Abstract
Importance
The management of lentigo maligna (LM) and LM melanoma (LMM) is challenging because of extensive subclinical spread and its occurrence on cosmetically-sensitive areas. Reflectance confocal microscopy (RCM) improves diagnostic accuracy for LM/LMM and can be used to delineate its margins.
Objectives
To evaluate whether handheld RCM with radial videomosaicking (HRCM-RV) offers accurate LM/LMM margin presurgical assessment.
Design
Prospective study involving consecutive patients with biopsy-proven LM/LMM located on the head-and-neck area who consulted for surgical management from March 2016 to March 2017.
Setting
Dermatology Service, Memorial Sloan Kettering Cancer Center, New York
Participants
Thirty-two patients were imaged using HRCM-RV. Ultimately, 23 LM/LMM underwent staged surgery and contributed to the analysis.
Main Outcomes and Measures
Clinical lesion size and area, LM/LMM area based on HRCM, surgical defect area predicted by HRCM, and observed surgical defect area. We also calculated the millimeters needed to achieve tumor clearance in each quadrant based on HRCM and compared them with the actual surgical margins.
Results
The mean surgical defect area predicted with HRCM-RV was 6.3 cm2 and the mean area of surgical excision with clear margins was 7.7 cm2. Overall, controlling for patient age and previous surgery, surgical margins were on average 0.76 mm larger than the HRCM-RV prediction (95% CI: 0.67– 0.84, p<0.001).
Conclusions and Relevance
HRCM-RV mapping of LM/LMM predicts similar, but slightly smaller, defects than staged excision. Thus, mapping of LM using HRCM-RV can result in sparing of healthy tissue by reducing the number of biopsies in clinically-uncertain areas and may be used to plan treatment of LM/LMM and counsel patients appropriately.
Keywords: reflectance confocal microscopy, handheld reflectance confocal microscopy, lentigo maligna, lentigo maligna melanoma, staged excision, surgery, margin control
Delineating the borders of lentigo maligna (LM) and LM melanoma (LMM) is challenging as they occur in heavily sun-damaged areas and may be hypo/amelanotic with subclinical spread not visible to the naked eye.1 This can lead to surgical margin underestimation, resulting in positive surgical margins and subsequent recurrences.2,3 Additionally, because LM/LMM occurs on cosmetically-sensitive areas such as the face and can have extensive subclinical spread, complete margin assessment and control with techniques such as Mohs micrographic surgery, staged excision or the spaghetti technique are of paramount importance. It is also known that the surgical margins needed to clear LM/LMM on the head and neck area are greater than traditional trunk and extremity melanomas.4–6
Dermoscopy and Wood’s lamp examination improve the delineation of LM/LMM margins.7 However, false-positives and false-negatives occur,8 and scouting biopsies are often performed.9,10 However, scouting biopsies provide static information that can be difficult to contextualize, as certain features such as melanocytic hyperplasia occur in both benign, sun-exposed skin and at the trailing edge of LM/LMM. Reflectance confocal microscopy (RCM) is a non-invasive imaging system that allows in vivo cellular evaluation of the epidermis and upper dermis.11,12 RCM has a sensitivity of 85% and specificity of 76% to diagnose LM/LMM,13 and has also been used to delineate its margins.14,15 Most of this work is based on traditional widefield-probe RCM which requires attaching a metal ring onto the skin and allows imaging areas up to 8×8mm. However, LM/LMM can be larger than 8mm and can occur at uneven surfaces where the metal ring may detach. Thus, mapping the LM/LMM margins with widefield-probe RCM can be a labor-intensive and time-consuming process (~1 hour per lesion).14 Conversely, handheld RCM (HRCM) can acquire images and videos along ad hoc arbitrarily freeform non-rastered paths and over large areas which the user determines in real-time, allowing a “live” approach to mapping margins over large areas. Although the HRCM allows a less restrictive imaging acquisition, the data regarding its use in diagnosing and mapping LM/LMM is limited,15,16 mainly due to the lack of orientation during image acquisition and lack of mosaicking capabilities in the HRCM software. However, with the advent of HRCM videomosaicking, it is now possible to transform dynamic videos into static videomosaics to help obtain architectural information.17–20
Herein, we describe a study of a novel imaging technique obtaining radial HRCM videos and videomosaics - from the LM/LMM center to the periphery - guided by the use of adhesive paper rings. The aim of this study was to evaluate whether radial imaging offers accurate presurgical assessment for LM/LMM margins and anticipates surgical defects.
