Abstract
Aldosterone production is initiated by angiotensin II (A-II) stimulation and activation of intracellular Ca2+ signaling. In aldosterone-producing adenoma (APA) cells, the activation of intracellular Ca2+ signaling is independent of the renin-angiotensin-aldosterone systems. The purpose of our study was to clarify molecular mechanisms of aldosterone production related to Ca2+ signaling. Transcriptome analysis revealed that the CALN1 gene encoding calneuron 1 had the strongest correlation with CYP11B2 among genes encoding Ca2+ binding proteins in APA. CALN1 modulation and synthetic or fluorescent compounds were used for functional studies in human adrenocortical carcinoma (HAC15) cells. CALN1 expression was 4.4-fold higher in APAs than non-functioning adrenocortical adenomas. CALN1 expression colocalized with CYP11B2 expression as investigated using immunohistochemistry in APA and zona glomerulosa of male rats fed by a low-salt diet. CALN1 expression was detected in the endoplasmic reticulum (ER) by using GFP-fused CALN1, CellLight ER-RFP and the corresponding antibodies. CALN1-overexpressing HAC15 cells showed increased Ca2+ in the ER and cytosol fluorescence based studies. Aldosterone production was potentiated in HAC15 cells by CALN1 expression, and dose-responsive inhibition with TMB-8 showed that CALN1-mediated Ca2+ storage in ER involved sarcoendoplasmic reticulum calcium transport ATPase. The silencing of CALN1 decreased Ca2+ in ER, and abrogated A-II or KCNJ5 T158A mediated aldosterone production in HAC15 cells. Increased CALN1 expression in APA was associated with elevated Ca2+ storage in ER and aldosterone over-production. Suppression of CALN1 expression prevented A-II or KCNJ5 T158A mediated aldosterone production in HAC15 cells, suggesting that CALN1 is a potential therapeutic target for excess aldosterone production.
Keywords: aldosterone-producing adenoma, CYP11B2, CALN1, Ca2+ signaling, angiotensin II
Introduction
Aldosterone is synthesized in the zona glomerulosa (ZG) of the normal adrenal gland primarily under regulation of the renin-angiotensin-aldosterone system 1. The binding of angiotensin II (A-II) to the angiotensin II type 1 receptor in the adrenal cortex triggers several intracellular signaling cascades including protein kinase C, calcium/calmodulin-dependent kinases, and mitogen-activated protein kinase (MAPK) 1-3. A-II stimulation cause cytosolic Ca2+ oscillations, which may be induced by its extrusion from ER through the inositol 1,4,5-trisphosphate receptor (IP3R) and inflow from outside the cells through Ca2+ channels in adrenal cells 4-6. The activation of intracellular signaling leads to aldosterone production via a series of steroidogenic enzymes including aldosterone synthase (CYP11B2), which catalyzes the final steps of aldosterone biosynthesis 1. A major site of aldosterone action is in the distal tubules of the kidney, where it is responsible for sodium homeostasis, resulting in blood pressure regulation.
Primary aldosteronism (PA), which is the most common cause of endocrine hypertension, results from the autonomous or inappropriate production of aldosterone from the adrenals 7. The rate of cardiovascular events in PA patients is higher than in patients with essential hypertension who have similar blood pressure and risk profiles 8, 9. PA is mainly classified into two subtypes: that caused by an aldosterone-producing adenoma (APA) or by bilateral idiopathic hyperaldosteronism (IHA) 7. Recently, exome-sequencing analyses demonstrated that 50 to 80% of APA harbored somatic mutations in KCNJ5, ATP1A1, ATP2B3, or CACNA1D 10-12. All of these mutations directly or indirectly activate intracellular Ca2+ signaling and CYP11B2 transcription 6, 10-12. Therefore, the activation of intracellular Ca2+ signaling is crucially important to produce aldosterone both in ZG and APA.
Ca2+ binding proteins (CaBP) play a pivotal role in the transduction of Ca2+ signaling 13. The binding of CaBPs with increased intracellular Ca2+ activate effector enzymes. Most CaBPs bind Ca2+ through the EF-hand motif, which consists of two alpha helices named E and F 14. The best-known CaBP, calmodulin, is expressed ubiquitously in most organs and tissues, and binds Ca2+ through four EF-hand domains. The binding induces several calmodulin kinases. 15. However, as we previously reported, the calmodulin kinase inhibitor dose not completely abrogate the increase in aldosterone production via the activation of Ca2+ signaling 6. Additionally, intracellular Ca2+ is stored in the ER and mitochondria, but the Ca2+ storage machinery has not been fully elucidated in APA.
In this study, we examined the gene expression levels of other CaBPs containing the EF-hand motif in APA, using a transcriptome analysis-based approach. Our results showed that the CALN1 gene encoding calneuron 1 was one of the most highly expressed genes and had the strongest correlation with CYP11B2 in APA. Importantly, CALN1 showed the highest global Ca2+ affinity among all neuronal calcium sensors with a KD of 180 nM 16. Therefore, we hypothesized that CALN1 plays a key role in aldosterone production in APA and the renin-angiotensin system. We demonstrated that APA and A-II stimulated cells had higher CALN1 expression, and CALN1 co-localized with CYP11B2. CALN1 was located in ER, its increased expression resulted in increased stored Ca2+ in ER, and it potentiated aldosterone production in human adrenocortical carcinoma (HAC15) cells.
Methods
Patients and tissue collection
The tissues of non-functioning adrenocortical adenoma (NFA, n=14) or APA (n=48) obtained at adrenalectomy were enrolled in this study, and stored at -80 °C until further use. First, 24 adrenal tumors including 5 NFA and 19 APA were examined by microarray analysis. Next, all of the tumors including 14 NFA and 48 APA were investigated by quantitative polymerase chain reaction (qPCR). Additional details including the diagnosis of APA and NFA are available in the online-only Data Supplement and our previous report 17. Our study was approved by the ethics committee of Hiroshima University, and written informed consent was obtained from all the patients.
Cell culture and Materials
The HAC15 cell line, a subclone of the H295R a human adrenocortical carcinoma cell 18, was provided by WE Rainey (University of Michigan, USA). The detail methods of cell culture were mentioned as previously reported and online-only Data Supplement 19.
