Abstract
The incidence of obesity is rapidly rising, increasing morbidity and mortality rates worldwide. Associated comorbidities include type 2 diabetes, heart disease, fatty liver disease, and cancer. The impact of excess fat on musculoskeletal health is still unclear, although it is associated with increased fracture risk and a decline in muscular function. The complexity of obesity makes understanding the etiology of bone and muscle abnormalities difficult. Exercise is an effective and commonly prescribed non-pharmacological treatment option, but it can be difficult or unsafe for the frail, elderly, and morbidly obese. Exercise alternatives, such as low-intensity vibration (LIV), have potential for improving musculoskeletal health, particularly in conditions with excess fat. LIV has been shown to influence bone marrow mesenchymal stem cell differentiation toward higher-order tissues (i.e., bone) and away from fat. While the exact mechanisms are not fully understood, recent studies utilizing LIV both at the bench and in the clinic have demonstrated its efficacy. Here, we discuss the current literature investigating the effects of obesity on bone, muscle, and bone marrow and how exercise and LIV can be used as effective treatments for combating the negative effects in the presence of excess fat.
Keywords: osteoporosis, diabetes, vibration, exercise, musculoskeletal
Introduction
Obesity rates are rising worldwide, especially in the United States, where more than one-third (78.6 million) of adults and 17% of children are considered obese.1 This overall prevalence is only projected to increase, reaching 42% by 2030.2,3 An increase in the morbidity and mortality associated with being obese, whether it is a result of nutrition (i.e., high caloric intake) or exacerbated by hormonal imbalances (i.e., estrogen deficiency in menopause), is a major clinical concern. Obesity is associated with a myriad of comorbidities, including type 2 diabetes,4 heart disease,5 stroke,6 metabolic syndrome,4 cancer,3 and increased susceptibility to infectious disease.7 In 2008 alone, these cumulatively resulted in obesity-related medical spending of approximately $147 billion in the United States.8. Consequently, physicians are faced with new challenges in treatment, diagnosis, and prognosis as we continue to learn more about the epidemiology of the disease. Extensive research in the last few years has clearly shown that obesity is a condition of chronic and mild inflammation. The mechanism of obesity-induced inflammation is thought to originate in the adipose tissue, which accumulate and hypertrophy with higher energy intake and lack of exercise.9,10 This inflammation has also been associated with insulin resistance, the basis for the development of type 2 diabetes.10 In fact, 90% of patients with type 2 diabetes are considered overweight or obese.11
Health complications due to altered endocrine and inflammatory function open the door to a range of chronic diseases. Growing evidence suggests that, in addition to these metabolic abnormalities, and in some cases as a results of the abnormalities, excess adipose tissue is linked with poor musculoskeletal health. It is reported that obese women face higher fracture-related morbidity than nonobese women and that there is a greater fracture incidence among diabetic versus healthy subjects.12,13 Obesity also leads to skeletal muscle insulin resistance, alterations in muscle size and composition,14 and the decline of muscular function in the elderly.15 As the obese population ages, it is imperative that musculoskeletal tissue systems are maintained, as age-related complications (i.e., osteoporosis and sarcopenia) increasingly wear on the skeleton. The extent to which excess fat influences these tissues is an area of study that requires further investigation to obtain a complete understanding of the negative consequences obesity imposes on bone and muscle.
It is essential that appropriate treatments are available and employed in the clinic, as the number of people affected by obesity and type 2 diabetes continues to rise and the costs for treatment invariably grow. Pharmacological agents, surgical procedures, and lifestyle interventions targeting diet and activity level are all options that can be effective but have limitations. Bariatric surgery successfully reduces adipose tissue and improves type 2 diabetes16 but is costly and invasive,17 making the procedure unfit for a large population. There are long-term and short-term U.S. Food and Drug Administration (FDA)-approved weight loss drugs, the majority of which are appetite suppressants,18 but these are often not void of side effects or do not maintain weight loss in the long term. Rosiglitazone is indicated for the treatment of type 2 diabetes and acts by sensitizing tissues to insulin; however, it has been associated with increased risk of cardiovascular disease, myocardial infarction, and heart failure.19,20. Other non-pharmacological interventions include dietary restrictions and increased physical activity; however, compliance is poor, and long-term weight loss is only achieved by a few.17 A low-cost, safe, and effective alternative that will be utilized by those with obesity remains an unmet need in the clinic. This review will focus on the impacts of obesity on bone and muscle tissues and the use of exercise and low-magnitude mechanical signals as an alternative and/or complement to existing treatment methodologies.
Impact of obesity on musculoskeletal tissue systems
Fat is an inhomogeneous tissue that contains a range of cell types, including adipocytes, fibroblasts, endothelial cells, immune cells, and adult stem cells (mesenchymal and hematopoietic) and their progenitors. In addition to storing energy, it is also an active endocrine organ that modulates adipokine (e.g., leptin, resistin, adiponectin) and inflammatory cytokine (e.g., TNFα, IL-6, TGF-β) release,21 affecting both local and systemic environments. Fat tissue plays a role in a range of physiological processes, including immune function,22 glucose homeostasis,23 and energy balance.24 Its signaling directly affects other tissue systems,25 including bone and muscle. Osteosarcopenic obesity syndrome (OSO), a recently defined condition, encompasses both the skeletal (osteoporosis) and muscular (sarcopenia) complications associated with excessive fat mass (obesity).26 Although OSO has thus far been primarily relevant to the elderly population, the whole-body impact of fat across age is important to consider. Not only does excess fat mass put increased physical loads on the musculoskeletal system, but its endocrine function also induces molecular challenges to bone and muscle. With greater research into the mechanisms of action on each system, we can work to develop preventative approaches to diseases, such as obesity, osteoporosis, diabetes, sarcopenia, and OSO.
Bone
It has been presumed that obesity would be associated with increased bone mass and concomitant protection against osteoporosis and fracture, achieved as an adaptive product of the increased skeletal loads imposed during locomotion.27,28. However, since non-load-bearing bones also show a similar response to obesity, this suggests that increased secretion of hormones and adipokines might also contribute to changes in bone mineral density (BMD).29,30 Still, more recent evidence suggests that obesity might negatively affect BMD and promote fracture risk. When evaluating the influence of increasing body fat in elderly patients with obesity, it was found that greater adiposity and adipokine levels were associated with reduced lean mass, lower BMD, and increased fracture risk.31 A clinical study assessing 400 obese women in relation to BMI and fracture risk found that even obese women with almost normal BMD had an elevated risk of fracture compared with obese women with higher BMD scores,32, suggesting that BMI does not always relate to fracture risk. Whether BMD is a predictor of fracture risk is still debatable. A meta-analysis performed by De Laet and colleagues concluded that the relationship between BMI and fracture risk is nonlinear and is dependent on the level of BMI. This analysis demonstrated that there is a marked increase in fracture risk with low BMI, but the relative risk for people with obesity and high BMI in not as clear.33 Furthermore, there is growing evidence to indicate that the risks may also be site specific and mediated not only by BMD but also bone structure, geometry, and patterns of falling.34 A study by Hsu et al., which looked at a cohort of Chinese men and women, showed that non-spine fractures were higher in patients with a higher percentage of body fat.35 Similarly, while obesity is shown to be protective against hip and pelvic fractures, it increases the risk of fracture at other anatomical sites, such as the humerus.36,37
In vivo rodent models have allowed for further examination of the complex relationship between bone and fat. Wei et al. showed that diet-induced obese mice that develop higher BMD compared with control regular diet–fed mice present with impaired bone remodeling characterized by decreased bone resorption, low bone turnover, and decreased insulin signaling in bone-forming osteoblasts.38. Some believe that the increased bone resorption overwhelms any positive effects that increased body weight may have, leading to eventual bone loss.39 Other studies in diet-induced obese mice showed that bone responds in two phases, with an initial positive response, in which visceral fat expansion leads to increased bone mass, followed by a second negative phase, where prolonged exposure to obesity results in impaired bone formation and bone metabolism.40 More specifically, deterioration of trabecular bone architecture occurs early and is followed by decreases in cortical bone density, suggesting that trabecular and cortical bone are differentially regulated in diet-induced obese mice.41
In addition to bearing weight and providing structural support, bone tissue is an endocrine target in which certain processes and properties (e.g., bone remodeling, mass, and material properties) are regulated by adipokine release, such as leptin from fat tissue.42,43 Leptin secretion, determined by the amount of body fat, is differentially related to bone depending on whether it acts centrally or peripherally. Central effects of leptin have been shown to have negative impacts on bone formation,44 while peripheral leptin has been shown to promote osteoblastogenesis and suppress osteoclastogenesis.45,46 While increases in abdominal adiposity are in large part the primary contributors of adipokines and hormones to bone health in obesity, a rise in bone marrow adiposity, also seen in obesity, could potentially have more direct impacts, especially on trabecular bone health.47
Bone marrow niche
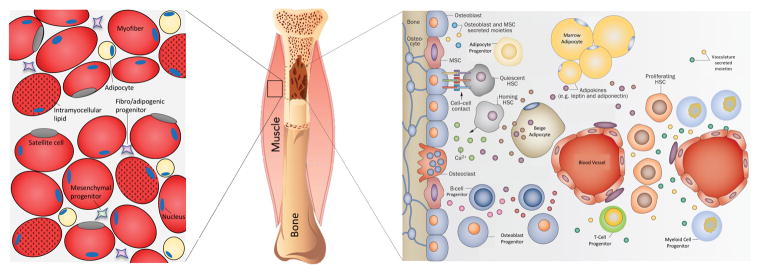
The bone marrow is a unique environment that modulates many systems, including bone and blood. It provides cellular niches that support the proliferation, self-renewal, and maintenance of stem cells, specifically hematopoietic (HSC) and mesenchymal (MSC) stem cells (Fig. 1).48 This dynamic environment also has access to a complex network of vasculature, allowing for exchange of endocrine signals and passage of cells into circulation,49 influencing the total body system. In the marrow there is a concentrated population of MSCs that has the potential to differentiate into bone, fat, skeletal muscle, and cartilage cell lineages,50 highlighting the therapeutic potential of these stem cells for tissue regeneration.51 Characterization of MSCs has been difficult owing to varying surface markers and differentiation potential, reliant upon species and strain of the host from which the cells are isolated.52 MSCs are even functionally different within the bone shaft itself, having distinct niches dependent on proximity to bone surfaces.53 Minimum characterization requirements include self-renewability, plastic adherence, and ability to differentiate into fat, bone, and cartilage lineages and are identified through a panel of cell surface markers: (CD105+ CD90+ CD73+ and CD45− CD34− CD11b− CD19− HLA-DR−).54 MSCs play critical roles in the marrow space, particularly in HSC homeostasis, expressing HSC maintenance genes and providing better homing capabilities for HSCs following lethal irradiation in mice.55 MSCs also regulate T cells56 and play a role in immunomodulation, influencing B-cells, natural killer cells, and dendritic cells.57 MSC function relies in part on local cues from the marrow microenvironment,55 and, considering the important regulatory role of MSCs within the bone marrow space, disruption of the niche is likely to influence MSC lineage commitment and neighboring cell populations.