Methods
Clinical information
Under an Institutional Review Board-approved protocol at Memorial Sloan Kettering Cancer Center, from March 2016 to March 2017 we conducted a prospective study involving consecutive patients with LM/LMM who consulted for surgical management in our dermatology service. We included patients who had biopsy-proven LM/LMM located in the head-and-neck area, and who were imaged using HRCM radial videomosaicking (HRCM-RV) prior to staged excision with complete circumferential margin control. We excluded LM/LMM not located in the head-and-neck area, patients who decided not to undergo staged-excision, and patients who pursued treatment elsewhere.
We collected patient demographic information, prior LM/LMM treatments, lesion location, Breslow depth, and number surgical stages. We calculated the clinical lesion size and area, the LM/LMM area based on HRCM, the surgical defect area predicted by HRCM, and the observed surgical defect area. To calculate the areas, we took digital images of the lesions and surgical defects using a Canon PowerShot G10 camera (Canon Inc, Tokyo, Japan) or the VeosDS3 system (Canfield Scientific, Parsippany, NJ). Using Mirror 7.5 software (Canfield Scientific, Parsippany, NJ) we retrieved the images and calculated the areas after image calibration, using either a ruler placed in the image field, anthropometric measurements, or annotations in the medical chart such as defect size. To ensure measurement accuracy, two calibration methods were used for each image.
Clinical margin delineation and confocal imaging
Clinical margins were determined using dermoscopy and Wood’s lamp. To facilitate RCM navigation and margin calculation, we placed adhesive paper rings (product number 1529; 3M, Flemington, NJ) surrounding this margin21 (Figure 1). In irregularly-shaped lesions, multiple paper rings were placed and eventually overlapped to allocate the entire lesion inside the paper rings and image its entire borders. Several paper ring diameters are available; however, all paper rings have a 2.5mm-wide rim, which was used to calculate the predicted surgical margins. We imaged the lesions with a handheld reflectance confocal microscope (Vivascope3000; Caliber ID, Rochester, NY) which has a lateral resolution of ~ 1µm, optical sectioning of ~ 3µm and a field of view (FOV) of 1×1mm., Two confocalists (MC with >5 years of experience, OY with 1 year of experience at the beginning of the study) performed image acquisition together for the first 10 cases, and later together or alone depending on their availability in the clinic.
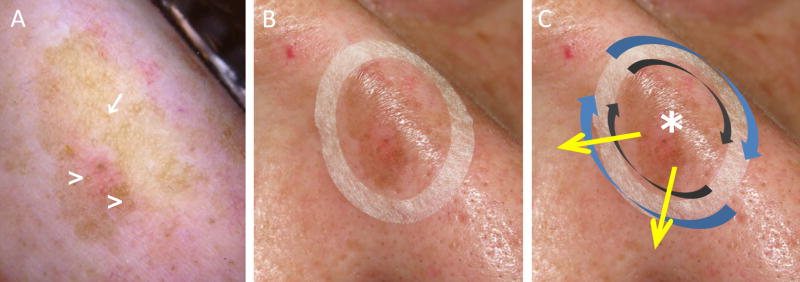
Figure 1.
Lentigo maligna margin determination using dermoscopy, Wood´s lamp examination and handheld reflectance confocal microscopy. A, Dermoscopy evaluation showing a pigmented lesion with ill-defined margins, asymmetric pigmented follicular openings (arrow) and circle within a circle (arrowheads). B, Paper ring placed outside the clinical margin determined with dermoscopy and Wood’s lamp to facilitate confocal navigation. C, Handheld reflectance confocal microscopy evaluation was performed by imaging initially the center to determine the cell morphology (asterisk), and later by imaging clockwise the peripheral margin inside the ring (grey arrows) and outside the ring (blue arrows). Radial videos were obtained (yellow arrows) in the areas where a higher degree of atypia was identified outside the paper ring to determine the LM/LMM subclinical extension.
Initially, we imaged the lesion center to identify the predominant melanoma cell morphology (large round, dendritic or pleomorphic).13 Next, we navigated clockwise along the ring’s inner margin to confirm the dermoscopy/Wood’s lamp margins. Then, we imaged along the outer ring margin to identify LM/LMM subclinical spread. When a positive area was identified outside the ring, we captured a video at the plane where the highest degree of atypia was identified and navigated radially from the lesion center toward the LM-positive area outside the ring until no LM features were observed. Radial videos were obtained in all the quadrants that were positive outside the paper ring.
To consider an area positive on HRCM, the features present in the LM algorithm (Table 1),13 validated to diagnose LM using widefield-probe RCM and HRCM, were used.13,16 When further evaluating the periphery, margins were considered positive for LM if any of the positive criteria of the LM algorithm was present, and if a single large round or a large dendritic cell was identified on RCM.15 Based on our previous experience and the results obtained when imaging complex LM/LMM cases,17 we also considered the margin positive if smaller atypical dendritic cells were observed continuing from the LM trailing edge (Table 1).
