics that eradicate commensal bacteria, and therefore are poised to bloom during antibiotic treatment (Erb Downward et al., 2013; Fan et al., 2015; Mason et al., 2012). This ability of fungi to rapidly accumulate after antibiotic induced eradication of intestinal bacteria was exploited to investigate whether commensal fungi – in isolation – can functionally recapitulate the protective benefits of enteric bacteria. Supplementing the drinking water with a previously described cocktail of broad-spectrum antibiotics efficiently eliminates recoverable anerobic and aerobic bacteria from the feces of mice housed in our specific pathogen free facility (Figure 1A) (Abt et al., 2012; Jiang et al., 2015). 16S rDNA copies were also sharply reduced (>400-fold) in the feces of antibiotic treated compared with control mice (Figure 1B), in agreement with prior studies demonstrating commercial rodent chow subsequently becomes the major contributor and source of residual bacterial 16S rDNA in mice receiving this antibiotic cocktail (Hill et al., 2010). In turn, Candida albicans efficiently establishes intestinal colonization among mice sustained on this broad-spectrum antibiotic cocktail (Figure 1C). Interestingly, despite high-density intestinal C. albicans colonization, mice gained weight at a comparable tempo compared with antibiotic treated controls not administered C. albicans (Figure 1D). Fungal intestinal colonization also occurs without disrupting the architecture and morphology of local tissues. Crypt height, goblet cell density and paneth cell granularity were each histologically unchanged throughout the small and large intestine among C. albicans colonized mice (Figure S1). Together, these results show C. albicans can replace intestinal bacteria in a commensal fashion without overt harmful consequences.
Figure 1. Candida albicans intestinal mono-colonization bypasses the protective necessity of commensal enteric bacteria.
(A) Recoverable bacterial colony forming units (CFUs) from feces of mice after supplementing the drinking water with an antibiotic cocktail (ABX) containing ampicillin, gentamicin, metronidazole, neomycin, vancomycin, compared with no-antibiotic treated conventional (CNV) controls housed under specific-pathogen free conditions.
(B) Bacterial-specific 16S rDNA qPCR of feces for mice described in panel (A) normalized to conventional (CNV) controls housed under specific-pathogen free conditions.
(C) Recoverable fungal CFUs in the feces (left) and each intestinal segment (right) for mice inoculated with C. albicans (CA) and maintained on ABX treatment for 14 days (ABX+CA), compared to ABX treated controls without CA administration (ABX).
(D) Weight gain after C. albicans inoculation among antibiotic treated mice (ABX+CA) compared with ABX only controls.
(E) Fungal-specific internal transcribed spacer (ITS-1) rDNA (left) and eukaryotic 18S rDNA (right) qPCR of feces for mice described in panel (C) normalized to germ-free (GF) controls. (E) Percent survival after DSS supplementation in the drinking water (for six days) for mice described in panels (A,C).
(F) Percent survival after influenza A PR8-OVA (Flu A) intranasal infection (6 × 104 PFUs) for mice described in panels (A,C).
(G) Total number of Flu A-specific KbOVA tetramer+ CD8 T cells (left), and IFN-γ+ CD8 T cells after in vitro OVA257–264 peptide stimulation (right), from lungs nine days after Flu A infection for mice described in panels (A,C).
*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, by Log-rank (Mantel-Cox) test (F,G) or nonparametric Kruskal-Wallis test with Dunn’s correction (H). Data are representative of at least two independent experiments each with similar results. Bars, mean ± s.e.m. L.o.D., limit of detection.
See also Figures S1–3.
Mice housed in our specific pathogen free facility are devoid of endogenous commensal fungi and noroviruses since the levels of these microbes measured in the feces by nucleic acid qPCR were comparable to the background observed for germ-free mice (Figure 1E and data not shown). We therefore reasoned that any in vivo shifts in disease susceptibility will reflect the biological properties of mono-colonization with this fungal species. Remarkably, C. albicans mono-colonization efficiently overturned mortality induced by DSS among antibiotic treated mice, with overall survival and average time-to-death rebounding to levels comparable to commensal bacteria replete conventional mice (Figure 1F). Commensal C. albicans also protects against DSS induced colonic shortening, intestinal permeability and inflammation compared with antibiotic treated control mice (Figure S2). Importantly, this protection cannot be explained by potential bacteria that persist despite antibiotic treatment since C. albicans mono-association also significantly improved survival of germ-free mice, which are highly susceptible to DSS induced intestinal injury (Kitajima et al., 2001) (Figure S3). Thus, enteric mono-colonization with a single fungal species can override the protective necessity of commensal bacteria in averting local tissue injury.
We next investigated the immune modulatory properties of commensal fungi in extra-intestinal tissues, given the active calibration of systemic immune cell responsiveness previously shown for intestinal bacteria (Abt et al., 2012; Clarke et al., 2010; Ichinohe et al., 2011). For these studies, influenza A virus with restricted tropism to respiratory tissues (Chaturvedi et al., 2015), and natural resistance to the antibiotics used to facilitate C. albicans colonization (Abt et al., 2012; Ichinohe et al., 2011), makes it an ideal pathogen to probe the extra-intestinal impacts of commensal fungi. We found C. albicans mono-colonization efficiently overturned the fatal susceptibility to influenza A virus infection amongst commensal bacteria depleted mice (Figure 1G). Commensal fungi also reversed the blunted accumulation and IFN-γ production of viral-specific CD8 T cells for mice treated with antibiotics, to levels comparable to commensal bacteria replete conventional mice housed under specific pathogen free conditions (Figure 1H). Collectively, these findings highlight C. albicans mono-colonization can replace the systemic benefits of commensal enteric bacteria.
