Abstract
Mesenchymal stem cells (MSCs) have been extensively used for cell therapies and tissue engineering. The current MSC strategy requires a large quantity of cells for such applications, which can be achieved through cell expansion in culture. In the body, stem cell fate is largely determined by their microenvironment, known as the niche. The complex and dynamic stem cell niche provides physical, mechanical, and chemical cues to collaboratively regulate cell activities. It remains a great challenge to maintain the properties of MSCs in culture. Constructing a microenvironment as an engineered stem cell niche in culture to maintain MSC phenotypes, properties, and functions is a viable strategy to address the issue. Here, we review the current understanding of MSC behavior in the bone marrow niche, describe different strategies to engineer an in vitro microenvironment for maintaining MSC properties and functions, and discuss previous findings on environmental factors critical to the modulation of MSC activities in engineered microenvironments.
Keywords: mesenchymal stem cell, niche, microenvironment, regenerative medicine, tissue engineering
Introduction
Mesenchymal stem cells (MSCs) were first described in a series of studies by Friedenstein in the 1960s.1,2 He identified the cell in bone marrow that is able to adhere to the surface of a tissue culture plate and generate skeletal tissues, including bone and cartilage, during heterotopic implantation.3–5 Cell populations with similar properties were also found in many other tissues, such as adipose tissue6,7 and umbilical cord blood.8,9 It was later confirmed that MSCs are multipotent, and, with appropriate induction signaling, the cell can differentiate into bone, cartilage, adipose, and muscle lineage cells in culture.10–12 In vivo, MSCs are tightly controlled by the stem cell niche/microenvironment to regulate their activities. Specifically, the microenvironment provides physical and biochemical cues to regulate MSC behavior.13 On the other hand, when cultured in vitro without the presence of regulatory cues, MSCs often behave and function differently. Changes of their phenotypic properties during cell culture increases the challenge to obtain high-quality cells for MSC-based therapies. It is therefore essential to develop approaches that can effectively retain MSC properties after the cell is isolated and cultured in vitro, and one of the most promising approaches is to construct a culture environment that imitates the MSC niche to enhance the quality of cultured cells.
Since bone marrow–derived MSCs are the most commonly used cell type that has been extensively studied among MSCs from different tissue sources, we focus the discussion here on bone marrow–derived MSCs and environmental cues identified in the bone marrow niche. We discuss challenges in maintaining MSC properties and functions in culture and describe different strategies that have been used to address the challenges.
Mesenchymal stem cell–based therapeutic applications
With the capability to proliferate in culture and differentiate into cells of multiple musculoskeletal lineages, MSCs have become a promising cell source for stem cell–based musculoskeletal tissue engineering and regenerative medicine.14,15 In preparation for applications, MSCs are harvested from adult tissues, such as bone marrow, expanded in cell culture, and then transplanted into the body or seeded in a three-dimensional biomaterial scaffold for generation of new tissue. During the process of tissue formation, MSCs are induced by chemical factors, such as growth factors, steroids, or small molecules, and often simultaneously stimulated by mechanical loading and circulated oxygen and nutrients provided in a bioreactor to differentiate into a tissue-specific cell types. For generation of musculoskeletal tissues in vitro, it is particularly critical to involve a bioreactor for cell culture, because the device can provide mechanical cues needed to mature an engineered tissue. For example, studies have shown that cyclic compression and tensile strain are able to facilitate cartilage16,17 and tendon/ligament formation, respectively.18,19 Mature engineered tissue can then be implanted to replace or repair damaged or degenerative tissue to restore its functions. The tissue engineering approach, as just described, has been used to generate musculoskeletal tissues, such as bone,20,21 cartilage,22,23 intervertebral disc,24–27 and ligament/tendon.28,29
Another application for MSCs is to transplant cells as a therapeutic agent for disease treatment. The procedure of transplantation can be performed with either cultured or uncultured MSCs suspended in a saline solution. After delivery to the site of injury, transplanted MSCs are induced to produce trophic factors, such as vascular endothelial growth factor (VEGF), transforming growth factor β (TGF-β), and platelet-derived growth factor (PDGF), to instruct local cells to participate in tissue repair and regeneration.30–32 For example, systematic or local administration of MSCs to treat acute myocardial infarction has been performed in animal models and in human patients.33,34 Results of these studies suggest that injected MSCs do not become cardiomyocytes but help to enhance compliance of scarred heart muscle for improving heart function.
Immunomodulation is one of the essential characteristics of MSCs that has been increasingly recognized as a unique feature for disease treatment. The immunomodulatory capacity of the cell can regulate immune activities of a recipient receiving MSC transplantation, which allows therapeutic applications to be performed in either an autologous or allogenous host without safety concerns and makes the cell very attractive for resolving inflammation and encouraging tissue repair.17 In fact, MSCs have been used in clinical trials to treat inflammatory diseases, such as graft-versus-host disease35,36 and inflammatory bowel disease.37 Recent evidence has shown that beneficial effects on tissue repair after MSC injection may result from immune modulation mediated by the cell rather than differentiation of the cell to regenerate tissue.38,39
When tissue inflammation occurs due to injury or insult, immune cells, such as neutrophils and macrophages, are recruited to the site of inflammation and induced to release chemotactic factors to attract endogenous or transplanted MSCs to move to the site. The mechanisms by which MSCs are induced to modulate immune cells are not ben fully understood, but a great deal has been learned about MSC immunomodulation in recent years. It is known that MSCs require cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin-1α (IL-1α) or interleukin-1β (IL-1β), to activate their immunomodulatory capability.40–42 The cell can also be activated by Toll-like receptor (TLR) ligands to exert immunomodulatory effects. Depending on the type of TLRs on the MSCs being activated, the cell can trigger either a pro- or anti-inflammatory response. Waterman et al. reported that TLR4-primed MSCs, classified as MSC1, are proinflammatory inducers, whereas TLR3-primed MSCs, classified as MSC2, are anti-inflammatory ones.43 To reduce inflammation, MSCs secrete immunomodulatory molecules, such as TGF-βB, nitric oxide (NO), indoleamine 2,3-dioxygenase (IDO), TNF-α-stimulated gene/protein 6, prostaglandin E2 (PGE2), IL-1 receptor antagonist, IL-10, and an antagonistic variant of chemokine C-C motif ligand 2 (CCL2), to regulate cells of the innate and adaptive immune systems.44–47 It has been shown that these molecules can suppress T cell proliferation1,13,15 and differentiation14 or induce apoptosis of the cell40 to modulate the immune response. On the other hand, MSCs can be induced to produce IL-6 and IL-8, which leads to an increase in proinflammatory response. In addition, MSCs can alter the balance between various T cell subsets to exert a protective effect by increasing anti-inflammatory TH2 cells and decreasing proinflammatory TH1 cells,48 modulate B cell proliferation,16 and inhibit IL-2–induced natural killer cell proliferation.49 It has also been reported that MSCs can reprogram macrophages and dendritic cells to produce more anti-inflammatory cytokines but fewer proinflammatory ones through the induction of IDO and PGE2.50,51
Current challenges of MSC-based therapies
While MSC-based therapies are promising for disease treatment, a number of challenges remain before the cell can be used in extensive clinical applications. The major issue lies in a need for MSC expansion after the cell is harvested from bone marrow. Because MSCs only account for approximately 0.01% of mononuclear cells in the bone marrow,52 expanding the cell in culture is almost always necessary to obtain a sufficient number of cells for subsequent applications. For example, millions of MSCs are required for most tissue engineering applications. Since the environment in culture is distinct from that in the body, cultured MSCs are inclined to alter their behavior and activities in response to the environmental change. For example, during cell culture, the production of stromal cell–derived factor-1 and IL-7 in MSCs was greatly reduced, an indication of loss of the capability to support hematopoiesis.53,54 It has also been shown that the expression of cell surface antigens on MSCs changes during cell culture. Qian et al. have demonstrated that uncultured MSCs do not express CD44 but begin to express the surface protein after being plated in culture; more than 90% of the cultured cells express CD44 in 8–10 days.55 In contrast to an increase in CD44, the expression of CD106 and CD271 on MSCs is decreased after the cell is harvested and cultured.56–58 The change in the expression of surface markers of MSCs during cell culture indicates that the MSC phenotype is tightly regulated by the microenvironment in culture, which has also been shown to affect migration, proliferation, and differentiation of the cell.59–61 In addition, a study conducted by Churchman et al. reported that the transcriptional profile of native MSCs is largely different from that of culture-expanded MSCs.62 They have further demonstrated that MSCs undergoing the procedure of cell culture downregulate the expression of osteogenic and adipogenic markers.
