Abstract
Background:
Millions of coastal inhabitants in Southeast Asia have been experiencing increasing sodium concentrations in their drinking-water sources, likely partially due to climate change. High (dietary) sodium intake has convincingly been proven to increase risk of hypertension; it remains unknown, however, whether consumption of sodium in drinking water could have similar effects on health.
Objectives:
We present the results of a cohort study in which we assessed the effects of drinking-water sodium (DWS) on blood pressure (BP) in coastal populations in Bangladesh.
Methods:
DWS, BP, and information on personal, lifestyle, and environmental factors were collected from 581 participants. We used generalized linear latent and mixed methods to model the effects of DWS on BP and assessed the associations between changes in DWS and BP when participants experienced changing sodium levels in water, switched from “conventional” ponds or tube wells to alternatives [managed aquifer recharge (MAR) and rainwater harvesting] that aimed to reduce sodium levels, or experienced a combination of these changes.
Results:
DWS concentrations were highly associated with BP after adjustments for confounding factors. Furthermore, for each reduction in sodium in drinking water, systolic/diastolic BP was lower on average by , and odds of hypertension were lower by 14%. However, MAR did not consistently lower sodium levels.
Conclusions:
DWS is an important source of daily sodium intake in salinity-affected areas and is a risk factor for hypertension. Considering the likely increasing trend in coastal salinity, prompt action is required. Because MAR showed variable effects, alternative technologies for providing reliable, safe, low-sodium fresh water should be developed alongside improvements in MAR and evaluated in “real-life” salinity-affected settings. https://doi.org/10.1289/EHP659
Introduction
Low-lying deltas, such as Bangladesh, have been experiencing increasing numbers of storm surges over recent decades, inundating densely populated coastal areas (Singh et al. 2000). This trend is believed to be associated with climate change and, in combination with sea level rise, may result in contamination of unprotected drinking-water sources, such as ponds and shallow tube wells, with saline water [Hoque et al. 2016; Institute of Water Modelling (IWM) 2014]. Changes in river flow from an upstream barrage, faulty management of polders, shrimp farming, and groundwater extraction may all contribute further to salinization (Mahmuduzzaman et al. 2014). Previously, we found a mean sodium concentration in drinking water of approximately (with extremes exceeding ) (Khan et al. 2014) in coastal areas; this contributes substantially to the daily sodium intake of coastal populations (Scheelbeek 2015). As a consequence, the World Health Organization (WHO)-recommended daily maximum sodium intake of (WHO 2012b) can easily be exceeded in the area solely by drinking of water. Climate change predictions, including sea level rise (Hijioka et al. 2014), suggest further exacerbation of salinity problems in the future.
High dietary salt intake from food is a major risk factor for raised blood pressure (BP) worldwide (Aburto et al. 2013; Elliott et al. 1996, 2007; Elliott and Stamler 2002; Pietinen et al. 1988). However, the effects of long-term consumption of substantial amounts of sodium through drinking water on population health remain unknown.
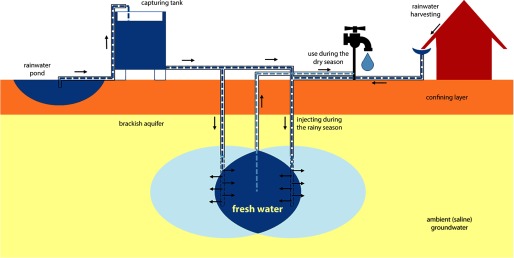
In the present study, we explored the relationship between drinking-water salinity and BP in a coastal population in Bangladesh. We looked at the relationship between BP and drinking-water sodium concentrations in individuals whose sodium intake fluctuated during the study period. Differences in sodium concentrations occurred because users consumed drinking water from different sources [pond, tube well, managed aquifer recharge (MAR) system (Figure 1), or rainwater] or because of seasonal fluctuation of drinking-water sodium concentrations in a single source (i.e., pond). Furthermore, some participants changed their drinking-water sources during the study period. It was expected that consumers switching from ponds and tube wells to MAR sources would experience a significant decrease in their drinking-water salinity, and the study assessed whether this occurred.
Figure 1.
Schematic overview of a managed aquifer recharge (MAR) system.
Methods
For this study, ethical clearance was obtained from the National Research Ethics Committee of the Bangladesh Medical Research Council.
