Abstract
In the modern era, a pathology report of thyroid carcinoma requires the inclusion of numerous prognostically relevant histopathologic features, e.g. the presence and extent of vascular and capsular invasion, extrathyroidal extension, the surgical margin status, and the characteristics of nodal metastasis. These pathologic features are crucial components of the initial risk stratification to determine the need for completion thyroidectomy and/or post-operative radioactive iodine ablation therapy. The current review aims to summarize the diagnostic criteria, the controversies, the prognostic impacts, and the challenges of these pathologic characteristics, focusing specifically on the parameters that are incorporated into the American Joint Committee on Cancer (AJCC) staging system, College of American Pathologists (CAP) reporting template, American Thyroid Association (ATA), and National Comprehensive Cancer Network (NCCN) guidelines.
Keywords: Extrathyroidal extension, lymphovascular invasion, AJCC staging
Introduction
In the modern era, a pathology report requires the inclusion of numerous prognostically relevant histopathologic tumor characteristics. Thyroid carcinoma is no exception to such rules. In addition to tumor type, variant and size, other features such as the presence and extent of capsular invasion (CI), vascular invasion (VI) and extrathyroidal extension (ETE), margin status, as well as the number and size of neck lymph node(s) with metastatic carcinoma, have been shown to provide additional prognostic information, and are now required in a standardized pathology report for thyroid carcinoma1, and in the initial risk stratification published by several renowned organizations, e.g. the American Thyroid Association (ATA), the National Comprehensive Cancer Network (NCCN) and the American Joint Committee for Cancer (AJCC) staging manual, to guide management and determine the need for radioactive iodine (RAI) ablation therapy2–4. Hence, it becomes increasingly important for pathologists to report these parameters, and for clinicians to understand the potential impacts of such on patients’ management. The current review intends to summarize the definition, diagnostic challenges, controversies, and the clinical impacts of each pathologic parameter which is incorporated into a routine pathology report of differentiated follicular derived carcinoma.
Extrathyroidal extension
Extrathyroidal extension, defined as tumor extension beyond the thyroid capsule into the adjacent soft tissue, is a common pathologic finding detected in 23.5% of all papillary thyroid carcinomas5. ETE has long been considered as an adverse prognostic factor with an increased risk of recurrence and mortality5–8. It can be further subdivided into two categories: 1) minimal (or microscopic) ETE, which is invasion into the immediate perithyroidal soft tissue, detected solely at microscopic level and not appreciated clinically or grossly at the time of surgery; and 2) extensive (or gross) ETE that is defined as gross direct extension of the carcinoma into strap muscles (e.g. sternohyoid, sternothyroid, thyrohyoid, and omohyoid muscles), subcutaneous tissue, adjacent viscera (e.g. larynx, trachea, and esophagus), or recurrent laryngeal nerve, and is typically established clinically by imaging or during the operation (Figure 1). These two categories of ETE bear different prognostic values: the risk of recurrence associated with minor extrathyroidal extension is approximately 3 to 9% 9–15, compared with 23 to 40% risk of recurrence in patients with gross ETE9, 10, 12–14, 16, 17. Furthermore, several recent studies have shown that microscopic ETE is not an independent predictor for persistent disease, recurrence free survival and disease specific survival (Figure 2)11, 12, 15, 18–20. Under the 2015 ATA guidelines, microscopic ETE is considered as a feature for intermediate risk lesion which may be subjected to post-operative radioactive iodine (RAI) treatment, while tumors with gross ETE are defined as high risk lesions and thus mandating a (near-)total thyroidectomy and post-operative RAI therapy2. Similarly, NCCN 2017 guidelines recommend completion thyroidectomy and post-operative RAI for lesions with gross ETE, while the administration of 30 mCi of 131I is considered optional for patients with a primary tumor of < 4 cm, clinical M0 and minor ETE 3. Histologically, the thyroid gland is devoid of a well-defined capsule in many areas along its periphery, and the follicles are often intermingled with adipose tissue or even skeletal muscle21. Therefore, the very definition of microscopic ETE is problematic and subjective, and a universally accepted pathologic criterion for ETE is lacking. In a recent study involving eleven expert endocrine pathologists, the following histologic diagnostic criteria have been utilized and recognized as microscopic ETE: perithyroidal fat involvement by 9 of 11 pathologists; perithyroidal skeletal muscle involvement by 11 of 11 pathologists, perithyroidal nerve involvement by 9 of 11 pathologists, and perithyroidal thick-wall vessel involvement by 7 of 11 pathologists. Additionally, in tumors arising from the isthmus, three of eleven pathologists did not consider involvement of skeletal muscle as evidence of microscopic ETE22. Not surprisingly, the interobserver agreement for minimal ETE was poor with a kappa value of 0.14 even among expert pathologists22.
Figure 1.