In addition to the changes in cell activities described above, the morphology of MSCs gradually switches from spindle-shaped to flat and well-spread during cell culture,63 indicating that MSCs undergo cellular senescence, proliferate slowly, and eventually stop growing.63,64 Cellular senescence that often occurs in cells after an extensive culture period results from shortening of telomere length and/or DNA damage due to accumulation of reactive oxygen species in cells.65–67 As a result of cellular senescence, MSCs tend to lose their multilineage differentiation potential. Studies have shown that senescent MSCs are less inducible for osteogenesis, adipogenesis, and chondrogenesis63,68 and less capable of immunomodulation than at early passages.69 These issues deriving from in vitro cell expansion greatly affect the quality of MSCs for therapies, which limits their applications for tissue regeneration or immunomodulation.
Since the behavior of MSCs in culture is regulated by the environment to which they are exposed, the undesired cell response is induced mostly by suboptimal culture conditions.70,71 One of the factors that may cause suboptimal culture conditions is the composition of culture medium. Basic culture medium composed of minimum essential medium alpha or Dulbecco's modified Eagle's medium (DMEM) and 5% to 20% of fetal bovine serum (FBS) is commonly used to support MSC growth.72,73 However, FBS is an animal product that contains proteins different from those in human bone marrow to which native MSCs are exposed. Other factors that may also lead to suboptimal culture conditions include dimensionality of the culture substrate and oxygen tension. While MSCs in bone marrow are grown in a three-dimensional environment with a low level of oxygen tension,74 the cell in culture is generally seeded on two-dimensional tissue culture plastics in an incubator with atmospheric oxygen levels.72 These discrepancies in the MSC growth environment between in vivo stem cell niches and in vitro culture conditions result in differences in cell behavior after native MSCs are harvested and plated in culture.
Stem cell niche
The concept of a stem cell niche was first introduced by Schofield in 1978.75 The niche refers to the unique microenvironment that forms both an anatomical location and a functional unit and where stem cells reside in vivo, encompassing a number of components, including stem cells, neighboring non-stem cells, the extracellular matrix (ECM), cell adhesion molecules, soluble factors, neural fibers, and vascular networks.76 For example, it has been reported that tenascin-C is a critical ECM molecule in the bone marrow niche required for hematopoietic regeneration, on the basis of evidence demonstrating that the expression of tenascin-C is dramatically increased during hematopoietic recovery after myeloablation.77 Hematopoietic stem cells in the niche require the aid of E-selectin, a cell adhesion molecule expressed on endothelial cells, to promote attachment to vascular networks for proliferation.78 In addition to the ECM and adhesion proteins, biological functions of stem cells are also regulated by their neighboring cells in the niche. For example, activities and hematopoietic functions of hematopoietic stem cells in the bone marrow are supported by the signals of soluble factors secreted from their surrounding osteoblasts and MSCs or by cell–cell interactions between these cells.79,80 It is clear that niche components work coordinately to regulate activities of stem cells.
Studies of mammalian stem cell niches have demonstrated that stem cells residing in the niche are maintained in a quiescent state to keep them from being depleted prematurely.81–83 Stem cells remain quiescent most of the time in the niche, regulated by coordinated signals from environmental factors; however, when disruption of the niche occurs, stem cells leave the quiescent state and begin to proliferate and/or differentiate, resulting in reduction in the number of cells in a stem cell pool.84 Since studies of MSCs and the bone marrow niche have just begun to emerge, much of the knowledge about the stem cell niche to date has not come directly from MSC studies. However, it is believed that MSCs may share similar behavior with hematopoietic stem cells in response to environmental cues in the bone marrow. In the following sections, we focus on research findings of MSC behavior in the bone marrow and strategies to precisely regulate MSC activities in culture.
Mesenchymal stem cells in the bone marrow niche
When first discovered in the 1960s, MSCs were described and defined on the basis of their properties and functions in cell culture or heterotopic sites of implantation.4 Recently, the activities of the cells and their regulation in the bone marrow have begun to be unveiled. For example, to study activities of MSCs in vivo, Muguruma et al. transplanted human MSCs into the intramedullary compartment of femoral bone in immunocompromised mice. Their results have shown that the transplanted cell can integrate with the host bone marrow and differentiate into pericytes, myofibroblasts, stromal cells, osteoblasts, and osteocytes in bone,85 suggesting that MSCs in the bone marrow niche can undergo lineage-specific differentiation to become a number of different cell types. However, there is an additional challenge associated with MSC identity to address: it is unclear, among the heterogeneous cell populations in the bone marrow, which is the MSCs and where the cells are located.
Recently, with the advancement of techniques in cell labeling and tracking, researchers have been able to mark and follow a cell population of interest in vivo to study functions and properties of MSCs directly in their bone marrow niche rather than in cell culture or at heterotopic sites of implantation. For example, Mendez-Ferrer et al. used green fluorescent protein to label a cell population that expresses nestin in murine bone marrow. Nestin-expressing bone marrow cells that colocalized with hematopoietic stem cells express all of the MSC characteristics and can remain quiescent in the bone marrow niche.80 Using the lineage-tracing method, they also revealed that nestin-expressing MSCs are able to contribute to bone remodeling by differentiating into osteoblasts, osteocytes, and chondrocytes. Other research groups have used similar strategies to study properties and activities of MSCs in vivo. Park et al. performed a study in which they demonstrated that, in response to tissue stress, Mx1-expressing bone marrow cells with MSC features migrate to injury sites and differentiate into osteoblasts during fracture healing.86 They further used a method based on single-cell transplantation to demonstrate that Mx1-expressing MSC-like cells yield progeny that can both preserve progenitor functions and express osteogenic capability. Besides nestin- and Mx1-expressing MSCs, Zhou et al. in another study identified a population of MSCs in bone marrow expressing leptin receptor (LepR).87 They demonstrated that LepR-expressing cells fulfill all of the criteria that define MSCs and are able to give rise to bone and fat in adult bone marrow. They further showed that LepR-expressing cells are normally quiescent in bone marrow but begin to proliferate to regenerate bone in response to injury. While these studies suggest that there may be at least three cell populations possessing MSC characteristics in the bone marrow, the findings that LepR-expressing cells are able to commit to the adipogenic lineage but nestin- or Mx1-expressing cells cannot indicate they may be different cell populations. Progress in identifying the cell with MSC behavior has been made in recent years. For example, a population of gremlin-1–expressing cells within mouse bone marrow was identified through a fate-mapping approach, and the cell is capable of self-renewal and generating osteoblasts, chondrocytes, and reticular marrow stromal cells but not adipocytes.88,89 Skeletal stem cells are induced by growth factors, such as bone morphogenetic protein-2 (BMP2) or VEGF, in the niche to specify the differentiation fate toward osteoblasts, chondrocytes, or stromal cells to maintain homeostasis of the skeletal system. Collectively, it is known that MSCs or MSC-like skeletal cells with different surface identities are a small fraction of bone marrow cells and normally remain quiescent in the niche until physiological needs, such as repair of tissue injury, arise; they then begin to proliferate and differentiate into a tissue-specific cell type.