A-II and A23187, Calcium ionophore, were purchased from Sigma Aldrich Co. Ltd. (St. Louis, MO, USA). The Fluo4-AM dye (Invitrogen, Carlsbad, CA, USA) was used to detect intracellular Ca2+ concentration. TMB-8, an inhibitor of sarcoendoplasmic reticulum calcium transport ATPase (SERCA), and 2APB, an inhibitor of inositol 1,4,5-triphosphate (IP3), were obtained from Wako (Osaka, Japan).
DNA extraction and genotyping
Genomic DNA from adrenal tissues and peripheral leucocytes was isolated by the DNeasy blood and tissue kit (Qiagen, Hilden, Germany), and stored at −20°C. PCR-based direct sequencing for KCNJ5, ATP1A1, ATP2B3, and CACNA1D was performed as previously described 19.
Lentiviral production and infection
Lentivirus production and infection in HAC15 cells were conducted as previously reported and online-only Data Supplement 6.
RNA extraction and qPCR assay
Total RNA extraction, reverse transcription and qPCR assay were performed as previously reported and online-only Data Supplement 17.
Western blot analysis
Cell lysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transfer, and blots processing were performed as previously reported and is in the online-only Data Supplement 20.
Intracellular localization of CALN1
pLVSIN-N-AcGFP with CALN1 was transduced in HAC15 cells. The ER and Golgi bodies were fluorescently labeled using CellLight ER-RFP (Thermo Fisher Scientific, Waltham, MA, USA) and CellLight Golgi-RFP (Thermo Fisher Scientific, Waltham, MA, USA), respectively, following the manufacturer's protocol. The fluorescence was detected using the BZ-9000 fluorescence microscope (KEYENCE, Itasca, IL, USA).
To investigate endogenous CALN1 expression, HAC15 cells were first incubated with CellLight ER-RFP for 24 hours. The cells were then fixed for 10 minutes in 4% paraformaldehyde, and 0.5% Triton X-100 was added to the fixed cells for 10 minutes. The primary antibody for CALN1 (Abcam, #ab117584, Cambridge, UK) and ProLong Gold Antifade Reagent with DAPI (Thermo Fisher Scientific, Waltham, MA, USA) were then applied.
Ca2+ concentration in ER and cytosol
The Ca2+ concentration in ER was detected by Calcium-measuring organelle-Entrapped Protein Indicator 1 (CEPIA) 21. pCMV R-CEPIA1er was purchased from Addgene (Cambridge, MA, USA). Transfection was performed using Polyethylenimine 40K (Polysciences, Inc., #23966, Warrington, PA, USA) in 70-80% confluent HAC15 cells. Forty-eight hours after transfection, pCDH-CALN1-MCS-EF1-Puro was infected into HAC15 cells. Forty-eight hours after the infection, the cells were incubated using DMEM/F12 with 0.1% Cosmic Calf serum for 24 hours. The medium was replaced with Hanks' Balanced Salt Solution without phenol red (Wako, Osaka, Japan) for 30 minutes, and the protein was detected at an excitation wavelength of 562 nm and an emission wavelength of 641 nm in a micro plate reader (Varioskan Flash, Thermo Fisher Scientific, Waltham, MA, USA).
After CALN1 transduction in HAC15 cells, the cells were incubated with Fluo4-AM for the detection of Ca2+ concentration in cytosol as previously described 6.
Immunohistochemistry
Rat adrenals were collected from isoflurane anesthetized rats perfused with sterile normal saline. Rat adrenals and human NFA and APA tissues were fixed in 4% buffered paraformaldehyde and embedded in paraffin. Four-micron sections were cut, processed and incubated with mouse monoclonal antibodies against rat or human CYP11B2 and CALN1 (Proteintech, #11477-1-AP, Rosemont, IL, USA) as previously described 22, 23.
Animals
Dahl Salt sensitive (SSjr) rats from the Department of pharmacology in-house colony were housed under standard condition including a 12 hour light cycle at University of Mississippi Medical Center. They were fed a standard phytosteroid-controlled rat chow with 0.2% Na+ (Teklad Global 16% protein rodent diet, Harlan Laboratories, Indianapolis, IN, USA) and tap water. At 8 weeks of age two groups, 4 males and 3-4 female rats received the regular chow (low-salt) or chow +0.9% saline to drink (high-salt) for 23 days before the adrenals were obtained. All animal protocols were approved by the Institutional Animal Care and Use Committee of the G.V. (Sonny) Montgomery Veterans Affairs Medical Center in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Statistical Analysis
Basic results were expressed as mean ± S.E. of at least three separate experiments in which each sample was assayed in triplicate or quadruplicate, and clinical results were expressed as mean ± S.D. Differences between two groups were analyzed for statistical significance by t-test and multiple groups were analyzed by one-way ANOVA followed by Bonferroni comparisons. The differences were considered to be significant at P<0.05. Analyses were performed using SPSS for Windows (release 24.0; SPSS Inc., Chicago, IL, USA).
Results
Transcriptome analysis of genes related to CaBP with EF-hand
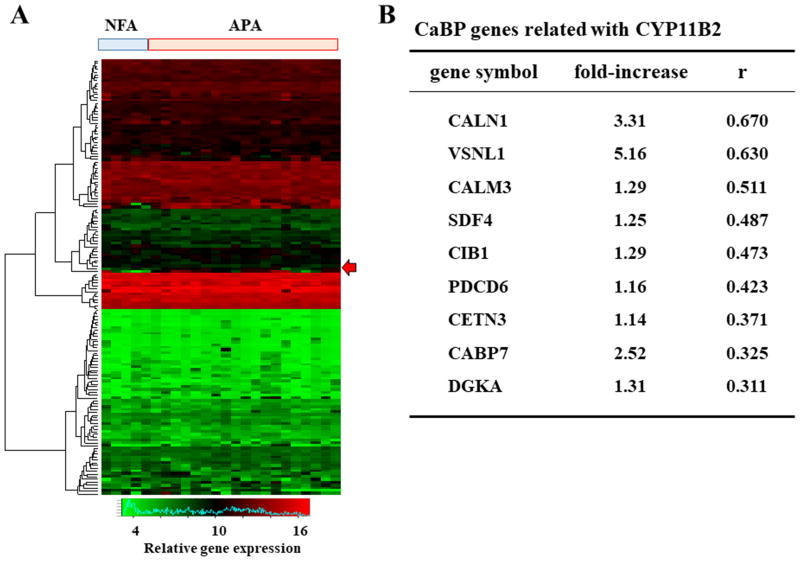
One hundred fifty-five genes encoding CaBPs with EF-hand were compared between NFAs (n=5) and APAs (n=19), and the heat map is depicted in Figure 1A. The most highly expressed among these was VSNL1 (Figure 1B). A previous study has revealed that VSNL1 protects APA cells from calcium-induced apoptosis 24. CALN1, which encodes calneuron 1, was the second highest expressed gene in the APAs, and showed the strongest correlation with CYP11B2 expression (Figure 1B). Furthermore, we showed all differentially expressed genes as well as genes encoding CaBPs between APAs and NFAs in Table S2 and S3.