S. cerevisiae intestinal mono-colonization overturns disease susceptibility induced by commensal bacteria depletion
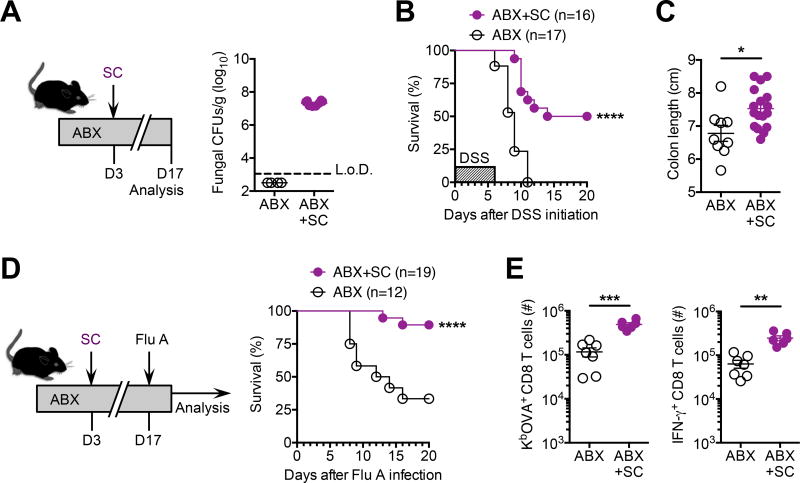
Saccharomyces cerevisiae, another yeast naturally found in the mammalian gut (Hoffmann et al., 2013; Sokol et al., 2017), was used to evaluate whether these protective benefits are shared by other species of commensal fungi. Similar to C. albicans, mice treated with the same cocktail of broad-spectrum antibiotics and then inoculated with S. cerevisiae consistently have high-density of this yeast in their feces (Figure 2A). In turn, the uniform mortality and colonic shortening induced by DSS among commensal bacteria depleted mice was dramatically improved in mice with S. cerevisiae mono-colonization (Figure 2B and 2C). Commensal S. cerevisiae also reduced susceptibility to intranasal influenza A virus infection among antibiotic treated mice (Figure 2D), which coincided with rebounded accumulation and IFN-γ production by protective viral-specific CD8 T cells (Figure 2E). These near identical benefits conferred by S. cerevisiae and C. albicans suggest universal protective properties shared across fungal species capable of mammalian host colonization.
Figure 2. Saccharomyces cerevisiae intestinal mono-colonization overturns disease susceptibility induced by commensal bacteria depletion.
(A) Recoverable fungal colony forming units (CFUs) in the feces of specific-pathogen free mice administered S. cerevisiae (SC) and maintained on drinking water supplemented with an antibiotic cocktail containing ampicillin, gentamicin, metronidazole, neomycin, vancomycin for 14 days (ABX+CA), compared with antibiotic treated controls without SC inoculation (ABX).
(B) Percent survival after DSS supplementation in the drinking water (for six days) for mice described in panel (A).
(C) Colon length after DSS treatment (for six days) for mice described in panel (A).
(D) Percent survival after influenza A PR8-OVA (Flu A) intranasal infection (6 × 104 PFUs) for mice described in panel (A).
(E) Total number of Flu A-specific KbOVA tetramer+ CD8 T cells (left), and IFN-γ+ CD8 T cells after in vitro OVA257–264 peptide stimulation (right), from lungs nine days after Flu A infection for mice described in panel (A).
*P < 0.05, **P < 0.01, ***P < 0.001, by ****P < 0.0001, Log-rank (Mantel-Cox) test (B,D) or unpaired t-test with Welch’s correction (C,E). Data are representative of at least two independent experiments each with similar results. Bars, mean ± s.e.m. L.o.D., limit of detection.
Protective benefits of enteric commensal fungi require persistent colonization
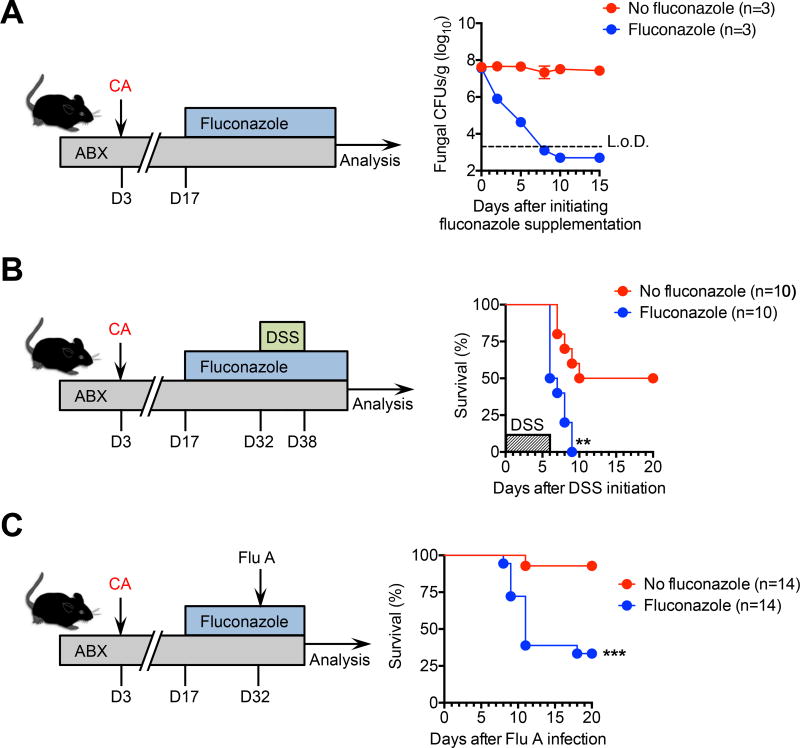
To investigate the durability of protection conferred by commensal fungi, the impacts of fungal eradication with the antimycotic agent, fluconazole, on protection against DSS colitis and respiratory influenza A virus infection were evaluated. We found recoverable fungi in the feces of C. albicans mono-colonized mice rapidly declined to undetectable levels 10 days after adding fluconazole to antibiotic-supplemented drinking water that is consistent with recent studies (Figure 3A) (Underhill and Lliev, 2014; Wheeler et al., 2016). Interestingly, fungal eradication efficiently abolished the improved survival and delayed time-to-death after DSS challenge conferred by C. albicans mono-colonization (Figure 3B). Protection against influenza A was similarly overturned following depletion of commensal fungi (Figure 3C). This necessity for persistent fungal colonization in protection against DSS colitis and influenza A virus infection is consistent with susceptibility to chemical colitis and allergic airway disease unleashed after fluconazole treatment of mice with a diverse repertoire of commensal microbe (Wheeler et al., 2016). However, this requirement for tonic presence of commensal fungi in systemic immune modulation contrast with the concept of “trained immunity” recently shown to be primed by invasive fungi (Cheng et al., 2014), which may reflect contextual differences in sensing fungi as intestinal microbes as opposed to pathogens in sterile tissues after parental injection.