Table 1.
Reflectance confocal microscopy features considered positive for lentigo maligna at the edges in this study
| RCM features | ||
|---|---|---|
| Guitera et al.13 | Major criteria | Non-edged dermal papillae |
| Round large pagetoid cells | ||
| Minor criteria | Nucleated cells in dermal papillae | |
| Nucleated cells in dermal papillae | ||
| Atypical cells at DEJ | ||
| Follicular localization of atypical cells | ||
| Broadened honeycomb pattern (negative feature) | ||
| Champin et al.15 | Single large round or a large dendritic cell | |
| Yélamos et al. (current study) | Atypical dendritic cell (any size) continuing from the LM trailing edge | |
Abbreviations: DEJ, dermal-epidermal junction; LM, lentigo maligna; RCM, reflectance confocal microscopy
Determination of subclinical tumor margins using HRCM radial videomosaicking
To calculate subclinical margins with HRCM-RV, the acquired videos were converted into videomosaics using an algorithm written in MATLAB (Mathwork, Natick, MA).18,20 Briefly, frames were extracted and then stitched together to create mosaics of the imaged area. Videomosaics were used to calculate the subsurface extension by measuring the distance between the paper ring inner part (clinical margin) and the furthest HRCM-positive finding identified (confocal margin) (Figure 2 and Video 1). To determine these measurements, two reference parameters were used: 1) the ring rim width of 2.5mm; 2) each HRCM FOV of 1×1mm, which equals 1000×1000 pixels. We overlaid these measurements on the clinical pictures and calculated the HRCM-predicted area. Margin determination was performed by a board-certified dermatologist with experience in RCM and dermatopathology (OY) by reviewing the radial videomosaics obtained in each quadrant.
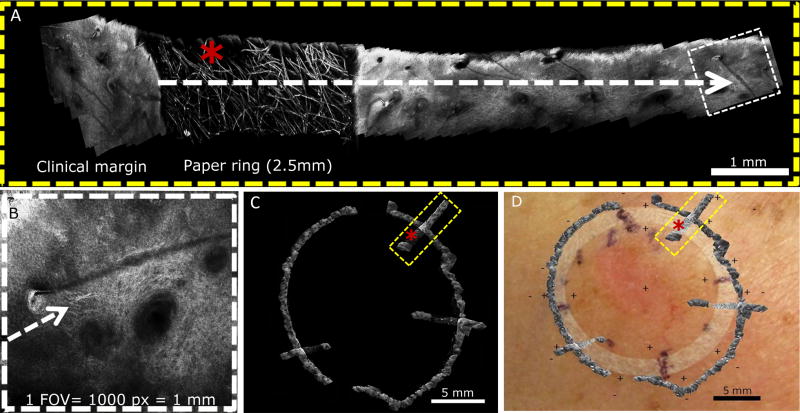
Figure 2.
Margin determination in case 3 using handheld reflectance confocal microscopy with radial videomosaicking. A, Videomosaic obtained from a video acquired at the 1 o’clock position from the center to the periphery (Video 1). Lentigo maligna extension was based on: 2.5 mm paper ring rim width (asterisk), and the field of view (FOV) of the handheld reflectance confocal microscope is 1mm, which equals 1000 pixels (panel B). B, Single FOV at the edge showing single dendritic cells continuing from the trailing edge of a lentigo maligna at the 1 o’clock position. C, Digital overlap of videomosaics depicting 3 radial videomosaics obtained in 3 different lesion quadrants. D, Videomosaic map digitally overlaid on clinical image to predict surgical margin based on 3mm histologic clearance.
Staged excision and determination of surgical margins using HRCM radial videomosaicking
According to our practice protocol and standard of care,4,22,23 our surgeons (AR, KN, EL) performed staged excisions blinded to HRCM calculations with an initial 5mm margin beyond the clinical margin, which the surgeons determined using dermoscopy and Wood’s lamp examination. After radial histopathologic sectioning, further excision stages guided by histopathology results were performed until reaching at least 3mm of histological clearance.4,22,23 Thus, prior to surgery and based on these parameters, we determined the predicted surgical defects based on HRCM-RV. It should be noted that a hypothetical LM/LMM extending just to – but not beyond – the outer edge of the paper ring would yield 2.5mm of histologic clearance rather than 3mm. However, we considered these 0.5mm negligible, as such variations occur depending on the surgical technique.24 If a quadrant was positive outside the ring on HRCM, we added 3mm to the margin calculated using HRCM-RV (eFigure 1in the Supplement).