Figure 1.
(A) A heat map was generated from the microarray analysis of non-functioning adenoma (NFA; n=5) and aldosterone-producing adenoma (APA; n=19) samples. Genes encoding Ca2+ binding protein (CaBP) with EF-hand were used in the analysis. Arrow expresses CALN1 expression. (B) The expressions of CaBP-encoding genes in APA were described in the comparison with those in NFA. The relationships between CaBP genes and CYP11B2 expression were tested by Pearson's analysis, and arranged by descending order of correlation coefficient.
Relationship between CALN1 and CYP11B2 expression in human tissues
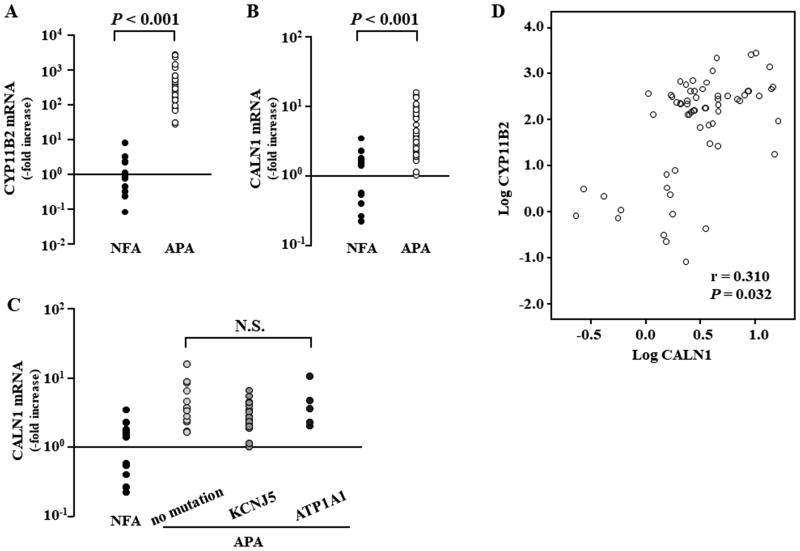
CYP11B2 expression was 535.1-fold higher in APAs than in NFAs (P<0.001, Figure 2A), and CALN1 expression was in 4.4-fold greater in APAs than in NFAs (P<0.001, Figure 2B). CALN1 mRNA expression levels was not different among APA genotypes such as no mutation (n=16), KCNJ5 mutation (n=27), and ATP1A1 mutation (n=5) (Figure 2C). CALN1 mRNA expression had a weak positive correlation with CYP11B2 mRNA expression in APA (r=0.310 and P=0.032, Figure 2D).
Figure 2.
CYP11B2 (A) and CALN1 (B) mRNA expression levels between non-functioning adrenocortical adenoma (NFA; n=14) and aldosterone-producing adenoma (APA; n=48) samples. (C) CALN1 mRNA expression levels were compared among different genotypes of APA such as no mutation, KCNJ5 mutation, and ATP1A1 mutation. (D) The CALN1 mRNA expression had positive relationship with CYP11B2 mRNA expression in APA (r = 0.310 and P = 0.032).
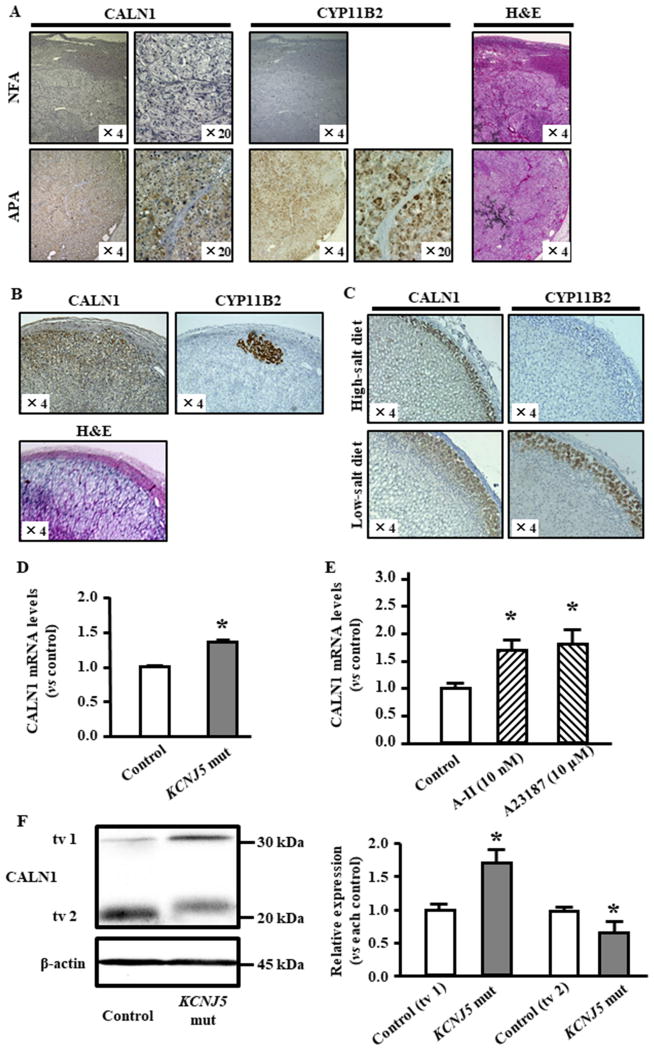
CALN1 expression was detected in APAs, but not in NFAs, and the expression appeared to be colocalized with CYP11B2 expression in APAs (Figure 3A). In addition, CALN1 was detected only in ZG cells in adrenal tissue adjacent to NFA (Figure 3B). Aldosterone-producing cell clusters (APCC) which are found by foci of CYP11B2 expressing cells in addition to normal CYP11B2 expression in ZG 25, and CALN1 expression was not only in APCC but also entire ZG in human (Figure 3B). Interestingly, CALN1 was not detected in the zona fasciculate (ZF) of the adrenal tissue (Figure 3B).