Three sub-districts in southwestern Bangladesh—Dacope, Batiaghata and Paikghaccha—(see Figure S1) were selected for this study because of high salinity levels in drinking water and an ongoing MAR construction project in the area [Netherlands Embassy in Dhaka 2014; Sultana et al. 2014; United Nations Children's Fund (UNICEF) 2014] (Figure 1).
Based on access and hydrological conditions, 25 villages were found suitable for MAR construction (Hasan 2012); six were prioritized based on water shortages. MAR systems in these villages were scheduled to become operational during the study period; however, some participants started drinking MAR water before the planned starting day of the scheme. All 303 families in the six MAR locations were invited to participate in the study. In addition, six other villages were randomly selected from the remaining 19 villages on the “waiting list”. All households in these villages (or a randomly selected maximum of 60 households in villages with households), comprising an additional 321 families, were invited to participate (see Figures S2 and S3).
Each adult within the selected households was numbered following the Kish-grid method (Kish 1949), and one adult household member was then invited for participation in the study. Invitees were excluded if they were unable to meet the data collector within 7 d following the first visit and were then replaced by a household member of the same sex (if possible) and closest in age. During the initial recruitment visit, data collectors explained the aim of the study, the reasons for selection, future use of proposed data collection, and the procedures and time frame for participation. After any questions of the potential participants were answered, written informed consent was obtained. Participants were followed up for 15 mo, during which three rounds of measurements were performed. Participants were not paid but were offered a free health consultation from local health assistants. Blinding for source was not possible, but data collectors and participants were unaware of sodium concentrations measured during the study. A total of 624 participants were invited to the study, of whom 581 (93%) took part.
Baseline data were collected in March 2013; the first follow-up data were collected in March 2014; and a second round of follow-up data were collected in May 2014.
Data collection—performed at the participant’s house—included systolic and diastolic BP, sodium concentration of each drinking water source used, and anthropometry. Interview data about lifestyle and environmental exposures were collected using an adapted version of the Non-Communicable Disease Risk Factor Survey Bangladesh (WHO 2011b), which was pretested before data collection. Furthermore, participants were asked about (family) history of hypertension and cardiovascular disease (see Supplemental Material, “Confounders and effect modifiers,” for a full list of covariates). BP was measured in the left arm (resting, with palm up) using an arm-type fully automatic sphygmomanometer type H1209 with an Accumax arm-cuff. Data collectors were trained using the WHO STEPwise approach to Surveillance (STEPS) protocol (WHO 2005). Participants were asked to refrain from eating, drinking, and hukka/gul (smokeless tobacco) use during the interview. For religious reasons, bare skin measurements were not always possible and were alternatively performed on thin and nonconstrictive clothing. If the first two BP measures differed by systolic/diastolic BP, a third measurement was taken, and the first was discarded. A 3-min break was observed between BP readings.
Samples of drinking water were collected after the interview. Each source consumed by the participants over the previous 2 wk was sampled using a plastic sampling bottle. Effects of changes in (drinking-water) sodium intake on BP were expected to be measurable after a few days up to a few weeks (Law et al. 1991; Van Vliet and Montani 2008); hence, participants were asked about the amount they had been drinking from each sampled source in the past two weeks and on which specific days. Based on this information, a weighted average of sodium exposure could be calculated in the case that multiple sources were consumed during the “window period”. In addition, cooking-water sources (if different from drinking-water sources) were sampled. The data collectors were careful not to touch the bottle neck with their fingers. First, the bottle was rinsed with water from the source to be sampled. When a water sample had to be taken from an open body of water, the data collector used the bucket/cup used by the family or, if not available, a small sampling cup. This cup was then attached to a rope and immersed into the water source, pulled up, and emptied into the sample bottle. All bottles were immediately sealed, labeled, and placed in an icebox.
Spot urine samples were collected from all participants, and 24-h urine samples were collected from a random subsample of participants (). This enabled us to develop an algorithm to estimate 24-h sodium excretion (usually regarded as an accurate proxy for sodium consumption) from morning spot urine samples, based on earlier algorithms developed by Brown et al. (2013), taking into account the age of the participant as well as potassium and chlorine concentrations in the spot sample (Scheelbeek 2015). The response rate was 100%, but for two participants, the 24-h urine volume was less than 500 mL, and these collections were disregarded.