Statistical analysis
Descriptive statistics, such as means, standard deviations, medians, minimum and maximum values, and relative frequencies, were used to describe the patient, lesion, imaging and surgical characteristics. Normality of interval scaled variables was assessed by graphical methods. The imaging data were assessed using two approaches: 1) a lesion-based approach, with each lesion contributing one pair of surgical and HRCM measures (total lesion area [cm2]); and 2) a radial quadrant-based approach with each lesion contributing 4 pairs of surgical and HRCM measures of length (millimeters) based on the distances calculated emanating from the clinical margin to the surgical and HRCM margins at perpendicular 90° points around the lesion. For the lesion-based approach, a comparison was performed between the defect area predicted by HRCM imaging and the final surgical margin Paired t-tests were used to assess differences in the estimated lesion area measured by RCM and surgical excision. To assess differences in radial length between surgical and RCM imaging, random effects models were used. All analyses were performed using Stata SE v14.1 (Stata Corportation, College Station, TX).
Results
Thirty-two patients consented to participate in the study and were imaged using HRCM-RV. Seven patients decided not to undergo surgery and three decided to have treatment elsewhere. Nineteen LM and 4 LMM (median Breslow 0.37mm) coming from 22 patients (12 men, 10 women) with a mean age of 68.95 years (SD 8.62, range: 46– 83 years) contributed to the analysis. None of the LM or LMM cases were upstaged after staged excision. Patient characteristics and imaging measurements are summarized in eTable 1 in the Supplement. The mean visualized clinical lesion maximum diameter was 1.86cm (SD=1.77, range: 0.5cm –4.9cm), and the mean area determined by clinical examination was 1.85cm2 (SD=1.77, range: 0.3 – 6.5cm2). The mean LM area based on HRCM was 3.9cm2 (SD=3.3, range: 1.1 – 16.0cm2). In all cases, HRCM confirmed the diagnosis of LM within the dermoscopy/Wood’s lamp margin. In 43.4% of quadrants (40 out of 92) HRCM identified LM beyond the clinical margin, extending 3.6mm on average (SD=2.6, range: 0.5 –11.0mm). The mean surgical defect area predicted with HRCM was smaller than the surgical margin area (6.34 cm2 versus 7.74 cm2, p=0.01) (Figure 3).
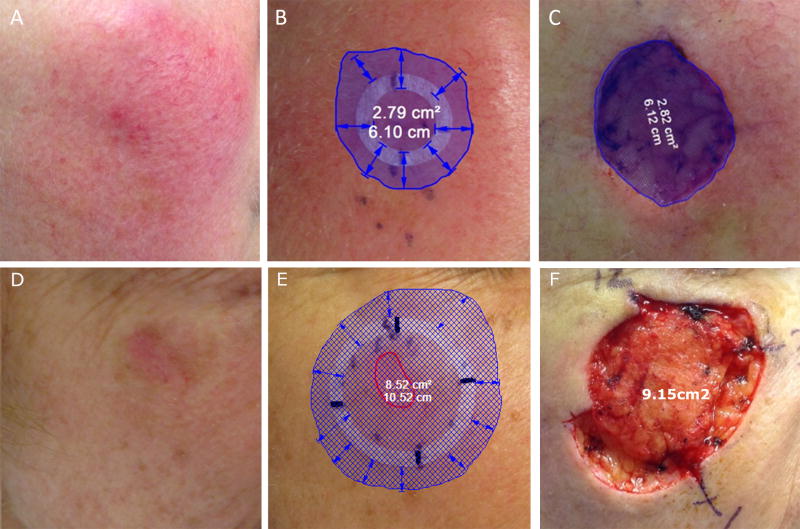
Figure 3.
Examples of lentigo maligna melanoma (case 2, panels A–C) and lentigo maligna (case 3, D–F) imaged with handheld reflectance confocal microscopy with radial videomosaicking and corresponding surgical defects after staged excision. A, Clinical image of case 2 showing an amelanotic ill-defined lentigo maligna melanoma (Breslow 0.39 mm) with a clinical size of 0.26 cm2 B, Estimated surgical margin after radial videomosaicking predicted a 2.79 cm2 defect. C, Final surgical defect was 2.82 cm2 after 2 stages of excision. D, Clinical image of case 3 showing a hypomelanotic ill-defined lentigo maligna with a clinical size of 0.54cm2. E, Estimated surgical margin after radial videomosaicking predicted an 8.52 cm2 defect. F, Final surgical defect was 9.15cm2 after 3 stages of excision.