Figure 3.
CALN1 expression in human, rat model and HAC15 cells. (A) CALN1 and CYP11B2 expression in APA and NFA. (B) CALN1 and CYP11B2 expression in adjacent adrenal tissue of NFA. (C) CALN1 and CYP11B2 immunohistochemistry of rat adrenal cortex. Effect of feeding high or low-salt chow on CALN1 and CYP11B2 expression level in adrenal gland of male Dahl Salt sensitive rats. (D) HAC15 cells harboring the KCNJ5 T158A mutation had significantly higher CALN1 expression than control cells. (E) CALN1 expression was measured in HAC15 cells with control, angiotensin II (A-II) stimulation, and A23187 (Calcium ionophore) stimulation. (F) CALN1 and β-actin were detected by western blotting analysis in HAC15 cells where CALN1 expression was knocked down. HAC15 cells with KCNJ5 T158A mutation had higher CALN1 transcript variant (tv1) and less transcript variant 2 (tv2) compared with control cells. CALN1 tv1 in KCNJ5 mut cells was 1.72-fold higher than in control cells. CALN1 tv2 in KCNJ5 mut cells was 0.65-fold decreased than in control cells. *P<0.05 vs. each control (n=3).
CALN1 expression in rat adrenal glands
Figure 3C are representative photomicrographs of sections from a low-salt and a high-salt fed male rat adrenal. The adrenal ZG of the low-salt fed rat comprised more and larger cells, most of which were immunoreactive for CYP11B2 by immunohistochemistry. In contrast, the ZG of high-salt fed rats was narrower, comprised smaller and fewer ZG cells with only some being moderately immunoreactive for CYP11B2. Female rats generally produce slightly more ZG and ZF steroids, but they are regulated in the same was by salt consumption 26.
The expression of CALN1 was detected only in the rat adrenal ZG (Figure 3C), as in human tissues (Figure 3B). CALN1 immunoreactivity faded in the inner ZG next to the transition zone comprising smaller and more densely packed cells between the ZG and ZF, and is coexpressed in cells expressing CYP11B2, also consistent with its expression the human adrenal.
CALN1 expression in HAC15 cells
Infection of HAC15 cells with lentivirus carrying the KCNJ5-T158A mutation potentiated basal aldosterone production 5.8-fold over the empty lentivirus infection (data not shown). HAC15 cells expressing the KCNJ5 mutation had 1.4-fold higher CALN1 mRNA levels than control HAC15 cells (P<0.05, Figure 3E). CALN1 expression was significantly elevated by A-II stimulation in HAC15 cells (1.7-fold increase, P<0.05, Figure 3E). Calcium ionophore, A23187, significantly stimulated CALN1 expression (1.8-fold increase, P<0.05, Figure 3E), suggesting that increased Ca2+ signaling up-regulates CALN1 expression.
The CALN1 protein levels were detected by western blotting in HAC15 cells harboring the KCNJ5 mutation. Interestingly, CALN1 tv1 expression was significantly increased, whereas CALN1 tv2 was attenuated by this KCNJ5 mutation (Figure 3F). In addition, CALN1 tv1 transduction in HAC15 cells resulted in higher CYP11B2 expression and aldosterone levels than CALN1 tv2 transduction (Figure S1AB). Hence, we used CALN1 tv1 transduction was used for functional analysis.
Intracellular localization of CALN1 and Ca2+ storage in ER
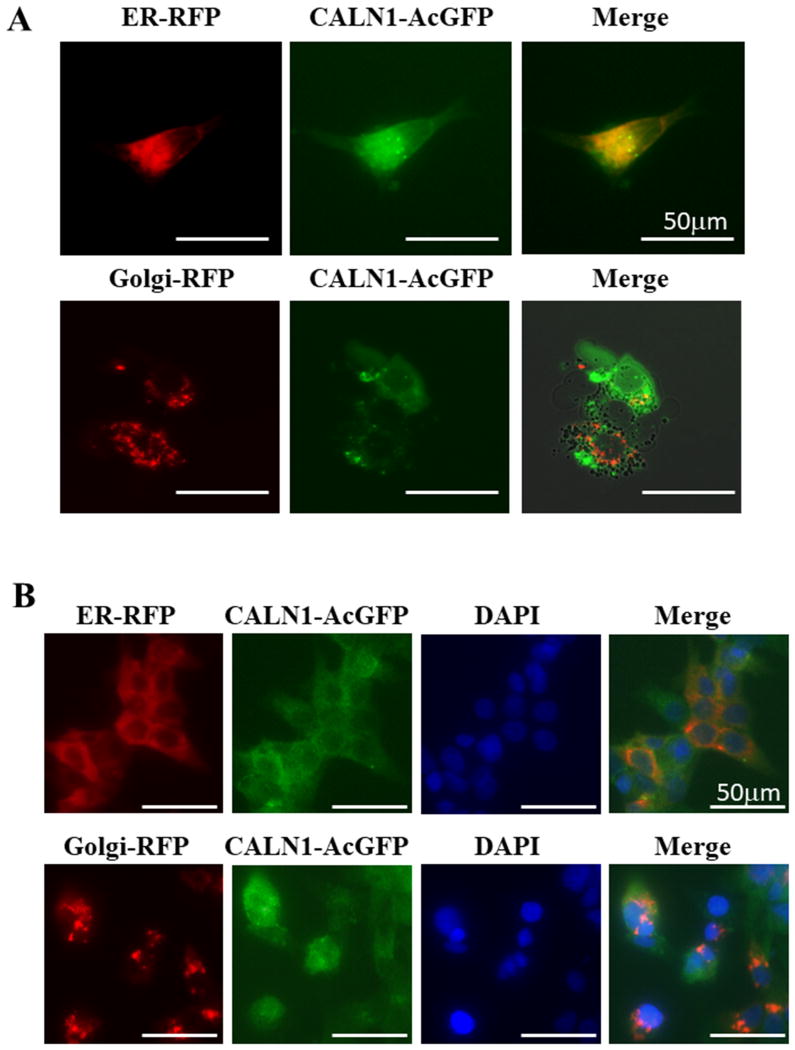
Previous study demonstrated that intracellular localization of CALN1 was likely to be in ER or Golgi bodies 27. We analyzed the intracellular localization of CALN1 using CellLight RFP to detect ER or Golgi in HAC15 cells transduced with pLVSIN-N-AcGFP with CALN1 (Figure 4A). Endogenous CALN1 expression was also visualized by staining with antibodies (Figure 4B). Taken together, our results confirm that CALN1 is located in ER, probably bound to the ER membrane through its tail-anchored transmembrane domain (TA domain) 28.