Drinking water sodium concentrations were measured using the atomic absorption flame photometry method with an air-acetylene flame (see Supplemental Material, “Confounders and effect modifiers”) and multiplied by self-reported water volume intake in glasses per day; data collectors measured the volume of presented glasses. Eighteen volunteers agreed to participate in a sub-study to assess the accuracy of self-reported drinking-water volume by pouring a glass of water in a container for each glass drunk. No material differences were observed between reported and actual fluid intake. A sensitivity analysis was performed using average fluid intake to assess any significant differences in the models by comparing the use of these two methods of estimating fluid intake. Arsenic concentrations in tube-well water, which plays an important role in water availability and water-related burden of disease in other parts of Bangladesh (Y Chen et al. 2011; Smith et al. 2000), were low in the tube wells located in the study villages. A nationwide survey [Department of Public Health Engineering and British Geological Survey (DPHE/BGS) 2001] revealed that in the study area, the arsenic levels of nearly all tube wells fell within the WHO guideline of (WHO 2011a); all were within the national guideline of (DPHE 2016), and hence, arsenic levels were not measured in the samples collected for this study.
We estimated dietary sodium from questionnaire data combined with sodium measurements from 20 local dishes. However, because there was limited correlation between dietary sodium and spot urine sodium concentration (), we also calculated the dietary component by subtracting estimated water sodium intake from estimated 24-h urinary sodium excretion. Sensitivity analysis showed some significant differences between both methods; the latter method was more accurate and was used for further analysis.
Details on confounders, effect modifiers, sample collections, and calculations of the intra-cluster correlation coefficient are given in the Supplemental Material (“Confounders and effect modifiers” and “Intra-Cluster Correlation Coefficient”).
We collected a complete set of baseline data, information on confounders, and effect modifiers for 581 individuals (93% of all people who were invited to participate in the study). Of these, 521 (83%) took part in the first follow-up a year later, of whom 14 were interviewed away from their homes, so no water sample could be collected; 507 participants (81%) were visited in the second follow-up (two months after the first follow-up), of whom 5 were interviewed away from their homes. All data collected at each of the data points were used in the statistical models (up to three measurements per individual). Study design and a flow chart with recruitment data are shown in the Supplemental Material (see Figures S2–S4). A (pseudo) experimental design—with MAR as the intervention—was ruled out in the design stage of the study because drinking-water sodium levels in MAR systems (measured in neighboring areas) showed large variations and did not consistently offer a lower-sodium alternative to pond or tube-well water for the population.
The main outcomes in this study were systolic and diastolic BP (mmHg). Hypertension was considered a secondary outcome. The latest definition of hypertension, developed by the Joint National Committee, was used (James et al. 2014): systolic/diastolic blood pressure for people and for those . The main exposure for BP-related outcomes was drinking-water sodium concentration.
We used generalized linear latent and mixed models (GLLAMMs) to analyze the association between blood pressure and drinking-water salinity over the three measured time points. Because the study was conducted in field settings, GLLAMMs were preferred to generalized linear mixed models (GLMMs) because GLLAMMs would allow us to account for unmeasured heterogeneity: GLLAMMs allow latent variables to be both discrete and to have a (multivariate) normal distribution (Skrondal and Rabe-Hesketh 2003). We used three consecutive regression models: Model 1 adjusted for age and sex; Model 2 additionally adjusted for physical activity, body mass index, and smoking; and Model 3 included Model 2 variables plus demographic factors, socioeconomic status, environmental and weather exposures (such as temperature), underlying diseases, education, religion, use of local stimulants, exposure to chemicals (such as pesticides), and estimated dietary salt intake. (see Supplemental Material, “Confounders and effect modifiers”).
One random effect per participant was used, and the models also accounted for the geographical location of the participants, assigning one random effect per village and sub-region. Models were used to identify the average effect of each decrease in water salinity over all participants and measurement periods. The linear predictor () in the GLLAMM was specified as where is the drinking-water sodium concentration; is the fixed effect parameter; , , and represent the three model levels (individual, village, and sub-region); corresponds to the baseline data collection round; is the last data collection round (follow-up 2); and the second term of the linear predictor is a collection of random effects, where η is the vector of latent variables, and represents the confounders that were adjusted for in each model.
We used mixed-effect logistic regression models (MLRMs) to analyze the odds of hypertension related to decreases in sodium concentration for all participants over the three measurement points. One random effect per person was used in both models as well as per village and sub-region.