Current strategies used to construct the bone marrow niche in vitro
To retain MSC functions and activities in vitro, intracellular and extracellular engineering strategies have been developed. The intracellular engineering strategy is mediated mainly through genetic modification,90 whereas the extracellular engineering strategy focuses on optimizing the culture condition.91 However, a genetically modified cell likely becomes mutated, raising a safety concern for clinical applications. An emerging strategy has been applied to construct an in vitro microenvironment imitating a stem cell niche to maintain properties of MSCs in culture. A number of approaches have been developed to achieve this goal, including modulation of oxygen and glucose, engineering of the ECM, optimization of stiffness of the culture substrate, co-culture of MSCs and other types of bone marrow cells, and administration of soluble factors.
Modulation of oxygen and glucose in culture
Mesenchymal stem cells in the bone marrow reside in a microenvironment with a low oxygen tension, typically ranging from 1% to 8%, depending on the distance from the vascular network.74 Because physiological oxygen tensions that MSCs are normally exposed to in vivo are way below levels of ambient oxygen, researchers have managed to culture the cell under hypoxic conditions to control cell metabolism in culture. It has been shown that maintaining MSCs under hypoxia decreases the accumulation of reactive oxygen species in the cell, which leads to a reduction in DNA damage and a delay in cellular senescence.92 The approach of culturing MSCs in hypoxic conditions also greatly extends the life span of the cell, contributing to an increase in the total number of accumulative population doublings.64,68 In addition to an impact on cell growth, hypoxic culture conditions also affect the transcriptome of MSCs. Basciano et al., in their study investigating effects of hypoxia on MSCs in long-term culture, demonstrated that hypoxia dictates transcriptional profiles of the cell to sustain the undifferentiated status and multipotent capacity.
There are several signaling pathways that have been identified as being involved in the hypoxia-induced regulation of MSC activities. Hypoxia-inducible factor-1α (HIF-1α) is a key molecule regulated in the oxygen-induced signaling pathways.93,94 For example, phosphorylation of AKT and p38 mitogen-activated protein kinase in MSCs is increased by hypoxic induction, in turn promoting HIF-1α to translocate from the cytosol to the nucleus to bind to the target DNA, which leads to enhanced chondrogenesis with an increase in the expression of collagen type 2 and sex-determining region Y-box 9 and proteoglycan production.95 Another study showed that MSCs under hypoxic conditions are directed toward the osteogenic lineage with upregulated expression of runt-related transcription factor 2 (RUNX2) and away from the adipogenic lineage with downregulated expression of peroxisome proliferator–activated receptor γ through differential regulation of the HIF-1α–mediated pathway.96 In addition to modulating multilineage differentiation, hypoxia is able to delay cellular senescence through increased production of macrophage migration inhibitory factor (MIF) and activation of AKT signaling.64 Among all the pathways related to metabolic regulation, the PI3K/AKT signaling pathway is considered the dominant one by which stem cell activities are modulated in response to induction of environmental factors, such as oxygen or glucose.97 In this signaling pathway, PI3K and AKT can activate mechanistic target of rapamycin (mTOR) and its downstream molecule HIF-1α or suppress forkhead box protein O (FOXO) as well as downstream reactive oxygen species. Several groups have demonstrated that the PI3K/AKT/mTOR and the PI3K/AKT/FOXO3a signaling pathway are associated with MSC survival in culture or animal models.98,99 It has also been demonstrated that activation of the PI3K/AKT signaling pathway is critical to increase hypoxia-induced proliferation and differentiation of MSCs into endothelial cells.100
Glucose is the major energy source in the human body and has been used in culture to grow a variety of cell types, including MSCs. Previous studies examining the general effects of glucose on non-stem cells have accumulated substantial information101 that can be shared to improve our understanding of MSC behavior regulated by glucose in the niche. The emerging interest in MSCs for therapies has resulted in cumulative research evidence to elucidate the importance of glucose on the regulation of MSC activities.102 Because the physiological level of glucose in the serum of a healthy individual is maintained at around 1 mg/mL by hormones, such as insulin and glucagon, the glucose concentration that MSCs are exposed to in the bone marrow niche should be around the same level. Therefore, if a glucose concentration in the niche or in culture is significantly changed due to diseases or experimental manipulation, the change of glucose concentration likely leads to alteration of MSC properties and activities. For example, culturing MSCs with 4.5 mg/mL high-glucose medium reduces their proliferation as well as induces premature cellular senescence.103,104 The concentration of glucose in culture has a big impact on multilineage differentiation. Osteogenesis of MSCs is upregulated in culture with high-glucose medium compared with low-glucose medium.105 Our group has demonstrated that culturing MSCs with high-glucose medium for cell expansion and then inducing them for chondrogenic differentiation decreases the chondrogenic capacity of MSCs by modulating the activities of protein kinase C and transforming growth factor receptor.106 Our findings are interesting because they not only suggest that, for effective cartilage formation in vitro, it is desirable to expand MSCs in low-glucose culture but also indicate that abnormality of the glucose level in the body, such as hyperglycemia, may impair the chondrogenic capacity of MSCs.
Glutamine is an essential amino acid for peptide synthesis, amino sugar synthesis, and nucleotide production and acts as a source of carbon for oxidation in some cells.107 Like glucose required for the maintenance and promotion of cell functions, glutamine is equally important for the same reason. Glutamine is a required additive to most culture media and is also used as an energy source in culture when glucose levels are low and energy demands are high. For example, when glucose metabolism is less efficient in hypoxic culture, cells may use glutamine for energy. Previous studies have demonstrated that, under normoxic conditions, glutamine is not used as an energy source by MSCs for cell growth;108,109 however, there is more glutamine consumption by MSCs in hypoxic culture than in normoxic culture.110
Engineering of the ECM
The ECM is a key component of the bone marrow niche.76 It provides adhesion molecules for MSC attachment and offers a three-dimensional environment as physical support for the cell. To build the niche ECM for MSCs in culture, researchers have used a number of biomaterials and a variety of fabrication methods to construct three-dimensional constructs/scaffolds for culture of the cell.111 Within a three-dimensional construct, MSCs are guided by architectural cues of the structure along with or without the support of adhesion molecules, such as fibronectin or arginylglycylaspartic acid, to adopt a three-dimensional spindle-shaped or spheroid-shaped cell morphology, respectively, which is different from a flattened cell morphology in monolayer culture. In general, MSCs maintained in a three-dimensional environment undergo slower cell proliferation and growth than those in monolayer culture,112,113 while a three-dimensional culture setup permits the cell to maintain or promote the multilineage differentiation capacity compared with a two-dimensional culture counterpart.114,115
Besides dimensionality of the culture substrate, MSCs require appropriate chemical cues generated through binding to ECM proteins to regulate their activities. Analysis of the ECM in bone marrow reveals that it comprises a variety of proteins, including fibronectin; laminin; collagen types 1, 3, 4, 5, and 6; and high-molecular-weight proteoglycans.116,117 While a number of studies, each focused on the effects of a particular matrix protein on regulation of MSC proliferation and differentiation, have been performed,118–121 the approach using a single matrix protein in these studies does not take the complex ECM composition of bone marrow into consideration. Instead of using a single matrix protein, Hashimoto et al. cultured MSCs in decellularized matrix containing complete ECM proteins derived from bone/bone marrow tissue to demonstrate that the matrix supports proliferation and enhances osteogenic differentiation of MSCs.122 Chen et al. also demonstrated that, by removing adherent bone marrow cells from a culture plate, a surface containing a number of ECM proteins can be obtained. The ECM-rich surface in culture plates promotes MSC proliferation while keeping the cell undifferentiated,123 indicating the importance of using complete niche ECM proteins to maintain MSC properties.