Figure 4.
Intracellular localization of CALN1 was evaluated by CellLight RFP tags for ER or Golgi and CALN1 with GFP. (A) pLVSIN-N-AcGFP with CALN1 was transduced in HAC15 cells and detected by a green color. The fluorescent label of ER and Golgi body were determined by CellLight ER-RFP (red) and CellLight Golgi-RFP (red). Scale bars represent 50 μm. (B) Immunofluorescence staining of native CALN1 in HAC15 cells by anti-CALN1 antibody (green). ER or Golgi-specific CellLight-RFP (red) was added and expressed in HAC15 cells to trace localization of each organelle. DAPI, which stained the nucleus, is shown as blue. Scale bars represent 50 μm.
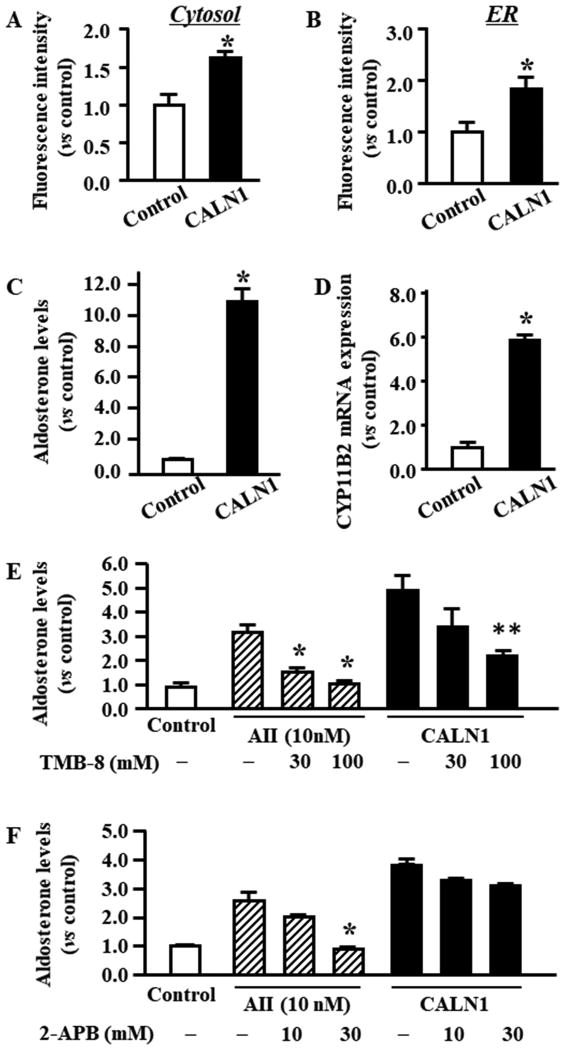
To examine the effects of CALN1 expression on Ca2+ levels in cytosol and ER, we used a specific dye, Fluo4-AM, and the R-CEPIA1er method, respectively. The CALN1 over-expressing cells had significantly higher Ca2+ levels in the cytosol and ER compared with control cells (Figure 5AB). Additionally, Ca2+ in ER was significantly increased by the KCNJ5 mutation in HAC15 cells (Figure S2).
Figure 5.
The effects of CALN1 transduction by lentiviral technique in HAC15 cells. (A) Fluo4-AM fluorescence, index of intracellular Ca2+, was compared between control and CALN1 cells. *P<0.05 vs. control (n=3). (B) R-CEPIAer was applied to compare intra-ER Ca2+ between control and CALN1 cells. *P<0.05 vs. control (n=3). (C) Aldosterone levels in media in HAC15 cells were compared between control and CALN1 cells. *P<0.05 vs. control (n=3). (D) CYP11B2 mRNA levels in control and CALN1 cells are shown. *P<0.05 vs. control (n=3). (E) The effects of TMB-8, an inhibitor of sarcoendoplasmic reticulum calcium transport ATPase (SERCA), on aldosterone production in HAC15 cells with angiotensin II (A-II) stimulation or CALN1 over-expression. *P<0.05 vs. HAC15 cells with A-II stimulation and without TMB-8 (n=3). **P<0.05 vs. HAC15 cells with CALN1 over-expression and without TMB-8 (n=3). (F) The analysis of 2-APB, an inhibitor of IP3, for aldosterone production in HAC15 cells mediated by A-II or CALN1 transduction. *P<0.05 vs. HAC15 cells with A-II stimulation and without 2-APB (n=3).
Effect of CALN1 over-expression on aldosterone production
Aldosterone levels in CALN1 over-expressing cells were 11.2-fold greater than those in control cells (P<0.05, Figure 5C and Figure S1A). In agreement with these results, CALN1 over-expressing cells had higher CYP11B2 expression than control cells (6.0-fold increase, P<0.05, Figure 5D and Figure S1B).
