Abstract
Background:
Heat waves are extreme weather events that have been associated with adverse health outcomes. However, there is limited knowledge of heat waves’ impact on population morbidity, such as emergency department (ED) visits.
Objectives:
We investigated associations between heat waves and ED visits for 17 outcomes in Atlanta over a 20-year period, 1993–2012.
Methods:
Associations were estimated using Poisson log-linear models controlling for continuous air temperature, dew-point temperature, day of week, holidays, and time trends. We defined heat waves as periods of consecutive days with temperatures beyond the 98th percentile of the temperature distribution over the period from 1945–2012. We considered six heat wave definitions using maximum, minimum, and average air temperatures and apparent temperatures. Associations by heat wave characteristics were examined.
Results:
Among all outcome-heat wave combinations, associations were strongest between ED visits for acute renal failure and heat waves defined by maximum apparent temperature at lag 0 [relative risk (RR) = 1.15; 95% confidence interval (CI): 1.03–1.29], ED visits for ischemic stroke and heat waves defined by minimum temperature at lag 0 (RR = 1.09; 95% CI: 1.02–1.17), and ED visits for intestinal infection and heat waves defined by average temperature at lag 1 (RR = 1.10; 95% CI: 1.00–1.21). ED visits for all internal causes were associated with heat waves defined by maximum temperature at lag 1 (RR = 1.02; 95% CI: 1.00, 1.04).
Conclusions:
Heat waves can confer additional risks of ED visits beyond those of daily air temperature, even in a region with high air-conditioning prevalence. https://doi.org/10.1289/EHP44
Introduction
Heat waves are extreme weather events that can exert notable impacts on the economy and public health (Field et al. 2014). Although the definition of a heat wave varies by country and region, it is commonly characterized by a period of sustained abnormally hot weather compared to historical observations (Meehl and Tebaldi 2004). In the United States, a heat wave is often identified as a period of two or more exceedingly hot days, but the temperature metric used and the definition of extreme temperature can vary (Anderson et al. 2013; Chen et al. 2015). While the occurrence of heat waves is mostly a natural phenomenon, human activities that contribute to climate change are thought to increase the severity of heat waves (Meehl et al. 2007). Additionally, projections from global climate models indicate that the number of severe heat waves is likely to increase in the future due to increased emissions of greenhouse gases and greater urban heat island effects (Duffy and Tebaldi 2012; Coumou et al. 2013).
Heat waves have been consistently associated with increased risk of mortality based on evidence from historical extreme events (Semenza et al. 1996) and recent epidemiological studies (Anderson and Bell 2011; D’Ippoliti et al. 2010; Hajat et al. 2006; Wang et al. 2015). High ambient temperature can cause heat-related illnesses such as heat exhaustion and heat stroke, or aggravate several common cardiovascular and pulmonary conditions (Borden and Cutter 2008; Bouchama et al. 2007; Wilker et al. 2012). In the United States, extreme heat accounted for about 31% of all the weather-related deaths during 2006 to 2010 (Berko et al. 2014). A large study of 43 cities in the United States estimated that the daily mortality rate during heat wave days was 3.7% higher on average than non–heat wave days during 1987–2005 (Anderson and Bell, 2011). Epidemiologic studies have shown that the association between high temperature and mortality has decreased over the past few decades; however, contemporary health risks are still substantial (Bobb et al. 2014; Davis et al. 2003; Gasparrini et al. 2015). The decrease may be attributed to successful adaptation and mitigation strategies, such as heat warning systems, communication campaigns that lead to behavior changes, and increases in air-conditioning prevalence (Boeckmann and Rohn 2014; Hondula et al. 2015).
The elderly and children have been identified as two susceptible populations for heat-related mortality and morbidity (Schifano et al. 2009; Vanos 2015). The elderly population is at a higher risk because of physiology and behavioral reasons, such as existing cardiovascular diseases, impaired kidney function, and living alone with limited social support (Kovats and Kristie 2006). Individuals who are confined to bed and unable to care for themselves may be at high risk of death during heat waves, possibly due to their limited access to emergency care (Hajat et al. 2006; Knowlton et al. 2009). Children are vulnerable in part because their renal systems are particularly stressed by a series of thermoregulatory adjustments under excessive heat (Xu et al. 2012), as well as their activity patterns (Vanos 2015).
While numerous studies worldwide have examined relationships between heat waves and mortality, fewer studies have examined associations between heat waves and morbidity using indicators such as hospital admissions and emergency department (ED) visits (reviewed by Li et al. 2015). In the United States, national studies of hospital admissions have relied on the Medicare database in which the at-risk population is restricted to those y old (Bobb et al. 2014; Gronlund et al. 2014). A study of ED visits in North Carolina from 2007 to 2011 found increased visits during heat wave days compared to non–heat wave days, especially among the elderly, adolescents, and people who had high occupational exposure to heat (Fuhrmann et al. 2016). Similar increases in ED visits were found during the 2006 heat wave in Paris (Josseran et al. 2009) and during the 2011 heat wave in Sydney, Australia (Schaffer et al. 2012). Time-series and case-crossover studies of ED visits and heat waves have also been conducted in Houston, Texas, United States (Zhang et al. 2015), Australia (Toloo et al 2014), and China (Sun et al. 2014).
The objective of this study was to estimate warm season associations between heat waves and daily ED visits in Atlanta, Georgia, during the period from 1993 to 2012. To our knowledge, our 20-year study is the longest among other ED visit time-series studies, and fills an important knowledge gap on the impacts of heat waves on population morbidity as measured by ED visits. In previous U.S. national studies, associations between heat waves and mortality are generally found to be weaker in Atlanta and in the southeastern United States than in other parts of the country (Anderson and Bell 2011; Davis et al. 2016). However, the southeastern United States tends to experience more intense heat waves with higher temperature and humidity than rest of the United States (Bonan 1997). Atlanta has also experienced rates of increase in heat wave frequency and duration that are higher than the national averages from 1961 to 2010 (Habeeb et al. 2015).
Here, we build upon previous work in Atlanta in which, similar to studies in other regions (Basu et al. 2012; Ghirardi et al. 2015; Petitte et al. 2016; Saha et al. 2015), we observed associations between continuous maximum temperature and maximum apparent temperature and ED visits for all internal causes, heat illness, fluid/electrolyte imbalances, renal diseases, asthma/wheeze, diabetes, and intestinal infections (Winquist et al. 2016). For the present study, we were specifically interested in the added effect of extreme heat over a sustained period beyond the continuous temperature–response relationships. In examining the added effect of extreme heat, we considered six heat wave definitions using various temperature metrics that can provide results relevant to public health intervention. While maximum temperature is the most commonly used temperature metric, we also considered minimum temperature and apparent temperature that may strongly influence the body’s warming and cooling mechanisms. Different temperature metrics are also less correlated at the extremes and occur at different hours of the day. Studies have shown that temperature metrics most associated with adverse health outcomes can vary across cities (Barnett et al. 2010; Davis et al. 2016).
Exposure to high heat is often avoidable. Our findings can play an important role in supporting local emergency preparedness, performing detailed risk assessment, and protecting public health (Ebi and Schmier 2005; Frumkin et al. 2008). For example, identification of heat metrics most associated with adverse health outcomes may result in more effective local warning systems. During extreme heat events, Atlanta provides cooling centers and free public pool access. Developing targeted communication strategies for specific subpopulations, such as outdoor workers, the elderly without air-conditioning, or those with preexisting medical conditions (as examined here), may further reduce the health impacts of extreme heat events.