The manner by which cells, including MSCs, are regulated after binding to ECM proteins through activation of integrins and their downstream signaling pathways has been well established.124 In general, each integrin molecule, composed of an αand a β subunit, can recognize different types of ECM proteins to activate a number of signaling molecules, such as focal adhesion kinase (FAK), vinculin, and paxillin,125 which in turn triggers the signaling pathways, including AKT, ERK, JNK, RhoA, and Rac1, to elicit cellular responses.126 Previous studies focusing on the ECM regulation of MSCs showed that laminin-5, vitronectin, fibronectin, and collagen type 1 play critical roles in regulating proliferation and differentiation of MSCs through the ERK or AKT signaling pathways.118–121
Optimization of stiffness of culture substrates
Given that physical properties of the niche affect MSC behavior,127 it is desirable to construct a culture microenvironment with properties similar or identical to those in the niche to maintain MSC activities. A pioneer study in this line of research by Engler et al. concluded that MSC fates are controlled by stiffness of the culture substrate, independent of chemical factors, based on their findings demonstrating that MSCs on a soft matrix are directed into the neurogenic lineage, whereas those on a rigid matrix are directed into the osteogenic lineage.128 One of the identified mechanisms involved in the regulation of stiffer matrices increasing osteogenic differentiation is via an increase in expression of integrin subunit α2 and the activities of Rho kinase, FAK, and ERK1/2.129,130 Recently, our group demonstrated that, besides than these identified pathways, osteogenesis of MSCs modulated by stiffness of a culture substrate can occur through activation of MIF and the AKT/YAP signaling pathway to regulate the expression of RUNX2.131 On the basis of the findings of a recent study, it seems that fate commitment of MSCs controlled by matrix stiffness is transient and can be manipulated. Schellenberg et al. reported that MSC differentiation toward the adipogenic or osteogenic lineages is increased on a soft or rigid substrate, respectively, but the propensity becomes less obvious after the cell is transferred to tissue culture plates.132 On the other hand, recent evidence has shown that MSCs are possess mechanical memory obtained from past physical environments that can influence MSC fate.133,134 Although details of the mechanism remain elusive, studies have revealed that MSCs possess mechanical memory through activities of microRNA-21.135
Other than directing lineage specification, matrix stiffness alters MSC division and the cell cycle. Winer et al. demonstrated that MSCs cultured on a soft substrate made of polyacrylamide gel with stiffness similar to that of bone marrow become quiescent by halting progression of the cell cycle but still remain responsive to chemical or physical stimulation.136 These findings may provide insight into how a niche property participates in the regulation that maintains MSCs in the quiescent state. In terms of regulation of cell mobility, it has also been shown that MSCs are inclined to migrate to a rigid substrate rather than a soft one as a result of more focal adhesion and larger traction forces being established between the cell and a rigid surface than with a soft one.137
Co-culture of MSCs and other bone marrow cells
Despite a great deal of research effort exploring the potential of MSCs for therapeutic applications, little has been done to investigate the origin of MSCs. Using the cell surface markers CD146, NG2, platelet-derived growth factor receptor β, or Stro-1 to label MSCs in vivo, the cell can be located be at perivascular sites in a number of tissues, such as bone marrow.138,139 With this finding, it was hypothesized that all MSCs are pericytes.140 However, recent research has demonstrated that pericytes do not behave as MSCs in vivo141 and that not all MSCs can act as pericytes,142 suggesting that MSCs and pericytes are likely to be different cell types residing at the same anatomical sites. In bone marrow, MSCs are located either on or around blood vessels, so it is suspected that MSCs and vascular endothelial cells (ECs) reciprocally regulate each other through paracrine factors and cell–cell interactions. These two types of cells have been co-cultured to investigate how MSC activities are affected by ECs, and the results have shown that interaction with ECs increases proliferation and survival of MSCs and directs the cell into the osteogenic lineage.143,144 To further enhance the regulation between the cells, Saleh et al. co-cultured MSCs and ECs in a spheroid cell pellet that imitates a three-dimensional microenvironment and demonstrated that these two cell types can self-assemble to form an organized structure in which the osteogenic capacity of MSCs is increased while their adipogenic capacity is decreased.145
Bone marrow is a complex tissue containing many different types of cells, including hematopoietic cells, immune inflammatory cells, osteoblasts, adipocytes, neural cells, and vascular cells. Other than ECs, MSCs also closely interact with bone cells. In a study investigating the influence of osteoblasts and osteocytes on osteogenic differentiation of MSCs, Birmingham et al. demonstrated that both osteoblasts and osteocytes direct MSCs toward the osteogenic lineage, with osteocytes being more efficient than osteoblasts at driving the induction.146 When co-cultured with adipocytes, MSCs begin to express adipogenic markers, lipoprotein lipase, and leptin as indications of undergoing adipogenesis.147 These findings suggest that cells of a specific tissue, such as bone or fat, are capable of instructing co-cultured MSCs for differential differentiation toward the cell lineage of the tissue. In the bone marrow niche, MSCs are surrounded by hematopoietic stem cells and their progeny, myeloid and lymphoid cells. While the interaction between hematopoietic stem cells and MSCs has been studied for years,148 mainly focusing on how MSCs as supporting cells provide regulatory signals to maintain functions and activities of hematopoietic stem cells,80,149 research exploring how hematopoietic stem cells support MSC activities or how myeloid and lymphoid cells regulate MSC functions have begun to accumulate.150 For example, when co-cultured with immune cells, certain functions of MSCs, such as the immunomodulation capacity, are turned on. A study by Anton et al. demonstrated that macrophages produce paracrine IL-8, CCL2, and CCL5, which stimulate MSCs to produce IL-6, CCL5, and CXCL10 and to increase their migratory capability in response to inflammation and tissue injury.151
Administration of soluble factors
Proteomic analysis of interstitial fluid or plasma of bone marrow using two-dimensional gel electrophoresis has shown that bone marrow contains a variety of soluble proteins secreted by MSCs and other types of cells.152–154 Since serum albumin precursors make up a huge portion of the proteins, it becomes challenging to quantitatively detect low-abundance proteins, such as growth factors or cytokines, using gel electrophoresis. Alternatively, using antibody arrays to overcome the challenge, Kovac et al. confirmed that there are a number of different cytokines and growth factors in bone marrow and further identified tissue inhibitor of matrix metalloproteinase-1 and leukemia inhibitory factor as being significantly abundant in plasma of the tissue.155 For applications of regenerative medicine, some of the previously identified soluble factors of bone marrow have been individually tested in MSC culture; however, most of these studies focus on determining effects of a single molecule of interest rather than a combination of multiple molecules on enhancing activities, such as proliferation or lineage-specific differentiation, of MSCs. For example, fibroblast growth factor-2 (FGF2) is one of the soluble factors in bone marrow and has been extensively studied for its capacity to stimulate proliferation and increase the chondrogenic capacity of MSCs by priming the cell or selecting a specific MSC subpopulation for differentiation induction.156,157 Considering MSCs in the niche are under the influence of a number of different soluble factors, only some of which have been identified, it would be ideal to expose MSCs to complete soluble factors of bone marrow in culture, if possible, to avoid the possibility of missing any chemical cues critical to retaining MSC properties in vitro.