A total of 92 pairs of radial measurements (4 per lesion, 1 per quadrant) were made from the clinical margin to the HRCM-predicted surgical margin. When evaluating the millimeters needed to be excised to achieve clearance, in 58 of the 92 quadrants (63.0%) there were no differences between the values predicted with HRCM and the observed surgical values. In 25 quadrants (27.2%), the defect was larger than the HRCM prediction (mean 2.24mm, range 1–6mm), and in 9 quadrants (9.8%) HRCM overestimated the margin by 1.27mm on average (range: 0.5–3mm). Overall, after controlling for patient age, previous surgery and clustering of observation within a given patient, surgical margins were on average 0.76mm larger than the HRCM prediction (95% CI: 0.67– 0.84, p<0.001) (Figure 4).
Figure 4.
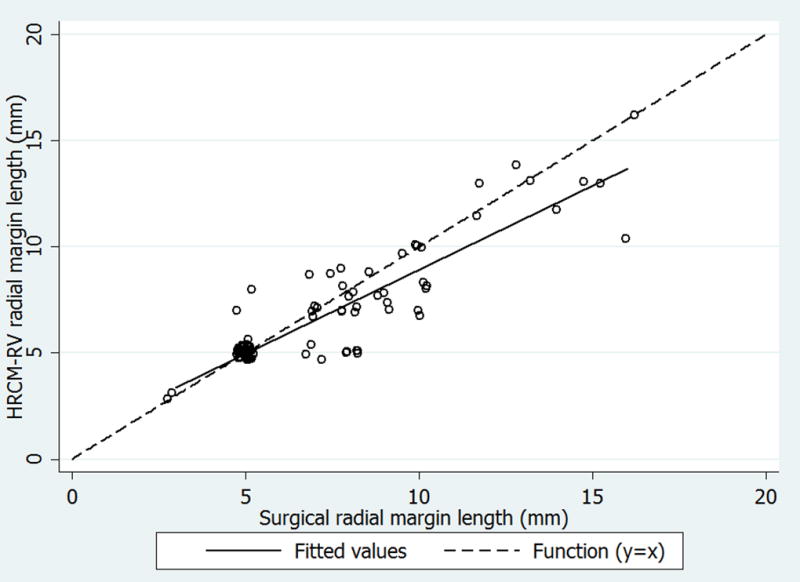
Plot depicting the paired observations (hollow circles) of the radial measurements of the surgical margins (mm) and handheld reflectance confocal microscopy (HRCM-RV) predicted margins (mm). There are 4 measures from each sample; one from each lesion quadrant measured from the center of the lesion to the perceived margin. The dashed line represents the linear function (y=x), where surgical measures and HRCM-RV are equal. The solid line represents the least squares fitted regression line for the relationship between the two measurement types. Hollow circles below the dotted line indicate observations where the surgical margin was greater than the HRCM-RV margin, hollow circles on the dotted line indicate similar estimates, and hollow circles above the dotted line indicate observations where HRCM-RV margins were larger than surgical margins. Observations were slightly offset to visualize overlapping data points. Overall, from our random effects regression model, controlling for patient age and previous surgery, surgical margins were on average 0.76 mm larger than the HRCM-RV prediction (95% CI: 0.67– 0.84, p<0.001).
Discussion
Although dermoscopy and Wood’s lamp have been used to guide LM excisions, in our study in ~40% of the quadrants RCM identified LM/LMM extending beyond the dermoscopic/Wood’s lamp margins. These results highlight that RCM improves LM/LMM margin evaluation12,14,15 and confirm the need for increased surgical margins to achieve clear margins.4,25–27 We used an imaging approach that parallels the design of staged excision with radial histopathologic sectioning, by imaging with HRCM radially from the center to the periphery. Our results suggest that HRCM-RV correlates well with surgical defects after staged excision of LM/LMM, although the average HRCM-RV predictions tend to be smaller than the actual defect. This can be due to difficulty assessing LM edges in a background of sun-damaged skin, more precise margin delineation with RCM, or errors in margin estimation.
In this study, to consider an area at the lesion edges positive for LM on RCM, we used the features present in the original LM diagnostic algorithm13, the presence of isolated large atypical cells at the edges,15 and, importantly, the presence of smaller atypical dendritic cells continuing from the LM trailing edge (Table 1). Because atypical melanocytes can occur in “healthy” sun-exposed skin, distinguishing LM from sun-reactive melanocytic hyperplasia can be challenging, both on confocal and histopathological evaluation.28 Thus, by considering smaller atypical cells as being positive for LM, one could argue that numerous false-positives may occur as they could correspond to activated melanocytes which frequently occur in sun-exposed skin. However, our findings suggest that in most quadrants HRCM predictions were equivalent to the final surgical margins or slightly smaller (0.76mm on average), and only in 9/92 quadrants we overcalled photodamage as LM. Interestingly, the cases with bigger differences came from previously-treated LM/LMM, suggesting that our approach performs better in non-recurrent LM/LMM (eTable 1 in the Supplement). Therefore, we believe that atypical melanocytes, regardless of their size, if evolving from a fully-fledged LM/LMM should be considered as positive as they may reflect the trailing edge of a LM/LMM (Figure 2, panel B).