Figure 1.
Schematic (adapted from Ref. 49) depicts the bone marrow cellular niche (right) and muscle cellular niche (left). The bone marrow is a dynamic heterogeneous environment and home to mesenchymal and hematopoietic stem cells. Mesenchymal stem cells regulate bone and fat systems in the marrow and support the hematopoietic niche. Hematopoietic stem cells regulate blood and immune cells, which can be compromised by obesity. The skeletal muscle microenvironment comprises multinucleated myofibers and stem cells, including satellite cells, mesenchymal progenitors, and fibro/adipogenic progenitors. Obesity results in increased intramyocellular lipid accumulation within myofibers and increased local adipocytes, likely through mesenchymal or fibro/adipogenic progenitor differentiation. This change is paralleled in the marrow, where increased marrow adipose tissue is characteristic of conditions with excess adiposity. Maintaining healthy levels of fat in bone and muscle may be achieved through mechanical stimulation intervention and biasing stem cell differentiation away from adipogenesis.
In the development of obesity, the expansion of fat depots is mediated through fat pad remodeling and adipocyte lineage commitment via MSC differentiation.58 Adipogenic differentiation is primarily driven by the expression of PPAR and C/EBP gene families59,60 and can occur in non-adipose tissues, including the marrow space. MSC function in this niche can be influenced by both local and systemic adipokine release and from the physical encroachment of adipocytes.61 Concurrent with increases in total body and visceral adiposity, high-fat diet models of obesity in rodents show increased marrow fat,47,62 increased commitment to adipogenesis, and alterations in MSC differentiation potential.63 MSC differentiation is also regulated by the Wnt signaling pathway,64–66 and it is understood that osteoblastogenesis and adipogenesis in the marrow generally maintain an inverse relationship. Adipocyte commitment is often associated with reduced bone formation, as seen in models of microgravity,67,68 ovarian hormone depletion,69,70 and aging.71. Through Wnt signaling or other osteogenic signaling pathways, it is plausible that MSC commitment to adipogenesis in the obese state can be controlled.
MSC populations also reside in tissues outside of the marrow72 and vary in functional capabilities depending on origin.73 However, MSCs derived from bone marrow and adipose tissue have been shown to share similar gene profiles and phenotypes, in both humans and mice.73,74. Bone marrow–derived MSCs can migrate and engraft in tissues outside of the bone and differentiate into tissue-specific cell types (e.g., myoblasts and adipocytes) under physiological conditions or if these cell populations solely originate from resident progenitors. Work in animal models has demonstrated bone marrow cell migration that is also influenced by both diabetes and rosiglitazone.75,76. Similarly, Ryden et al. showed that the obese condition influences bone marrow cell migration and behavior in humans. Following bone marrow and blood stem cell transplantations, there was increased donor cell engraftment in the fat pads of obese individuals compared with nonobese subjects.77 This work suggests that fat pad––and perhaps other tissue––formation and function is not derived solely from local progenitor pools and that bone marrow–derived cells and/or bone marrow–derived MSCs play a role in these tissues.
Obesity-induced MSC differentiation toward the adipogenic lineage leads to characteristic fat depots in the marrow cavity. Bone marrow adipose tissue (MAT) is distinct in comparison to white and brown fat depots.78 While development of a fatty marrow is a known consequence of aging and osteoporosis, the obese condition accelerates the fatty marrow phenotype, as has been demonstrated in both rodent and human marrow.79,80 A plausible reason for MAT deposition is an abnormally high commitment of MSCs into adipocytes due to biased lineage commitment towards adipogenesis and the inability to differentiate into other cell types, such as the osteoblastogenic lineage. This inverse bone and fat relationship has been seen in models of osteoporosis81 but is not so clear in obesity models. Nevertheless, high levels of MAT observed in the aged population,82,83 obesity,84 and postmenopausal women80 have the potential to alter marrow homeostasis and crowd resident marrow cell populations, including immune and stem cells, and may have negative effects on hematopoiesis resulting from paracrine signaling.81 This is an area of heavy research as we continue to learn more about the role of MAT in the pathogenesis of obesity.
Bone marrow hematopoietic stem cells give rise to all blood cells in the body and have been largely studied in the context of metabolic disorders owing to the impacts on the immune system. People with obesity have an increased risk and greater severity of various infections and illnesses, suggesting an immune system that is inferior to that of people without obesity.85,86. To a degree, this is a result of the development of chronic systemic inflammation, releasing increased proinflammatory cytokines into circulation, such as IL-1, IL-6 and TNF-α, that then promote a cascade of immune cell responses.87 Similar changes, including inflammation and compromised stem cell populations, can also be seen within the bone marrow niche as fat encroaches into that space.87–89
Rodent models of obesity, including diet-induced, leptin/leptin receptor knockout, and estrogen-deficient models, have expanded our understanding of the impacts of excess fat on immune systems, specifically hematopoiesis and bone marrow HSCs. Studies have shown that 16 weeks on a 60% kcal high-fat diet as well as in the ob/ob mouse leads to increased HSC progenitor populations concurrent with increased leukocytosis.90,91 Interestingly, short-term, 1-week exposure to the same diet results in a transient reduction in primitive HSCs followed by a later increase.91 Several studies also reported on the imbalance of mature leukocyte (i.e., blood cell) populations caused by obesity. For example, some reported that long-term exposure to high-fat diet leads to aging of the thymus concurrent with defective T cell production.83 Similarly, a different study showed a suppression of bone marrow B lymphopoiesis concurrent with deterioration of trabecular bone mass in obese mice.82 Other studies with diet-induced obesity have shown expansion of circulating and marrow leukocytes, including lymphocytes, data that are more consistent with reports of circulating leukocytes in obese humans.92,93 The collective literature on the adaptive immune cell populations in obesity are, however, inconsistent and warrant further research. Nevertheless, the general consensus is that white adipose tissues in obesity are permissive and recruit macrophages from the circulation and bone marrow, fueling the chronic inflammatory state.90,94
It is believed that obesity might also influence immunity through direct alterations to the HSC and HSC progenitors that maintain the mature immune populations. In fact, HSC behavior has been shown to be primed by states of obesity-induced chronic inflammation to bias lineage commitment and their proliferative and self-renewal capabilities. More specifically, LSK+ HSCs from mice on a 45% kcal high-fat diet show reduced proliferation when removed from their obese in vivo environments. When these same obese HSCs were transplanted into a lean animal, they exhibited reduced reconstitution capabilities.95 Additionally, when repopulating a lean, irradiated animal, obese HSCs have a biased differentiation towards myeloid cell types.91 These studies suggest that, once the HSC and/or HSC progenitor is primed by the disease state, its altered behavior cannot be reversed, even when transplanted into a lean host environment. Thus, considering the complex signaling cascades initiated by adipose tissue and obesity-related factors (e.g., cytokines, hormones), the obese condition may alter bone marrow stem cell behavior, including their migration and contributions of bone marrow–derived cells to tissues outside of the bone.