It is desirable to have a chemically defined medium with the complete soluble proteins of the bone marrow for MSC culture, but it remains challenging to achieve this goal. Therefore, the strategy of creating a culture environment including the key soluble factors available for regulation of MSC activities seems to be a feasible alternative solution. Researchers have used the method of conditioned medium or Transwell plates to culture MSCs, allowing the cell to be exposed to a combination of soluble factors. For example, using endothelial cell–conditioned medium, Kaigler et al. demonstrated that BMP2, among all the proteins secreted by endothelial cells in culture, is the most potent factor that increases osteogenesis of MSCs.158 Another study by Saleh et al. has shown that secreted soluble factors from ECs activate several candidate signaling pathways, including FGF, Wnt, BMP, and Notch, to enhance proliferation and osteogenic differentiation of MSCs.159 Using Transwell cultures, in which MSCs and ECs are cultured on separate layers, hepatocyte growth factor (HGF)150 and endothelin 1160 were identified as being involved in the regulation of MSC activity. In addition to the molecules produced by ECs described above, different soluble factors have also been identified through studies in which MSCs are co-cultured with other types of bone marrow cells or treated with conditioned medium from these cells. For example, BMP2 and BMP6 are produced by hematopoietic stem cells in co-culture to direct MSC fate and lineage decisions.161 Another study has shown that Lin-expressing hematopoietic cells secrete TNF-α; PDGF-β; WNT1, 4, 6, 7A, and 10A; and secreted frizzled-related peptide-3 and −5 to stimulate proliferation, induce osteogenic differentiation, and inhibit cellular senescence of MSCs in coculture.162 While it provides an opportunity to simultaneously access to a number of paracrine factors in culture, the approach of co-culture or conditioned medium still falls short of retrieving the complete soluble factors of the bone marrow. Recently, our group extracted total soluble factors from human bone marrow to treat MSCs in culture and found that bone marrow extract improves proliferation, cellular senescence, and multilineage differentiation of MSCs. We have further identified lipocalin-2 and prolactin as key molecules that are responsible for enhancing MSC activities in culture as well as in an animal model of bone repair.163
Conclusions
Two general areas of MSC research have developed since the discovery of MSCs.164 One is an understanding of the biology of MSCs. In this line of research, researchers have studied MSCs to determine the origin of the cells, characterize their behavior in vivo, and identify the mechanisms that regulate their activities.164 The other is to utilize the cell for therapeutic applications. MSCs have been evaluated through cell transplantation or tissue engineering in clinical trials for treating osteogenesis imperfecta, cartilage defects, myocardial infarction, liver cirrhosis, and inflammatory diseases.165 Recently, genome editing has been applied to stem cell therapies. Different genome editing techniques capable of enhancing functions of MSCs have been evaluated, and the success has made MSC-based therapies even more attractive for treating diseases. For example, Chang et al. utilized transcription activator-like effector nuclease (TALEN) to modify MSCs for inducible secretion of HGF to enhance angiogenesis as a potential treatment for vascular diseases.166
Since the current practices using MSCs for basic science research or translational applications often involve the step of cell expansion, it is essential that, when maintained ex vivo, MSCs are able to retain the phenotypes, functions, and properties as in their tissue niches. Future studies should focus on analyzing the effects of environmental factors of the stem cell niche on MSCs and determining the underlying mechanisms induced by the factors that regulate MSC activities. The knowledge gained from these studies can be used to help researchers develop viable strategies (Fig. 1) to maintain MSC phenotypes and retain their properties in cell culture. For example, it seems to be feasible to establish a culture environment with a well-defined composition of soluble factors, ECM molecules, and oxygen tensions that mimics that of the bone marrow niche to culture MSCs (Fig. 2). A strategy to create an engineered stem cell niche–like environment in vitro can be applied to basic science studies that aim to understand MSC biology as well as to translational research that aims to enhance MSC activities, such as lineage-specific differentiation and immunomodulation, for clinical applications.
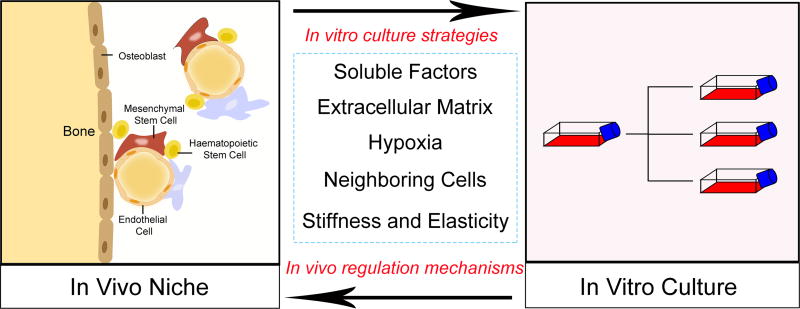
Figure 1.
Strategies proposed to advance methods for in vitro MSC culture. The knowledge gained through studies of the bone marrow niche can be used to help researchers develop viable strategies to maintain MSC properties in culture. On the other hand, MSC culture constructed on the basis of the bone marrow niche can provide a reliable in vitro model to study mechanisms involved in the interaction between MSCs and their microenvironment components.
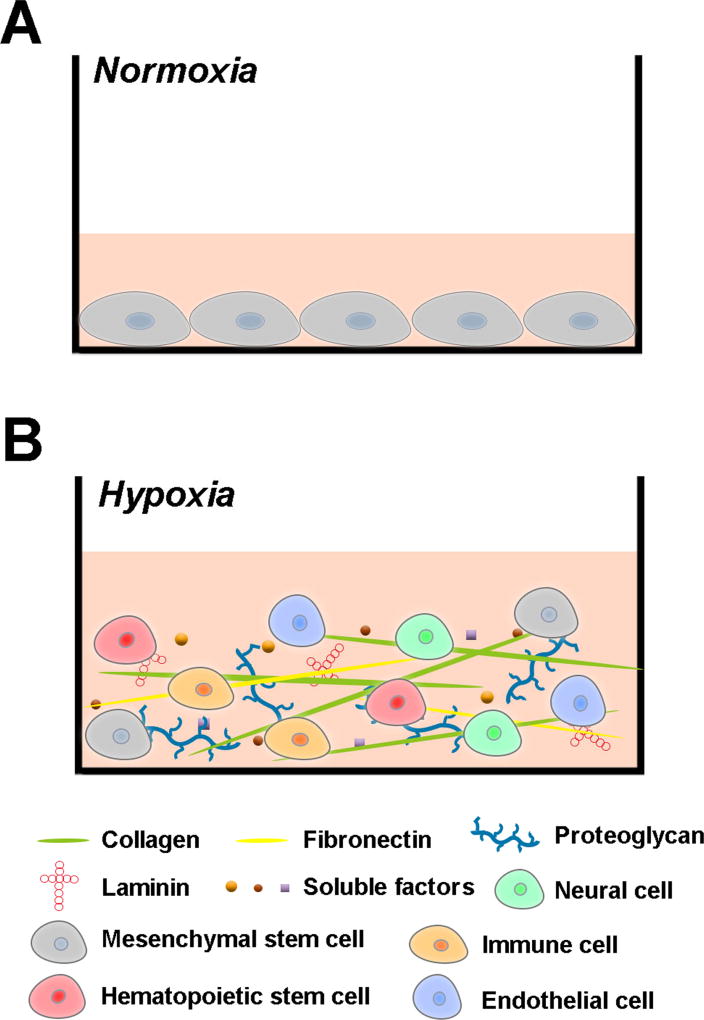
Figure 2.
Illustration of an engineered MSC microenvironment mimicking the bone marrow niche. Compared with the conventional culture under normoxia (A), the niche-like culture with a well-defined composition of soluble factors, ECM molecules, and cells under hypoxia (B) can be used to maintain MSC properties and functions.