We investigated the effects of TMB-8 and 2-APB on aldosterone production in HAC15 cells over-expressing CALN1. TMB-8 showed a dose-responsive inhibition of aldosterone production mediated by A-II and CALN1 in HAC15 cells (Figure 5E). 2-APB significantly inhibited aldosterone production in response to A-II, but did not suppress aldosterone production in HAC15 cells over-expressing CALN1 (Figure 5F).
Silencing of CALN1 expression in HAC15 cells
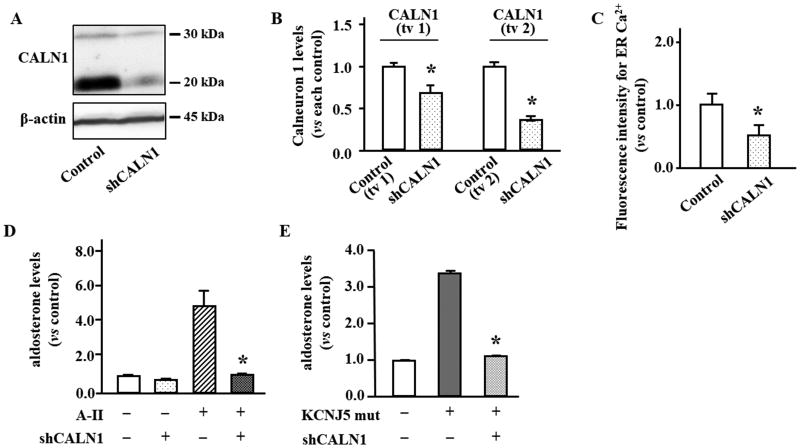
CALN1 was silenced using a lentivirus delivering shRNA-CALN1 in HAC15 cells (shCALN1 cells) and resulted in a decreased CALN1 tv1 and tv2 protein expression by 32% and 47%, respectively (Figure 6AB). shCALN1 cells had significantly less Ca2+ in ER compared with control cells (P<0.05, Figure 6C). The suppression of CALN1 expression significantly abrogated A-II and KCNJ5 T158A mediated aldosterone production in HAC15 cells (Figure 6DE).
Figure 6.
The effects of CALN1 silencing by lentiviral technique in HAC15 cells. (A) Expression of transcript variant 1 (tv1) and 2 (tv2) of CALN1 and β-actin were detected by western blotting analysis in HAC15 cells with shRNA-CALN1 (shCALN1). (B) The band intensity in the scanned image was adjusted using that of β-actin and expressed as fold change vs. each control. *P<0.05 vs. each control (n=3). (C) R-CEPIAer was applied to compare intra-ER Ca2+ concentration between control and shCALN1 cells. *P<0.05 vs. control (n=3). (D) The effects of shCALN1 on aldosterone levels in HAC15 cells mediated by angiotensin II (A-II). *P<0.05 vs. HAC15 cells mediated by A-II (n=3). (E) Increase of aldosterone levels in HAC15 cells with the KCNJ5 T158A mutation was abrogated by shCALN1. *P<0.05 vs. HAC15 cells with the KCNJ5 T158A mutation (n=3).
Discussion
We demonstrated that expression of CALN1 correlated strongly with that of CYP11B2 and was limited to the normal ZG of human and rat adrenal, and that CALN1 co-localized with CYP11B2 in APA. CALN1 is located in ER and increases Ca2+ in ER and cytosol in HAC15 cells. Both A-II and the KCNJ5 mutation potentiated CALN1 expression, and increased CALN1 expression led to increased aldosterone production in HAC15 cells. In contrast, CALN1 suppression significantly decreased A-II or KCNJ5 mutation mediated aldosterone production in HAC15 cells.
We are the first to clarify that CALN1 is a key molecule for the regulation of Ca2+ storage in the ER of adrenal ZG cells. It is well known that the ER stores abundant Ca2+, which is released into the cytosol when a Ca2+ signaling pathway is activated 1. The transfer of Ca2+ by calmodulin in cytosol has been studied well 29. Our results regarding SERCA function in ER suggest that CALN1 collaborates with SERCA to transfer Ca2+ from the cytosol to the ER in aldosterone-producing adrenal cells. CALN1 binds to ER by its TA domain and has four EF hands in the cytosol which may sense cytosolic Ca2+ 28. Taken together with data from previous structural analysis, our results indicate that the increased in CALN1 expression provides greater opportunity to bind cytosol Ca2+, resulting in enhancement of the SERCA function to increase Ca2+ storage in the ER. According to a previous study, excess Ca2+ in ER leads to Ca2+ efflux through IP3R without IP3 stimulation 30. In fact, an IP3 inhibitor did not suppress the aldosterone production in HAC15 cells over-expressing CALN1 (Figure 5F). Taking our results together with previous reports, we postulate that increased CALN1 expression lead to the accumulation of Ca2+ in ER via collaboration with SERCA, and the increased Ca2+ leaking into the cytosol stimulates aldosterone production in APAs (Figure S3).
CALN1 is a potential therapeutic target to mitigate excess of aldosterone production, because KCNJ5 T158A mediated aldosterone production was found to be inhibited by silencing CALN1 mRNA. Likewise, this study was performed to identify a key molecule for Ca2+ signaling in APA and lead to our finding that CALN1 had the strongest correlation with CYP11B2 among CaBPs and was co-localized with CYP11B2 in APAs. In considering therapeutic targets of aldosterone excess, we must evaluate the adverse effects on cortisol production. In contrast to CYP11B2 inhibitors that are not totally selective 31, CALN1 suppression is unlikely to inhibit cortisol production, because it is specific to the ZG of the human, as well as rat adrenals. Furthermore, CALN1 expression is limited to the brain and adrenal gland according to RNA-Seq analysis 32. Compounds targeting CALN1 that do not cross the blood brain barrier might be useful to mitigate aldosterone excess.
The aldosterone production mediated by A-II was also abrogated by the silencing of CALN1 in this study. Cytosolic Ca2+ increases in response to A-II stimulation with subsequent accumulation in the ER 1. A-II also initiates IP3 signaling. Therefore, the increased Ca2+ in ER would lead to an increase in Ca2+ released into the cytosol by IP3 signal via A-II stimulation in adrenal cells 33. Ca2+ storage in ER was decreased in adrenal cells in which CALN1 was silenced, reducing aldosterone production by A-II stimulation. Therefore, a CALN1 antagonist would also be a selective therapeutic target in patients with renin-induced hyperaldosteronism such as renal artery stenosis.