To further explore the relationship between changes in drinking-water salinity and BP, an additional analysis was performed to assess the differences in sodium concentrations and associated differences in BP for each individual (comparing baseline to follow-up 1 and comparing follow-up 1 to follow-up 2). Before data collection, it was decided to allocate all participants experiencing a decrease of in their drinking-water sodium between two time points (approximately sodium intake through water per day, based on estimated intake of ) to a sodium decrease (dNa) group. Those who experienced no or minor changes in sodium concentration () were allocated to the reference group, and those who experienced an increase in sodium were allocated to the sodium increase (iNa) group. The three groups represented three hypothetical situations: A “do-nothing scenario” (an expected increase in salinity in the future), a “business-as-usual” scenario (representing the current situation), and an “intervention scenario” (successful rollout of low-salinity drinking water options), respectively. For this within-person analysis, we used GLMMs to analyze differences in BP with respect to changes in drinking-water sodium scenarios using the same three-step modeling approach described above.
Because participants changed their drinking water sources at different points during the study period, some crossed over between sodium-change and control groups when comparing two consecutive years and two measurements in the same dry season. Sensitivity analyses were performed including and excluding participants with various combinations of crossover patterns.
Analyses were performed in STATA® version 13.1 (StataCorp LLC) and RStudio® version 3.0.1 (RStudio).
Results
Baseline characteristics for all study participants and stratified by baseline sodium concentration are shown in Table 1. Participants who drank water with low sodium concentrations were more often from a higher socioeconomic class and were on average more educated than participants who drank water with higher sodium concentrations; additionally, a significant difference was found in physical activity between low-, intermediate-, and high-sodium water drinkers. Those drinking low-sodium water at baseline were more likely to be former smokers.
Table 1.
Baseline characteristics of all study participants and stratified by drinking water sodium concentrations.
| Characteristic | Drinking water sodium concentration at baseline | All | |||
|---|---|---|---|---|---|
| () | () | () | |||
| Age [median (interquartile range)] | 39 (31–51) | 37 (30–47) | 37 (27–46) | 38 (29–48) | |
| Male (%) | 50.3 | 49.0 | 41.5 | 47.4 | |
| Hours physical work/day (median) | 8 | 8 | 8 | 8 | |
| Work-related physical activity | |||||
| Light/sedentary work (%) | 23.2 | 18.6 | 12.2 | 18.9 | 0.001a |
| Moderately heavy workload (%) | 44.4 | 46.8 | 53.5 | 47.6 | |
| Heavy workload (%) | 32.3 | 34.6 | 34.3 | 33.6 | |
| Body mass index (mean) | 21.3 | 20.5 | 20.7 | 21.4 | |
| Smoking | |||||
| Never smoked (%) | 70.7 | 75.8 | 73.9 | 73.6 | 0.044a |
| Former smoker (%) | 10.5 | 3.5 | 3.9 | 6.1 | |
| Current smoker (%) | 18.8 | 20.7 | 22.3 | 20.4 | |
| Marital Status | |||||
| Married (%) | 82.3 | 88.4 | 89.2 | 86.5 | |
| Single (%) | 8.8 | 5.6 | 6.9 | 7.1 | |
| Separated/widowed (%) | 8.8 | 6.1 | 3.9 | 6.5 | |
| Religion | |||||
| Muslim (%) | 37.0 | 42.6 | 32.3 | 38.2 | |
| Hindu (%) | 63.0 | 57.4 | 67.7 | 61.8 | |
| Size of household (mean) | 4.4 | 4.3 | 4.2 | 4.3 | |
| Education | |||||
| No education/illiterate (%) | 25.4 | 22.1 | 20.0 | 22.9 | 0.007a |
| Primary school (%) | 23.2 | 34.7 | 43.1 | 32.6 | |
| Secondary school or higher (%) | 51.4 | 43.2 | 36.9 | 44.5 | |
| Socioeconomic status | |||||
| Lowest tertile (%) | 35.9 | 38.4 | 30.0 | 35.4 | a |
| Intermediate tertile (%) | 19.3 | 34.9 | 45.4 | 32.0 | |
| Highest tertile (%) | 44.8 | 26.8 | 24.6 | 32.6 | |
| Salt intake per adult family member ()b | 123 | 120 | 120 | 121 | |
Pearson chi-squared test.
Based on total salt used by the family per month/number of adult family members.