Skeletal muscle
Fatty infiltration of muscle tissue (myosteatosis) is a strong indicator of decreased muscular function and mobility96 and is likely to be accelerated by a systemic fat insult. Changes in muscular composition, specifically increased non-contractile tissue, directly affect the muscle tissue97 and significantly alter overall functional capabilities of muscle. Intramyocellular lipid and ectopic adipocytes are found in a range of conditions with decreased muscle mass and strength, including aged muscle,98 disuse,99 and muscular dystrophies, such as Duchene muscular dystrophy (DMD).100 Fatty muscle has also been identified in the pathogenesis of obesity101 and linked to poor muscular mechanical properties102 and insulin resistance.103 Importantly, myosteatosis accelerates with age, and fatty infiltration in the thigh muscle is associated with increased risk of hip fracture in the elderly.104 Diet-induced obesity in the mouse has also led to impaired muscular regenerative capabilities following injury, specifically impaired satellite cell function and delayed myogenic gene expression.105
Intermuscular adipocytes likely originate from fibro/adipogenic progenitors (FAPs), which readily differentiate into adipocytes, lack myogenic potential, and are influenced by cues in the muscular niche.106,107 Mesenchymal progenitors,73 pericytes,108 satellite cells,109,110 and PDGFRα+ PW1+ interstitial cells (PICs)111 have also been identified as muscular stem cell populations having adipogenic potential. Local increases in adipogenesis and alterations in the muscular niche could negatively regulate muscle regeneration through altering stem cell function, proliferation, or differentiation. Controlling adipogenic differentiation in skeletal muscle may be achievable through upregulation of the Wnt signaling pathway,112,113 similar to mechanisms occurring within the bone marrow cavity.114
The accumulation of fat metabolites within the muscle results in metabolic abnormalities, including interrupted insulin signaling96 and glucose transport activity.115 Elevated levels of intramyocellular lipids are a direct indication of insulin sensitivity and/or resistance,116 which is commonly seen in obesity and can lead to type 2 diabetes. Insulin resistance has long been thought to develop via increased fatty oxidation leading to inactivation of pyruvate dehydrogenase activity and reduced glucose oxidation.117 However, a recent hypothesis suggests that muscular insulin resistance results from the accumulation of lipids within the muscle cells and activation of the θ isoform of protein kinase C (PKC-θ),118,119 inhibiting insulin signaling and glycogen synthesis. The metabolite responsible for the activation of the PKC-θ pathway is believed to be diacylglycerol (DAG), a fat intermediate that is a precursor to triglycerides. Previous studies showed transient increases in DAG due to lipid infusion in the skeletal muscle of both rats120 and humans,121 and elevated DAG concentrations were found in humans that are obese and have type 2 diabetes.121 While data suggest that DAG leads to insulin resistance, it should be noted that DAG plays a complex role in the muscle, and higher concentrations of this metabolite do not necessarily translate to impaired insulin signaling. For example, total myocellular DAG content was found to be greater in well-trained athletes who also had higher insulin sensitivity compared with obese, sedentary adults.122 This echoes the “athlete’s paradox:” well-trained endurance athletes often have higher levels of skeletal muscle adiposity in the form of triglycerides.123
Combating obesity with mechanical signals
Exercise as a treatment modality
Exercise is a desirable treatment modality for obesity and type 2 diabetes because it is a non-pharmacological means of catabolizing fat tissue and building muscle and bone. A strong musculoskeletal system is imperative for preventing falls and fractures, particularly in the elderly and for maintaining quality of life. The public health recommendation of physical activity for adults is to perform a minimum of 150 min of moderate-intensity aerobic exercise per week (or other variations) to achieve health benefits.124. Various exercise regimes, from walking to high-intensity exercise, have been clinically shown to provide health benefits related to the prevention or treatment of obesity and type 2 diabetes and building muscle and bone tissues.125–127
Nevertheless, exercise is often not effective as a weight loss intervention. While short-term weight loss may be achieved through exercise, patients often relapse and regain the weight that was lost. Importantly, regular physical activity is critical for maintaining long-term weight loss,128 which will in turn prevent the recurrence of obesity-related health complications. Compliance with exercise intervention is a major concern,89 and it is often the case that this intervention is considered too strenuous or time-consuming for patients to incorporate this type of treatment into a permanent lifestyle change. Lastly, successful exercise interventions in the clinic are often coupled with dietary restrictions,129 making it difficult to tease apart the benefits of exercise from diet. While exercise is seemingly effective in catabolizing fat and building muscle and bone, its effectiveness in long-term weight maintenance is likely to require a simultaneous healthy diet.
Epidemiological studies have shown that exercise can prevent and improve obesity and related complications, including diabetes. A study by Helmrich et al. showed that, among 5990 male students, the risk of developing diabetes was reduced by 6% for every 500-kcal increase in weekly exercise over a period of 14 years.130 Exercise also protects from cardiovascular disease, reduces blood pressure and bad cholesterol, improves insulin sensitivity,131 and protects from non-alcoholic fatty liver disease,132 which are all complications associated with obesity. Interestingly, weight loss interventions, including diet-induced interventions or bariatric surgery, have been shown to induce bone loss in overweight and obese patients.133,134. It should be noted that the inclusion of exercise training with these weight loss programs significantly ameliorated bone loss in this population.135
The influence of exercise on bone has been extensively shown in both rodent and human models to improve skeletal outcomes that are affected by obesity and diabetes, including bone mineral density and structural, material, and biomechanical properties.136,137 More recently, voluntary exercise has been shown to influence the bone marrow microenvironment in diet-induced obese mice, mainly by reducing fat accumulation in the bone marrow.62 Interestingly, while exercise reduced MAT in these obese mice, it simultaneously increased bone mass. When challenged by PPAR-γ agonists, such as rosiglitazone, which induces MAT in mice, exercise was also able to suppress MAT and induce bone formation.138 This was also observed in ovariectomized rats given access to treadmill running: a reduction in MAT and prevention of bone loss.139 While this inverse bone-to-fat relationship has been observed in several models, it is not always the case, as evidenced in certain mouse strains, such as C3H mice, which experience an increase in both bone and MAT.78,140 However, while there was a suppression of PPAR-γ, master regulator of adipogenesis, they did not observe an increase in RUNX2, master regulator of osteoblastogenesis, indicating that this relationship might not always be one-to-one. These data suggest that marrow health can be mitigated by exercise when challenged not only by diet but also by hormonal and pharmacological insults.
The effects of exercise on bone marrow stem cells in obesity are, however, not fully understood. A recent study reported that exercised lean mice exhibit increased bone marrow MSCs concurrent with enhanced osteogenic differentiation potential and inhibition of adipogenic properties.141 Similarly, MSCs isolated from lean mice that underwent climbing exercise had limited adipocyte differentiation and increased osteogenic potential.142 One study investigated the response of obese bone marrow hematopoietic cells to exercise and have found that obese animals have a suppressed immune response to high-intensity bouts of exercise compared with lean animals; however, the effects on HSCs are still not understood.143 With greater knowledge of the exact influence of fat burden on these bone marrow stem cells, we can begin to ask more questions about how exercise might be used to change their behavior, particularly in the obese condition.
The effects of exercise on skeletal muscle are also well-established, benefiting muscular composition and glucose metabolism. Exercise is anabolic to muscle144 and reduces both muscular fat content in humans101,145 and muscle triglycerides in animal models of high-fat diet.146 Importantly, the reduction of ectopic lipid is associated with improved insulin resistance,145 which aids in curbing the progression of glucose intolerance. Aerobic exercise combined with weight loss was effective in reducing lipid in the thigh muscle and improving glucose tolerance in aged men.147 Duncan et al. also demonstrated that improved insulin sensitivity in humans can be achieved through exercise without any weight loss.148 Additionally, a lack of physical activity is associated with increased intermuscular adipose tissue in humans with type 2 diabetes,149 emphasizing the importance of exercise at any level in maintaining musculoskeletal health and preventing the onset of type 2 diabetes. In addition to muscle and bone, exercise can have profound impacts on other load-bearing tissues. Exercise is commonly prescribed by clinicians to alleviate the symptoms of osteoarthritis;150 however, the intensity of the exercise can be a concern, because extreme loads can have detrimental consequences on cartilage health.151 Strenuous treadmill running in rats and cyclic loading of tibias in mice, for example, have been shown to induce cartilage degeneration in the knee.152,153 Likewise, the absence of mechanical stimulation, such as in cases of extended bedrest, has been shown to induce cartilage thinning.154 Moderate exercise, however, in the form of cycling, rope skipping, and light jogging, has been shown to improve knee cartilage glycosaminoglycan content in patients at high risk of developing knee OA.155 As with bone and muscle, previous research suggests that obesity induces degenerative changes in cartilage tissue. Although the mechanism by which obesity induces cartilage degeneration is still unclear, research suggests that altered joint biomechanics and elevated secretion of proinflammatory cytokines and adipokines contribute to increased osteoarthritis risk.156 With regard to using exercise as an intervention, wheel running was shown to reduce the progression of osteoarthritis in obese mice while also disrupting the clustering of inflammatory cytokine expression compared with sedentary animals fed a high-fat diet.157
Low-intensity vibration as an exercise surrogate or adjunct therapy
While exercise may in many cases be a desirable method for treating metabolic disorders, certain groups of people may require safer or less strenuous alternatives. The elderly or those with osteopenia, sarcopenia, or osteoporosis are at great risk of falls158 and fracture.159. The recommended moderate-intensity exercise may be undesirable as it may precipitate a fall and fracture, and such events in the elderly population can significantly reduce quality of life, both socially and physically.159,160 Those who suffer from motion-restricting musculoskeletal disorders or severe musculoskeletal pain may find a less intensive alternative to exercise attractive, and patients who are morbidly obese may be bedridden or otherwise physically incapable of exercise owing to physical limitations.