Figure 3. Persistent fungal intestinal colonization is required for maintaining their protective benefits.
(A) Fungal colony forming units (CFUs) in the feces with or without supplementing fluconazole (FLUC) to specific-pathogen free mice previously administered C. albicans (CA) and maintained on drinking water supplemented with an antibiotic cocktail (ABX) containing ampicillin, gentamicin, metronidazole, neomycin, and vancomycin.
(B) Percent survival after DSS supplementation in the drinking water (for six days) for mice described in panel (A).
(C) Percent survival after influenza A PR8-OVA (Flu A) intranasal infection (6 × 104 PFUs) for mice described in panel (A).
*P < 0.05, **P < 0.001, by Log-rank (Mantel-Cox) test. Data are representative of at least two independent experiments each with similar results. Bars, mean ± s.e.m. L.o.D., limit of detection.
Mannans mediate the protective benefits conferred by commensal intestinal fungi
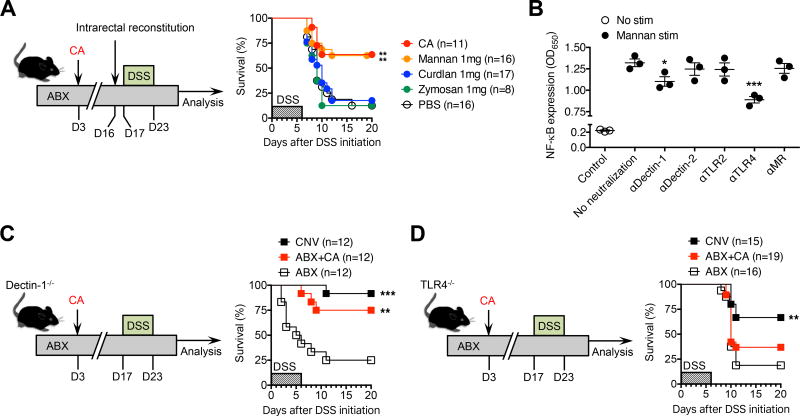
This requirement for persistent intestinal colonization by commensal fungi, combined with the functional overlap between C. albicans and S. cerevisiae, led us to investigate the molecular basis for these fungal-driven immunological shifts. Since mannans are a major cell wall component shared by nearly all fungi (Klis et al., 2006; Ruiz-Herrera et al., 2006), we evaluated if this fungal moiety could functionally replace the protective benefits of intact fungi in the absence of commensal bacteria. Intrarectal administration was employed to mimic the high-density commensal fungi colonization observed in the lower intestinal tract. Remarkably, susceptibility to DSS and influenza A virus infection among antibiotic treated mice were each mitigated by mannan reconstitution, with survival and accumulation of protective immune cells restored to levels comparable to C. albicans colonized controls (Figure 4A and S4). By contrast, intrarectal inoculation with other fungal cell wall components such as curdlan or zymosan failed to improve mortality among antibiotic treated mice (Figure 4A). Thus, the protective benefits of commensal fungi are recapitulated by mannans, a highly conserved fungal cell wall constituent. Fungal derived mannans can stimulate host cells through a variety of microbial pattern recognition receptors. For example, mannan induced TNF-α production is sharply reduced among TLR2 or TLR4-deficient macrophage cells (Jouault et al., 2003; Netea et al., 2006; Tada et al., 2002), and the ability of mannan-rich fungal moieties such as zymosan to activate host cells is blunted in the absence of dectin-1 (Brown et al., 2002; Taylor et al., 2007). The stimulation of other proinflammatory cytokines such as IL-17 by mannan is dependent on the presence of the mannose receptor CD206, and can be further amplified through co-activation of TLR2 (van de Veerdonk et al., 2009). More recently, an essential role has been demonstrated for dectin-2 in mediating responsiveness of bone marrow derived dendritic cells to mannans (Saijo et al., 2010). A potential explanation for these apparently discordant results may be heterogeneity in the mannan preparation or cell activation markers evaluated. Therefore, to investigate the necessity of each host receptor in recognizing our purified, biologically active mannan preparation, we evaluated how neutralizing each molecule impacts mannan stimulation in vitro. For these studies, we used the RAW-Blue 264.7 macrophage NF-κB reporter cell line that are responsive to stimulation through a wide variety classical microbial pattern recognition receptors including toll-like receptors and c-type lectins (i.e., dectin-1, dectin-2, mannose receptor) (Bi et al., 2010; Ying et al., 2015). Interestingly, neutralization of dectin-1 and TLR4 each significantly reduced mannan induced NF-κB. By contrast, mannan stimulated cell activation was not significantly impacted by antibody blockade against TLR2, dectin-2 or the mannose receptor (Figure 4B).
Figure 4. Protective benefits of commensal fungi are mediated by mannans and through TLR4 dependent pathways.
(A) Percent survival after DSS supplementation (for six days) among specific-pathogen free mice maintained on drinking water supplemented with an antibiotic cocktail (ABX) containing ampicillin, gentamicin, metronidazole, neomycin, vancomycin, and administered C. albicans (CA) three days after initiating antibiotic treatment, or intrarectally administered mannan, curdlan, zymosan or saline (PBS) every other day starting one day prior to DSS challenge.
(B) NF-kB expression induced among RAW-blue macrophages after incubation with or without mannan in the presence or absence of the indicated neutralizing antibodies.