Another explanation why our HRCM-predicted defects were smaller than the surgical defects could be that HRCM provides better margin delineation. Although our staged excision is very conservative in terms of tissue sparing, several factors can influence the amount of tissue excised: the thickness of the pen used to do the marking, the use of a magnifying glass to draw the marking, or where the incision is performed (inside or outside the skin marking).24 These limitations are not present when digitally diagramming the margins on an image. However, we acknowledge that in quadrants where we did not identify LM outside the paper ring, we may have underestimated the margins by considering a quadrant negative if 2.5mm of clearance were obtained. However, the clinical relevance of such small differences is unknown, and other margin-controlled surgical procedures may choose to use less than 3mm of histological clearance for negative margins.5 Therefore, if HRCM-RV were used to guide surgery, it could be the starting point of margin-controlled surgical procedures as it predicted a smaller and not a larger defect. This is important because the presence of false-positives could lead to negative, but wider than necessary, margins.
We observed 9 false-positive quadrants which corresponded to areas of photodamage with small dendritic cells on RCM. The overestimation was by 1.27 mm on average (range 0.5–3mm), which represents an additional ~6 to 37% margin relative to the real margin size. Previous studies have shown that variations due to the surgical technique can range from 8 to 45%, when margins from 2 to 10mm are drawn on the skin.24 Thus, it is reassuring that overestimation only occurred in <10% of quadrants and by a range that occurs in standard surgical procedures. To overcome variation in margin estimation due to wound retraction after electrocoagulation, specimen shrinkage after formalin fixation,29 or inaccuracy in the digital margin calculation, we performed 2 methods of image calibration, margin estimation, and videomosaic quadrant size estimation, which have not been described in previous studies assessing the LM/LMM margins.7,8,14,15 However, further studies involving multiple readers with varying levels of experience are needed to validate these results, since our study included only two confocalists who acquired data and one who analyzed the results.
Our approach combines two major innovations - adhesive rings and videomosaics - to guide navigation and calculate the surgical margins. A common challenge when using HRCM is identifying the exact area on the skin being imaged. Recently, adhesive rings have been utilized to define the area of interest when imaging with HRCM.19,21,30 Adhesive rings are inexpensive, can be adapted to the shape of the lesion by slightly bending them or placing multiple rings outside the lesion margins, and, because the fibers can be identified on HRCM, can easily facilitate orientation thus allowing a single operator to perform the image acquisition, as opposed to the 2 operators needed with other LM mapping techniques using HRCM.15 We used commercially-available paper rings with a 2.5-mm-wide rim to calculate the surgical margins by combining them with videomosaicking; however, it is also possible to customize the diameter and rim width by cutting adhesive plastic,30 thus permitting tailored HRCM navigation and margin estimation.
Another challenge when using HRCM is the absence of architectural information due to lack of mosaicking capabilities in its native software. The ability to observe and examine architectural information in large fields of view is necessary, similar to that in pathology. For the newer HRCM with video acquisition, an algorithm has been developed to transform videos into videomosaics.17,18 In the current study, we have used radial videomosaicking to help delineate the LM/LMM margins. However, the current limitations of videomosaicking are that videomosaicking is not integrated in the HRCM software and that the video post-processing takes ~10 minutes per 1 minute of each video. Nevertheless, each radial video is generally ~20 second-long, making the whole HRCM-RV process ~30 minute-long. Therefore, our next phase of research will be on increasing the speed of post-processing and integrating videomosaicking into the native HRCM software, to obtain "on-the-fly" videomosaics. This would allow faster margin estimation both at the patient bedside and in the operation room. In the meantime, a simplified and less precise method to perform our radial imaging approach could be counting in real-time the number of positive FOV outside the paper ring while imaging radially from the center to the periphery.
Conclusion
Our results suggest that HRCM-RV margin mapping correlates well with histology, and can predict the subclinical extension and presurgical margins. LM/LMM management can benefit from non-invasively imaging radially, since evaluating a given LM/LMM from the center toward the periphery illustrates its trailing edge as it dissipates into melanocytic hyperplasia,4 a feature that can be difficult to contextualize when seen in an isolated sample such as a scouting biopsy. Thus, we believe our approach can result in sparing of healthy tissue by reducing the number of biopsies in clinically-uncertain areas. Furthermore, predicting the LM/LMM size and the estimated surgical defect may enable the clinician to decide the most adequate treatment, plan the surgical reconstruction in advance, and counsel the patient appropriately.