Although mechanical signals delivered through high-impact (strains > 1000 με, or 0.1% strain) exercises with fewer cycles (< 1000 cycles per day), such as resistance training, are anabolic to the skeleton, studies show that low-impact signals (strains < 100 με) delivered over many repeating cycles (>1 × 105) might also impart similar benefits.161 Low-intensity vibration (LIV) is a low-magnitude (< 1 g where g is earth’s gravitational field at 9.81 m/s2), high-frequency (30–90 Hz) mechanical signal typically delivered through the lower extremities that gets transmitted through the skeleton. LIV biases stem cell differentiation toward osteoblastogenesis and away from adipogenesis, making this intervention a noninvasive means of building bone and muscle and preventing the formation of fat––and giving it the potential to be used as a surrogate for exercise. Compared with many exercise regimes, LIV can be less time-consuming and easier to use and has the potential to increase compliance. LIV is typically delivered in brief (< 30 min) bouts five to seven days a week and only requires a person to stand on the plate for the duration of the treatment. Most importantly, the delivery of LIV is safe for up to several hours of use each day, as designated by the International Standards Organization.162 LIV does not put people at risk of fracture in the same way that moderate-intensity exercise may, and, unlike high-intensity vibration, LIV is not damaging to the skeleton nor does it cause musculoskeletal pain.
In humans, LIV has been an effective therapeutic intervention for a variety of musculoskeletal disorders, including building trabecular bone in the tibia of children with Duchenne muscular dystrophy,163 reducing low back pain,164 preventing bone loss165 and increasing muscular strength and function166,167 in postmenopausal women, improving balance168 in patients who underwent bed rest, and increasing both muscle and bone mass in young women with low bone mineral density.169 These clinical trials successfully utilized LIV intervention without any severe adverse events and targeted a range of musculoskeletal conditions in different age groups. The anabolic potential of LIV has also been demonstrated in animal models through increasing the cross-sectional area of muscle fibers,170 preserving trabecular bone loss,171,172, and biasing MSC differentiation toward osteoblastogenesis.173 Considering bone, fat, and muscle tissues all arise from a common progenitor, these studies support the idea that LIV can be utilized as a protection mechanism in the presence of a high-fat environment through promoting bone and muscle anabolism and preventing adipogenesis.
LIV in models of obesity
The influence of whole-body vibration on MSCs and its ability to bias the stem cell away from adipogenesis provides the foundation for a new focus of investigation involving the prevention of obesity and type 2 diabetes.173 While still a relatively new area of research, much of the literature has largely focused on in vitro and rodent models. There are currently a variety of vibration protocols with different frequencies, magnitudes, and durations being tested for medical applications; however, our focus here is on the use of lower-intensity vibration protocols utilizing low magnitudes and high frequencies for short exposure durations.
One of the earliest reports of LIV’s influence on obesity used a high-fat diet–induced obesity model in C57BL/6 mice to show that 12 weeks of LIV (0.2 × g, 90 Hz, 15 min/day) treatment reduced subcutaneous and visceral fat in the obese animals concurrent with increased bone marrow MSCs with decreased adipogenic differentiation capability.173 Further testing in a similar animal model was able to show that LIV (0.2 × g, 90 Hz, 15 min/day) had the ability to restore bone mass and bone marrow and peripheral immune populations (B and T cells) that were initially depleted by the high-fat diet.82 More recently, evaluation of bone, muscle, and fat systems with vibration was performed in an ovariectomized mouse model, which displayed both a systemic fat burden and osteopenia. These studies showed that LIV (0.2–0.3 × g, 90Hz, 15–30 min/day) was able to partially protect against estrogen-deficient bone loss and reduce the presence of adipocytes or adipogenic gene expression in bone marrow and muscle tissues.70,174
Whole-body vibration of different intensity but relatively low magnitude (0.13–0.68 × g, 5.6–13 Hz) was shown to improve exercise performance and fatigue while also preventing fat accumulation in obese mice.175 Vibration treatment was also tested and proved positive in a more severe obesity model. Application of vibration (0.5 × g, 45 Hz, 60 min/day) for 12 weeks in female db/db mice ameliorated insulin resistance, showing the potential for the treatment of type 2 diabetes.176 In male db/db mice, McGee-Lawrence et al. showed that 3 months of whole-body vibration is comparable to the effects of treadmill exercise for that same duration, specifically in mitigating adipocyte hypertrophy in visceral fat pads, attenuating insulin resistance, and increasing skeletal muscle fiber diameter and serum osteocalcin levels.177 A recent clinical evaluation of overweight prepubertal boys demonstrated that 10 weeks of whole-body vibration (1.9–6.2 × g, 30–40 Hz, 3–20 min/day) with dynamic lower and upper body training improved their bone mineral content and bone mineral density through a reduction in bone-resorptive activity.178 These studies highlight the potential of low-intensity whole-body vibration for treatment of obesity-related complications; however, they also highlight the considerable uncertainties regarding the mechanisms of these mechanical signals, especially in the complicated pathogenesis of obesity.
Preliminary data in our lab has shown that low-intensity vibration stimulates increased cartilage thickness in high-fat animals compared to high-fat diet controls, presumably leaving these animals better equipped to endure the potential onset of obesity-induced osteoarthritis.179 Our results contrast with recent research that reports that repeated exposure to low-intensity vibration induced marked degeneration of murine knee joints, including meniscal tears and focal damage to the cartilage surface within the medial joint compartment.180 Of the numerous small animal models70,82,171,174,181,182 and human studies164,168,183–185 that have utilized LIV, no damage to connective tissues, including bone, cartilage, tendon, intervertebral disc, muscle, or ligament, has been observed. It is important to note that CD-1 mice were used in this study, and when repeated in the C57BL/6 strain, no joint degeneration due to LIV was observed.186 Therefore, genetic differences may be a source of contrasting results and warrant further investigation.
While recent evidence is promising, LIV is a subtle signal that is not always effective in improving or maintaining the musculoskeletal system. Whole-body vibration treatment given to an elderly (> 80years) population resulted in no reversal of poor bone mineral density,187 and mechanical intervention in general is thought to be less effective in older populations owing to the decreased number and responsiveness of stem cells to mechanical signals. Slatkovska et al. also saw no improvement in bone outcomes in postmenopausal women subject to vibration treatment for 12 months;188 however, these subjects did not all have osteoporotic bone at the start of the study. Severe systemic burdens, such as menopause or obesity, may alter cell responsiveness to mechanical signals and thus the ability of LIV to bias stem cell differentiation away from adipogenesis.
It should also be noted that animal models of obesity, especially in the mouse, vary significantly and are likely to lead to inclusive findings regarding the effectiveness of vibration. There are a range of mutations and dietary models that affect metabolism and the musculoskeletal system differently, all which are limited when modeling obesity after humans. Two of the most common and robust genetic strains include mutations in the leptin hormone (ob) and leptin receptor (db) genes. There are a range of other transgenic models targeting the tubby, Carboxypeptidase E, Mc3r, Mc4r, and Pomc genes, among others, and obesity can also be induced via pharmacological agents (e.g., gold thioglucose model). Dietary approaches typically include diets high in carbohydrates or fat, which can originate from various sources (e.g., animal lard, plant oils, beef tallow, coconut fat, fish oil) and vary in fat fraction (20–60%).189 High-fat diets in the range of 40–60% kcal from fat are commonly used models and mimic the progression of insulin resistance and glucose intolerance seen in humans.190 Data from transgenic strains or mutation models must be interpreted with care, since it may be unclear whether the cause was the genetic background or the obese phenotype, and there is certainly a difference between the effects of diet versus the effects of obesity on the body system. The effectiveness of vibration treatment in improving bone and muscle maybe reliant on the initial condition of the subjects, chosen animal models, or methods of analysis, and LIV may be more effective in humans and animals that have a pre-existing detriment.