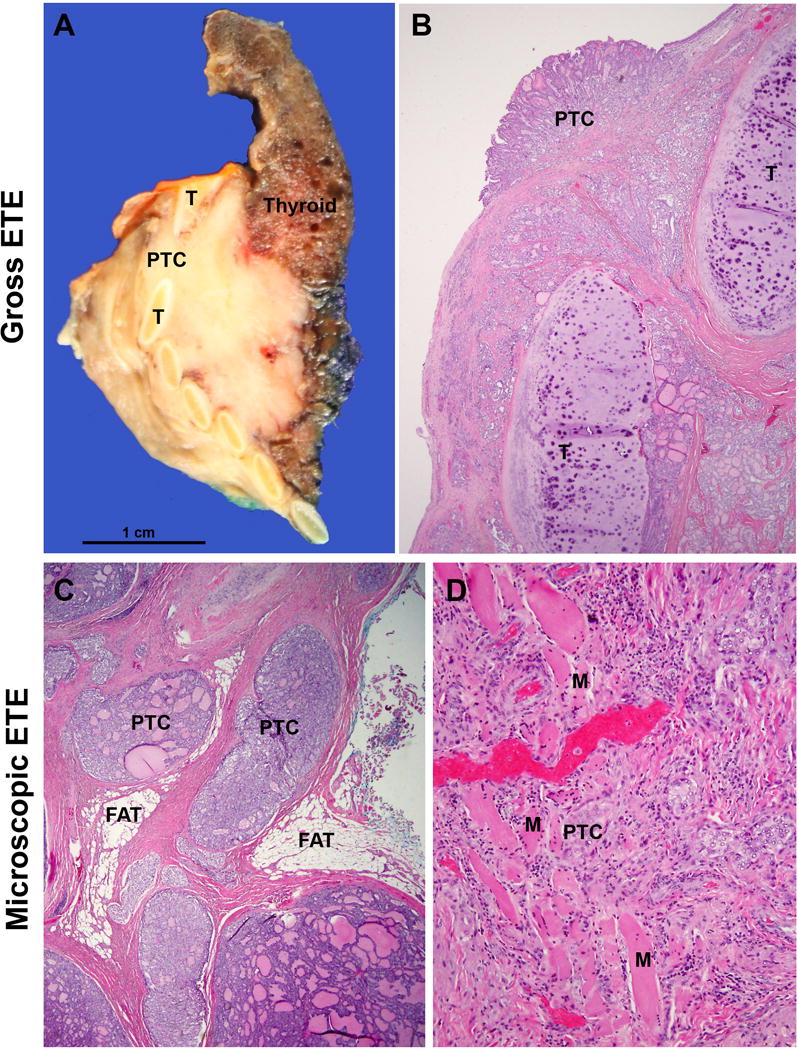
Extrathyroidal extension (ETE). (A/B) Gross ETE: A patient with papillary thyroid carcinoma (PTC) with pre-operative radiological evidence of invasion into trachea. Macroscopically (A), an infiltrative firm beige mass was present involving thyroid gland proper and infiltrating between tracheal rings (T). Microscopically, the carcinoma infiltrated in between tracheal cartilage and was present at the tracheal mucosal surface. This tumor should be staged as pT4a based on gross ETE into trachea. (C/D) Microscopic (minimal) ETE: tumor nests were present in between perithyroidal adipose tissue (FAT, panel C) or skeletal muscle (M, panel D).
Figure 2.

Adverse outcome (defined as the presence of disease at last follow-up) according to degree of extra-thyroid extension (ETE) in papillary thyroid carcinoma cases with adequate follow-up. Only patients with gross ETE (15 cases) had an adverse outcome. There was no survival difference between patients without ETE (11 cases) and those with microscopic (micro) ETE (31 cases). Reprinted with permission from reference 20.
In the seventh edition of AJCC staging system, a tumor with minimal ETE is staged as pT3 regardless of tumor size, while gross ETE beyond strap muscles is staged as T4 disease 23. The facts that microscopic ETE is associated with poor interobserver agreement and does not affect recurrence and survival raise concerns of whether microscopic ETE alone is sufficient to upstage a patient as pT3. Therefore, in the upcoming AJCC eighth edition that will be implemented on January 1st, 2018, microscopic ETE has been removed entirely from the staging system of differentiated thyroid carcinoma4. Gross ETE invading strap muscles only by a tumor of any size will be staged as pT3b, while gross ETE with invasion into subcutaneous soft tissue, larynx, trachea, esophagus, or recurrent laryngeal nerve as pT4a.