Materials and Methods
Data Sources
For the period from 1993 to 2004, individual records of ED visits were obtained from hospitals within the 20-county Atlanta metropolitan area; for the period from 2005 to 2012, ED visit data were obtained from the Georgia Hospital Association. A comparison of data for the 2002–2004 period indicated minimal differences by hospital in visits captured between the two data sources. ED records from both data sources included admission date, and primary and secondary International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes (U.S. Dept. Health and Human Services 1991). We calculated daily counts of ED visits for 17 adverse health outcomes of interest; outcomes were the same as those analyzed previously by Winquist et al. (2016), and represented outcomes associated with heat in previous studies. The outcomes were defined either by the primary ICD-9 codes only, or by the presence of the selected codes in any of the diagnosis variables (i.e., primary or secondary). Inclusion of secondary diagnoses for some outcomes was based on the finding that stronger associations between temperature and these outcomes were observed when secondary diagnoses were included (Winquist et al. 2016). The outcomes were defined as follows: fluid and electrolyte imbalance (primary ICD-9 code 276), all renal diseases (primary ICD-9 codes 580–593), nephritis and nephrotic syndrome (primary ICD-9 codes 580–589), acute renal failure (primary ICD-9 code 584), all circulatory system diseases (primary or secondary ICD-9 codes 390–459), hypertension (primary or secondary ICD-9 codes 401–405), ischemic heart disease (primary or secondary ICD-9 codes 410–414), dysrhythmia (primary or secondary ICD-9 code 427), congestive heart failure (primary or secondary ICD-9 code 428), ischemic stroke (primary or secondary ICD-9 codes 433–437), all respiratory system diseases (primary ICD-9 codes 460–519), pneumonia (primary ICD-9 codes 480–486), chronic obstructive pulmonary disease (primary or secondary ICD-9 codes 491, 492, and 496), asthma/wheeze (primary ICD-9 codes 493 or 786.07); diabetes (primary ICD-9 codes 249 and 250), and intestinal infections (primary ICD-9 codes 001–009). We also considered all internal causes (ICD-9 codes 001–799).
Weather data for Atlanta were obtained from the National Climatic Data Center for the first-order weather station located at the Atlanta Hartsfield International Airport. These data were used to calculate daily metrics (i.e., maximum, minimum, average) of dry bulb temperature (i.e., MAXT, MINT, AVGT), apparent temperature (MAXAT, MINAT, AVGAT), and dew-point temperature. Apparent temperature is a measure that combines temperature and humidity in the metric (Steadman 1984), defined as , where is ambient air temperature () and is water vapor pressure (kPa). There is no universally recognized definition of heat wave; however, a heat wave event should reflect duration and intensity of extreme heat. We examined six heat wave metrics. We first identified heat wave periods with consecutive days with daily maximum, minimum, or average temperature or apparent temperature beyond the 98th percentiles. The 98th percentile threshold values were determined based on the distributions of year-round daily maximum, minimum, and average temperatures and apparent temperature over all available station records in Atlanta during 1945–2012. Heat wave days were then defined as days within each heat wave period except the first day to only capture effects of sustained heat over days. Hence, we treated the first day of a heat wave period as a non–heat wave day. We also characterized heat waves according to their duration, timing, and intensity. For duration, heat wave days were categorized as being the first, second, third, or later day within each heat wave. For timing, each heat wave was categorized as being the first, second, or later heat wave within each year. Finally, the intensity of a heat wave was characterized by the average temperature across days of the heat wave, using the temperature metric that defined the heat wave.
Statistical Analysis
We assessed the increase in risk of ED visits during heat wave days compared to non–heat wave days, using a Poisson log-linear model, allowing for over-dispersion. We restricted the analysis to warm seasons (May to September). The primary model was specified as:
where is the expected number of ED visits for health outcome on day ; is the log relative risk for ED visits on heat wave days vs. non–heat wave days; is 0 when day is a non–heat wave day (including the first day of every heat wave), and 1 when day is the second or later days in heat wave under definition ; is the temperature (in Celsius) with the same metric used in heat wave definition on day , modeled as a smooth function using natural cubic splines (ns) with 4 degrees of freedom to account for possible nonlinear relationships with ED visits; is the maximum dew-point temperature (in Celsius) on day to capture the strongest level of human discomfort during the day, but it is not included in models with heat waves defined using apparent temperature, as these models already include control for continuous apparent temperature (via the term), which incorporates a measure of humidity; is the categorical variable for day of the week on day ; includes dichotomous variables that indicate days on which federal holidays are observed; denotes hospital indicators to account for hospitals’ contributions to the total ED visits, coded 1 when hospital contributes ED visits on day ; and ns(DATEt) includes a common smooth function of day of the warm season with monthly knots across years, and a year-specific linear function for day of the warm season to capture between-year differences (Winquist et al 2016). We used a larger degrees of freedom to model calendar date per year compared to previous time-series analyses of daily mortality or hospital admissions among the elderly because ED visits can exhibit finer-scale temporal trends (e.g., over a few weeks compared to months). For example, ED visits for respiratory diseases can peak during the first few weeks in early fall due to a back-to-school effect among the pediatric population. The use of indicators to account for hospitals’ contributions to the total ED visit count and the nonlinear temporal trend may minimize potential bias in the assessment of acute heat wave effects resulting from the change in the ED visit data source between 2004 and 2005. We examined heat waves lagged up to 3 days while controlling for temperature at the same lag. Models for each outcome, heat wave definition, and lag combination were fitted separately.
Sensitivity analyses were conducted by increasing the degrees of freedom for continuous temperature from 4 to 6, replacing maximum dew-point temperature ( by minimum or average dew-point temperature or by relative humidity, and considering defining using different metrics that are not the same as the heat wave definition.
To examine associations by heat wave characteristics, the main heat wave indicator ( was replaced by categorical variables for heat wave duration or timing with non–heat wave days serving as the reference; all other covariates remained the same. We also assessed the effects of heat wave intensity by replacing with the average temperature during the heat wave for heat wave days and a value of 0 for non–heat wave days. Hence, in the intensity analysis, heat wave effects were based on continuous exposure metrics.
Results
Table 1 presents descriptive statistics of the ED visits. This study included a total of 9,856,015 ED visits to Atlanta metropolitan area hospitals during 1993–2012, of which 6,994,110 had primary ICD-9 codes indicating internal causes. The overall mean daily count of ED visits for internal causes was 2,286, with the overall mean daily counts of cause-specific ED visits ranging from six for acute renal failure to 622 for all circulatory diseases. For most outcomes, the mean daily counts during heat waves defined by minimum temperature were the highest, while those during heat waves defined by maximum temperature were the lowest. The mean daily counts during heat waves defined by daily temperature were similar to those defined by apparent temperature using daily maximum, minimum, or average.
Table 1.