Summary: mechanism of LIV and implications
While it is understood that a mechanical signal of low magnitude (< 1×g) is necessary for the safe application of vibration, there is currently no consensus on the signal parameters that are most beneficial in improving bone or muscle tissues or biasing stem cell differentiation. It is likely that the frequency, magnitude, and duration of exposure is specific to the target tissue––the signal most anabolic to bone in postmenopausal osteoporosis may differ from the signal most anabolic to muscle in the obese condition. The incorporation of a refractory period is a parameter of recent interest that may be more important in influencing cellular outcomes than the signal magnitude, frequency, or total duration of exposure. Previous work in rats shows that a refractory period incorporated between bouts of the dynamic loading of bone allows for the restoration of mechanosensitivity and ultimately leads to increased bone formation.191 Other groups also found that incorporating a rest period between loads resulted in a heightened cellular response.192,193 Work by Sen et al. demonstrated that a 3-h refractory period was more successful in suppressing MSC adipogenesis in vitro than either a 1-h period or the absence of a refractory period.194 Allowing a period of rest may enable appropriate cellular adaptations to become more sensitive to or more primed for responding to the mechanical signal. This idea can also be translated to exercise in humans––when you exercise rather than how you exercise may be a determining factor in your body response.
While the mechanism by which LIV influences bone, muscle, and fat is not completely understood, biased differentiation towards the osteoblastic lineage resulting from mechanical stimulation is thought to come at the expense of MSC differentiation towards adipogenesis (Table 1).173,195,196 Recent work regarding the understanding of how MSCs recognize a low-magnitude mechanical cue and transform the signal into a behavioral adaptation has shown that the signal elicits an acute cellular response and reorganization of focal adhesions and the cytoskeleton.197,198 Uzer and colleagues showed that LIV activates a coupling between the cell nucleus and cytoskeleton (termed the LINC complex) that initiates intracellular signaling.198 Recent studies also suggest that LIV may bias stem cell differentiation through sclerostin (SOST), which regulates adipogenesis through inhibiting Wnt signaling.66 Vibration has been shown to suppress SOST both in culture and in vivo.199,200 Additional research is needed to gain a complete understanding of the mechanosensitive signaling pathways triggered by LIV and the consequential stem cell behavior. Understanding the mechanism of LIV-biased stem cell differentiation and the implications of the signal parameters––whether it is magnitude, frequency, duration of exposure, or incorporation of a refractory period–– will allow for optimized treatment and the development of targeted therapies.
Table 1.
Summary of the bone, muscle, and marrow systems in response to obesity and the impacts of exercise and LIV in the presence of excess fat
| Obesity/adipose burden | Exercise | LIV | |
|---|---|---|---|
| Bone | - Decreases bone resorption38 - Leads to low bone turnover38 - Short-term diet-induced obesity (DIO) leads to increased bone mass40 - Long-term DIO leads to impaired bone formation40 - Trabecular bone deterioration occurs before cortical bone41 - Peripheral leptin promotes osteoblastogenesis and suppresses ostecolastogenesis45,46 - Central leptin negatively affects bone formation44 |
- Improves bone mineral density, bone structure, and biomechanics in rodents and humans136,137 - Ameliorates bone loss in patients who have undergone bariatric surgery or other weight loss135 |
- Restores bone mass in DIO82 - Protects from bone loss in OVX174,70 - Reduces bone-resorptive activity177 - 10 weeks of vibration treatment improved bone mineral density and bone mineral content in overweight prepubertal boys178 - Whole-body vibration treatment showed no improvement in bone in elderly population and postmenopausal women187,188 |
|
| |||
| Muscle | - Myosteatosis is an indication of decreased muscular function and mobility96, 97 - Fatty muscle is linked to poor mechanical properties and insulin resistance102,103 - DIO in the mouse impairs muscular regenerative capabilities following injury105 |
- Reduces muscular fat content in humans101,145 - Reduces thigh muscle lipid content and improves glucose tolerance in humans when combined with weight loss147 - Improves insulin sensitivity148 - Reduces muscle triglycerides in high-fat diet animal models146 - Anabolic to muscle in obese adults144 |
- Increases muscular strength and function in postmenopausal women166,167 - Increases cross-sectional area of muscle fibers in mice170 - Reduces adipogenic gene expression in the muscle of OVX mice174 - Improves insulin resistance in db/db mice176,177 - Increases fiber diameter in db/db mice177 |
|
| |||
| Bone marrow | - Increases marrow adipose tissue62,47,79,80,82–84 - Long-term DIO leads to increased HSC progenitors90,91 - Short-term DIO leads to transient reduction followed by increase in HSC progenitors91 - Decreases and/or increases in B and T cells82,87, 92,93 - Increases recruitment of BM macrophages to white adipose tissue90, 94 - Primes BM HSCs to promote inflammation91 |
- Reduces MAT in DIO62,139 - Reduces PPAR-γ expression136 - Increases BM MSCs with increased osteogenic potential in DIO141 - MSCs from exercised mice have limited adipocyte differentiation142 |
- Increases BM MSCs with decreased adipogenic potential in DIO173 - Restores BM B and T cells in DIO82 |
In summary, low-magnitude mechanical signals show potential to be used as an adjunct therapy or alternative to more expensive, invasive, or strenuous treatment methods. Importantly, the use of this exercise surrogate is particularly valuable for patients that suffer from musculoskeletal pain, frailty, or physical limitations due to obesity. Through biasing MSC differentiation and influencing the bone marrow space, LIV can alter obesity related complications, including impaired immunity, ectopic fat formation in muscle and bone tissues, and insulin resistance, ultimately delaying the onset of type 2 diabetes or even improving the existing condition.
Acknowledgments
This work was supported by grants from the NIH, including #AR-43498 and #EB-14351. Danielle M. Frechette and Divya Krishnamoorthy contributed equal effort in the conception and drafting of this review, with substantial help from Clinton T. Rubin. M. Ete Chan, and Vihitaben Patel. Tee Pamon contributed a significant effort in revising the concepts and integrity of the manuscript.
Footnotes
Competing interests
CTR is a founder of Marodyne Medical and BTT Health Systems, as well as the holder of several patents related to healthcare applications of low-intensity vibration and low-magnitude mechanical signals. The other authors have no competing interests to declare.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Weiss BM, Vogl DT, Berger NA, et al. Trimming the fat: obesity and hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:1152–1160. doi: 10.1038/bmt.2012.201. [DOI] [PubMed] [Google Scholar]
- 4.Cara JF, Chaiken RL. Type 2 diabetes and the metabolic syndrome in children and adolescents. Curr Diab Rep. 2006;6:241–250. doi: 10.1007/s11892-006-0041-8. [DOI] [PubMed] [Google Scholar]
- 5.Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997;96:3248–3250. doi: 10.1161/01.cir.96.9.3248. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell AB, Cole JW, McArdle PF, et al. Obesity increases risk of ischemic stroke in young adults. Stroke. 2015;46:1690–1692. doi: 10.1161/STROKEAHA.115.008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235:1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein EA, Trogdon JG, Cohen JW, et al. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai T, Ogasawara J, Kizaki T, et al. The effects of exercise training on obesity-induced dysregulated expression of adipokines in white adipose tissue. Int J Endocrinol. 2013;2013:801743. doi: 10.1155/2013/801743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 11.Eckel RH, Kahn SE, Ferrannini E, et al. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? J Clin Endocrinol Metab. 2011;96:1654–1663. doi: 10.1210/jc.2011-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Compston JE, Flahive J, Hooven FH, et al. Obesity, health-care utilization, and health-related quality of life after fracture in postmenopausal women: Global Longitudinal Study of Osteoporosis in Women (GLOW) Calcif Tissue Int. 2014;94:223–231. doi: 10.1007/s00223-013-9801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napoli N, Strotmeyer ES, Ensrud KE, et al. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia. 2014;57:2057–2065. doi: 10.1007/s00125-014-3289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafortuna CL, Tresoldi D, Rizzo G. Influence of body adiposity on structural characteristics of skeletal muscle in men and women. Clin Physiol Funct Imaging. 2014;34:47–55. doi: 10.1111/cpf.12062. [DOI] [PubMed] [Google Scholar]
- 15.Rolland Y, Lauwers-Cances V, Cristini C, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr. 2009;89:1895–1900. doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. e245. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 17.Encinosa WE, Bernard DM, Steiner CA, et al. Use and costs of bariatric surgery and prescription weight-loss medications. Health affairs. 2005;24:1039–1046. doi: 10.1377/hlthaff.24.4.1039. [DOI] [PubMed] [Google Scholar]
- 18.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malinowski JM, Bolesta S. Rosiglitazone in the treatment of type 2 diabetes mellitus: a critical review. Clin Ther. 2000;22:1151–1168. doi: 10.1016/s0149-2918(00)83060-x. discussion 1149–1150. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Yang L, Zhai SD. Risk of cardiovascular disease and all-cause mortality among diabetic patients prescribed rosiglitazone or pioglitazone: a meta-analysis of retrospective cohort studies. Chin Med J (Engl) 2012;125:4301–4306. [PubMed] [Google Scholar]