In summary, recent evidence has shown that microscopic or minimal ETE bears limited prognostic significance (i.e. does not impart worse outcome than absence of ETE), and may not be a reproducible histologic feature. In comparison, carcinomas with gross ETE are associated with high risk of recurrence, and are considered as high stage (at least pT3) and high risk lesions, which should trigger additional therapy, e.g. completion thyroidectomy and post-operative RAI. Therefore, it is crucial for the pathologists and clinicians to realize the difference between microscopic and gross ETE. A knee jerk response from the clinicians to consider completion thyroidectomy and post-operative RAI to a pathologic report mentioning microscopic ETE only may subject the patient to unnecessary treatment especially in PTC cases20.
Margin assessment
The margin status of a surgical resection for differentiated thyroid carcinoma is a mandatory reporting element in the current College of American Pathologists (CAP) guidelines1, and can be divided into three categories: a R0 resection (microscopically negative margin), a R1 resection (grossly complete resection with microscopically positive margin), and a R2 resection (grossly positive margin or incomplete resection)4. Histologically, a positive margin is defined by the presence of tumor cells at the inked tissue border and/or the outer aspect of the thyroid gland (Figure 3)24–27. The reported frequency of microscopically positive margin is between 6.1% and 14.1%24–27. Several recent studies have shown that microscopically positive margin is not an independent predictor for recurrence and disease free survival, especially after adjusting for tumor stage and ETE25–27. Taken these into consideration, the current ATA guideline has only included incomplete R2 resection into the risk stratification as a feature of high risk lesions2. In contrast, NCCN 2017 guideline has included any positive resection margin as one of the criteria along with large tumor (> 4 cm), gross ETE, macroscopic nodal metastasis, macroscopic multifocal disease, and vascular invasion to recommend completion thyroidectomy3.
Figure 3.

Microscopic positive resection margin (i.e. R1 resection). Arrows: tumor cells with thermal artifact are present at the inked tissue edge. The lesion is an encapsulated follicular variant of papillary thyroid carcinoma.
Interestingly, Lang et al. has shown that a microscopic positive posterior margin was an independent predictor for recurrence free survival with a 23-fold risk of recurrence, while a positive anterior margin did not pose a significant risk for recurrence25. Hence, it may be prudent to ink the anterior and posterior margins differently when processing thyroid specimens in pathology laboratories and to document the status of anterior and posterior margins separately in the pathology report.
Characteristics of regional nodal metastases
Regional lymph node metastasis is a common finding in papillary thyroid carcinoma, being detected in up to 80% of patients as a result of extensive neck dissection and meticulous histological examination28. Most studies have shown that regional nodal metastasis, especially lateral neck nodal disease, carries prognostic significance4, 28–32. The impact of nodal metastases on disease specific survival and recurrence free survival are relatively small, and are most evident in older patients30–32. Nodal metastases are incorporated into AJCC tumor, nodal disease, and distant metastasis (TNM) staging and prognostic stage group, and can be further divided into central (pN1a) and lateral compartment involvement (pN1b) 4, 29.
Recently, increasing evidence has shown that various characteristics of nodal metastases, e.g. number, size, and extranodal extension (ENE), may provide additional prognostic information. Thus, detailed features of nodal disease ought to be included in the pathology report, and be considered in risk stratification and staging system18, 28, 33–39. A recent meta-analysis by Randolph et al. has shown that small volume subclinical microscopic pathologic N1 disease, i.e. five or fewer subcentimeter metastatic lymph nodes, conveys little prognostic impact on recurrence free survival and disease specific survival in PTC, compared with clinically evident macroscopic nodal disease involving more than 5 lymph nodes, especially those with ENE 28. Taken this data into consideration, the NCCN 2017 guidelines no longer recommend completion thyroidectomy and post-operative RAI in small volume pN1a disease, i.e. < 5 involved nodes with metastasis < 2 mm in largest dimension 3. Histologic features of the nodal metastasis that has been incorporated in the ATA initial risk stratifications included number of involved lymph nodes (> 5 is considered as intermediate risk) and size of the metastatic lymph nodes (≥ 3 cm as high risk).
Extranodal extension is not an uncommon finding, being reported in up to 12% of PTC overall and 33% of nodal metastatic PTC18, 36. Similar to ETE, a well-defined consensus histologic diagnostic criterion for ENE is currently lacking1, 40. A recent study by Du et al. has shown that involvement of perinodal adipose tissue appears to be the most consistent diagnostic criteria of ENE, being considered by eleven participating endocrine pathologists as ENE40. However, the overall agreement in diagnosing ENE is only fair with a kappa value of 0.35 among expert pathologists40. Nevertheless, studies have repeatedly demonstrated the association between ENE and persistent and/or recurrence disease18, 28, 33–38. Hence, it is important to document ENE in the pathology report of a differentiated thyroid carcinoma.
Taken these recent data into consideration, the upcoming AJCC 8th edition has recommended recording detailed histologic features of nodal metastasis in the pathologic report, including number of involved lymph nodes, number of lymph nodes sampled, location of the involved lymph nodes, size of the largest involved lymph node, size of metastatic foci within involved lymph node, and the presence of extranodal extension4.