Descriptive statistics for daily emergency department (ED) visits based during May to September in Atlanta, 1993–2012.
| Outcome | ICD-9 Code(s) | Total ED visits | Mean daily ED visits | Mean daily ED visits, during heat waves defined by: | |||||
|---|---|---|---|---|---|---|---|---|---|
| MAXT | MINT | AVGT | MAXAT | MINAT | AVGAT | ||||
| All ED visits | All | 9,856,015 | 3,220 | 2,570 | 3,249 | 2,827 | 2,664 | 3,193 | 2,969 |
| All internal causes | 001–799 | 6,994,110 | 2,286 | 1,831 | 2,366 | 2,043 | 1,909 | 2,332 | 2,165 |
| Fluid and electrolytea imbalance | 276 | 66,369 | 22 | 22 | 27 | 24 | 23 | 26 | 25 |
| All renal diseasea | 580–593 | 140,678 | 46 | 43 | 56 | 49 | 46 | 56 | 52 |
| Nephritis and nephrotic syndromea | 580–589 | 22,412 | 7 | 9 | 11 | 10 | 9 | 11 | 11 |
| Acute renal failurea | 584 | 19,274 | 6 | 8 | 10 | 9 | 8 | 10 | 10 |
| All circulatory system diseaseb | 390–459 | 1,905,253 | 622 | 533 | 761 | 634 | 573 | 740 | 694 |
| Hypertensionb | 401–405 | 1,501,108 | 490 | 431 | 624 | 517 | 464 | 605 | 568 |
| Ischemic heart diseaseb | 410–414 | 367,013 | 120 | 102 | 145 | 123 | 110 | 143 | 134 |
| Dysrhythmiab | 427 | 285,998 | 93 | 79 | 115 | 96 | 86 | 113 | 105 |
| Congestive heart failureb | 428 | 227,586 | 74 | 62 | 90 | 74 | 67 | 88 | 82 |
| Ischemic strokeb | 433–437 | 71,302 | 23 | 20 | 27 | 23 | 22 | 27 | 25 |
| All respiratory system diseasea | 460–519 | 900,570 | 294 | 207 | 253 | 224 | 206 | 253 | 232 |
| Pneumoniaa | 480–486 | 90,587 | 29 | 20 | 26 | 22 | 20 | 25 | 23 |
| Chronic obstructive pulmonary diseaseb | 491–492, 496 | 224,127 | 73 | 61 | 87 | 73 | 65 | 85 | 79 |
| Asthma/wheezea | 493 or 7896.09/.07 | 177,020 | 58 | 43 | 49 | 44 | 41 | 51 | 47 |
| Diabetes mellitusa | 250 or 249 | 70,076 | 22 | 20 | 27 | 23 | 21 | 28 | 25 |
| Intestinal infectiona | 001–009 | 30,610 | 10 | 10 | 12 | 11 | 10 | 11 | 11 |
Note: Mean daily visits are calculated across the entire study period and during heat wave days. Heat waves are defined as periods of consecutive days with temperature (T) or apparent temperature (at) exceeding the 98th percentile using daily maximum (MAX), minimum (MIN), or average (AVG). ED visits occurring on the first day of each heat wave period are excluded from the summary to only reflect visits occurring during sustained heat over 2 or more days.
primary ICD-9 codes only.
presence of the selected ICD-9 codes in any of the diagnoses (i.e. primary and secondary).
Table 2 presents a summary of the heat waves occurring in Atlanta from 1993–2012, according to the six different heat wave definitions. Heat waves defined by maximum temperature ( consecutive days exceeding the 98th percentile threshold of ) had the fewest heat wave days overall ( days) with average durations of 3.1 days per heat wave. All heat wave definitions had a median duration of 2 days. Heat wave days defined by minimum temperature had the most heat waves overall ( days). Table S1 shows the pairwise concordance and discordance between heat wave days defined using different heat wave metrics. Overall, there was only moderate concordance across the different definitions. The concordance percent ranged from 25% for heat waves defined by maximum temperature and minimum apparent temperature to 65% for heat waves defined by maximum apparent temperature and average temperature.
Table 2.
Descriptive statistics of heat wave characteristics during May to September in Atlanta, Georgia, 1993–2012.
| Heat wave definitions | 98th percentilea threshold temperature () | Total number of heat wave days | Average number of heat waves per year | Average duration of heat waves (after first day, in days) | Mean temperature during heat waves () |
|---|---|---|---|---|---|
| MAXT | 35.0 | 91 | 1.5 | 3.1 | 36.6 |
| MINT | 23.3 | 232 | 3.2 | 3.6 | 24.2 |
| AVGT | 28.9 | 123 | 1.8 | 3.5 | 30.2 |
| MAXAT | 35.6 | 109 | 1.9 | 3.0 | 37.2 |
| MINAT | 26.1 | 96 | 1.8 | 2.7 | 27.2 |
| AVGAT | 30.6 | 118 | 1.8 | 3.3 | 31.9 |
Note: Heat wave periods are defined as periods of consecutive days with temperature (T) or apparent temperature (at) exceeding the 98th percentile using daily maximum (MAX), minimum (MIN), or average (AVG). The first day of each heat wave period is excluded from the summary to only reflect characteristics occurring during sustained heat over 2 or more days.
Thresholds are determined among records from 1945 to 2012.
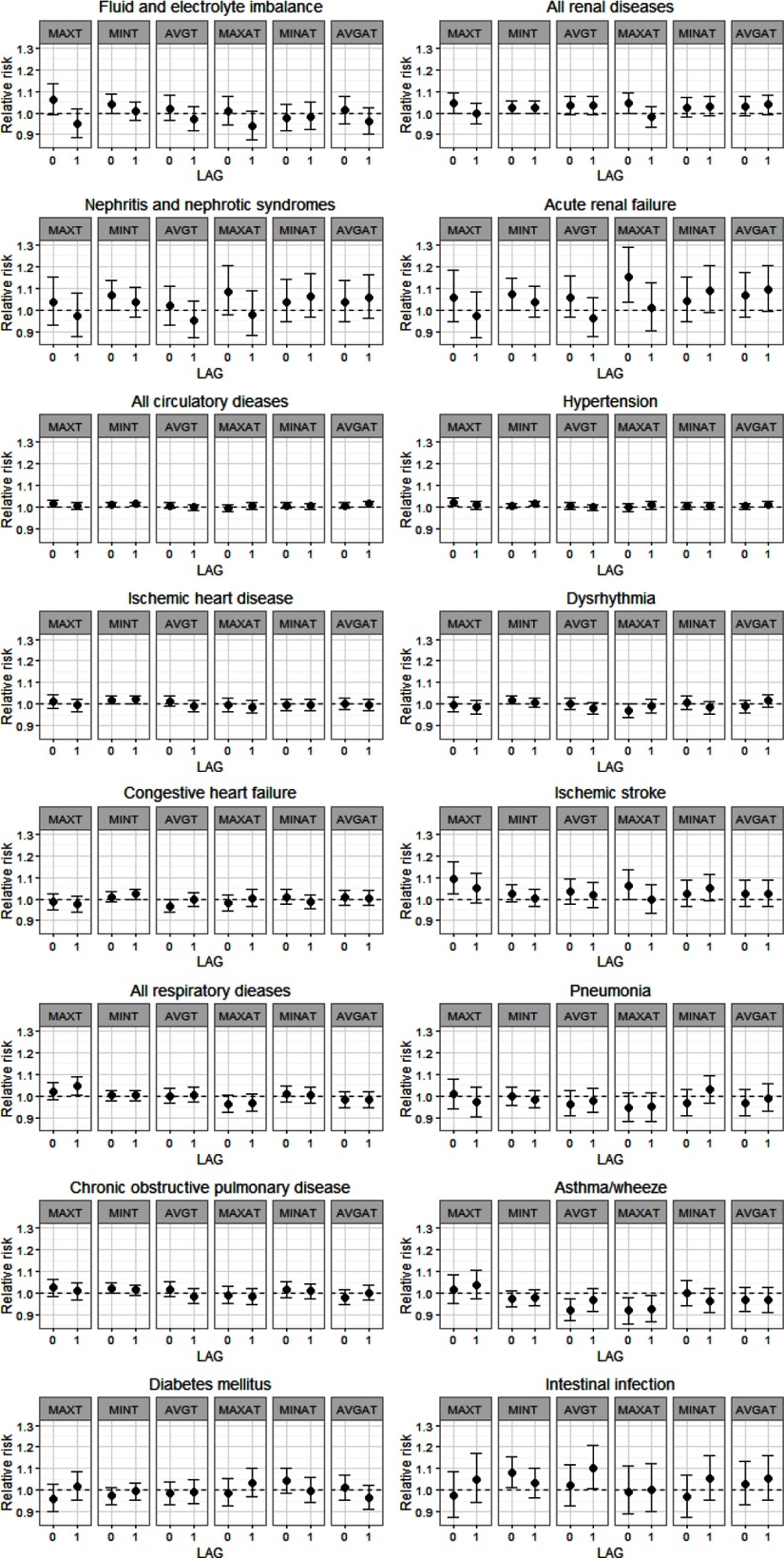