- 21.Palermo A, Tuccinardi D, Defeudis G, et al. BMI and BMD: The Potential Interplay between Obesity and Bone Fragility. Int J Environ Res Public Health. 2016:13. doi: 10.3390/ijerph13060544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffaut C, Galitzky J, Lafontan M, et al. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord G. Role of leptin in immunology. Nutr Rev. 2002;60:S35–38. doi: 10.1301/002966402320634913. discussion S68–84, 85–37. [DOI] [PubMed] [Google Scholar]
- 25.Rajala MW, Scherer PE. Minireview: The adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 26.Ilich JZ, Kelly OJ, Inglis JE. Osteosarcopenic Obesity Syndrome: What Is It and How Can It Be Identified and Diagnosed? Curr Gerontol Geriatr Res. 2016;2016:7325973. doi: 10.1155/2016/7325973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson DT, Zhang Y, Hannan MT, et al. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8:567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 28.Villareal DT, Apovian CM, Kushner RF, et al. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228. [DOI] [PubMed] [Google Scholar]
- 29.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimitri P, Wales JK, Bishop N. Adipokines, bone-derived factors and bone turnover in obese children; evidence for altered fat-bone signalling resulting in reduced bone mass. Bone. 2011;48:189–196. doi: 10.1016/j.bone.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 31.Aguirre L, Napoli N, Waters D, et al. Increasing adiposity is associated with higher adipokine levels and lower bone mineral density in obese older adults. J Clin Endocrinol Metab. 2014;99:3290–3297. doi: 10.1210/jc.2013-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cawsey S, Padwal R, Sharma AM, et al. Women with severe obesity and relatively low bone mineral density have increased fracture risk. Osteoporos Int. 2015;26:103–111. doi: 10.1007/s00198-014-2833-z. [DOI] [PubMed] [Google Scholar]
- 33.De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporosis Int. 2005;16:1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 34.Compston JE, Flahive J, Hosmer DW, et al. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW) J Bone Miner Res. 2014;29:487–493. doi: 10.1002/jbmr.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu YH, Venners SA, Terwedow HA, et al. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–154. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- 36.Gnudi S, Sitta E, Lisi L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J Bone Miner Metab. 2009;27:479–484. doi: 10.1007/s00774-009-0056-8. [DOI] [PubMed] [Google Scholar]
- 37.Prieto-Alhambra D, Premaor MO, Fina Aviles F, et al. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27:294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 38.Wei J, Ferron M, Clarke CJ, et al. Bone-specific insulin resistance disrupts whole-body glucose homeostasis via decreased osteocalcin activation. J Clin Invest. 2014;124:1–13. doi: 10.1172/JCI72323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192:292–297. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- 40.Lecka-Czernik B, Stechschulte LA, Czernik PJ, et al. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol. 2015;410:35–41. doi: 10.1016/j.mce.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Fujita Y, Watanabe K, Maki K. Serum leptin levels negatively correlate with trabecular bone mineral density in high-fat diet-induced obesity mice. J Musculoskelet Neuronal Interact. 2012;12:84–94. [PubMed] [Google Scholar]
- 42.Magni P, Dozio E, Galliera E, et al. Molecular aspects of adipokine-bone interactions. Curr Mol Med. 2010;10:522–532. doi: 10.2174/1566524011009060522. [DOI] [PubMed] [Google Scholar]
- 43.Greco EA, Lenzi A, Migliaccio S. The obesity of bone. Ther Adv Endocrinol Metab. 2015;6:273–286. doi: 10.1177/2042018815611004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 45.Holloway WR, Collier FM, Aitken CJ, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 46.Burguera B, Hofbauer LC, Thomas T, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–3553. doi: 10.1210/endo.142.8.8346. [DOI] [PubMed] [Google Scholar]
- 47.Doucette CR, Horowitz MC, Berry R, et al. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J Cell Physiol. 2015;230:2032–2037. doi: 10.1002/jcp.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10:737–748. doi: 10.1038/nrendo.2014.169. [DOI] [PubMed] [Google Scholar]
- 50.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 51.Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218. doi: 10.1155/2013/496218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 53.Siclari VA, Zhu J, Akiyama K, et al. Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone. 2013;53:575–586. doi: 10.1016/j.bone.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 55.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benvenuto F, Ferrari S, Gerdoni E, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25:1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]
- 57.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 58.Hu X, Chen LL, Zheng J, et al. Increases in systemic and local stress: a probable mechanism of visceral fat accumulation and insulin resistance in adult catch-up growth rats? Experimental biology and medicine. 2013;238:57–65. doi: 10.1258/ebm.2012.012207. [DOI] [PubMed] [Google Scholar]
- 59.Thorn SL, Gollob MH, Harper ME, et al. Chronic AMPK activity dysregulation produces myocardial insulin resistance in the human Arg302Gln-PRKAG2 glycogen storage disease mouse model. EJNMMI Res. 2013;3:48. doi: 10.1186/2191-219X-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–253. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naveiras O, Nardi V, Wenzel PL, et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Styner M, Thompson WR, Galior K, et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu CL, Diekman BO, Jain D, et al. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: the effects of free fatty acids. Int J Obes (Lond) 2013;37:1079–1087. doi: 10.1038/ijo.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ross SE, Hemati N, Longo KA, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 65.Wright WS, Longo KA, Dolinsky VW, et al. Wnt10b inhibits obesity in ob/ob and agouti mice. Diabetes. 2007;56:295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- 66.Fairfield H, Falank C, Harris E, et al. The Skeletal Cell-Derived Molecule Sclerostin Drives Bone Marrow Adipogenesis. J Cell Physiol. 2017 doi: 10.1002/jcp.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 68.Ahdjoudj S, Lasmoles F, Holy X, et al. Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma. J Bone Miner Res. 2002;17:668–677. doi: 10.1359/jbmr.2002.17.4.668. [DOI] [PubMed] [Google Scholar]
- 69.Benayahu D, Shur I, Ben-Eliyahu S. Hormonal changes affect the bone and bone marrow cells in a rat model. J Cell Biochem. 2000;79:407–415. doi: 10.1002/1097-4644(20001201)79:3<407::aid-jcb60>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 70.Krishnamoorthy D, Frechette DM, Adler BJ, et al. Marrow adipogenesis and bone loss that parallels estrogen deficiency is slowed by low-intensity mechanical signals. Osteoporos Int. 2016;27:747–756. doi: 10.1007/s00198-015-3289-5. [DOI] [PubMed] [Google Scholar]
- 71.Meunier P, Aaron J, Edouard C, et al. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 72.Wang CY, Liao JK. A Mouse Model of Diet-Induced Obesity and Insulin Resistance. Methods Mol Biol. 2012;821:421–433. doi: 10.1007/978-1-61779-430-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of cell science. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 74.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 75.Crossno JT, Jr, Majka SM, Grazia T, et al. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang C, Seifert RA, Bowen-Pope DF, et al. Diabetes and aging alter bone marrow contributions to tissue maintenance. Int J Physiol Pathophysiol Pharmacol. 2009;2:20–28. [PMC free article] [PubMed] [Google Scholar]
- 77.Ryden M, Uzunel M, Hard JL, et al. Transplanted Bone Marrow-Derived Cells Contribute to Human Adipogenesis. Cell Metab. 2015;22:408–417. doi: 10.1016/j.cmet.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 78.Scheller EL, Rosen CJ. What’s the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adler BJ, Green DE, Pagnotti GM, et al. High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLoS One. 2014;9:e90639. doi: 10.1371/journal.pone.0090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity. 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Menagh PJ, Turner RT, Jump DB, et al. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. 2010;25:757–768. doi: 10.1359/jbmr.091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan ME, Adler BJ, Green DE, et al. Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:4855–4863. doi: 10.1096/fj.12-209841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang H, Youm YH, Vandanmagsar B, et al. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Griffith JF, Yeung DK, Ma HT, et al. Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging. 2012;36:225–230. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 85.Dossett LA, Dageforde LA, Swenson BR, et al. Obesity and site-specific nosocomial infection risk in the intensive care unit. Surg Infect (Larchmt) 2009;10:137–142. doi: 10.1089/sur.2008.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morgan OW, Bramley A, Fowlkes A, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5:e9694. doi: 10.1371/journal.pone.0009694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cortez M, Carmo LS, Rogero MM, et al. A high-fat diet increases IL-1, IL-6, and TNF-alpha production by increasing NF-kappaB and attenuating PPAR-gamma expression in bone marrow mesenchymal stem cells. Inflammation. 2013;36:379–386. doi: 10.1007/s10753-012-9557-z. [DOI] [PubMed] [Google Scholar]
- 88.Halade GV, El Jamali A, Williams PJ, et al. Obesity-mediated inflammatory microenvironment stimulates osteoclastogenesis and bone loss in mice. Exp Gerontol. 2011;46:43–52. doi: 10.1016/j.exger.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laharrague P, Fontanilles AM, Tkaczuk J, et al. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur Cytokine Netw. 2000;11:634–639. [PubMed] [Google Scholar]