Supplementary Material
Mp4-format video acquired at the 1 o'clock position from the center to the periphery of the lesion in case 3. This video was converted into a videomosaic to calculate the lesion margins (see Figure 2A).
KEY POINTS.
Question: Is handheld reflectance confocal microscopy with radial videomosaicking (HRCM-RV) accurate to non-invasively delineate the subclinical lentigo maligna (LM) and LM melanoma (LMM) borders and estimate its margins?
Findings: In this prospective study we evaluated 23 LM/LMM and showed that overall, controlling for patient age and previous surgery, surgical margins were on average 0.76 mm larger than the HRCM-RV prediction (95% CI: 0.67– 0.84, p<0.001).
Meaning: HRCM-RV correlates well with histology and can be useful to plan treatment and counsel patients accordingly.
Acknowledgments
Funding/Support: This research was funded by the NIH/NIBIB grant R01EB020029, and partially by the NIH/NCI Cancer Center Support Grant P30 CA008748 and the Beca Excelencia Fundación Piel Sana (Dr Yélamos).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
| Funding/Sponsor was involved? | ||
|---|---|---|
| Design and conduct of the study | Yes__ | No X |
| Collection, management, analysis and interpretation of data | Yes__ | No X |
| Preparation, review, or approval of the manuscript | Yes__ | No X |
| Decision to submit the manuscript for publication | Yes__ | No X |
Footnotes
Author Contributions: Drs Yélamos and Rossi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Yélamos, Cordova, Rossi. Acquisition, analysis, and interpretation of data: Cordova, Yélamos, Dusza, Lee, Rossi, Nehal, Kose. Drafting of the manuscript: Yélamos, Rossi, Blank, Dusza, Rajadhyaksha, Kose. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dusza. Obtained funding: Yélamos, Rajadhyaksha. Administrative, technical, or material support: Kose, Blank, Lee. Study supervision: Rossi, Rajadhyaksha.
Financial Disclosure: Milind Rajadhyaksha is a former employee of and owns equity in Caliber Imaging and Diagnostics (formerly Lucid Inc.), the company that manufactures and sells the Vivascope confocal microscope. The Vivascope is the commercial version of an original laboratory prototype that he had developed at Massachusetts General Hospital, Harvard Medical School.
The other authors have no disclosures or conflicts of interest to report.
References
- 1.Star P, Guitera P. Lentigo Maligna, Macules of the Face, and Lesions on Sun-Damaged Skin: Confocal Makes the Difference. Dermatol Clin. 2016;34(4):421–429. doi: 10.1016/j.det.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Osborne JE, Hutchinson PE. A follow-up study to investigate the efficacy of initial treatment of lentigo maligna with surgical excision. Br J Plast Surg. 2002;55(8):611–615. doi: 10.1054/bjps.2002.3967. [DOI] [PubMed] [Google Scholar]
- 3.Pitman GH, Kopf AW, Bart RS, Casson PR. Treatment of lentigo maligna and lentigo maligna melanoma. J Dermatol Surg Oncol. 1979;5(9):727–737. doi: 10.1111/j.1524-4725.1979.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 4.Hazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: A retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58(1):142–148. doi: 10.1016/j.jaad.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma) J Am Acad Dermatol. 2011;64(1):113–118. doi: 10.1016/j.jaad.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72(5):840–850. doi: 10.1016/j.jaad.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Robinson JK. Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Arch Dermatol. 2004;140(9):1095–1100. doi: 10.1001/archderm.140.9.1095. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SB, Varma R, Raimer D, et al. Utility of Wood's light in margin determination of melanoma in situ after excisional biopsy. Dermatol Surg. 2015;41(5):572–578. doi: 10.1097/DSS.0000000000000345. [DOI] [PubMed] [Google Scholar]
- 9.Dengel L, Turza K, Noland MMB, Patterson JW, Slingluff CL. Skin Mapping With Punch Biopsies for Defining Margins in Melanoma: When You Don't Know How Far to Go. Annals of Surgical Oncology. 2008;15(11):3028–3035. doi: 10.1245/s10434-008-0138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeneby TT, Chang B, Bucky LP. Ultraviolet-assisted punch biopsy mapping for lentigo maligna melanoma. Ann Plast Surg. 2001;46(5):495–499. doi: 10.1097/00000637-200105000-00007. discussion 499–500. [DOI] [PubMed] [Google Scholar]