ED visits for all internal causes were associated with heat wave days defined by maximum temperature at lag 0 [relative risk (RR) = 1.02; confidence interval (CI): 1.00, 1.03) and lag 1 (RR = 1.02; 95% CI: 1.00, 1.04), controlling for continuous maximum temperature at the same lag. Observed associations between heat wave days and all other outcomes are presented in Figure 1; these associations consider heat waves at lags 0 and 1, controlling for continuous temperature at the same lag. RR estimates and 95% CIs for all ED outcomes by heat wave metrics are also given in Table S2. Among renal diseases, we observed significant heat wave associations for nephritis and nephrotic syndrome (lag 0, minimum temperature RR = 1.07; 95% CI: 1.00, 1.14) and acute renal failure (lag 0, minimum temperature RR = 1.07; 95% CI: 1.00, 1.15; lag 0, maximum apparent temperature RR = 1.15; 95% CI: 1.03, 1.29). Significant associations were also detected for total circulatory diseases (lag 0, minimum temperature RR = 1.01; 95% CI: 1.00, 1.02; lag 1, minimum temperature RR = 1.01; 95% CI: 1.00, 1.02), as well as specific causes, including: hypertension (lag 0, maximum temperature RR = 1.02; 95% CI: 1.00, 1.04; lag 1, minimum temperature RR = 1.02; 95% CI: 1.01, 1.03), ischemic heart disease (lag 0 minimum temperature RR = 1.02; 95% CI: 1.00, 1.04; lag 1, minimum temperature RR = 1.02; 95% CI: 1.00, 1.04), dysrhythmia (lag 0, minimum temperature RR = 1.02; 95% CI: 1.00, 1.04), congestive heart failure (lag 1, minimum temperature RR = 1.02; 95% CI: 1.00, 1.05), and ischemic stroke (lag 0, maximum temperature RR = 1.09; 95% CI: 1.02, 1.17). Associations of heat waves and ED visits for intestinal infections were also significant (lag 0, minimum temperature RR = 1.08; 95% CI: 1.01, 1.16; lag 1 average temperature RR = 1.10; 95% CI: 1.00, 1.21). We found no positive significant associations between heat waves and ED visits for total and most respiratory outcomes. Associations at lag 2 and lag 3 were weaker and mostly null for all outcomes, except for diabetes mellitus (lag 2, maximum temperature RR = 1.08; 95% CI: 1.01, 1.15).
Figure 1.
Estimated relative risks and 95% confidence intervals for emergency department (ED) visits associated with heat wave days compared to non–heat wave days. Heat waves were defined as periods of consecutive days with temperature (T) or apparent temperature (AT) exceeding the 98th percentile using daily maximum (MAX), minimum (MIN), or average (AVG) temperature. The first day of each heat wave period was considered as a non–heat wave day.
Figure S1 presents results of the sensitivity analysis of varying the continuous temperature metric in the health model for lag 0 heat wave exposures defined using maximum temperature, minimum temperature, or maximum apparent temperature. While most RRs are robust against the choice of the continuous temperature metric, when the continuous temperature metric is different from the heat wave definition, we observed stronger associations for some outcomes. This may be due to residual confounding, such that the heat wave association no longer presents the added effect beyond the continuous temperature. Figure S2 presents results of the sensitivity analyses by replacing the continuous maximum dew-point temperature in the health model with minimum or average dew-point temperature, as well as with maximum, average, or minimum relative humidity. For lag 0 and lag 1 heat wave metrics defined using maximum or minimum temperature, we found the heat wave and ED visit associations to be robust against the choice of confounders. In a few cases for heat waves defined using maximum temperature, additional significant associations were detected when relative humidity was used. This may be due to residual confounding because the magnitude of relative humidity may not fully capture human discomfort as compared to dew-point temperature. Associations by the order of days in each heat wave, defined using maximum or minimum temperature, and the sequence of the heat wave within a year and ED visit outcomes at lag 0 and lag 1 are given in Tables S3 and S4. For most outcomes, the associations were similar in magnitude across different heat wave days. However, stronger associations at later compared to earlier days in a heat wave were found for intestinal infection (day 3, lag 0, minimum temperature RR = 1.16; 95% CI: 1.04, 1.29), acute renal failure (day 4, lag 1, minimum temperature RR = 1.17; 95% CI: 1.04, 1.31), and ischemic stroke (day 4, lag 1, maximum temperature RR = 1.17; 95% CI: 1.02, 1.34). We found that associations were stronger for heat waves occurring later than those occurring earlier within a year for some outcomes, including acute renal failure (third or later heat wave, lag 0, maximum temperature RR = 1.15; 95% CI: 1.01, 1.31) and ischemic stroke (second heat wave, lag 0, maximum temperature RR = 1.13; 95% CI: 1.04, 1.22).
Table 3 presents associations between heat wave intensity (as measured by average temperature during a heat wave) and ED visits for selected outcomes at lag 0 and lag 1, using heat waves defined by minimum or maximum temperature. Estimates for all heat wave metrics are given in Table S5. A increase in temperature during a heat wave was associated with a RR of 1.0025 (lag 0, maximum temperature, 95% CI: 1.0007, 1.0044) for ischemic stroke, a RR of 1.0029 (lag 0, minimum temperature, 95% CI: 1.0001, 1.0057) for acute renal failure, and a RR of 1.0032 (lag 1, minimum temperature, 95% CI: 1.0004, 1.0060) for intestinal infection. These results indicate a potential exposure–response relationship for heat wave intensity.
Table 3.