- 90.Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singer K, DelProposto J, Morris DL, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3:664–675. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trottier MD, Naaz A, Li Y, et al. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc Natl Acad Sci U S A. 2012;109:7622–7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van der Weerd K, Dik WA, Schrijver B, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012;61:401–408. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bai Y, Sun Q. Macrophage recruitment in obese adipose tissue. Obes Rev. 2015;16:127–136. doi: 10.1111/obr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van den Berg SM, Seijkens TT, Kusters PJ, et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. FASEB J. 2016;30:1779–1788. doi: 10.1096/fj.201500175. [DOI] [PubMed] [Google Scholar]
- 96.Addison O, Marcus RL, Lastayo PC, et al. Intermuscular fat: a review of the consequences and causes. International journal of endocrinology. 2014;2014:309570. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 98.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cree MG, Paddon-Jones D, Newcomer BR, et al. Twenty-eight-day bed rest with hypercortisolemia induces peripheral insulin resistance and increases intramuscular triglycerides. Metabolism. 2010;59:703–710. doi: 10.1016/j.metabol.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tyler KL. Origins and early descriptions of “Duchenne muscular dystrophy”. Muscle Nerve. 2003;28:402–422. doi: 10.1002/mus.10435. [DOI] [PubMed] [Google Scholar]
- 101.Goodpaster BH, Theriault R, Watkins SC, et al. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 102.Choi SJ, Files DC, Zhang T, et al. Intramyocellular Lipid and Impaired Myofiber Contraction in Normal Weight and Obese Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71:557–564. doi: 10.1093/gerona/glv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goodpaster BH, Thaete FL, Simoneau JA, et al. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 104.Lang T, Cauley JA, Tylavsky F, et al. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25:513–519. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.D’Souza DM, Trajcevski KE, Al-Sajee D, et al. Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. Physiol Rep. 2015:3. doi: 10.14814/phy2.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Uezumi A, Fukada S, Yamamoto N, et al. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 108.Birbrair A, Zhang T, Wang ZM, et al. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22:2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rossi CA, Pozzobon M, Ditadi A, et al. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PLoS One. 2010;5:e8523. doi: 10.1371/journal.pone.0008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. Journal of cell science. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- 111.Pannerec A, Formicola L, Besson V, et al. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140:2879–2891. doi: 10.1242/dev.089326. [DOI] [PubMed] [Google Scholar]
- 112.Taylor-Jones JM, McGehee RE, Rando TA, et al. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev. 2002;123:649–661. doi: 10.1016/s0047-6374(01)00411-0. [DOI] [PubMed] [Google Scholar]
- 113.Prestwich TC, Macdougald OA. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007;19:612–617. doi: 10.1016/j.ceb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamrick M, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Frontiers in Endocrinology. 2016;7:69. doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 116.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Randle PJ, Garland PB, Hales CN, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 118.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 119.Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16:400–402. doi: 10.1038/nm0410-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yu CL, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 121.Szendroedi J, Yoshimura T, Phielix E, et al. Role of diacylglycerol activation of PKC theta in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci U S A. 2014;111:9597–9602. doi: 10.1073/pnas.1409229111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Amati F, Dube JJ, Alvarez-Carnero E, et al. Skeletal Muscle Triglycerides, Diacylglycerols, and Ceramides in Insulin Resistance Another Paradox in Endurance-Trained Athletes? Diabetes. 2011;60:2588–2597. doi: 10.2337/db10-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goodpaster BH, He J, Watkins S, et al. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 124.Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: executive summary. Nutr Rev. 2009;67:114–120. doi: 10.1111/j.1753-4887.2008.00136.x. [DOI] [PubMed] [Google Scholar]
- 125.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. Jama. 1999;282:1433–1439. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 126.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 127.Chae JS, Kang R, Kwak JH, et al. Supervised exercise program, BMI, and risk of type 2 diabetes in subjects with normal or impaired fasting glucose. Diabetes Care. 2012;35:1680–1685. doi: 10.2337/dc11-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dalsky GP, Stocke KS, Ehsani AA, et al. Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med. 1988;108:824–828. doi: 10.7326/0003-4819-108-6-824. [DOI] [PubMed] [Google Scholar]
- 129.Krousel-Wood MA, Berger L, Jiang X, et al. Does home-based exercise improve body mass index in patients with type 2 diabetes? Results of a feasibility trial. Diabetes Res Clin Pract. 2008;79:230–236. doi: 10.1016/j.diabres.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 130.Helmrich SP, Ragland DR, Leung RW, et al. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. N Engl J Med. 1991;325:147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- 131.Myers J. Cardiology patient pages. Exercise and cardiovascular health. Circulation. 2003;107:e2–5. doi: 10.1161/01.cir.0000048890.59383.8d. [DOI] [PubMed] [Google Scholar]
- 132.Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 133.Zibellini J, Seimon RV, Lee CM, et al. Does Diet-Induced Weight Loss Lead to Bone Loss in Overweight or Obese Adults? A Systematic Review and Meta-Analysis of Clinical Trials. J Bone Miner Res. 2015;30:2168–2178. doi: 10.1002/jbmr.2564. [DOI] [PubMed] [Google Scholar]
- 134.Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–1065. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 135.Beavers DP, Beavers KM, Loeser RF, et al. The independent and combined effects of intensive weight loss and exercise training on bone mineral density in overweight and obese older adults with osteoarthritis. Osteoarthritis Cartilage. 2014;22:726–733. doi: 10.1016/j.joca.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hinton PS, Shankar K, Eaton LM, et al. Obesity-related changes in bone structural and material properties in hyperphagic OLETF rats and protection by voluntary wheel running. Metabolism. 2015;64:905–916. doi: 10.1016/j.metabol.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 137.Foster-Schubert KE, Alfano CM, Duggan CR, et al. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20:1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Styner M, Pagnotti GM, Galior K, et al. Exercise Regulation of Marrow Fat in the Setting of PPARgamma Agonist Treatment in Female C57BL/6 Mice. Endocrinology. 2015;156:2753–2761. doi: 10.1210/en.2015-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen Y, Wang S, Bu S, et al. Treadmill training prevents bone loss by inhibition of PPARgamma expression but not promoting of Runx2 expression in ovariectomized rats. Eur J Appl Physiol. 2011;111:1759–1767. doi: 10.1007/s00421-010-1820-0. [DOI] [PubMed] [Google Scholar]
- 140.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, et al. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maredziak M, Smieszek A, Chrzastek K, et al. Physical Activity Increases the Total Number of Bone-Marrow-Derived Mesenchymal Stem Cells, Enhances Their Osteogenic Potential, and Inhibits Their Adipogenic Properties. Stem Cells Int. 2015;2015:379093. doi: 10.1155/2015/379093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Menuki K, Mori T, Sakai A, et al. Climbing exercise enhances osteoblast differentiation and inhibits adipogenic differentiation with high expression of PTH/PTHrP receptor in bone marrow cells. Bone. 2008;43:613–620. doi: 10.1016/j.bone.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 143.Kostrycki IMFMNWG, Donato YH, dos Santos AB, Rhoden CR, Ludwig MS, Heck TG. Hematological Response of Acute Exercise in Obese Mice: the Obesity Attenuation Effect on Leaukocytes Response. Journal of Exersice Physiology Online. 2016;19:85–93. [Google Scholar]
- 144.Villareal DT, Smith GI, Sinacore DR, et al. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity (Silver Spring) 2011;19:312–318. doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Petersen KF, Dufour S, Morino K, et al. Reversal of muscle insulin resistance by weight reduction in young, lean, insulin-resistant offspring of parents with type 2 diabetes. Proc Natl Acad Sci U S A. 2012;109:8236–8240. doi: 10.1073/pnas.1205675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu J, Zheng J, Liu XF, et al. Exercise improved lipid metabolism and insulin sensitivity in rats fed a high-fat diet by regulating glucose transporter 4 (GLUT4) and musclin expression. Braz J Med Biol Res. 2016;49:e5129. doi: 10.1590/1414-431X20165129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prior SJ, Joseph LJ, Brandauer J, et al. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007;92:880–886. doi: 10.1210/jc.2006-2113. [DOI] [PubMed] [Google Scholar]
- 148.Hellinger FJ, Encinosa WE. Inappropriate drug combinations among privately insured patients with HIV disease. Med Care. 2005;43:III53–62. doi: 10.1097/01.mlr.0000175630.68791.cd. [DOI] [PubMed] [Google Scholar]
- 149.Tuttle LJ, Sinacore DR, Cade WT, et al. Lower Physical Activity Is Associated With Higher Intermuscular Adipose Tissue in People With Type 2 Diabetes and Peripheral Neuropathy. Phys Ther. 2011;91:923–930. doi: 10.2522/ptj.20100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 151.Spector TD, Harris PA, Hart DJ, et al. Risk of osteoarthritis associated with long-term weight-bearing sports: a radiologic survey of the hips and knees in female ex-athletes and population controls. Arthritis Rheum. 1996;39:988–995. doi: 10.1002/art.1780390616. [DOI] [PubMed] [Google Scholar]