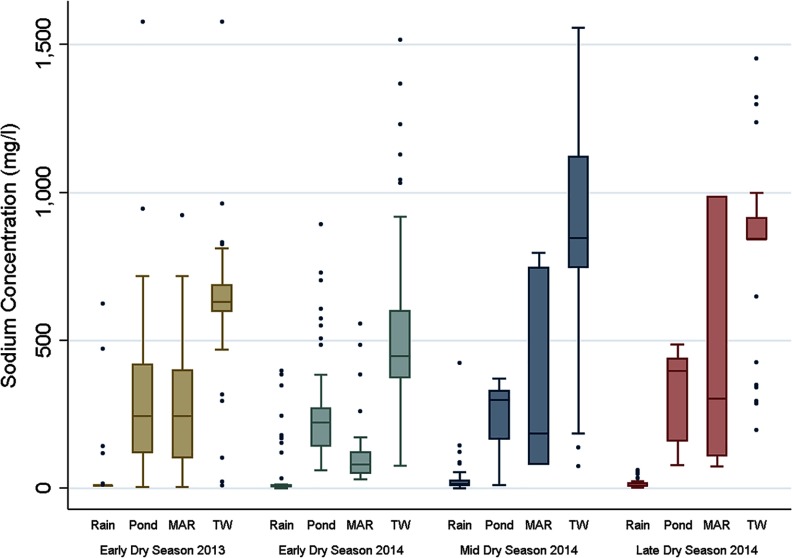
The sodium measurements showed high sodium concentrations in several drinking-water sources including some of the MAR sources; however, large variations were present within each type of source (Figure 1). We found a gradual increase in sodium concentration over the course of the dry season. Median sodium concentrations of pond and MAR sources were toward the end of the dry season, whereas median sodium concentrations in tube wells exceeded . Again, we found extremes (Figure 2). Some rainwater users mixed their rainwater with water from other sources to prolong the period of rainwater use. Toward the end of the dry season, only those with large amounts of storage space (and hence more likely to consume unmixed rainwater) still reported rainwater as the main drinking-water source, which explains the high outliers in sodium concentrations in “rainwater” in the early dry-season measurements.
Figure 2.
Sodium concentration (mg/L) per source and per measurement period [rain, pond, managed aquifer recharge (MAR), and tube well (TW)].
Adjusted GLLAMMs showed significantly lower systolic and diastolic blood pressures with decreasing drinking-water sodium concentrations: after adjustments for several confounding factors, the models showed that for each decrease in drinking-water sodium, systolic BP was lower on average by (95% CI: 0.71, 1.20), and diastolic blood pressure was lower on average by (95% CI: 0.38, 0.76). Small differences were observed between men and women (Table 2).
Table 2.
Generalized linear latent and mixed models for systolic and diastolic blood pressure per lower water salinity.
| Blood pressure | Model 1: Adjusted for age and sex | Model 2: Adjusted for age, sex, physical activity, smoking, BMI | Model 3: Adjusted for multiple confoundersa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference in BP | 95% CI | Difference in BP | 95% CI | Difference in BP | 95% CI | ||||
| Systolic | |||||||||
| decrease (All) | |||||||||
| Women | |||||||||
| Men | |||||||||
| Diastolic | |||||||||
| decrease (All) | |||||||||
| Women | 0.006 | 0.001 | |||||||
| Men | |||||||||
Note: Covers baseline, follow-up 1, and follow-up 2 measurements for each participant; one random effect per person, village, and subdistrict. BMI, body mass index; BP, blood pressure; CI, confidence interval.
Adjusted for age; sex; physical activity; smoking status; BMI; maximum daily temperature; underlying disease; marital status; religion; number household members; education; use of paan, hukka, and gul; water treatment; dietary salt intake; socioeconomic status; exposure to insecticides and chemical manure; and important changes in life.
Mixed effect logistic regression models showed that for each 100 g/mL decrease in drinking-water sodium concentration, the odds of hypertension were lower by 13.8% (95% CI: 7.4, 20.6) (Table 3).
Table 3.