Relative risk (RR) estimates and 95% confidence interval (CI) for selected emergency department (ED) visit outcomes associated with a increase in heat wave average temperature in Atlanta, Georgia, 1993–2012.
| Outcome | Heat wave | Lag 0 RR (95% CI) | Lag 1 RR (95% CI) |
|---|---|---|---|
| Nephritis and nephrotic syndrome | MAXT | 1.0009 (0.9980, 1.0038) | 0.9993 (0.9964, 1.0022) |
| MINT | 1.0027 (1.0000, 1.0054) | 1.0015 (0.9988, 1.0042) | |
| Acute renal failure | MAXT | 1.0015 (0.9984, 1.0046) | 0.9993 (0.9963, 1.0023) |
| MINT | 1.0029 (1.0001, 1.0057) | 1.0015 (0.9987, 1.0044) | |
| Hypertension | MAXT | 1.0006 (1.0001, 1.0011) | 1.0002 (0.9997, 1.0007) |
| MINT | 1.0003 (0.9999, 1.0007) | 1.0007 (1.0003, 1.0011) | |
| Ischemic heart disease | MAXT | 1.0003 (0.9994, 1.0011) | 0.9998 (0.9990, 1.0007) |
| MINT | 1.0008 (1.0000, 1.0015) | 1.0009 (1.0001, 1.0016) | |
| Ischemic stroke | MAXT | 1.0025 (1.0007, 1.0044) | 1.0013 (0.9994, 1.0031) |
| MINT | 1.0011 (0.9994, 1.0027) | 1.0002 (0.9985, 1.0019) | |
| Intestinal infection | MAXT | 0.9992 (0.9962, 1.0023) | 1.0014 (0.9983, 1.0044) |
| MINT | 1.0032 (1.0004, 1.0060) | 1.0013 (0.9985, 1.0041) |
Note: Heat waves are defined as periods of consecutive days with minimum temperature (MINT) or maximum temperature (MAXT) exceeding the 98th percentile. The reference period includes any non-heat wave day and the first day of every heat wave period. The exposure metric for days during a specific heat wave is the average temperature of the heat wave, while reference days are assigned a value of zero.
Discussion
In this 20-year time-series analysis of sustained extreme heat exposures and daily ED visits in Atlanta, we found the strongest evidence of significant associations for renal and circulatory outcomes, particularly acute renal failure and ischemic stroke (lags 0 and 1), and intestinal infections among the outcomes examined. When exposed to extreme heat, acute thermoregulatory adjustments accelerate heat loss in the body (Libert et al. 1988). Acute renal failure can happen when the adjustment produces stress on the renal system. The kidney is mainly responsible for maintaining the balance of body fluid and electrolyte (Karmarkar and MacNab 2012). Several studies have found significant association between heat wave and hospitalization for renal diseases (Bobb et al. 2014; Fletcher et al. 2012; Hansen et al. 2008) Among the limited studies that examined cause-specific ED visits in relation to heat waves, Knowlton et al. (2009) found that ED visits for acute renal failure were higher during the 2006 California heat wave compared with reference periods before and after the heat wave (RR = 1.15; 95% CI: 1.11–1.19). During three heat warning events in North Carolina, Fuhrmann et al. (2016) also found significant increases in ED visits for acute renal failure with percent excess visits ranging from 28% to 34%. These previous estimates are similar to those obtained in our current 20-year time-series analysis in Atlanta. Regarding associations between heat waves and intestinal infection, sustained heat may enhance environmental bacterial growth conditions. High temperature has been associated with bacillary dysentery cases in China (Zhang et al. 2008) and incidence of hospital admissions for infectious gastroenteritis and inflammatory bowel disease (Manser et al. 2013). Xu et al. (2012) also found high temperature to be associated with pediatric ED visits for intestinal infections in Brisbane, Australia.
Epidemiologic studies have consistently found that cardiovascular, cerebrovascular, and respiratory illnesses account for a large proportion of increased mortality and hospital admissions during heat waves (Fouillet et al. 2006; Kovats and Kristie 2006; Michelozzi et al. 2009). However, heat wave studies assessing ED visits for these diseases have shown contradictory findings. In studies in New York and Taiwan, the number of ED visits for cardiovascular and respiratory illness were significantly higher during heat wave days compared to non–heat wave days, especially among the elderly (Lin et al. 2009; Wang et al. 2012). Some studies did not observe such associations (Hansen et al. 2008; Zacharias et al. 2014). One study in Europe observed that high temperature had a positive impact on respiratory admissions, but not for cardiovascular admissions (Michelozzi et al. 2009); similarly, Basu et al. (2012) found both positive and negative associations between temperature and ED visits for different circulatory and respiratory diseases. Heterogeneity in associations may be due to differences in population composition, geographical location, outcome and heat wave definitions, and population resilience. Our previous study in Atlanta examining associations between continuous daily maximum temperature and ED visits also identified several associations for cardiovascular and respiratory conditions (Winquist et al. 2016). Here we found that sustained high heat does confer additional risks over the risks associated with high continuous temperature for several circulatory diseases in our study region in the southeastern United States.
We examined six definitions for heat waves using daily maximum, minimum, and average of temperature or apparent temperature. The greater frequency of minimum temperature heat waves is likely associated with positive trends in low-level moisture in our study area that in turn increases the frequency of days with high minimum temperatures (Dai 2006; Brown and DeGaetano 2013). This is because water vapor is a greenhouse gas and can increase nighttime minimum temperature.
We found that observed associations between heat waves and ED visits can be sensitive to the temperature metric used. For example, the strongest associations of heat waves and ED visits were observed when minimum or maximum temperature was used to define heat waves rather than average temperature. One reason for this observation may be that heat waves defined using minimum temperature were more frequently observed in our data set leading to higher power compared to other heat wave metrics. It is also possible that minimum temperature represented sustained heat stress that was not alleviated during the evening. Several studies have examined the health effects of daily minimum temperature. A study of mortality and heat stress in Houston, Texas, found that daily minimum temperature provided better model fit compared to other temperature metrics (Heaton et al. 2014). Kalkstein and Davis (1989) also found minimum temperature to be associated with mortality in several U.S. cites. We found that heat waves defined using minimum or maximum temperature compared to average temperature were more likely to be associated with ED visits. Because the concordance between heat wave definitions is only moderate, the different heat wave metrics may represent different heat stress characteristics. This warrants further examination for other health outcomes, timing of exposure during the day (Davis et al. 2016), and in additional geographical regions.
We did not evaluate the association between heat waves and heat illness (ICD-9 code 992) due to model convergence issues, although this outcome had a very strong association with continuous maximum temperature in previous analyses (Winquist et al. 2016). The definition of heat illness ED visits includes outcomes such as heat stroke, heat syncope, heat cramps, and heat exhaustion that can arise from various activities (Nelson et al. 2011). In our 20-year study period, there were only a total of 9,155 heat illness ED visits, and 23.2% occurred during heat waves defined using minimum temperature. Heat stroke is a life-threatening condition (Leon and Helwig 2010). The admission rate and case fatality rate have been reported to be substantially higher for heat stroke ED visits than any other type of ED visit (Wu et al. 2014). Hence, quantifying the added effect of heat waves on heat illness should be considered in future studies with a longer time-series or larger study population.
There are several considerations when interpreting the results of this study. First, similar to other heat wave and morbidity studies (Hajat et al. 2014; Sun et al. 2014), we chose to estimate the additional effect of heat wave beyond daily high temperature, but some studies reported heat wave effects that include the effect of high temperature (e.g., by not controlling for continuous temperature in the models) (Bobb et al. 2014; Toloo et al. 2014; Zhang et al. 2015). We also did not consider the first day of a heat wave period as added temperature effect in order to only capture sustained heat effects over 2 or more days. Specifically, we assumed that the first day of a heat wave is no different from another hot day. This approach differs from previous studies and may impact comparability with other studies. We evaluated the relative importance of the added and sustained heat wave effect by calculating the pseudo-R2 for the nonlinear daily temperature effect and the heat wave effect for three outcomes at lag 0: acute renal failure (minimum temperature), ischemic stroke (maximum temperature), and intestinal infection (minimum temperature). R2 measures the variation in ED visits explained by each covariate. The corresponding daily temperature/heat wave R2 for these three outcomes are 0.2%/0.01%, 0.009%/0.02%, and 0.09%/0.04%. Hence, temperature explains more of the variability in daily ED visits for acute renal failure than heat waves, while heat waves explain more of the variability in daily ED visits for ischemic stroke and intestinal infection than temperature.