Vascular invasion
Vascular invasion was first described in thyroid malignancy by Graham 41 in 1924. Although three groups have shown that the mere existence of VI, regardless of the extent, entails a substantial risk of distant metastasis, a larger body of literature have shown that the extent (rather than the existence) of vascular invasion is a prognostic factor in low grade thyroid carcinomas42–47. Based on these evidences, the current ATA and NCCN guideline, as well as the upcoming 4th edition of WHO classification, have advocated to encapsulated follicular and Hurthle cell carcinoma with extensive vascular invasion from those with capsular invasion only and/or focal (a few foci) of vascular invasion 3, 48, 49. Tumors with minimal VI (defined as a few microscopic foci by NCCN, or less than 5 foci by ATA guidelines) are associated with a low risk of recurrence (0 – 5%) with an overall similar outcome to those without VI in low grade thyroid carcinoma42–46. Fortunately, extensive VI appears to be a relatively rare event in differentiated thyroid carcinoma, being present in 8.7% of well-differentiated encapsulated thyroid carcinomas in one study46. When it occurs, extensive vascular invasion is an independent predictor of recurrence, is associated with 42% risk of distant metastasis, and is frequently observed in patients who died of thyroid carcinoma19, 43, 46, 50. Based on these emerging evidences, the most recent ATA guidelines have classified an intrathyroidal follicular thyroid carcinoma with minimal VI as low risk lesion, which does not command a completion thyroidectomy and RAI ablation therapy2. Similarly, the NCCN 2017 guidelines have defined minimal vascular invasion as a few (one to four foci) of VI, and does not mandate RAI administration in an intrathyroidal well defined follicular or Hurthle cell carcinoma with minimal VI3. On the other hand, the presence of extensive vascular invasion (>4 foci) in a differentiated thyroid carcinoma will place the patient into the high risk group, which mandates total thyroidectomy and post-operative RAI therapy2, 3. In Hurthle cell carcinoma, tumors with extensive VI (≥ 4 foci) or extrathyroidal VI were associated with a different RNA expression profile compared with minimally invasive Hurthle cell carcinoma including those with focal VI (< 4 foci), suggesting that the molecular signatures might be different according to the extent of VI 51. Hence, it is crucial for pathologists to reliably evaluate and report the presence and extent of vascular invasion in low grade thyroid carcinoma in order to direct risk stratification and subsequent clinical treatment decisions.
However, the very definition of vascular invasion has been surrounded by controversies. The initial definition of VI delineated in the authorative 1992 and 2015 AFIP fascicles 52, 53 and adopted in the majority of the published literature mandated the following two conditions to be qualified for VI: (1) the involved blood vessels has to be located within or outside of the tumor capsule; and (2) the intravascular polypoid tumor growth must be attached to the vessel wall, admixed with fibrin or covered by endothelium (Figure 4). Areas of VI that are closely adjacent to one another are typically counted as separate foci 46. On the other hand, a recent study published by Mete and Asa did not consider tumor protrusion into vascular space lined by endothelial cells alone as a diagnostic criterion for VI, but rather required fibrin thrombus adherent to intravascular tumor54. This is based on the idea that intra-vascular endothelial lined tumor is separated from the bloodstream by endothelial cells, while thrombus formation is indicative of host response and coagulation pathway activation54. When this more stringent criterion of VI is applied, the incidence of VI in differentiated thyroid carcinoma decreased drastically from 7–62%46, 47, 55–57 to 3%54, while the risk of distant metastasis in association with the mere existence of VI becomes 35%. This latter approach may fail to identify a significant proportion of thyroid tumors with VI, focal or extensive, that should be classified as carcinoma based on the presence of invasion, and that may benefit from appropriate risk stratification and/or additional therapies.
Figure 4.

Vascular invasion. Tumor emboli covered by endothelial cells are present within capsular vasculature (A), which may be associated with fibrin thrombus (B, arrow heads).
Capsular invasion
The CAP guidelines and AFIP fascicle define capsular invasion as a tumor that completely transgressed the tumor capsule beyond the outer contour with or without neocapsule formation or satellite nodule lying outside of the tumor capsule (Figure 5) 1, 53. Some authorities have advised to use the term minimally invasive to describe those carcinomas with microscopic capsular invasion only (regardless of number of CI foci), since their metastatic rate is close to 045, 46, 58–61. In contrast, widely invasive follicular carcinoma was used for carcinoma with grossly apparent invasion into the thyroid and peri-thyroidal soft tissue (i.e. ETE)1 and is associated with a high risk of distant metastasis 62. The existence of invasion, including CI, is the histologic characteristics which distinguish and differentiate certain types of malignant thyroid tumors from their non-malignant counterparts, e.g. follicular carcinoma from follicular adenoma, Hurthle cell carcinoma from Hurthle cell adenoma, as well as encapsulated follicular variant of papillary thyroid carcinoma with invasion from noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP)63. However, as multiple studies have shown that carcinomas with only CI, regardless of extent, is associated with an overall excellent prognosis44, 46, 58, 64–66, CI was not a factor being considered in risk stratification in ATA and NCCN guidelines2, 3.