CALN1 expression was detected in the ZG of the human adrenal gland even where CYP11B2 was not detected by immunohistochemistry. Additionally, the adrenal ZG of rats consuming a high-salt diet which had low plasma renin and aldosterone concentrations as expected, also had some expression of CALN1. CALN1 is basally expressed in ZG cells even when aldosterone production is low. It appears that CYP11B2 expression is regulated by CALN1 expression and Ca2+ signaling induced by A-II or a mutation driving aldosterone production, all of which, to date, cause an increase in intracellular Ca2+ mobilization.
Previous studies indicate that CALN1 has a role in the trans-Golgi network or transferring the vesicles in the Golgi bodies or ER of the brain 27. Although we investigated the expressions of genes related with trans-Golgi network between APA and NFA, we found no differences (data not shown). This is not surprising because, unlike neurons or glia cells, secretory granules or vesicles are unremarkable in adrenocortical or other primarily steroidogenic cells. CALN1 appears to have different roles in the adrenal gland and brain.
This study has a limitation. CALN1 has two transcript variants, in whom the sequences are identical from exon 2 to 6 while exon 1 is transcribed only in tv 1. Therefore, specific primers to differentiate between the two variants might not be available. However, we separated them by western blotting, so a protein assay might be more useful for functional analysis compared to the mRNA assay. We showed that tv 1 was the stronger promoter of aldosterone production of between the two variants (Figure 3F and Figure S1AB). Thus, we could further clarify more important roles of CALN1 function for aldosterone production.
Perspectives
Since patients with PA have an increased risk of cardiovascular and cerebrovascular complications compared with those with essential hypertension 8, 9, all of somatic mutations which drive aldosterone production directly or indirectly activate intracellular Ca2+ signaling and CYP11B2 transcription 6, 10-12. The elucidation of the molecular mechanisms of aldosterone production related with Ca2+ signaling pathway is crucially important for the development of therapeutic agents for PA. We demonstrated that CALN1 expression was enhanced in APA and after A-II stimulation in HAC15 cells or ZG of rats. The increased CALN1 expression was associated with increased Ca2+ storage in ER and cytosol, resulted in aldosterone over-production. Suppression of CALN1 expression abrogated A-II or KCNJ5 T158A-mediated aldosterone production in HAC15 cells. CALN1 suppression is unlikely to inhibit cortisol production, because it is specific to the ZG of the human, as well as rat adrenals. Taken together, CALN1 is a potential selective therapeutic target for excess of aldosterone production in patients with APA and renin-induced hyperaldosteronism.
Supplementary Material
Novelty and Significance.
1) What Is New?
CALN1, calneuron 1, expression strongly correlated with CYP11B2 expression and was limited to the normal zona glomerulosa (ZG) of human and rat adrenal, and that CALN1 co-localized with CYP11B2 in APA. CALN1 located in endoplasmic reticulum (ER) and increases Ca2+ in ER and cytosol in HAC15 cells. Both Angiotensin II (A-II) and the KCNJ5 mutation potentiated CALN1 expression, and increased CALN1 expression led to increased aldosterone production in HAC15 cells. CALN1 suppression significantly decreased A-II or KCNJ5 mutation mediated aldosterone production in HAC15 cells.
2) What Is Relevant?
Activation of intracellular Ca2+ signaling is important pathway to stimulate aldosterone production in APA and renin-angiotensin system. CALN1 is a potential selective therapeutic target for excess of aldosterone production in patients with APA and renin-induced hyperaldosteronism.
3) Summary
CALN1 expression correlated with CYP11B2 expression in APA and ZG of human and rat adrenal. Increased CALN1 expression in APA was associated with elevated Ca2+ storage in ER and aldosterone over-production. Suppression of CALN1 expression prevented A-II or KCNJ5 T158A mediated aldosterone production in HAC15 cells.
Acknowledgments
This work was partly carried out with the kind cooperation of the Analysis Center of Life Science, Hiroshima University and the Program of the network-type joint Usage/Research Center for Radiation Disaster Medical Science of Hiroshima University, Nagasaki University and Fukushima Medical University.
Sources of Funding: This study was financially supported by JSPS KAKENHI Grant Number JP17K09883 (K.O.), Japan Heart Foundation Dr. Hiroshi Irisawa & Dr.Aya Irisawa Memorial Research Grant (K.O.), SENSHIN Medical Research Foundation (K.O.), National Heart, Lung and Blood Institute grant R01 HL27255 (C.-E.GS.), and the National Institute of General Medical Sciences under Award Number 1U54GM115428 (C.-E.GS.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure statements: The authors have nothing to disclose.