Acknowledgments
The research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR064803. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. [PubMed] [Google Scholar]
- 2.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. Journal of embryology and experimental morphology. 1966;16:381–390. [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell and tissue kinetics. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein A, Kuralesova AI. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation. 1971;12:99–108. doi: 10.1097/00007890-197108000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and tissue kinetics. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 6.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 7.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 9.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem cells. 2004;22:1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 10.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. Journal of cellular biochemistry. 1997;64:295–312. [PubMed] [Google Scholar]
- 11.Johnstone B, Hering TM, Caplan AI, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental cell research. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochimica et biophysica acta. 2014;1840:2506–2519. doi: 10.1016/j.bbagen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. The international journal of biochemistry & cell biology. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen C, Gordeladze J, Noel D. Tissue engineering through autologous mesenchymal stem cells. Current opinion in biotechnology. 2004;15:406–410. doi: 10.1016/j.copbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Huang AH, Farrell MJ, Kim M, et al. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. European cells & materials. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Conor CJ, Case N, Guilak F. Mechanical regulation of chondrogenesis. Stem cell research & therapy. 2013;4:61. doi: 10.1186/scrt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youngstrom DW, Rajpar I, Kaplan DL, et al. A bioreactor system for in vitro tendon differentiation and tendon tissue engineering. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2015;33:911–918. doi: 10.1002/jor.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grier WG, Moy AS, Harley BA. Cyclic tensile strain enhances human mesenchymal stem cell Smad 2/3 activation and tenogenic differentiation in anisotropic collagen-glycosaminoglycan scaffolds. European cells & materials. 2017;33:227–239. doi: 10.22203/eCM.v033a14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meinel L, Karageorgiou V, Hofmann S, et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. Journal of biomedical materials research. Part A. 2004;71:25–34. doi: 10.1002/jbm.a.30117. [DOI] [PubMed] [Google Scholar]
- 21.Yamada Y, Nakamura S, Ito K, et al. Injectable bone tissue engineering using expanded mesenchymal stem cells. Stem cells. 2013;31:572–580. doi: 10.1002/stem.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li WJ, Tuli R, Okafor C, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Li WJ, Jiang YJ, Tuan RS. Cell-nanofiber-based cartilage tissue engineering using improved cell seeding, growth factor, and bioreactor technologies. Tissue engineering. Part A. 2008;14:639–648. doi: 10.1089/tea.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iu J, Massicotte E, Li SQ, et al. In Vitro Generated Intervertebral Discs: Towards Engineering Tissue Integration. Tissue engineering. Part A. 2017 doi: 10.1089/ten.TEA.2016.0433. [DOI] [PubMed] [Google Scholar]
- 25.Smith LJ, Gorth DJ, Showalter BL, et al. In vitro characterization of a stem-cell-seeded triple-interpenetrating-network hydrogel for functional regeneration of the nucleus pulposus. Tissue engineering. Part A. 2014;20:1841–1849. doi: 10.1089/ten.tea.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai TL, Nelson BC, Anderson PA, et al. Intervertebral disc and stem cells cocultured in biomimetic extracellular matrix stimulated by cyclic compression in perfusion bioreactor. The spine journal : official journal of the North American Spine Society. 2014;14:2127–2140. doi: 10.1016/j.spinee.2013.11.062. [DOI] [PubMed] [Google Scholar]
- 27.Hudson KD, Bonassar LJ. Hypoxic Expansion of Human Mesenchymal Stem Cells Enhances Three-Dimensional Maturation of Tissue-Engineered Intervertebral Discs. Tissue engineering. Part A. 2017;23:293–300. doi: 10.1089/ten.TEA.2016.0270. [DOI] [PubMed] [Google Scholar]
- 28.Barber JG, Handorf AM, Allee TJ, et al. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue engineering. Part A. 2013;19:1265–1274. doi: 10.1089/ten.tea.2010.0538. [DOI] [PubMed] [Google Scholar]
- 29.Czaplewski SK, Tsai TL, Duenwald-Kuehl SE, et al. Tenogenic differentiation of human induced pluripotent stem cell-derived mesenchymal stem cells dictated by properties of braided submicron fibrous scaffolds. Biomaterials. 2014;35:6907–6917. doi: 10.1016/j.biomaterials.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Fu Y, Karbaat L, Wu L, et al. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue engineering. Part B, Reviews. 2017 doi: 10.1089/ten.TEB.2016.0365. [DOI] [PubMed] [Google Scholar]
- 31.Konala VB, Mamidi MK, Bhonde R, et al. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13–24. doi: 10.1016/j.jcyt.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of cellular biochemistry. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 33.Berry MF, Engler AJ, Woo YJ, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. American journal of physiology. Heart and circulatory physiology. 2006;290:H2196–2203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 34.Otto Beitnes J, Oie E, Shahdadfar A, et al. Intramyocardial injections of human mesenchymal stem cells following acute myocardial infarction modulate scar formation and improve left ventricular function. Cell transplantation. 2012;21:1697–1709. doi: 10.3727/096368911X627462. [DOI] [PubMed] [Google Scholar]
- 35.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 36.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 37.Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 38.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Experimental & molecular medicine. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunology and cell biology. 2013;91:19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 40.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell stem cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 41.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 42.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 43.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PloS one. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell stem cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi Y, Su J, Roberts AI, et al. How mesenchymal stem cells interact with tissue immune responses. Trends in immunology. 2012;33:136–143. doi: 10.1016/j.it.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature medicine. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature immunology. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 48.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell death & disease. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spaggiari GM, Abdelrazik H, Becchetti F, et al. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 51.Vasandan AB, Jahnavi S, Shashank C, et al. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Scientific reports. 2016;6:38308. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, et al. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transplantation proceedings. 2013;45:434–439. doi: 10.1016/j.transproceed.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 53.Pinho S, Lacombe J, Hanoun M, et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of experimental medicine. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall SR, Jiang Y, Leary E, et al. Identification and isolation of small CD44-negative mesenchymal stem/progenitor cells from human bone marrow using elutriation and polychromatic flow cytometry. Stem cells translational medicine. 2013;2:567–578. doi: 10.5966/sctm.2012-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qian H, Le Blanc K, Sigvardsson M. Primary mesenchymal stem and progenitor cells from bone marrow lack expression of CD44 protein. The Journal of biological chemistry. 2012;287:25795–25807. doi: 10.1074/jbc.M112.339622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis and rheumatism. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 57.Quirici N, Soligo D, Bossolasco P, et al. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Experimental hematology. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- 58.Jung EM, Kwon O, Kwon KS, et al. Evidences for correlation between the reduced VCAM-1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells. Biochemical and biophysical research communications. 2011;404:463–469. doi: 10.1016/j.bbrc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Zhu H, Mitsuhashi N, Klein A, et al. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 60.Yang MC, Chi NH, Chou NK, et al. The influence of rat mesenchymal stem cell CD44 surface markers on cell growth, fibronectin expression, and cardiomyogenic differentiation on silk fibroin - Hyaluronic acid cardiac patches. Biomaterials. 2010;31:854–862. doi: 10.1016/j.biomaterials.2009.09.096. [DOI] [PubMed] [Google Scholar]
- 61.Mikami Y, Ishii Y, Watanabe N, et al. CD271/p75(NTR) inhibits the differentiation of mesenchymal stem cells into osteogenic, adipogenic, chondrogenic, and myogenic lineages. Stem cells and development. 2011;20:901–913. doi: 10.1089/scd.2010.0299. [DOI] [PubMed] [Google Scholar]