Figure 5.

Common forms of capsular invasion. A: Hurthle cell carcinoma showing the typical mushroom like shape invasion (ci) into the full thickness of the capsule (c). Note the presence of a new fibrous capsule at the leading edge of the invasive tumor bud (arrow). B: Hurthle cell carcinoma in a different patient displaying capsular invasion in the form of a tumor nodule (ci) lying immediately outside an intact capsule (c). The point of entry into the capsule is not apparent at this particular level. This is not an uncommon finding.
Conclusions
The field of thyroid pathology has evolved dramatically over the past several decades. Several major changes occurred, including incorporation of numerous detailed pathologic characteristics (e.g. the presence and extent of VI and ETE) into the pathology report. These shifts in the characterization of thyroid carcinoma have led to the refining of risk stratification and de-escalation of therapy in differentiated thyroid carcinoma. The prognosis and therapy of thyroid carcinomas can be better delineated if a meticulous microscopic analysis is performed. An accurate assessment of the extent of VI for example is crucial. Hence, it is important for pathologists to meticulously report on histopathologic features other than tumor type such as extent of invasion in order to better predict tumor spread and survival and assist clinical decision making.
Acknowledgments
Funding: Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DR BIN XU (Orcid ID : 0000-0003-4638-9835)
Disclosure: No competing financial interests exist for all contributory authors.
Author Contributions:
Bin Xu: Drafting and revision of the review.
Ronald Ghossein: Drafting and revision of the review.
References
- 1.Seethala RR, Asa SL, Carty SE, et al. Protocol for the examination of specimens from patients with carcinomas of the thyroid gland. College of american pathologists; College of American Pathologist; 2016. [Google Scholar]
- 2.Haugen BRM, Alexander EK, Bible KC, et al. 2015 american thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddad RI, Lydiatt WM, Bischoff L, et al. Nccn clinical practive guidelines in oncology (nccn guidelines): Thyroid carcinoma. Version 1.2017. National Comprehensive Cancer Network; 2017. [Google Scholar]
- 4.Amin MB, Edge SB, GF L, et al. Ajcc cancer staging manual Eighth edition. New York: Springer Nature; 2017. [Google Scholar]
- 5.Ortiz S, Rodriguez JM, Soria T, et al. Extrathyroid spread in papillary carcinoma of the thyroid: Clinicopathological and prognostic study. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001;124:261–265. doi: 10.1067/mhn.2001.113141. [DOI] [PubMed] [Google Scholar]
- 6.Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. Differentiated carcinoma of the thyroid with extrathyroidal extension. American journal of surgery. 1995;170:467–470. doi: 10.1016/s0002-9610(99)80331-6. [DOI] [PubMed] [Google Scholar]
- 7.Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J. Papillary carcinoma of the thyroid. A clinicopathologic study of 241 cases treated at the university of florence, italy. Cancer. 1985;55:805–828. doi: 10.1002/1097-0142(19850215)55:4<805::aid-cncr2820550419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 8.McConahey WM, Hay ID, Woolner LB, van Heerden JA, Taylor WF. Papillary thyroid cancer treated at the mayo clinic, 1946 through 1970: Initial manifestations, pathologic findings, therapy, and outcome. Mayo Clinic proceedings. 1986;61:978–996. doi: 10.1016/s0025-6196(12)62641-x. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, Tomoda C, Uruno T, et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: Massive but not minimal extension affects the relapse-free survival. World journal of surgery. 2006;30:780–786. doi: 10.1007/s00268-005-0270-z. [DOI] [PubMed] [Google Scholar]
- 10.Jukkola A, Bloigu R, Ebeling T, Salmela P, Blanco G. Prognostic factors in differentiated thyroid carcinomas and their implications for current staging classifications. Endocrine-related cancer. 2004;11:571–579. doi: 10.1677/erc.1.00826. [DOI] [PubMed] [Google Scholar]
- 11.Nixon IJ, Ganly I, Patel S, et al. The impact of microscopic extrathyroid extension on outcome in patients with clinical t1 and t2 well-differentiated thyroid cancer. Surgery. 2011;150:1242–1249. doi: 10.1016/j.surg.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radowsky JS, Howard RS, Burch HB, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid : official journal of the American Thyroid Association. 2014;24:241–244. doi: 10.1089/thy.2012.0567. [DOI] [PubMed] [Google Scholar]