(C) Percent survival after DSS supplementation (for six days) among conventional (CNV) dectin-1 deficient mice housed in specific-pathogen free conditions, administered antibiotics (ABX), or C. albicans (CA) inoculated three days after initiating antibiotic treatment (ABX+CA).
(D) Percent survival after DSS supplementation (for six days) among conventional (CNV) TLR4 deficient mice housed in specific-pathogen free conditions, administered antibiotics (ABX), or C. albicans (CA) inoculated three days after initiating antibiotic treatment (ABX+CA).
*P < 0.05, **P < 0.01, ***P < 0.001, by one-way ANOVA with Holm-Sidak’s correction (B) or Log-rank (Mantel-Cox) test (C,D). Data are representative of at least two independent experiments each with similar results. Bars, mean ± s.e.m.
See also Figure S4.
We next addressed the in vivo necessity of dectin-1 and TLR4 in mediating the protective benefits of C. albicans by evaluating the impact of fungal colonization on susceptibility to DSS induced intestinal injury among mice with targeted defects in these molecules. Similar to our findings for isogeneic WT mice on the C57BL/6 background, susceptibility to DSS was sharply increased after antibiotic induced eradication of commensal bacteria in both dectin-1-deficient and TLR4-deficient mice (Figure 4C, 4D). Interestingly, C. albicans mono-colonization efficiently overturned DSS induced mortality among dectin-1 deficient mice (Figure 4C). By contrast, the protective benefits of fungal colonization against DSS were sharply reduced among TLR4 deficient mice, with only marginally improved survival amongst C. albicans colonized mice compared with antibiotic treated controls (P = 0.90) (Figure 4D). Thus, TLR4 plays essential non-redundant roles for conferring the protective benefits of commensal C. albicans that bypass the protective necessity of enteric bacteria through fungal specific mannans.
DISCUSSION
Tonic stimulation by commensal bacteria is increasingly recognized to improve many aspects of host health (Abt et al., 2012; Chung and Kasper, 2010; Hooper et al., 2012; Ichinohe et al., 2011; Rakoff-Nahoum et al., 2004; Round and Mazmanian, 2009). Here we show that susceptibility to intestinal injury and extra-intestinal infection caused by absent bacteria is overturned with fungal colonization. Antifungal administration eliminates the beneficial impacts of commensal fungi, in agreement with the necessity for endogenous enteric fungi to protect against intestinal injury or airway inflammation in the presence of commensal bacteria (Wheeler et al., 2016). These benefits of commensal fungi, when evaluated in the absence of intestinal bacteria, are in sharp contrast to their deleterious roles in exacerbating intestinal injury in dectin-1 deficient mice (Iliev et al., 2012). This discrepancy likely reflects additional stimulation by commensal bacteria or differences in commensal bacteria composition among dectin-1 deficient mice (Tang et al., 2015), or discordant features of the fungal “mycobiome” across institutions (Iliev et al., 2012). By exploiting the absence of detectable endogenous fungi in our facility, we further demonstrate that individual fungal species, in isolation, can take the place of commensal bacteria in positively calibrating local and systemic immunity.
The protective benefits of commensal fungi are mediated by mannan, a highly conserved structural component of fungal cell walls. These results parallel the biological properties of commensal bacteria conferred by their principal molecular constituents. For example, lipotechoic acid or LPS administration, in lieu of live commensal bacteria, can each avert DSS-induced mortality (Rakoff-Nahoum et al., 2004). Likewise, peptidoglycan, LPS, CpG or poly(I:C) reconstitution augments systemic antimicrobial immunity among antibiotic treated mice (Abt et al., 2012; Ichinohe et al., 2011). These benefits of commensal bacteria conferred by their individual structural components, require host microbial pattern recognition receptors as LPS-mediated protection against DSS colitis is abolished in absence of TLR4 (Rakoff-Nahoum et al., 2004). Interestingly however, host recognition of mannans has been demonstrated to occur with considerably more functional redundancy spanning multiple pattern recognition receptors including TLR2, TLR4, dectin-1, dectin-2, DC-SIGN, mincle and the mannose receptor (Hardison and Brown, 2012; Jouault et al., 2003; Netea et al., 2008; Nigou et al., 2008; Saijo et al., 2010). While these conclusions have been shown using complementary models of in vitro stimulation with purified mannans or parenteral infection with invasive fungal pathogens, our data show an essential role for TLR4 in sensing commensal fungi in vivo.
In the broader biological context, we show the protective benefits of commensal microbes are not limited to bacteria and viruses, but also shared by enteric fungi. Host sensing of type I interferons is essential for the immune modulatory properties of commensal murine norovirus (Kernbauer et al., 2014), and macrophage cells show diminished interferon responsiveness in the absence of commensal bacteria (Abt et al., 2012). Considering invasive fungi also induce type I interferons (Biondo et al., 2011; Bourgeois et al., 2011; del Fresno et al., 2013), it is tempting to speculate that diverse commensal microbial types – spanning fungi, bacteria and viruses – all converge to stimulate type I interferon production that potentiates inflammasome-dependent IL-1β and IL-18 activation to positively calibrate beneficial host responses (Fang et al., 2014; Fernandes-Alnemri et al., 2010; Henry et al., 2007; Ichinohe et al., 2011). Interestingly, IFN-β supplementation can blunt C. albicans induced pro-IL-1β and IL-1β release by LPS primed macrophage cells, highlighting that type I interferons can also suppress inflammasome activation causing increased susceptibility to invasive fungal infection (Guarda et al., 2011). Therefore, further investigating how host receptors that sense intestinal microbes can facilitate their protective benefits yet simultaneously suppress their potential for invasive infection may unveil the fundamental dichotomy between commensal and pathogenic microbes.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Sing Sing Way (singsing.way@cchmc.org)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Age- and sex-matched wild-type, Dectin-1−/− and TLR4−/− mice on the C57BL/6 background were purchased from Charles River and housed under specific pathogen-free conditions. Germ-free C57BL/6 mice were maintained in gnotobiotic isolator units, exclusively fed autoclaved chow and water, and routinely monitored to ensure the absence of microbial contamination. All experiments were conducted under Cincinnati Children’s Hospital Animal Care and Use Committee approved protocols.