References
- 1.Bollag WB. Regulation of aldosterone synthesis and secretion. Compr Physiol. 2014;4:1017–1055. doi: 10.1002/cphy.c130037. [DOI] [PubMed] [Google Scholar]
- 2.Hunyady L, Gáborik Z, Shah BH, Jagadeesh G, Clark AJ, Catt KJ. Structural determinants of agonist-induced signaling and regulation of the angiotensin at1 receptor. Mol Cell Endocrinol. 2004;217:89–100. doi: 10.1016/j.mce.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Otis M, Campbell S, Payet MD, Gallo-Payet N. Angiotensin ii stimulates protein synthesis and inhibits proliferation in primary cultures of rat adrenal glomerulosa cells. Endocrinology. 2005;146:633–642. doi: 10.1210/en.2004-0935. [DOI] [PubMed] [Google Scholar]
- 4.Spat A, Rohacs T, Horvath A, Szabadkai G, Enyedi P. The role of voltage-dependent calcium channels in angiotensin-stimulated glomerulosa cells. Endocr Res. 1996;22:569–576. doi: 10.1080/07435809609043748. [DOI] [PubMed] [Google Scholar]
- 5.Oki K, Plonczynski MW, Lam ML, Gomez-Sanchez EP, Gomez-Sanchez CE. The potassium channel, kir3.4 participates in angiotensin ii-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology. 2012;153:4328–4335. doi: 10.1210/en.2012-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oki K, Plonczynski MW, Luis Lam M, Gomez-Sanchez EP, Gomez-Sanchez CE. Potassium channel mutant kcnj5 t158a expression in hac-15 cells increases aldosterone synthesis. Endocrinology. 2012;153:1774–1782. doi: 10.1210/en.2011-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF., Jr The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 8.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. Journal of the American College of Cardiology. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833. doi: 10.1210/jc.2013-2805. [DOI] [PubMed] [Google Scholar]
- 10.Choi M, Scholl UI, Yue P, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azizan EA, Poulsen H, Tuluc P, et al. Somatic mutations in atp1a1 and cacna1d underlie a common subtype of adrenal hypertension. Nat Genet. 2013;45:1055–1060. doi: 10.1038/ng.2716. [DOI] [PubMed] [Google Scholar]
- 12.Beuschlein F, Boulkroun S, Osswald A, et al. Somatic mutations in atp1a1 and atp2b3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444. 444e441–442. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- 13.Haynes LP, McCue HV, Burgoyne RD. Evolution and functional diversity of the calcium binding proteins (cabps) Front Mol Neurosci. 2012;5:9. doi: 10.3389/fnmol.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zamparelli C, Ilari A, Verzili D, Giangiacomo L, Colotti G, Pascarella S, Chiancone E. Structure-function relationships in sorcin, a member of the penta ef-hand family. Interaction of sorcin fragments with the ryanodine receptor and an escherichia coli model system. Biochemistry. 2000;39:658–666. doi: 10.1021/bi991648v. [DOI] [PubMed] [Google Scholar]
- 15.Chin D, Means AR. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 16.Mikhaylova M, Reddy PP, Munsch T, Landgraf P, Suman SK, Smalla KH, Gundelfinger ED, Sharma Y, Kreutz MR. Calneurons provide a calcium threshold for trans-golgi network to plasma membrane trafficking. Proc Natl Acad Sci U S A. 2009;106:9093–9098. doi: 10.1073/pnas.0903001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshii Y, Oki K, Gomez-Sanchez CE, Ohno H, Itcho K, Kobuke K, Yoneda M. Hypomethylation of cyp11b2 in aldosterone-producing adenoma. Hypertension. 2016;68:1432–1437. doi: 10.1161/HYPERTENSIONAHA.116.08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmar J, Key RE, Rainey WE. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab. 2008;93:4542–4546. doi: 10.1210/jc.2008-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto R, Oki K, Yoneda M, Gomez-Sanchez CE, Ohno H, Kobuke K, Itcho K, Kohno N. Gonadotropin-releasing hormone stimulate aldosterone production in a subset of aldosterone-producing adenoma. Medicine (Baltimore) 2016;95:e3659. doi: 10.1097/MD.0000000000003659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagano G, Ohno H, Oki K, Kobuke K, Shiwa T, Yoneda M, Kohno N. Activation of classical brown adipocytes in the adult human perirenal depot is highly correlated with prdm16-ehmt1 complex expression. PLoS One. 2015;10:e0122584. doi: 10.1371/journal.pone.0122584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M. Imaging intraorganellar ca2+ at subcellular resolution using cepia. Nat Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero DG, Yanes LL, de Rodriguez AF, Plonczynski MW, Welsh BL, Reckelhoff JF, Gomez-Sanchez EP, Gomez-Sanchez CE. Disabled-2 is expressed in adrenal zona glomerulosa and is involved in aldosterone secretion. Endocrinology. 2007;148:2644–2652. doi: 10.1210/en.2006-1509. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human cyp11b1 and cyp11b2. Mol Cell Endocrinol. 2014;383:111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams TA, Monticone S, Crudo V, Warth R, Veglio F, Mulatero P. Visinin-like 1 is upregulated in aldosterone-producing adenomas with kcnj5 mutations and protects from calcium-induced apoptosis. Hypertension. 2012;59:833–839. doi: 10.1161/HYPERTENSIONAHA.111.188532. [DOI] [PubMed] [Google Scholar]
- 25.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 26.Griffing GT, Melby JC, Holbrook M, Wilson TC, Wyss MJ. Adrenocorticosteroid excretion in salt-sensitive and salt-resistant spontaneously hypertensive rats. Steroids. 1992;57:90–94. doi: 10.1016/0039-128x(92)90036-9. [DOI] [PubMed] [Google Scholar]
- 27.Hradsky J, Raghuram V, Reddy PP, Navarro G, Hupe M, Casado V, McCormick PJ, Sharma Y, Kreutz MR, Mikhaylova M. Post-translational membrane insertion of tail-anchored transmembrane ef-hand ca2+ sensor calneurons requires the trc40/asna1 protein chaperone. J Biol Chem. 2011;286:36762–36776. doi: 10.1074/jbc.M111.280339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCue HV, Haynes LP, Burgoyne RD. The diversity of calcium sensor proteins in the regulation of neuronal function. Cold Spring Harb Perspect Biol. 2010;2:a004085. doi: 10.1101/cshperspect.a004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Condon JC, Pezzi V, Drummond BM, Yin S, Rainey WE. Calmodulin-dependent kinase i regulates adrenal cell expression of aldosterone synthase. Endocrinology. 2002;143:3651–3657. doi: 10.1210/en.2001-211359. [DOI] [PubMed] [Google Scholar]
- 30.Bittremieux M, Parys JB, Pinton P, Bultynck G. Er functions of oncogenes and tumor suppressors: Modulators of intracellular ca(2+) signaling. Biochim Biophys Acta. 2016;1863:1364–1378. doi: 10.1016/j.bbamcr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Bernhardt R. The potential of targeting cyp11b. Expert Opin Ther Targets. 2016;20:923–934. doi: 10.1517/14728222.2016.1151873. [DOI] [PubMed] [Google Scholar]
- 32.Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;13:397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shelat SG, Flanagan-Cato LM, Fluharty SJ. Glucocorticoid and mineralocorticoid regulation of angiotensin ii type 1 receptor binding and inositol triphosphate formation in wb cells. J Endocrinol. 1999;162:381–391. doi: 10.1677/joe.0.1620381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.