- 152.Franciozi CE, Tarini VA, Reginato RD, et al. Gradual strenuous running regimen predisposes to osteoarthritis due to cartilage cell death and altered levels of glycosaminoglycans. Osteoarthritis Cartilage. 2013;21:965–972. doi: 10.1016/j.joca.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 153.Ko FC, Dragomir C, Plumb DA, et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 2013;65:1569–1578. doi: 10.1002/art.37906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liphardt AM, Mundermann A, Koo S, et al. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthr Cartilage. 2009;17:1598–1603. doi: 10.1016/j.joca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 155.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52:3507–3514. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]
- 156.Griffin TM, Fermor B, Huebner JL, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12:R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Griffin TM, Huebner JL, Kraus VB, et al. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 2012;64:443–453. doi: 10.1002/art.33332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wickham C, Cooper C, Margetts BM, et al. Muscle strength, activity, housing and the risk of falls in elderly people. Age and ageing. 1989;18:47–51. doi: 10.1093/ageing/18.1.47. [DOI] [PubMed] [Google Scholar]
- 159.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 160.Randell AG, Nguyen TV, Bhalerao N, et al. Deterioration in quality of life following hip fracture: a prospective study. Osteoporos Int. 2000;11:460–466. doi: 10.1007/s001980070115. [DOI] [PubMed] [Google Scholar]
- 161.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16:482–489. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 162.I.S. Organization, editor ISO 2631/1. 1985. Evaluation of Human Exposure to Whole-Body Vibration. [Google Scholar]
- 163.Ward K, Alsop C, Caulton J, et al. Low magnitude mechanical loading is osteogenic in children with disabling conditions. Journal of Bone and Mineral Research. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 164.Holguin N, Muir J, Rubin C, et al. Short applications of very low-magnitude vibrations attenuate expansion of the intervertebral disc during extended bed rest. Spine J. 2009;9:470–477. doi: 10.1016/j.spinee.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 165.Rubin C, Recker R, Cullen D, et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343–351. doi: 10.1359/JBMR.0301251. [DOI] [PubMed] [Google Scholar]
- 166.Dutra MC, de Oliveira ML, Marin RV, et al. Whole-body vibration improves neuromuscular parameters and functional capacity in osteopenic postmenopausal women. Menopause. 2016;23:870–875. doi: 10.1097/GME.0000000000000644. [DOI] [PubMed] [Google Scholar]
- 167.Verschueren SMP, Roelants M, Delecluse C, et al. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. Journal of Bone and Mineral Research. 2004;19:352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 168.Muir J, Judex S, Qin YX, et al. Postural instability caused by extended bed rest is alleviated by brief daily exposure to low magnitude mechanical signals. Gait & posture. 2011;33:429–435. doi: 10.1016/j.gaitpost.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gilsanz V, Wren TA, Sanchez M, et al. Low-level, high-frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res. 2006;21:1464–1474. doi: 10.1359/jbmr.060612. [DOI] [PubMed] [Google Scholar]
- 170.Xie LQ, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056–1062. doi: 10.1152/japplphysiol.00764.2007. [DOI] [PubMed] [Google Scholar]
- 171.Pagnotti GM, Adler BJ, Green DE, et al. Low magnitude mechanical signals mitigate osteopenia without compromising longevity in an aged murine model of spontaneous granulosa cell ovarian cancer. Bone. 2012;51:570–577. doi: 10.1016/j.bone.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ozcivici E, Luu YK, Rubin CT, et al. Low-level vibrations retain bone marrow’s osteogenic potential and augment recovery of trabecular bone during reambulation. PLoS One. 2010;5:e11178. doi: 10.1371/journal.pone.0011178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Frechette DM, Krishnamoorthy D, Adler BJ, et al. Diminished satellite cells and elevated adipogenic gene expression in muscle as caused by ovariectomy are averted by low-magnitude mechanical signals. J Appl Physiol (1985) 2015;119:27–36. doi: 10.1152/japplphysiol.01020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huang CC, Tseng TL, Huang WC, et al. Whole-body vibration training effect on physical performance and obesity in mice. Int J Med Sci. 2014;11:1218–1227. doi: 10.7150/ijms.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu Y, Zhai M, Guo F, et al. Whole Body Vibration Improves Insulin Resistance in db/db Mice: Amelioration of Lipid Accumulation and Oxidative Stress. Appl Biochem Biotechnol. 2016;179:819–829. doi: 10.1007/s12010-016-2033-8. [DOI] [PubMed] [Google Scholar]
- 177.McGee-Lawrence ME, Wenger KH, Misra S, et al. Whole-body vibration mimics the metabolic effects of exercise in male leptin receptor deficient mice. Endocrinology. 2017 doi: 10.1210/en.2016-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Erceg DN, Anderson LJ, Nickles CM, et al. Changes in Bone Biomarkers, BMC, and Insulin Resistance Following a 10-Week Whole Body Vibration Exercise Program in Overweight Latino Boys. Int J Med Sci. 2015;12:494–501. doi: 10.7150/ijms.11364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pamon T, VB, Chan M, Krzesaj P, Rubin C. High fat diet compromises articular cartilage thickness relative to body weight, while low intensity mechanical signals protect this relationship, building cartilage relative to body size. Presented at American Society of Bone and Mineral Research, Washington State Convention Center.2015. [Google Scholar]
- 180.McCann MR, Patel P, Pest MA, et al. Repeated exposure to high-frequency low-amplitude vibration induces degeneration of murine intervertebral discs and knee joints. Arthritis Rheumatol. 2015;67:2164–2175. doi: 10.1002/art.39154. [DOI] [PubMed] [Google Scholar]
- 181.Holguin N, Uzer G, Chiang FP, et al. Brief daily exposure to low-intensity vibration mitigates the degradation of the intervertebral disc in a frequency-specific manner. J Appl Physiol (1985) 2011;111:1846–1853. doi: 10.1152/japplphysiol.00846.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chan ME, Uzer G, Rubin CT. The potential benefits and inherent risks of vibration as a non-drug therapy for the prevention and treatment of osteoporosis. Curr Osteoporos Rep. 2013;11:36–44. doi: 10.1007/s11914-012-0132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kiel DP, Hannan MT, Barton BA, et al. Low-Magnitude Mechanical Stimulation to Improve Bone Density in Persons of Advanced Age: A Randomized, Placebo-Controlled Trial. J Bone Miner Res. 2015;30:1319–1328. doi: 10.1002/jbmr.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Leonard MB, Shults J, Long J, et al. Effect of Low-Magnitude Mechanical Stimuli on Bone Density and Structure in Pediatric Crohn’s Disease: A Randomized Placebo-Controlled Trial. J Bone Miner Res. 2016;31:1177–1188. doi: 10.1002/jbmr.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Asselin P, Spungen AM, Muir JW, et al. Transmission of low-intensity vibration through the axial skeleton of persons with spinal cord injury as a potential intervention for preservation of bone quantity and quality. J Spinal Cord Med. 2011;34:52–59. doi: 10.1179/107902610x12886261091758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kerr GJ, McCann MR, Branch JK, et al. C57BL/6 mice are resistant to joint degeneration induced by whole-body vibration. Osteoarthritis Cartilage. 2017;25:421–425. doi: 10.1016/j.joca.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 187.Gomez-Cabello A, Gonzalez-Aguero A, Morales S, et al. Effects of a short-term whole body vibration intervention on bone mass and structure in elderly people. J Sci Med Sport. 2014;17:160–164. doi: 10.1016/j.jsams.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 188.Slatkovska L, Alibhai SM, Beyene J, et al. Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial. Ann Intern Med. 2011;155:668–679. W205. doi: 10.7326/0003-4819-155-10-201111150-00005. [DOI] [PubMed] [Google Scholar]
- 189.Carroll L, Voisey J, van Daal A. Mouse models of obesity. Clin Dermatol. 2004;22:345–349. doi: 10.1016/j.clindermatol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 190.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 191.Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204:3389–3399. doi: 10.1242/jeb.204.19.3389. [DOI] [PubMed] [Google Scholar]
- 192.Batra NN, Li YJ, Yellowley CE, et al. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. Journal of biomechanics. 2005;38:1909–1917. doi: 10.1016/j.jbiomech.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 193.Srinivasan S, Weimer DA, Agans SC, et al. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17:1613–1620. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sen B, Xie Z, Case N, et al. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. J Biomech. 2011;44:593–599. doi: 10.1016/j.jbiomech.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.David V, Martin A, Lafage-Proust MH, et al. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 196.Sen B, Xie Z, Case N, et al. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sen B, Guilluy C, Xie Z, et al. Mechanically induced focal adhesion assembly amplifies anti-adipogenic pathways in mesenchymal stem cells. Stem Cells. 2011;29:1829–1836. doi: 10.1002/stem.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Uzer G, Thompson WR, Sen B, et al. Cell Mechanosensitivity to Extremely Low-Magnitude Signals Is Enabled by a LINCed Nucleus. Stem Cells. 2015;33:2063–2076. doi: 10.1002/stem.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gnyubkin V, Guignandon A, Laroche N, et al. High-acceleration whole body vibration stimulates cortical bone accrual and increases bone mineral content in growing mice. J Biomech. 2016;49:1899–1908. doi: 10.1016/j.jbiomech.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 200.Thompson WR, Uzer G, Brobst KE, et al. Osteocyte specific responses to soluble and mechanical stimuli in a stem cell derived culture model. Sci Rep. 2015;5:11049. doi: 10.1038/srep11049. [DOI] [PMC free article] [PubMed] [Google Scholar]