Fungi
The commonly used C. albicans wild-type laboratory strain SC5314 was a kind gift from Dr. Daniel H. Kaplan (University of Minnesota) (Igyarto et al., 2011). S. cerevisiae strain MYA797 was purchased from the ATCC.
Viruses
Influenza A virus expressing OVA257–264 (SIINFEKL) peptide (OVA-Influenza A) derived from the PR8 H1N1 strain was a kind gift from Drs. Thomas Moran (Ichan School of Medicine at Mount Sinai) and Paul Thomas (Saint Jude Children’s Research Hospital).
METHOD DETAILS
Experimental replication, randomization and blinding
For each experiment, age and sex-matched groups of mice were randomly allocated to experimental groups. Each experiment was independently performed at least twice to ensure reproducibility. Histological scoring was performed by a board-certified veterinarian pathologist (T.A.) in a double blinded fashion. Sample sizes per group in each experiment reflect ≥80% power to detect a Cohen’s effect size of 1.5 with an α error probability of 0.05 (G*Power 3.1).
Antibiotic, antimycotic and DSS treatment
To eradicate commensal bacteria, filter-sterilized drinking water was supplemented with ampicillin (0.5 mg/mL, Sigma), gentamicin (0.5 mg/mL, Sigma), metronidazole (0.5 mg/mL Sigma), neomycin (0.5 mg/mL, Sigma), vancomycin (0.25 mg/mL, MP Biomedicals) and sucralose (4 mg/mL, Sigma) (Abt et al., 2012; Elahi et al., 2013). For depletion of intestinal C. albicans, the antibiotic cocktail was supplemented with fluconazole (0.5 mg/mL, Sigma) (Iliev et al., 2012). To induce intestinal injury, the drinking water of mice was supplemented with DSS (40,000 kDa, Alfa Aesar) for 6 days with or without the antibiotic cocktail, and then received untreated or antibiotic supplemented drinking water for the remainder of the experiment. Wild-type mice were administered 3% DSS. To account for the increased DSS susceptibility that occurs in the absence of dectin-1 or TLR4, or among gnotobiotic germ-free mice (Iliev et al., 2012; Kitajima et al., 2001; Rakoff-Nahoum et al., 2004), 2% DSS was used for comparing the impacts of fungal colonization and/or commensal bacteria eradication in these animals.
Fungal colonization
Fungi were cultured the day prior in yeast extract-peptone-adenine-dextrose media at 30°C (200 rpm). The following day, the culture was washed and suspended in sterile saline. Antibiotic treated mice were administered an oral lavage of 106 fungal CFUs (in 30 µL phosphate-buffered saline) via P200 micropipette (Xin et al., 2014).
Recoverable bacterial or fungal burden
Tissues were sterilely collected, homogenized, and serial dilutions of each homogenate (into PBS) were spread onto brain heart infusion media (Sigma) agar plates. For isolating fungi, brain heart infusion media used for agar plates were supplemented with ampicillin (2.5 µg/mL, Sigma), gentamicin (2.5 µg /mL, Sigma), metronidazole (2.5 µg /mL Sigma), neomycin (2.5 µg/mL, Sigma), vancomycin (1.25 µg /mL, MP Biomedicals). Colony forming units were enumerated after incubation for 24 hours at 37°C.
DNA/RNA isolation and qPCR
Bacterial DNA was isolated using the QIAamp DNA stool mini kit (Qiagen) according to the manufacturer’s instructions. For isolating fungal DNA, individual fecal pellets were suspended in 50 mM Tris buffer (pH 7.5) supplemented with 1 mM EDTA, 0.2% β-mercaptoethanol and 1000 units/ml of lyticase (Sigma), incubated at 37°C for 30 minutes to disrupt fungal cells as described (Iliev et al., 2012) prior to processing through the QIAamp DNA stool mini kit (Qiagen). Viral RNA was extracted with the QIAamp Viral RNA Mini Kit (QIAGEN) according to manufacturer’s instructions. qPCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems) using the following primers: Bacterial 16S, Forward 5’-ACTCCTACGGGAGGCAGCAGT-3’, Reverse 5’-ATTACCGCGGCTGCTGGC-3’; Fungal ITS1-2, Forward 5’-CTTGGTCATTTAGAGGAAGTAA-3, Reverse 5’-GCTGCGTTCTTCATCGATGC-3’; Eukaryotic 18S, Forward 5’ ATTGGAGGGCAAGTCTGGTG-3’, Reverse 5’-CCGATCCCTAGTCGGCATAG-3’; MNV RdRp, Forward 5’-CCAAAGTGGGAT AGAAATGGTAGTC-3’, Reverse 5’-TCACTCATCCTCATTCACAAAGACT-3’.
Intestinal permeability
Fasting was initiated and maintained throughout the experiment starting 2 hours prior to intragastric gavage with 0.6 mg/g body weight of FITC-labeled dextran solution (from 100 mg/mL, FD4, Sigma). After 4 hours, blood from the retro-orbital sinus was collected in heparinized tubes and serum analysis for FITC concentration was performed with a fluorescence spectrophotometer (Synergy HTX, Biotek) at an excitation wavelength of 485 nm and emission wavelength of 528 nm. Standard curves were obtained by diluting FITC–dextran in saline.
Influenza A infection
OVA-Influenza A virus was grown and titered in Madin-Darby canine kidney epithelial cell monolayers, and stored at −70°C. For infection, individual virus aliquots were thawed, diluted in PBS, and administered intranasally (6 × 104 PFUs in 30 µL) to mice anesthetized with xylazine and ketamine (Ichinohe et al., 2011).
Microscopy
Hematoxylin and eosin (H&E) or alcian blue/periodic acid-Schiff (AB/PAS) staining of intestinal tissue was performed by the Pathology Core at Cincinnati Children’s Hospital. Sections were imaged on a Nikon Eclipse 80i microscope. Slides were analyzed using ImageJ software. Villi width was measured where the base of the villi meets the crypt; at least 50 villi per mouse were measured for villi width. Paneth cell granules were counted in at least 30 crypts per mouse (Kernbauer et al., 2014). Mean values were calculated for each mouse and used as individual data points. The histological scoring for DSS treated mice was graded on a severity scale of 1–5 for epithelial ulceration, inflammatory cell infiltration, and edema.