- 11.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104(6):946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 12.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers Surg Med. 2016 doi: 10.1002/lsm.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130(8):2080–2091. doi: 10.1038/jid.2010.84. [DOI] [PubMed] [Google Scholar]
- 14.Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149(6):692–698. doi: 10.1001/jamadermatol.2013.2301. [DOI] [PubMed] [Google Scholar]
- 15.Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40(3):247–256. doi: 10.1111/dsu.12432. [DOI] [PubMed] [Google Scholar]
- 16.Menge TD, Hibler BP, Cordova MA, Nehal KS, Rossi AM. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): A prospective study. J Am Acad Dermatol. 2016 doi: 10.1016/j.jaad.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Hibler B, Yélamos O, Cordova M, et al. Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna. Cutis. 2017;99(5):346–352. [PMC free article] [PubMed] [Google Scholar]
- 18.Kose K, Cordova M, Duffy M, Flores ES, Brooks DH, Rajadhyaksha M. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171(5):1239–1241. doi: 10.1111/bjd.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yélamos O, Hibler B, Cordova M, et al. Handheld Reflectance Confocal Microscopy for the Detection of Recurrent Extramammary Paget Disease. JAMA Dermatol. 2017;153(7):1–5. doi: 10.1001/jamadermatol.2017.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kose K, Gou M, Yélamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesion. Proceedings of SPIE Photonics West. 2017 [Google Scholar]
- 21.Marino ML, Rogers T, Sierra Gil H, Rajadhyaksha M, Cordova MA, Marghoob AA. Improving lesion localization when imaging with handheld reflectance confocal microscope. Skin Res Technol. 2016 doi: 10.1111/srt.12280. [DOI] [PubMed] [Google Scholar]
- 22.Joyce KM, Joyce CW, Jones DM, et al. An Assessment of Histological Margins and Recurrence of Melanoma In Situ. Plastic and Reconstructive Surgery Global Open. 2015;3(2):e301. doi: 10.1097/GOX.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire LK, Disa JJ, Lee EH, Busam KJ, Nehal KS. Melanoma of the lentigo maligna subtype: diagnostic challenges and current treatment paradigms. Plast Reconstr Surg. 2012;129(2):288e–299e. doi: 10.1097/PRS.0b013e31823aeb72. [DOI] [PubMed] [Google Scholar]
- 24.Lalla R, Brown TL, Griffiths RW. Where to draw the line: the error in marking surgical excision margins defined. Br J Plast Surg. 2003;56(6):603–606. doi: 10.1016/s0007-1226(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 25.Felton S, Taylor RS, Srivastava D. Excision Margins for Melanoma In Situ on the Head and Neck. Dermatol Surg. 2016;42(3):327–334. doi: 10.1097/DSS.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 26.Moyer JS, Rudy S, Boonstra PS, et al. Efficacy of Staged Excision With Permanent Section Margin Control for Cutaneous Head and Neck Melanoma. JAMA Dermatol. 2017;153(3):282–288. doi: 10.1001/jamadermatol.2016.4603. [DOI] [PubMed] [Google Scholar]
- 27.Connolly KL, Busam KJ, Nehal KS. Optimizing Outcomes for Cutaneous Head and Neck Melanoma. JAMA Dermatol. 2017;153(3):267–268. doi: 10.1001/jamadermatol.2016.4604. [DOI] [PubMed] [Google Scholar]
- 28.Prieto VG, Argenyi ZB, Barnhill RL, et al. Are en face frozen sections accurate for diagnosing margin status in melanocytic lesions? Am J Clin Pathol. 2003;120(2):203–208. doi: 10.1309/J1Q0-V35E-UTMV-R193. [DOI] [PubMed] [Google Scholar]
- 29.Blasdale C, Charlton FG, Weatherhead SC, Ormond P, Lawrence CM. Effect of tissue shrinkage on histological tumour-free margin after excision of basal cell carcinoma. Br J Dermatol. 2010;162(3):607–610. doi: 10.1111/j.1365-2133.2009.09577.x. [DOI] [PubMed] [Google Scholar]
- 30.Ali FR, Craythorne EE. Gummed rings as the outer marker of microscopically examined tissue (GROMMETs) as mapping adjuncts to in vivo reflectance confocal microscopy (RCM) J Am Acad Dermatol. 2016;75(3):e103–104. doi: 10.1016/j.jaad.2016.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mp4-format video acquired at the 1 o'clock position from the center to the periphery of the lesion in case 3. This video was converted into a videomosaic to calculate the lesion margins (see Figure 2A).