- 13.Riemann B, Kramer JA, Schmid KW, et al. Risk stratification of patients with locally aggressive differentiated thyroid cancer. Results of the msds trial. Nuklearmedizin. 2010;49:79–84. doi: 10.3413/nukmed-0302. [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Tomoda C, Uruno T, et al. Minimal extrathyroid extension does not affect the relapse-free survival of patients with papillary thyroid carcinoma measuring 4 cm or less over the age of 45 years. Surg Today. 2006;36:12–18. doi: 10.1007/s00595-005-3090-8. [DOI] [PubMed] [Google Scholar]
- 15.Shin JH, Ha TK, Park HK, et al. Implication of minimal extrathyroidal extension as a prognostic factor in papillary thyroid carcinoma. International journal of surgery. 2013;11:944–947. doi: 10.1016/j.ijsu.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima M, Ito Y, Hirokawa M, Miya A, Shimizu K, Miyauchi A. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World journal of surgery. 2010;34:3007–3014. doi: 10.1007/s00268-010-0776-x. [DOI] [PubMed] [Google Scholar]
- 17.Xu B, Ibrahimpasic T, Wang LY, et al. Clinico-pathologic features of fatal non-anaplastic follicular cell-derived thyroid carcinomas. Thyroid : official journal of the American Thyroid Association. 2016 doi: 10.1089/thy.2016.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JW, Roh JL, Gong G, et al. Extent of extrathyroidal extension as a significant predictor of nodal metastasis and extranodal extension in patients with papillary thyroid carcinoma. Annals of surgical oncology. 2017;24:460–468. doi: 10.1245/s10434-016-5594-4. [DOI] [PubMed] [Google Scholar]
- 19.Xu B, Ibrahimpasic T, Wang L, et al. Clinicopathologic features of fatal non-anaplastic follicular cell-derived thyroid carcinomas. Thyroid : official journal of the American Thyroid Association. 2016;26:1588–1597. doi: 10.1089/thy.2016.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rivera M, Ricarte-Filho J, Tuttle RM, et al. Molecular, morphologic, and outcome analysis of thyroid carcinomas according to degree of extrathyroid extension. Thyroid : official journal of the American Thyroid Association. 2010;20:1085–1093. doi: 10.1089/thy.2010.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghossein R. Problems and controversies in the histopathology of thyroid carcinomas of follicular cell origin. Archives of pathology & laboratory medicine. 2009;133:683–691. doi: 10.5858/133.5.683. [DOI] [PubMed] [Google Scholar]
- 22.Su HK, Wenig BM, Haser GC, et al. Inter-observer variation in the pathologic identification of minimal extrathyroidal extension in papillary thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2016;26:512–517. doi: 10.1089/thy.2015.0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Ajcc cancer staging manual. Springer; 2009. [Google Scholar]
- 24.Hong CM, Ahn BC, Park JY, Jeong SY, Lee SW, Lee J. Prognostic implications of microscopic involvement of surgical resection margin in patients with differentiated papillary thyroid cancer after high-dose radioactive iodine ablation. Ann Nucl Med. 2012;26:311–318. doi: 10.1007/s12149-012-0574-7. [DOI] [PubMed] [Google Scholar]
- 25.Lang BH, Shek TW, Wan KY. Does microscopically involved margin increase disease recurrence after curative surgery in papillary thyroid carcinoma? Journal of surgical oncology. 2016;113:635–639. doi: 10.1002/jso.24194. [DOI] [PubMed] [Google Scholar]
- 26.Kluijfhout WP, Pasternak JD, Kwon JS, et al. Microscopic positive tumor margin does not increase the risk of recurrence in patients with t1-t2 well-differentiated thyroid cancer. Annals of surgical oncology. 2016;23:1446–1451. doi: 10.1245/s10434-015-4998-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang LY, Ghossein R, Palmer FL, et al. Microscopic positive margins in differentiated thyroid cancer is not an independent predictor of local failure. Thyroid : official journal of the American Thyroid Association. 2015;25:993–998. doi: 10.1089/thy.2015.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid : official journal of the American Thyroid Association. 2012;22:1144–1152. doi: 10.1089/thy.2012.0043. [DOI] [PubMed] [Google Scholar]
- 29.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Torotti A. Ajcc cancer staging manual Seventh edition. New York: Springer-Verlag; 2010. [Google Scholar]
- 30.Nixon IJ, Wang LY, Palmer FL, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery. 2014;156:137–146. doi: 10.1016/j.surg.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Tran Cao HS, Johnston LE, Chang DC, Bouvet M. A critical analysis of the american joint committee on cancer (ajcc) staging system for differentiated thyroid carcinoma in young patients on the basis of the surveillance, epidemiology, and end results (seer) registry. Surgery. 2012;152:145–151. doi: 10.1016/j.surg.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhattacharyya N. A population-based analysis of survival factors in differentiated and medullary thyroid carcinoma. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003;128:115–123. doi: 10.1067/mhn.2003.2. [DOI] [PubMed] [Google Scholar]