Isolation of lymphocytes from lung parenchyma
Lungs were minced into small pieces with a razor and digested with collagenase D (1 mg/mL, Sigma), DNAse (0.1 mg/mL, Sigma) in DMEM (Gibco) supplemented with 10% (vol/vol) FBS, 1% (vol/vol) L-glutamine (Cellgro), 1% (vol/vol) penicillin-streptomycin (Cellgro) and 10 mM HEPES (Cellgro) for 60 minutes (37°C, 200 rpm), and then mashed through a 70 µm filter. Residual red blood cells were lysed with hypertonic solution (10 mM HKCO3, 16mM NH4Cl, pH 7.3) prior to tetramer staining or cytokine stimulation.
Tetramer staining and flow cytometry
Single cell suspensions from the lung or mediastinal lymph node were incubated with brilliant violet 421-conjugated OVA257–264:H2-Kb tetramer (60 minutes, 25°C) prior to staining with the following fluorophore-conjugated antibodies purchased from eBioscience: FITC anti-mouse CD4 (clone GK1.5), APC anti-mouse CD8α (clone 53-6.7), PE-Cy5 anti-mouse CD11b (clone M1/70), PE-Cy5 anti-mouse CD11c (clone N418), PE-Cy5 anti-mouse F4/80 (clone BM8), PE-Cy5 anti-mouse B220 (clone RA3-6B2), eFluor 450 anti-mouse IFN-γ (clone XMG1.2). Samples were acquired on a BD FACSCanto and analyzed with FlowJo software (Treestar). OVA257–264:H-2Kb specific CD8 T cells were gated on lymphocytes, single cells, B cell and myeloid (B220, CD11b, CD11c, F4/80) negative, CD8+CD4− tetramer positive cells.
Cytokine production
Single cell suspensions from the lung were stimulated with 50 µM OVA257–264 peptide in media supplemented with BD GolgiPlug (BD Biosciences) according to manufacturer’s instructions for 4–5 hours at 37°C.
Rectal inoculation with fungal cell wall moieties
Mice were intrarectally administered the indicated dosage of mannan (Sigma), curdlan (Wako) or zymosan (Sigma) suspended in 50 µl saline after anesthetization with xylazine and ketamine beginning one day prior to DSS or influenza A challenge, and re-administered every other day thereafter.
RAW-Blue stimulation and quantification of NF-κB activity
RAW-Blue cells (InvivoGen) cells are derived from the murine RAW 264.7 macrophages with chromosomal integration of a secreted embryonic alkaline phosphatase reporter construct induced by NF-κB. 5 × 104 RAW-Blue cells were seeded 24 hours prior to addition of the indicated blocking antibody. One hour thereafter, mannan (Sigma) was added at a final concentration of 500 µg/mL. Supernatants were collected after 24 hours to quantify NF-κB activity by colorimetric assay using QUANTI-Blue reagent (InvivoGen).
QUANTIFICATION AND STATISTICAL ANALYSIS
The number (n) of individual animals used per group are described in each individual figure panel, or shown by individual data points that represents the results from an individual animal (Figures 1, 2, S1, S2, S3 and S4) or individual well for cell stimulation assays (Figure 4). The number of replicate experiments are described in each figure legend. All statistics and data distribution analysis were performed with Prism (GraphPad). The unpaired two-tailed Student’s t-test with Welch’s correction was used to compare differences between two groups (Ruxton, 2006). The non-parametric Kruskal-Wallis test with Dunn’s correction, one-way ANOVA with Holm-Sidak’s correction or Mann-Whitney U test was used to evaluate experiments containing more than two groups depending on the distribution pattern of the data. Survival curves were analyzed by the Log-rank (Mantel-Cox) test. The upper threshold for statistical significance for all experiments was set at P < 0.05.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| FITC anti-mouse CD4 (clone GK1.5) | eBioscience | Cat#11-0041-85 |
| APC anti-mouse CD8α (clone 53-6.7) | eBioscience | Cat#17-0081-83 |
| PE-Cy5 anti-mouse CD11b (clone M1/70) | eBioscience | Cat#15-0112-83 |
| PE-Cy5 anti-mouse CD11c (clone N418) | eBioscience | Cat#15-0114-82 |
| PE-Cy5 anti-mouse F4/80 (clone BM8) | eBioscience | Cat#15-4801-82 |
| PE-Cy5 anti-mouse B220 (clone RA3-6B2) | eBioscience | Cat#15-0452-83 |
| eFluor 450 anti-mouse IFN-γ (clone XMG1.2) | eBioscience | Cat#48-7311-82 |
| Anti-mouse Dectin-2 (clone D2.11E4) | Thermo Fisher Scientific | Cat#MA1-82675 |