Second, the study is restricted to the Atlanta metropolitan area, and the results may not apply to other areas. For example, the prevalence of air-conditioning in Atlanta is higher than some other locations in the United States. The Atlanta metropolitan area has an air-conditioning prevalence of 94%, according to the 2011 American Housing Survey (Donovan et al. 2013), that likely modifies Atlanta residents’ personal exposures to ambient heat in a way that dampens the impacts of heat waves on health. However, high prevalence does not necessarily lead to high utilization rate due to economic constraints (Hayden et al. 2011).
Third, in the epidemiologic model, we controlled for the continuous temperature using the same metric as the heat wave. This may not fully control for the effects of temperature if different continuous temperature metrics have independent health impacts. For example, the observed associations with heat waves defined by minimum temperature may be due to the continuous effect of maximum temperature that is only partially controlled for by the inclusion of minimum temperature in the model.
Fourth, we did not control for air pollution as a confounder, as done in some studies (Benmarhnia et al. 2014; Schwartz and Dockery 1992; Tong et al. 2010). Ambient air pollution concentrations, such as fine particulate matter and ozone, may be higher during heat waves as a result of increased emissions due to higher electricity demands, and from increased formation of secondary pollutants due to favorable meteorological conditions. By not including daily air pollution concentration in the health model, our estimated heat wave associations include the effects that are potentially mediated through increases in air pollution (Buckley et al. 2014).
Finally, we examined various heat wave definitions, exposure lags, and different aggregations of health outcomes without formally accounting for multiple testing. Some statistically significant associations, as indicated by the RRs and 95% CIs excluding 1, may be due to type I error. However, we note that across lags, we found a larger number of significant associations at lags 0 and 1 compared to longer lags, which supports our a priori hypothesis that the adverse health impacts of sustained high heat is acute.
Conclusions
Our results support the hypothesis that heat wave events are associated with increased morbidity as measured by ED visits, even in an area with high air-conditioning prevalence. Prolonged heat exposure can confer added adverse health impacts beyond the risk due to higher daily temperature, particularly for renal diseases, cardiovascular diseases, and intestinal infection. We found some evidence that longer heat wave duration, later timing in the year, and higher heat wave intensity were associated with higher risks. Associations of heat waves with ED visits can be sensitive to heat wave definitions, and we found stronger and more frequent associations when heat waves are defined using minimum or maximum temperatures compared to average temperature. Local heat warning systems typically include daily maximum temperature and heat index as criteria. Our findings suggest that minimum or nighttime temperatures may also be useful for some adverse health outcomes.
Supplemental Material
Acknowledgments
This research was supported by the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under Award R21ES023763. This research was also made possible by grants from the U.S. Environmental Protection Agency (U.S. EPA; R82921301, RD834799), NIEHS (R01ES11294), and the Electric Power Research Institute (EP-P27723/C13172 and EP-P4353/C2124). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. EPA. Further, U.S. EPA does not endorse the purchase of any commercial products or services mentioned in this manuscript.
References
- Anderson BG, Bell ML. 2011. Heat waves in the United States: Mortality risk during heat waves and effect modification by heat wave characteristics in 43 U.S. communities. Environ Health Perspect 119(2):210–218, PMID: 23934704, 10.1289/ehp.1206273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GB, Bell ML, Peng RD. 2013. Methods to calculate the heat index as an exposure metric in environmental health research. Environ Health Perspect 121(10):1111–1119, PMID: 23934704, 10.1289/ehp.1206273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, Tong S, Clements AC. 2010. What measure of temperature is the best predictor of mortality. Environ Res 110(6):604–611, PMID: 20519131, 10.1016/j.envres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Basu R, Pearson D, Malig B, Broadwin R, Green R. 2012. The effect of high ambient temperature on emergency room visits. Epidemiology 23(6):813–820, PMID: 23007039, 10.1097/EDE.0b013e31826b7f97. [DOI] [PubMed] [Google Scholar]
- Benmarhnia T, Oulhote Y, Petit C, Lapostolle A, Chauvin P, Zmirou-Navier D, et al. 2014. Chronic air pollution and social deprivation as modifiers of the association between high temperature and daily mortality. Environ Health 13(1):53, PMID: 24941876, 10.1186/1476-069X-13-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berko J, Ingram DD, Saha S, Parker JD. 2014. Deaths attributed to heat, cold, and other weather events in the United States, 2006–2010. Natl Health Stat. Report 76:1–15, PMID: 25073563, https://www.cdc.gov/nchs/data/nhsr/nhsr076.pdf [accessed 19 May 2017]. [PubMed] [Google Scholar]
- Bobb JF, Peng RD, Bell ML, Dominici F. 2014. Heat-related mortality and adaptation to heat in the United States. Environ Health Perspect 122(8):811–816, PMID: 24780880, 10.1289/ehp.1307392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Obermeyer Z, Wang Y, Dominici F. 2014. Cause-specific risk of hospital admission related to extreme heat in older adults. JAMA 312(24):2659–2667, PMID: 25536257, 10.1001/jama.2014.15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckmann M, Rohn I. 2014. Is planned adaptation to heat reducing heat-related mortality and illness? A systematic review. BMC Public Health 14:1112, PMID: 25349109, 10.1186/1471-2458-14-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonan GB. 1997. Effects of land use on the climate of the United States. Clim Change 37(3):449–486, 10.1023/A:1005305708775. [DOI] [Google Scholar]
- Borden KA, Cutter SL. 2008. Spatial patterns of natural hazards mortality in the United States. Int J Health Geogr 7:64, PMID: 19091058, 10.1186/1476-072X-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchama A, Dehbi M, Mohamed G, Matthies F, Shoukri M, Menne B. 2007. Prognostic factors in heat wave related deaths: A meta-analysis. Arch Intern Med 167(20):2170–2176, PMID: 17698676, 10.1001/archinte.167.20.ira70009. [DOI] [PubMed] [Google Scholar]
- Brown PJ, DeGaetano AT. 2013. Trends in U.S. surface humidity, 1930–2010. J Appl. Meteorol. Clim 52:147–163, 10.1175/JAMC-D-12-035.1. [DOI] [Google Scholar]
- Buckley JP, Samet JM, Richardson DB. 2014. Commentary: Does air pollution confound studies of temperature? Epidemiology 25(2):242–245, PMID: 24487206, 10.1097/EDE.0000000000000051. [DOI] [PubMed] [Google Scholar]
- Chen K, Bi J, Chen J, Chen X, Huang L, Zhou L. 2015. Influence of heat wave definitions to the added effect of heat waves on daily mortality in Nanjing, China. Sci Total Environ 506-507:18–25, PMID: 25460935, 10.1016/j.scitotenv.2014.10.092. [DOI] [PubMed] [Google Scholar]
- Coumou D, Robinson A, Rahmstorf S. 2013. Global increase in record-breaking monthly-mean temperatures. Clim. Change 118(3):771–782, 10.1007/s10584-012-0668-1. [DOI] [Google Scholar]
- Dai A. 2006. Recent climatology, variability, and trends in global surface humidity. J Climate 19:3589–3606, 10.1175/JCLI3816.1. [DOI] [Google Scholar]
- Davis RE, Knappenberger PC, Michaels PJ, Novicoff WM. 2003. Changing heat-related mortality in the United States. Environ Health Perspect 111(14):1712–1718, PMID: 14594620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Hondula DM, Patel AP. 2016. Temperature observation time and type influence estimates of heat-related mortality in seven U.S. cities. Environ Health Perspect 124(6):795–804, PMID: 26636734, 10.1289/ehp.1509946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ippoliti D, Michelozzi P, Marino C, De’Donato F, Menne B, Katsouyanni K, et al. 2010. The impact of heat waves on mortality in 9 European cities: Results from the EuroHEAT project. Environ Heal 9:37, 10.1186/1476-069X-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan S, Jones M, Pritzker P, Gallagher PD. 2013. General characteristics by units in structure—All occupied units. In: 2011 American Housing Survey, Geography: Atlanta-Sandy Springs-Marietta, GA AHS Area, http://www2.census.gov/programs-surveys/ahs/2011/Atlanta.xls [accessed 15 May 2017]. [Google Scholar]
- Duffy PB, Tebaldi C. 2012. Increasing prevalence of extreme summer temperature in the U.S. Climatic Change 111(2):487–495, 10.1007/s10584-012-0396-6. [DOI] [Google Scholar]
- Ebi KL, Schmier JK. 2005. A stitch in time: Improving public health early warning systems for extreme weather events. Epidemiol Rev 27:115–121, PMID: 15958432, 10.1093/epirev/mxi006. [DOI] [PubMed] [Google Scholar]
- Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, et al. 2014. Climate Change 2014: Impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. In: Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK:Cambridge University Press.