- 62.Churchman SM, Ponchel F, Boxall SA, et al. Transcriptional profile of native CD271+ multipotential stromal cells: evidence for multiple fates, with prominent osteogenic and Wnt pathway signaling activity. Arthritis and rheumatism. 2012;64:2632–2643. doi: 10.1002/art.34434. [DOI] [PubMed] [Google Scholar]
- 63.Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PloS one. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palumbo S, Tsai TL, Li WJ. Macrophage migration inhibitory factor regulates AKT signaling in hypoxic culture to modulate senescence of human mesenchymal stem cells. Stem cells and development. 2014;23:852–865. doi: 10.1089/scd.2013.0294. [DOI] [PubMed] [Google Scholar]
- 65.Riou JF, Guittat L, Mailliet P, et al. Cell senescence and telomere shortening induced by a new series of specific G-quadruplex DNA ligands. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2672–2677. doi: 10.1073/pnas.052698099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herbig U, Jobling WA, Chen BP, et al. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a) Molecular cell. 2004;14:501–513. doi: 10.1016/s1097-2765(04)00256-4. [DOI] [PubMed] [Google Scholar]
- 67.von Zglinicki T, Saretzki G, Ladhoff J, et al. Human cell senescence as a DNA damage response. Mechanisms of ageing and development. 2005;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 68.Tsai CC, Chen YJ, Yew TL, et al. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A–p21 by HIF-TWIST. Blood. 2011;117:459–469. doi: 10.1182/blood-2010-05-287508. [DOI] [PubMed] [Google Scholar]
- 69.Li XY, Ding J, Zheng ZH, et al. Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Molecular medicine reports. 2012;6:1183–1189. doi: 10.3892/mmr.2012.1039. [DOI] [PubMed] [Google Scholar]
- 70.Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem cells. 2004;22:675–682. doi: 10.1634/stemcells.22-5-675. [DOI] [PubMed] [Google Scholar]
- 71.Kuilman T, Michaloglou C, Mooi WJ, et al. The essence of senescence. Genes & development. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sotiropoulou PA, Perez SA, Salagianni M, et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura S, Yamada Y, Baba S, et al. Culture medium study of human mesenchymal stem cells for practical use of tissue engineering and regenerative medicine. Bio-medical materials and engineering. 2008;18:129–136. [PubMed] [Google Scholar]
- 74.Spencer JA, Ferraro F, Roussakis E, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 76.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nature reviews. Molecular cell biology. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura-Ishizu A, Okuno Y, Omatsu Y, et al. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119:5429–5437. doi: 10.1182/blood-2011-11-393645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 79.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 80.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leishman E, Howard JM, Garcia GE, et al. Foxp1 maintains hair follicle stem cell quiescence through regulation of Fgf18. Development. 2013;140:3809–3818. doi: 10.1242/dev.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nature reviews. Molecular cell biology. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chakkalakal JV, Jones KM, Basson MA, et al. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muguruma Y, Yahata T, Miyatake H, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- 86.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell stem cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell stem cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Worthley DL, Churchill M, Compton JT, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chan CK, Seo EY, Chen JY, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biondi M, Ungaro F, Quaglia F, et al. Controlled drug delivery in tissue engineering. Advanced drug delivery reviews. 2008;60:229–242. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 91.Haque N, Kasim NH, Rahman MT. Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. International journal of biological sciences. 2015;11:324–334. doi: 10.7150/ijbs.10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buravkova LB, Rylova YV, Andreeva ER, et al. Low ATP level is sufficient to maintain the uncommitted state of multipotent mesenchymal stem cells. Biochimica et biophysica acta. 2013;1830:4418–4425. doi: 10.1016/j.bbagen.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 93.Palomaki S, Pietila M, Laitinen S, et al. HIF-1alpha is upregulated in human mesenchymal stem cells. Stem cells. 2013;31:1902–1909. doi: 10.1002/stem.1435. [DOI] [PubMed] [Google Scholar]
- 94.Park IH, Kim KH, Choi HK, et al. Constitutive stabilization of hypoxia-inducible factor alpha selectively promotes the self-renewal of mesenchymal progenitors and maintains mesenchymal stromal cells in an undifferentiated state. Experimental & molecular medicine. 2013;45:e44. doi: 10.1038/emm.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanichai M, Ferguson D, Prendergast PJ, et al. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. Journal of cellular physiology. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 96.Wagegg M, Gaber T, Lohanatha FL, et al. Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PloS one. 2012;7:e46483. doi: 10.1371/journal.pone.0046483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nature reviews. Molecular cell biology. 2014;15:243–256. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xia W, Xie C, Jiang M, et al. Improved survival of mesenchymal stem cells by macrophage migration inhibitory factor. Molecular and cellular biochemistry. 2015;404:11–24. doi: 10.1007/s11010-015-2361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lv B, Li F, Han J, et al. Hif-1alpha Overexpression Improves Transplanted Bone Mesenchymal Stem Cells Survival in Rat MCAO Stroke Model. Frontiers in molecular neuroscience. 2017;10:80. doi: 10.3389/fnmol.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sheng L, Mao X, Yu Q, et al. Effect of the PI3K/AKT signaling pathway on hypoxia-induced proliferation and differentiation of bone marrow-derived mesenchymal stem cells. Experimental and therapeutic medicine. 2017;13:55–62. doi: 10.3892/etm.2016.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Palm W, Thompson CB. Nutrient acquisition strategies of mammalian cells. Nature. 2017;546:234–242. doi: 10.1038/nature22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Blazer S, Khankin E, Segev Y, et al. High glucose-induced replicative senescence: point of no return and effect of telomerase. Biochemical and biophysical research communications. 2002;296:93–101. doi: 10.1016/s0006-291x(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 104.Chang TC, Hsu MF, Wu KK. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PloS one. 2015;10:e0126537. doi: 10.1371/journal.pone.0126537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li YM, Schilling T, Benisch P, et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochemical and biophysical research communications. 2007;363:209–215. doi: 10.1016/j.bbrc.2007.08.161. [DOI] [PubMed] [Google Scholar]
- 106.Tsai TL, Manner PA, Li WJ. Regulation of mesenchymal stem cell chondrogenesis by glucose through protein kinase C/transforming growth factor signaling. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:368–376. doi: 10.1016/j.joca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 107.Newsholme P, Procopio J, Lima MM, et al. Glutamine and glutamate--their central role in cell metabolism and function. Cell biochemistry and function. 2003;21:1–9. doi: 10.1002/cbf.1003. [DOI] [PubMed] [Google Scholar]
- 108.Schop D, Janssen FW, van Rijn LD, et al. Growth, metabolism, and growth inhibitors of mesenchymal stem cells. Tissue engineering. Part A. 2009;15:1877–1886. doi: 10.1089/ten.tea.2008.0345. [DOI] [PubMed] [Google Scholar]
- 109.Shinde V, Perumal Srinivasan S, Henry M, et al. Comparison of a teratogenic transcriptome-based predictive test based on human embryonic versus inducible pluripotent stem cells. Stem cell research & therapy. 2016;7:190. doi: 10.1186/s13287-016-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dos Santos F, Andrade PZ, Boura JS, et al. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. Journal of cellular physiology. 2010;223:27–35. doi: 10.1002/jcp.21987. [DOI] [PubMed] [Google Scholar]
- 111.Saleh FA, Frith JE, Lee JA, et al. Three-dimensional in vitro culture techniques for mesenchymal stem cells. Methods in molecular biology. 2012;916:31–45. doi: 10.1007/978-1-61779-980-8_4. [DOI] [PubMed] [Google Scholar]
- 112.Bartosh TJ, Ylostalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Papadimitropoulos A, Piccinini E, Brachat S, et al. Expansion of human mesenchymal stromal cells from fresh bone marrow in a 3D scaffold-based system under direct perfusion. PloS one. 2014;9:e102359. doi: 10.1371/journal.pone.0102359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han S, Zhao Y, Xiao Z, et al. The three-dimensional collagen scaffold improves the stemness of rat bone marrow mesenchymal stem cells. Journal of genetics and genomics = Yi chuan xue bao. 2012;39:633–641. doi: 10.1016/j.jgg.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 115.Li Z, Tian X, Yuan Y, et al. Effect of cell culture using chitosan membranes on stemness marker genes in mesenchymal stem cells. Molecular medicine reports. 2013;7:1945–1949. doi: 10.3892/mmr.2013.1423. [DOI] [PubMed] [Google Scholar]
- 116.Hamilton R, Campbell FR. Immunochemical localization of extracellular materials in bone marrow of rats. The Anatomical record. 1991;231:218–224. doi: 10.1002/ar.1092310210. [DOI] [PubMed] [Google Scholar]
- 117.Klein G. The extracellular matrix of the hematopoietic microenvironment. Experientia. 1995;51:914–926. doi: 10.1007/BF01921741. [DOI] [PubMed] [Google Scholar]