| Anti-mouse TLR4/MD2 (clone MTS510) | Hycult Biotech | Cat#HM1029-FS |
| Anti-mouse Dectin-1 (clone R1-8g7) | InvivoGen | Cat#mabg-mdect |
| Anti-mouse TLR2 (clone C9A12) | InvivoGen | Cat#mabg-mtlr2 |
| Anti-mouse Mannose Receptor (clone 15-2) | Abcam | Cat#ab8918 |
| Fungal and Virus Strains | ||
| Candida albicans SC5314 | (Igyarto et al., 2011) | N/A |
| Saccharomyces cerevisiae Sb49 | ATCC | ATCC MYA797 |
| Influenza A H1N1 strain PR8 | (Moltedo et al., 2009) | N/A |
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Ampicillin | Sigma-Aldrich | Cat#A0166-100G |
| Gentamicin | Sigma-Aldrich | Cat#G3632-25G |
| Metronidazole | Sigma-Aldrich | Cat#M3761-100G |
| Neomycin | Sigma-Aldrich | Cat#N6386-100G |
| Vancomycin | MP Biomedical | Cat#0219554005-5g |
| Sucralose | Sigma-Aldrich | Cat#69293-100G |
| Fluconazole | Sigma-Aldrich | Cat#PHR1160-1G |
| FITC-dextran | Sigma-Aldrich | Cat#FD4-1G |
| Mannan | Sigma-Aldrich | Cat#M7504-5G |
| Curdlan | Wako Chemicals | Cat#034-09901 |
| Zymosan | Sigma-Aldrich | Cat#Z4250-5G |
| Dehydrated Culture Media: Brain Heart Infusion | Thermo Fisher Scientific | Cat#B11060 |
| QUANTI-Blue | InvivoGen | Cat#raw-sp |
| OVA257–264 peptide | United Biochemical Research, Inc. | N/A |
| BD GolgiPlug (Brefeldin A solution) | BD Biosciences | Cat#555029 |
| Lyticase | Sigma-Aldrich | Cat#L4025-100KU |
| Critical Commercial Assays | ||
| QIAamp DNA Stool Mini Kit | QIAGEN | Cat#51504 |
| QIAamp Viral RNA Mini Kit | QIAGEN | Cat#52904 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Raw-Blue Macrophage Cells | InvivoGen | Cat#raw-sp |
| Madin-Darby canine kidney Cells (line MDCK.2) | American Type Culture Collection | CRL-2936 |
| Experimental Models: Organisms/Strains | ||
| Oligonucleotides | ||
| Bacterial 16S, F: 5’-ACTCCTACGGGAGGCAGCAGT-3’; R: 5’-ATTACCGCGGCTGCTGGC-3’ | Thermo Fisher Scientific | N/A |
| Fungal ITS-1, F: 5’-CTTGGTCATTTAGAGGAAGTAA-3’; R: 5’-GCTGCGTTCTTCATCGATGC-3’ | Thermo Fisher Scientific | N/A |
| Eukaryotic 18S, F: 5’-ATTGGAGGGCAAGTCTGGTG-3’; R: 5’- CCGATCCCTAGTCGGCATAG-3’ | Thermo Fisher Scientific | N/A |
| MNV RdRp, F: 5’-CCAAAGTGGGATAGAAATGGTAGTC-3’, R: 5’-TCACTCATCCTCATTCACAAAGACT-3’ | Thermo Fisher Scientific | N/A |
| Recombinant DNA | ||
| Software and Algorithms | ||
| Prism 6.07 | GraphPad | https://www.graphpad.com/ |
| FlowJo 9.9.6 | Treestar | https://www.flowjo.com/solutions/flowjo |
| G*Power 3.1 | G*Power | http://gpower.software.informer.com/3.1/ |
| Other | ||
| OVA257–264:H2-Kb Tetramer | NIH Tetramer Core | N/A |
Supplementary Material
HIGHLIGHTS.
Commensal fungi functionally replace intestinal bacteria in mitigating tissue injury
Commensal fungi positively calibrate the activation of protective CD8 T cells
Protective benefits of commensal fungi require persistent intestinal colonization
Fungal cell-wall mannans recapitulate the protective benefits of commensal fung
Acknowledgments
We thank all members of the Deshmukh lab, Haslam lab, and Qualls lab for their useful comments and suggestions. We also thank the NIH Tetramer Core Facility for providing OVA257–264:H2-Kb MHC class I tetramer and monomers, Drs. Jason Jiang and Christina Quigley for providing expertise on murine norovirus. This work was supported by NIH Grants F30-DK107199 and T32-GM63483 (T.T.J.), K08-DK093784 and Crohn’s and Colitis Foundation of America/Janssen/AGA (T.A.), R21-AI123089, R21-AI128932 and DP1-AI131080 (S.S.W.). This work was also supported by NIH grant P30-DK078392 Pathology Core of the Digestive Diseases Research Core Center in Cincinnati.” T.A. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and is a Pew Scholar in the Biomedical Sciences, supported by the Pew Charitable Trust. S.S.W. is supported by the HHMI Faculty Scholars program and an Investigator in the Pathogenesis Award from the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
All authors participated in the design or execution of the experiments. T.T.J and S.S.W. wrote the manuscript with editorial input from all the authors.
None of the authors of this manuscript have a financial interest related to this work.