- 33.Wu MH, Shen WT, Gosnell J, Duh QY. Prognostic significance of extranodal extension of regional lymph node metastasis in papillary thyroid cancer. Head & neck. 2015;37:1336–1343. doi: 10.1002/hed.23747. [DOI] [PubMed] [Google Scholar]
- 34.Alpert EH, Wenig BM, Dewey EH, Su HK, Dos Reis L, Urken ML. Size distribution of metastatic lymph nodes with extranodal extension in patients with papillary thyroid cancer: A pilot study. Thyroid : official journal of the American Thyroid Association. 2015;25:238–241. doi: 10.1089/thy.2014.0392. [DOI] [PubMed] [Google Scholar]
- 35.Moritani S. Impact of invasive extranodal extension on the prognosis of patients with papillary thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2014;24:1779–1783. doi: 10.1089/thy.2014.0167. [DOI] [PubMed] [Google Scholar]
- 36.Lango M, Flieder D, Arrangoiz R, et al. Extranodal extension of metastatic papillary thyroid carcinoma: Correlation with biochemical endpoints, nodal persistence, and systemic disease progression. Thyroid : official journal of the American Thyroid Association. 2013;23:1099–1105. doi: 10.1089/thy.2013.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito Y, Hirokawa M, Jikuzono T, et al. Extranodal tumor extension to adjacent organs predicts a worse cause-specific survival in patients with papillary thyroid carcinoma. World journal of surgery. 2007;31:1194–1201. doi: 10.1007/s00268-007-9042-2. [DOI] [PubMed] [Google Scholar]
- 38.Asanuma K, Kusama R, Maruyama M, Fujimori M, Amano J. Macroscopic extranodal invasion is a risk factor for tumor recurrence in papillary thyroid cancer. Cancer letters. 2001;164:85–89. doi: 10.1016/s0304-3835(00)00698-4. [DOI] [PubMed] [Google Scholar]
- 39.Ricarte-Filho J, Ganly I, Rivera M, et al. Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic braf, the number of nodal metastases, and extra-nodal extension. Thyroid : official journal of the American Thyroid Association. 2012;22:575–584. doi: 10.1089/thy.2011.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du E, Wenig BM, Su HK, et al. Inter-observer variation in the pathologic identification of extranodal extension in nodal metastasis from papillary thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2016;26:816–819. doi: 10.1089/thy.2015.0551. [DOI] [PubMed] [Google Scholar]
- 41.Graham A. Malignant epithelial tumors of the thyroid, with special reference to blood vessels. Surg Gynecol Obstet. 1924;39:781–790. [Google Scholar]
- 42.Collini P, Sampietro G, Pilotti S. Extensive vascular invasion is a marker of risk of relapse in encapsulated non-hurthle cell follicular carcinoma of the thyroid gland: A clinicopathological study of 18 consecutive cases from a single institution with a 11-year median follow-up. Histopathology. 2004;44:35–39. doi: 10.1111/j.1365-2559.2004.01729.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghossein RA, Hiltzik DH, Carlson DL, et al. Prognostic factors of recurrence in encapsulated hurthle cell carcinoma of the thyroid gland: A clinicopathologic study of 50 cases. Cancer. 2006;106:1669–1676. doi: 10.1002/cncr.21825. [DOI] [PubMed] [Google Scholar]
- 44.Ito Y, Hirokawa M, Masuoka H, et al. Prognostic factors of minimally invasive follicular thyroid carcinoma: Extensive vascular invasion significantly affects patient prognosis. Endocrine journal. 2013;60:637–642. doi: 10.1507/endocrj.ej12-0419. [DOI] [PubMed] [Google Scholar]
- 45.Lang W, Choritz H, Hundeshagen H. Risk factors in follicular thyroid carcinomas. A retrospective follow-up study covering a 14-year period with emphasis on morphological findings. The American journal of surgical pathology. 1986;10:246–255. [PubMed] [Google Scholar]
- 46.Xu B, Wang L, Tuttle RM, Ganly I, Ghossein R. Prognostic impact of extent of vascular invasion in low-grade encapsulated follicular cell-derived thyroid carcinomas: A clinicopathologic study of 276 cases. Human pathology. 2015;46:1789–1798. doi: 10.1016/j.humpath.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao J, Hu JL, Chen C, et al. Vascular invasion is an independent prognostic factor for distant recurrence-free survival in papillary thyroid carcinoma: A matched-case comparative study. J Clin Pathol. 2016;69:872–877. doi: 10.1136/jclinpath-2015-203547. [DOI] [PubMed] [Google Scholar]