- Fletcher BA, Lin S, Fitzgerald EF, Hwang SA. 2012. Association of summer temperatures with hospital admissions for renal diseases in New York State: A case-crossover study. Am J Epidemiol 175(9):907–916, PMID: 22455834, 10.1093/aje/kwr417. [DOI] [PubMed] [Google Scholar]
- Fouillet A, Rey G, Laurent F, Pavillon G, Bellec S, Guihenneuc-Jouyaux C. et al. 2006. Excess mortality related to the August 2003 heat wave in France. Int Arch Occup Environ Health 80(1):16–24, PMID: 16523319, 10.1007/s00420-006-0089-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frumkin H, Hess J, Luber G, Malilay J, McGeehin M. 2008. Climate change: The public health response. Am J Public Health 98(3):435–445, PMID: 18235058, 10.2105/AJPH.2007.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann CM, Sugg MM, Konrad CE, Waller A. 2016. Impact of extreme heat events on emergency department visits in North Carolina (2007–2011). J. Community Health 41(1):146–156, PMID: 26289379, 10.1007/s10900-015-0080-7. [DOI] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Kinney PL, Petkova EP, Lavigne E. et al. 2015. Temporal variation in heat-mortality associations: a multicountry study. Environ Health Perspect 123(11):1200–1207, PMID: 25933359, 10.1289/ehp.1409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi L, Bisoffi G, Mirandola R, Ricci G, Baccini M. 2015. The impact of heat on an emergency department in Italy: Attributable visits among children, adults, and the elderly during the warm season. PloS One 10(10):e0141054, PMID: 26513471, 10.1371/journal.pone.0141054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronlund CJ, Zanobetti A, Schwartz JD, Wellenius GA, O’Neill MS. 2014. Heat, heat waves, and hospital admissions among the elderly in the United States 1992–2006. Environ Health Perspect 122(11):1187–1193, PMID: 24905551, 10.1289/ehp.1206132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb D, Vargo J, Stone B. 2015. Rising heat wave trends in large US cities. Nat Hazards 76(3):1651–1665, 10.1007/s11069-014-1563-z. [DOI] [Google Scholar]
- Hajat S, Armstrong B, Baccini M, Biggeri A, Bisanti L, Russo A, et al. 2006. Impact of high temperatures on mortality: Is there an added heat wave effect? Epidemiology 17(6):632–638, PMID: 17003686, 10.1097/01.ede.0000239688.70829.63. [DOI] [PubMed] [Google Scholar]
- Hajat S, Vardoulakis S, Heaviside C, Eggen B. 2014. Climate change effects on human health: Projections of temperature-related mortality for the UK during the 2020s, 2050s and 2080s. J Epidemiol Community Health 68(7):641–648, PMID: 24493740, 10.1136/jech-2013-202449. [DOI] [PubMed] [Google Scholar]
- Hansen AL, Bi P, Ryan P, Nitschke M, Pisaniello D, Tucker G. 2008. The effect of heat waves on hospital admissions for renal disease in a temperate city of Australia. Int J Epidemiol 37(6):1359–1365, PMID: 18710886, 10.1093/ije/dyn165. [DOI] [PubMed] [Google Scholar]
- Hayden MH, Brenkert-Smith H, Wilhelmi OV. 2011. Differential adaptive capacity to extreme heat: a Phoenix, Arizona, Case Study. Weather Clim Soc 3:269–280. [Google Scholar]
- Heaton MJ, Sain SR, Greasby TA, Uejio CK, Hayden MH, Monaghan AJ, et al. 2014. Characterizing urban vulnerability to heat stress using a spatially varying coefficient model. Spat Spatiotemporal Epidemiol 8:23–33, PMID: 24606992, 10.1016/j.sste.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Hondula DM, Balling RC, Vanos JK Jr, Georgescu M. 2015. Rising temperatures, human health, and the role of adaptation. Curr Clim Change Rep 1(3):144–154, 10.1007/s40641-015-0016-4. [DOI] [Google Scholar]
- Josseran L, Caillère N, Brun-Ney D, Rottner J, Filleul L, Brucker G, et al. 2009. Syndromic surveillance and heat wave morbidity: A pilot study based on emergency departments in France. BMC Med Inform Decis Mak 9:14, PMID: 19232122, 10.1186/1472-6947-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkstein LS, Davis RS. 1989. Weather and human mortality: An evaluation of demographic and interregional responses in the United States. Ann Assoc Am Geogr 79(1):44–64, 10.1111/j.1467-8306.1989.tb00249.x. [DOI] [Google Scholar]
- Karmarkar S, MacNab R. 2012. Fluid and electrolyte problems in renal dysfunction. Anaesth Intensive Care Med 13(7):332–335, 10.1016/j.mpaic.2012.04.011. [DOI] [Google Scholar]
- Knowlton K, Rotkin-Ellman M, King G, Margolis HG, Smith D, Solomon G, et al. 2009. The 2006 California heat wave: Impacts on hospitalizations and emergency department visits. Environ Health Perspect 117(1):61–67, PMID: 19165388, 10.1289/ehp.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats RS, Kristie LE. 2006. Heatwaves and public health in Europe. Eur. Eur J Public Health 16(6):592–599, PMID: 16644927, 10.1093/eurpub/ckl049. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. 2010. Mechanisms and modulators of temperature regulation heat stroke: role of the systemic Inflammatory response. J Appl Physiol 109(6):1980–1988, PMID: 20522730, 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Li M, Gu S, Bi P, Yang J, Liu Q. 2015. Heat waves and morbidity: Current knowledge and further direction-a comprehensive literature review. Int J Environ Res Public Health 12(5):5256–5283, PMID: 25993103, 10.3390/ijerph120505256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert JP, Amoros C, Di Nisi J, Muzet A, Fukuda H, Ehrhart J. 1988. Thermoregulatory adjustments during continuous heat exposure. Eur J Appl Physiol Occup Physiol 57(4):499–506, PMID: 3396564, 10.1007/BF00417999. [DOI] [PubMed] [Google Scholar]
- Lin S, Luo M, Walker RJ, Liu X, Hwang SA, Chinery R. 2009. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology 20(5):738–746, PMID: 19593155, 10.1097/EDE.0b013e3181ad5522. [DOI] [PubMed] [Google Scholar]
- Manser CN, Paul M, Rogler G, Held L, Frei T. 2013. Heat waves, incidence of infectious gastroenteritis, and relapse rates of inflammatory bowel disease: A retrospective controlled observational study. Am J Gastroenterol 108(9):1480–1485, PMID: 23939628, 10.1038/ajg.2013.186. [DOI] [PubMed] [Google Scholar]
- Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, et al. 2007. Global climate projections. In: Climate Change 2007: The Physical Science Basis. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, et al., eds. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK:Cambridge University Press. [Google Scholar]
- Meehl GA, Tebaldi C. 2004. More intense, more frequent, and longer lasting heat waves in the 21st century. Science 305(5686):994–997, PMID: 15310900, 10.1126/science.1098704. [DOI] [PubMed] [Google Scholar]
- Michelozzi P, Accetta G, De Sario M, D’Ippoliti D, Marino C, Baccini M, et al. 2009. High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am J Respir Crit Care Med 179(5):383–389, PMID: 19060232, 10.1164/rccm.200802-217OC. [DOI] [PubMed] [Google Scholar]
- Nelson NG, Collins CL, Comstock D, McKenzie LB. 2011. Exertional heat-related injuries treated in emergency departments in the U.S., 1997–2006. Am J Prev Med 40(1):54–60, PMID: 21146768, 10.1016/j.amepre.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Petitti DB, Hondula DM, Yang S, Harlan SL, Chowell G. 2016. Multiple trigger points for quantifying heat-health impacts: New evidence from a hot climate. Environ Health Perspect 124(2):176–183, PMID: 26219102, 10.1289/ehp.1409119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Brock JW, Vaidyanathan A, Easterling DR, Luber G. 2015. Spatial variation in hyperthermia emergency department visits among those with employer-based insurance in the United States—A case-crossover analysis. Environ Health 14:20, PMID: 25888865, 10.1186/s12940-015-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer A, Muscatello D, Broome R, Corbett S, Smith W. 2012. Emergency department visits, ambulance calls, and mortality associated with an exceptional heat wave in Sydney, Australia, 2011: A time-series analysis. Environ Health 11(1):3, PMID: 22273155, 10.1186/1476-069X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifano P, Cappai G, De Sario M, Michelozzi P, Marino C, Bargagli AM, et al. 2009. Susceptibility to heat wave-related mortality: a follow-up study of a cohort of elderly in Rome. Environ Health 8:50, PMID: 19909505, 10.1186/1476-069X-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Dockery DW. 1992. Increased mortality in Philadelphia associated with daily air pollution concentrations. Am Rev Respir Dis 145(3):600–604, PMID: 1546841, 10.1164/ajrccm/145.3.600. [DOI] [PubMed] [Google Scholar]
- Semenza JC, Rubin CH, Falter KH, Selanikio JD, Flanders WD, Howe HL, et al. 1996. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med 335(2):84–90, PMID: 8649494, 10.1056/NEJM199607113350203. [DOI] [PubMed] [Google Scholar]
- Steadman RG. 1984. A universal scale of apparent temperature. J Climate Appl Meteor 23(12):1674–1687, http://dx.doi.org/10.1175/1520-0450(1984)023%3C1674:AUSOAT%3E2.0.CO;2. [Google Scholar]
- Sun X, Sun Q, Yang M, Zhou X, Li X, Yu A, et al. 2014. Effects of temperature and heat waves on emergency department visits and emergency ambulance dispatches in Pudong New Area, China: A time series analysis. Environ Health 13:76, PMID: 25273545, 10.1186/1476-069X-13-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toloo GS, Yu W, Aitken P, FitzGerald G, Tong S. 2014. The impact of heatwaves on emergency department visits in Brisbane, Australia: A time series study. Crit Care 18(2):R69, PMID: 24716581, 10.1186/cc13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong S, Ren C, Becker N. 2010. Excess deaths during the 2004 heatwave in Brisbane, Australia. Int J Biometeorol 54(4):393–400, PMID: 20049484, 10.1007/s00484-009-0290-8. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. 1991. The International Classification of Diseases, Ninth revision, Clinical Modification: ICD-9-CM. 4th ed, Washington, DC:U.S. Department of Health and Human Services. [Google Scholar]
- Vanos JK. 2015. Children's health and vulnerability in outdoor microclimates: A comprehensive review. Environ Int 76:1–15, PMID: 25497108, 10.1016/j.envint.2014.11.016. [DOI] [PubMed] [Google Scholar]
- Wang XY, Guo Y, FitzGerald G, Aitken P, Tippett V, Chen D, et al. 2015. The impacts of heatwaves on mortality differ with different study periods: A multi-city time series investigation. PLoS One 10(7):e0134233, PMID: 26217945, 10.1371/journal.pone.0134233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Lin YK, Chuang CY, Li MH, Chou CH, Liao CH, et al. 2012. Associating emergency room visits with first and prolonged extreme temperature event in Taiwan: A population-based cohort study. Sci Total Environ 416:97–104, PMID: 22209370, 10.1016/j.scitotenv.2011.11.073. [DOI] [PubMed] [Google Scholar]
- Wilker EH, Yeh G, Wellenius GA, Davis RB, Phillips RS, Mittleman MA. 2012. Ambient temperature and biomarkers of heart failure: A repeated measures analysis. Environ Health Perspect 120(8):1083–1087, PMID: 22588803, 10.1289/ehp.1104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Grundstein A, Chang HH, Hess J, Sarnat SE. 2016. Warm season temperatures and emergency department visits in Atlanta, Georgia. Environ Res 147:314–323, PMID: 26922412, 10.1016/j.envres.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Brady JE, Rosenberg H, Li G. 2014. Emergency department visits for heat stroke in the United States, 2009 and 2010. Inj Epidemiol 1(1):8, PMID: 27747667, 10.1186/2197-1714-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Sheffield PE, Hu W, Su H, Yu W, Qi X, et al. 2012. Climate change and children’s health–a call for research on what works to protect children. Int J Environ Res Public Health 9(9):3298–3316, PMID: 23202687, 10.3390/ijerph9093298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias S, Koppe C, Mücke H-G. 2014. Influence of heat waves on ischemic heart diseases in Germany. Climate 2(3):133–152, 10.3390/cli2030133. [DOI] [Google Scholar]
- Zhang Y, Bi P, Hiller JE. 2008. Weather and the transmission of bacillary dysentery in Jinan, northern China: A time-series analysis. Public Health Rep 123(1):61–66, PMID: 18348481, 10.1177/003335490812300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Chen TH, Begley CE. 2015. Impact of the 2011 heat wave on mortality and emergency department visits in Houston, Texas. Environ Health 14:11, PMID: 25627975, 10.1186/1476-069X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.