References
- Abt MC, Osborne LC, Monticelli LA, Doering TA, Alenghat T, Sonnenberg GF, Paley MA, Antenus M, Williams KL, Erikson J, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi L, Gojestani S, Wu W, Hsu Y-MS, Zhu J, Ariizumi K, Lin X. CARD9 mediates dectin-2-induced IκBα kinase ubiquitination leading to activation of NF-κB in response to stimulation by the hyphal form of Candida albicans. Journal of Biological Chemistry. 2010;285:25969–25977. doi: 10.1074/jbc.M110.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondo C, Signorino G, Costa A, Midiri A, Gerace E, Galbo R, Bellantoni A, Malara A, Beninati C, Teti G, Mancuso G. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol. 2011;41:1969–1979. doi: 10.1002/eji.201141490. [DOI] [PubMed] [Google Scholar]
- Bourgeois C, Majer O, Frohner IE, Lesiak-Markowicz I, Hildering KS, Glaser W, Stockinger S, Decker T, Akira S, Muller M, Kuchler K. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J Immunol. 2011;186:3104–3112. doi: 10.4049/jimmunol.1002599. [DOI] [PubMed] [Google Scholar]
- Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major β-glucan receptor on macrophages. Journal of Experimental medicine. 2002;196:407–412. doi: 10.1084/jem.20020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi V, Ertelt JM, Jiang TT, Kinder JM, Xin L, Owens KJ, Jones HN, Way SS. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J Clin Invest. 2015;125:1713–1725. doi: 10.1172/JCI78578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-C, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A. mTOR-and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H, Kasper DL. Microbiota-stimulated immune mechanisms to maintain gut homeostasis. Current opinion in immunology. 2010;22:455–460. doi: 10.1016/j.coi.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Fresno C, Soulat D, Roth S, Blazek K, Udalova I, Sancho D, Ruland J, Ardavín C. Interferon-β production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans. Immunity. 2013;38:1176–1186. doi: 10.1016/j.immuni.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb Downward JR, Falkowski NR, Mason KL, Muraglia R, Huffnagle GB. Modulation of post-antibiotic bacterial community reassembly and host response by Candida albicans. Sci Rep. 2013;3:2191. doi: 10.1038/srep02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D, Coughlin LA, Neubauer MM, Kim J, Kim MS, Zhan X, Simms-Waldrip TR, Xie Y, Hooper LV, Koh AY. Activation of HIF-1alpha and LL-37 by commensal bacteria inhibits Candida albicans colonization. Nat Med. 2015;21:808–814. doi: 10.1038/nm.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Hara H, Sakai S, Hernandez-Cuellar E, Mitsuyama M, Kawamura I, Tsuchiya K. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect Immun. 2014;82:2310–2317. doi: 10.1128/IAI.01572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu J-W, Juliana C, Solorzano L, Kang S, Wu J, Datta P, McCormick M, Huang L, McDermott E. The AIM2 inflammasome is critical for innate immunity against Francisella tularensis. Nature Immunology. 2010;11:385. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, Tschopp J. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. Journal of Experimental Medicine. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal immunology. 2010;3:148. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, Wu GD, Lewis JD, Bushman FD. Archaea and fungi of the human gut microbiome: correlations with diet and bacterial residents. PLoS One. 2013;8:e66019. doi: 10.1371/journal.pone.0066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang TT, Chaturvedi V, Ertelt JM, Xin L, Clark DR, Kinder JM, Way SS. Commensal enteric bacteria lipopolysaccharide impairs host defense against disseminated Candida albicans fungal infection. Mucosal Immunol. 2015;8:886–895. doi: 10.1038/mi.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, Akira S, Poulain D. Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis. 2003;188:165–172. doi: 10.1086/375784. [DOI] [PubMed] [Google Scholar]
- Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516:94–98. doi: 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S, Morimoto M, Sagara E, Shimizu C, Ikeda Y. Dextran sodium sulfate-induced colitis in germ-free IQI/Jic mice. Experimental animals. 2001;50:387–395. doi: 10.1538/expanim.50.387. [DOI] [PubMed] [Google Scholar]
- Klis FM, Boorsma A, De Groot PW. Cell wall construction in Saccharomyces cerevisiae. Yeast. 2006;23:185–202. doi: 10.1002/yea.1349. [DOI] [PubMed] [Google Scholar]
- Mason KL, Erb Downward JR, Mason KD, Falkowski NR, Eaton KA, Kao JY, Young VB, Huffnagle GB. Candida albicans and bacterial microbiota interactions in the cecum during recolonization following broad-spectrum antibiotic therapy. Infect Immun. 2012;80:3371–3380. doi: 10.1128/IAI.00449-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltedo B, Lopez CB, Pazos M, Becker MI, Hermesh T, Moran TM. Cutting edge: stealth influenza virus replication precedes the initiation of adaptive immunity. J Immunol. 2009;183:3569–3573. doi: 10.4049/jimmunol.0900091. [DOI] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008;6:67–78. doi: 10.1038/nrmicro1815. [DOI] [PubMed] [Google Scholar]
- Netea MG, Gow NA, Munro CA, Bates S, Collins C, Ferwerda G, Hobson RP, Bertram G, Hughes HB, Jansen T. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. Journal of Clinical Investigation. 2006;116:1642. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigou J, Vasselon T, Ray A, Constant P, Gilleron M, Besra GS, Sutcliffe I, Tiraby G, Puzo G. Mannan chain length controls lipoglycans signaling via and binding to TLR2. J Immunol. 2008;180:6696–6702. doi: 10.4049/jimmunol.180.10.6696. [DOI] [PubMed] [Google Scholar]
- Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J, Elorza MV, Valentin E, Sentandreu R. Molecular organization of the cell wall of Candida albicans and its relation to pathogenicity. FEMS Yeast Res. 2006;6:14–29. doi: 10.1111/j.1567-1364.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- Ruxton GD. The unequal variance t-test is an underused alternative to Student's t-test and the Mann-Whitney U test. Behavioral Ecology. 2006;17:688–690. [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung S-h. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, Ohno N, Tamura H, Shibata Ki, Akashi S. Saccharomyces cerevisiae-and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14-and Toll-like receptor 4-dependent manner. Microbiology and immunology. 2002;46:503–512. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Tang C, Kamiya T, Liu Y, Kadoki M, Kakuta S, Oshima K, Hattori M, Takeshita K, Kanai T, Saijo S, et al. Inhibition of Dectin-1 Signaling Ameliorates Colitis by Inducing Lactobacillus-Mediated Regulatory T Cell Expansion in the Intestine. Cell Host Microbe. 2015;18:183–197. doi: 10.1016/j.chom.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nature immunology. 2007;8:31. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill DM, Lliev LD. The mycobiota: interactions between commensal fungi and the host immune system. Nature Reviews Immunology. 2014;14:405–416. doi: 10.1038/nri3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng S-C, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell host & microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Wheeler ML, Limon JJ, Bar AS, Leal CA, Gargus M, Tang J, Brown J, Funari VA, Wang HL, Crother TR, et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe. 2016;19:865–873. doi: 10.1016/j.chom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Jiang TT, Chaturvedi V, Kinder JM, Ertelt JM, Rowe JH, Steinbrecher KA, Way SS. Commensal microbes drive intestinal inflammation by IL-17-producing CD4+ T cells through ICOSL and OX40L costimulation in the absence of B7-1 and B7-2. Proc Natl Acad Sci U S A. 2014;111:10672–10677. doi: 10.1073/pnas.1402336111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H, Kang Y, Zhang H, Zhao D, Xia J, Lu Z, Wang H, Xu F, Shi L. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. The Journal of Immunology. 2015;194:1239–1251. doi: 10.4049/jimmunol.1402088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.