- 48.Lloyd RV, Osamura RY, Kloppel G, Rosai J. Who classification of tumours of endocrine organs. Lyon: International Agency for Research on Cancer (IARC); 2017. [Google Scholar]
- 49.Haugen BR, Sawka AM, Alexander EK, et al. American thyroid association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid : official journal of the American Thyroid Association. 2017;27:481–483. doi: 10.1089/thy.2016.0628. [DOI] [PubMed] [Google Scholar]
- 50.Xu B, Tuttle RM, Sabra MM, Ganly I, Ghossein R. Primary thyroid carcinoma with low-risk histology and distant metastases: Clinicopathologic and molecular characteristics. Thyroid : official journal of the American Thyroid Association. 2017 doi: 10.1089/thy.2016.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganly I, Ricarte Filho J, Eng S, et al. Genomic dissection of hurthle cell carcinoma reveals a unique class of thyroid malignancy. The Journal of clinical endocrinology and metabolism. 2013;98:E962–972. doi: 10.1210/jc.2012-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosai JCM, Delellis RA. Tumors of the thyroid gland. Washington, DC: Armed Forces Institute of Pathology; 1992. [Google Scholar]
- 53.Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, T G. Tumor of the thyroid and parathyroid gland (afip atlas of tumor pathology series 4) Silver Spring, MD: American Registry of Pathology Press; 2015. p. 606. [Google Scholar]
- 54.Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol. 2011;24:1545–1552. doi: 10.1038/modpathol.2011.119. [DOI] [PubMed] [Google Scholar]
- 55.Kim HJ, Sung JY, Oh YL, et al. Association of vascular invasion with increased mortality in patients with minimally invasive follicular thyroid carcinoma but not widely invasive follicular thyroid carcinoma. Head & neck. 2014;36:1695–1700. doi: 10.1002/hed.23511. [DOI] [PubMed] [Google Scholar]
- 56.Wreesmann VB, Nixon IJ, Rivera M, et al. Prognostic value of vascular invasion in well-differentiated papillary thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2015;25:503–508. doi: 10.1089/thy.2015.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Falvo L, Catania A, D’Andrea V, Marzullo A, Giustiniani MC, De Antoni E. Prognostic importance of histologic vascular invasion in papillary thyroid carcinoma. Annals of surgery. 2005;241:640–646. doi: 10.1097/01.sla.0000157317.60536.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Heerden JA, Hay ID, Goellner JR, et al. Follicular thyroid carcinoma with capsular invasion alone: A nonthreatening malignancy. Surgery. 1992;112:1130–1136. discussion 1136–1138. [PubMed] [Google Scholar]
- 59.Huang CC, Hsueh C, Liu FH, Chao TC, Lin JD. Diagnostic and therapeutic strategies for minimally and widely invasive follicular thyroid carcinomas. Surgical oncology. 2011;20:1–6. doi: 10.1016/j.suronc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 60.O’Neill CJ, Vaughan L, Learoyd DL, Sidhu SB, Delbridge LW, Sywak MS. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2011;37:181–185. doi: 10.1016/j.ejso.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Goldstein NS, Czako P, Neill JS. Metastatic minimally invasive (encapsulated) follicular and hurthle cell thyroid carcinoma: A study of 34 patients. Mod Pathol. 2000;13:123–130. doi: 10.1038/modpathol.3880023. [DOI] [PubMed] [Google Scholar]
- 62.Collini P, Sampietro G, Rosai J, Pilotti S. Minimally invasive (encapsulated) follicular carcinoma of the thyroid gland is the low-risk counterpart of widely invasive follicular carcinoma but not of insular carcinoma. Virchows Archiv : an international journal of pathology. 2003;442:71–76. doi: 10.1007/s00428-002-0701-2. [DOI] [PubMed] [Google Scholar]
- 63.Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA oncology. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sugino K, Kameyama K, Ito K, et al. Outcomes and prognostic factors of 251 patients with minimally invasive follicular thyroid carcinoma. Thyroid : official journal of the American Thyroid Association. 2012;22:798–804. doi: 10.1089/thy.2012.0051. [DOI] [PubMed] [Google Scholar]
- 65.Thompson LD, Wieneke JA, Paal E, Frommelt RA, Adair CF, Heffess CS. A clinicopathologic study of minimally invasive follicular carcinoma of the thyroid gland with a review of the english literature. Cancer. 2001;91:505–524. doi: 10.1002/1097-0142(20010201)91:3<505::aid-cncr1029>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Dionigi G, Kraimps JL, Schmid KW, et al. Minimally invasive follicular thyroid cancer (miftc)–a consensus report of the european society of endocrine surgeons (eses) Langenbeck’s archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2014;399:165–184. doi: 10.1007/s00423-013-1140-z. [DOI] [PubMed] [Google Scholar]