- 118.Klees RF, Salasznyk RM, Kingsley K, et al. Laminin-5 induces osteogenic gene expression in human mesenchymal stem cells through an ERK-dependent pathway. Molecular biology of the cell. 2005;16:881–890. doi: 10.1091/mbc.E04-08-0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kundu AK, Putnam AJ. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochemical and biophysical research communications. 2006;347:347–357. doi: 10.1016/j.bbrc.2006.06.110. [DOI] [PubMed] [Google Scholar]
- 120.Tsai KS, Kao SY, Wang CY, et al. Type I collagen promotes proliferation and osteogenesis of human mesenchymal stem cells via activation of ERK and Akt pathways. Journal of biomedical materials research. Part A. 2010;94:673–682. doi: 10.1002/jbm.a.32693. [DOI] [PubMed] [Google Scholar]
- 121.Singh P, Schwarzbauer JE. Fibronectin and stem cell differentiation - lessons from chondrogenesis. Journal of cell science. 2012;125:3703–3712. doi: 10.1242/jcs.095786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hashimoto Y, Funamoto S, Kimura T, et al. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials. 2011;32:7060–7067. doi: 10.1016/j.biomaterials.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 123.Chen XD, Dusevich V, Feng JQ, et al. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 124.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–163. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gahmberg CG, Fagerholm SC, Nurmi SM, et al. Regulation of integrin activity and signalling. Biochimica et biophysica acta. 2009;1790:431–444. doi: 10.1016/j.bbagen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Askari JA, Tynan CJ, Webb SE, et al. Focal adhesions are sites of integrin extension. The Journal of cell biology. 2010;188:891–903. doi: 10.1083/jcb.200907174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nature reviews. Molecular cell biology. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 128.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 129.Shih YR, Tseng KF, Lai HY, et al. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 130.Lv H, Li L, Sun M, et al. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem cell research & therapy. 2015;6:103. doi: 10.1186/s13287-015-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan H, Zhou Y, Lee MS, et al. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta biomaterialia. 2016;42:247–257. doi: 10.1016/j.actbio.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schellenberg A, Joussen S, Moser K, et al. Matrix elasticity, replicative senescence and DNA methylation patterns of mesenchymal stem cells. Biomaterials. 2014;35:6351–6358. doi: 10.1016/j.biomaterials.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 133.Yang C, Tibbitt MW, Basta L, et al. Mechanical memory and dosing influence stem cell fate. Nature materials. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peng T, Liu L, MacLean AL, et al. A mathematical model of mechanotransduction reveals how mechanical memory regulates mesenchymal stem cell fate decisions. BMC systems biology. 2017;11:55. doi: 10.1186/s12918-017-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li CX, Talele NP, Boo S, et al. MicroRNA-21 preserves the fibrotic mechanical memory of mesenchymal stem cells. Nature materials. 2017;16:379–389. doi: 10.1038/nmat4780. [DOI] [PubMed] [Google Scholar]
- 136.Winer JP, Janmey PA, McCormick ME, et al. Bone marrow-derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue engineering. Part A. 2009;15:147–154. doi: 10.1089/ten.tea.2007.0388. [DOI] [PubMed] [Google Scholar]
- 137.Plotnikov SV, Pasapera AM, Sabass B, et al. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell. 2012;151:1513–1527. doi: 10.1016/j.cell.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 139.Tormin A, Li O, Brune JC, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Caplan AI. All MSCs are pericytes? Cell stem cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 141.Guimaraes-Camboa N, Cattaneo P, Sun Y, et al. Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell stem cell. 2017;20:345–359. e345. doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blocki A, Wang Y, Koch M, et al. Not all MSCs can act as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem cells and development. 2013;22:2347–2355. doi: 10.1089/scd.2012.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Q, Wang Z. Influence of mesenchymal stem cells with endothelial progenitor cells in co-culture on osteogenesis and angiogenesis: an in vitro study. Archives of medical research. 2013;44:504–513. doi: 10.1016/j.arcmed.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 144.Gershovich JG, Dahlin RL, Kasper FK, et al. Enhanced osteogenesis in cocultures with human mesenchymal stem cells and endothelial cells on polymeric microfiber scaffolds. Tissue engineering. Part A. 2013;19:2565–2576. doi: 10.1089/ten.tea.2013.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saleh FA, Whyte M, Genever PG. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. European cells & materials. 2011;22:242–257. doi: 10.22203/ecm.v022a19. discussion 257. [DOI] [PubMed] [Google Scholar]
- 146.Birmingham E, Niebur GL, McHugh PE, et al. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. European cells & materials. 2012;23:13–27. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]
- 147.Clabaut A, Delplace S, Chauveau C, et al. Human osteoblasts derived from mesenchymal stem cells express adipogenic markers upon coculture with bone marrow adipocytes. Differentiation; research in biological diversity. 2010;80:40–45. doi: 10.1016/j.diff.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 148.Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends in immunology. 2014;35:32–37. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.He Q, Scott Swindle C, Wan C, et al. Enhanced Hematopoietic Stem Cell Self-Renewal-Promoting Ability of Clonal Primary Mesenchymal Stromal/Stem cells Versus Their Osteogenic Progeny. Stem cells. 2017;35:473–484. doi: 10.1002/stem.2481. [DOI] [PubMed] [Google Scholar]
- 150.Chen QH, Liu AR, Qiu HB, et al. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem cell research & therapy. 2015;6:44. doi: 10.1186/s13287-015-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Anton K, Banerjee D, Glod J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PloS one. 2012;7:e35036. doi: 10.1371/journal.pone.0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen C, Lorimore SA, Evans CA, et al. A proteomic analysis of murine bone marrow and its response to ionizing radiation. Proteomics. 2005;5:4254–4263. doi: 10.1002/pmic.200401295. [DOI] [PubMed] [Google Scholar]
- 153.Wang W, Gou L, Xie G, et al. Proteomic analysis of interstitial fluid in bone marrow identified that peroxiredoxin 2 regulates H(2)O(2) level of bone marrow during aging. Journal of proteome research. 2010;9:3812–3819. doi: 10.1021/pr901180w. [DOI] [PubMed] [Google Scholar]
- 154.An N, Janech MG, Bland AM, et al. Proteomic analysis of murine bone marrow niche microenvironment identifies thioredoxin as a novel agent for radioprotection and for enhancing donor cell reconstitution. Experimental hematology. 2013;41:944–956. doi: 10.1016/j.exphem.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kovac M, Vaskova M, Petrackova D, et al. Cytokines, growth, and environment factors in bone marrow plasma of acute lymphoblastic leukemia pediatric patients. European cytokine network. 2014;25:8–13. doi: 10.1684/ecn.2014.0348. [DOI] [PubMed] [Google Scholar]
- 156.Solchaga LA, Penick K, Porter JD, et al. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. Journal of cellular physiology. 2005;203:398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 157.Handorf AM, Li WJ. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PloS one. 2011;6:e22887. doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaigler D, Krebsbach PH, West ER, et al. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:665–667. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 159.Saleh FA, Whyte M, Ashton P, et al. Regulation of mesenchymal stem cell activity by endothelial cells. Stem cells and development. 2011;20:391–403. doi: 10.1089/scd.2010.0168. [DOI] [PubMed] [Google Scholar]
- 160.Tsai TL, Wang B, Squire MW, et al. Endothelial cells direct human mesenchymal stem cells for osteo- and chondro-lineage differentiation through endothelin-1 and AKT signaling. Stem cell research & therapy. 2015;6:88. doi: 10.1186/s13287-015-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jung Y, Song J, Shiozawa Y, et al. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem cells. 2008;26:2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhou S. Paracrine effects of haematopoietic cells on human mesenchymal stem cells. Scientific reports. 2015;5:10573. doi: 10.1038/srep10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tsai TL, Li WJ. Identification of Bone Marrow-Derived Soluble Factors Regulating Human Mesenchymal Stem Cells for Bone Regeneration. Stem cell reports. 2017;8:387–400. doi: 10.1016/j.stemcr.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bianco P. "Mesenchymal" stem cells. Annual review of cell and developmental biology. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 165.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. The Korean journal of internal medicine. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chang HK, Kim PH, Cho HM, et al. Inducible HGF-secreting Human Umbilical Cord Blood-derived MSCs Produced via TALEN-mediated Genome Editing Promoted Angiogenesis. Molecular therapy : the journal of the American Society of Gene Therapy. 2016;24:1644–1654. doi: 10.1038/mt.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]