Mixed logistic regression models for hypertension per decrease in water salinity.
| Hypertension | Model 1: Adjusted for age and sex | Model 2: Adjusted for age, sex, physical activity, smoking, BMI | Model 3: Adjusted for multiple confoundersa | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||||
| (All) | 0.901 | 0.005 | 0.84/0.97 | 0.962 | 0.339 | 0.88/1.04 | 0.862 | 0.79/0.93 | |
| Women | 0.877 | 0.011 | 0.79/0.97 | 0.935 | 0.224 | 0.83/1.04 | 0.855 | 0.004 | 0.77/0.95 |
| Men | 0.909 | 0.075 | 0.82/1.01 | 0.971 | 0.599 | 086/1.09 | 0.847 | 0.011 | 0.75/0.96 |
Notes: Covers baseline, follow-up 1 and follow-up 2 measurements for each participant; one random effect per person, village, and subdistrict. BMI, body mass index; CI, confidence interval.
Adjusted for age; sex; physical activity; smoking status; BMI; maximum daily temperature; underlying disease; marital status; religion; number household members; education; use of paan, hukka and gul; water treatment; dietary salt intake; socioeconomic status; exposure to insecticides and chemical manure; and important changes in life.
In the between-year comparison, the results of the analyzing the sodium difference groups showed a significant decrease in blood pressure in the dNa group and a significant increase in blood pressure in the iNa group compared with the participants who did not experience changes in sodium concentration. Differences were smaller in the within-year comparison. Further details can be found in the Supplemental Material (“Results Generalized Linear Mixed Models”; see also Tables S1 and S2) and in Figure S5.
Discussion
Our study confirms that sodium concentrations in ponds, tube wells, and some MAR systems are extremely high, this problem is hypothesized to be partly related to climate change. We found evidence for a direct relationship between drinking water sodium and BP; moreover, the sodium group analysis suggests reversibility of BP response if an alternative lower-salinity source of drinking water is used instead of a high-salinity source. The results are in line with previous dietary sodium (reduction) studies, although the effect for water sodium found here is somewhat larger than has been reported for sodium in food (Elliott et al. 1996; He et al. 2013; Pietinen et al. 1988; Sacks et al. 2001). This phenomenon might be partly explained by the way imbibed sodium is absorbed in the body compared with sodium consumed through food (Lifshitz and Wapnir 1985). The absorption mechanisms from water have been investigated, for example, in the context of optimizing rehydration for athletes, mostly in studies with small sample sizes and low study power. [e.g., (Shirreffs et al. 1996)]. It has been hypothesized that sodium absorption mechanisms depend on the sodium concentration in the rehydration solution (water) and that they differ from absorption mechanisms following rehydration through (sodium-rich) foods (Lifshitz and Wapnir 1985; Shirreffs et al. 1996). The greater between-year than within-year differences may indicate that the effects of high drinking-water sodium on BP are relatively long lasting.
The observed decreases in BP in the dNa group are also in line with previous food-sodium studies: successful lowering of BP through decreased salt intake from foods has been extensively documented in several randomized controlled trials (e.g., He et al. 2013). Animal studies have investigated the reversibility of BP changes through manipulation of sodium in drinking water and found similar results (Lenel et al. 1948; Sapirstein et al. 1950).
This is the first cohort study on drinking water sodium and blood pressure in (nonpregnant) adults in a salinity-affected coastal area. Although several other studies on drinking-water sodium were performed in in the last 3 decades of the 20th century—mainly analyzing the salinizing effects of certain water softeners (e.g., Calabrese and Tuthill 1985; Hofman et al. 1980; Luft et al. 1990; Schorr et al. 1996; Tuthill and Calabrese 1989)—these studies evaluated much lower sodium concentrations. Furthermore, these studies investigated human-made drinking-water salinity, whereas in the present study, we address a serious environmental health problem. The high drinking-water sodium concentrations described here are of particular importance because they affect millions of people living in poor coastal areas where there are often no or very limited alternative drinking-water sources available for consumption.
The strengths of our study include the real-world setting and the addition of a pseudo-experimental design to examine the effects on BP of a low-cost and practicable method to reduce the salinity of drinking water. Although the study was performed in southwest Bangladesh, our findings may be more widely generalizable to other deltaic areas in southeast Asia (Hoque et al. 2016; Hoque and Butler 2015).
Previous studies in Bangladesh, where arsenic pollution plays an important role, have linked drinking-water arsenic to cardiovascular diseases and mortality (Chen Y et al. 2011), but mixed results were found regarding the association between arsenic exposure and hypertension (Abhyankar et al. 2012; Chen CJ et al. 2007; Chen Y et al. 2007). In our study area, arsenic levels in drinking water were generally low, and it was therefore very unlikely that arsenic formed a confounder in the detected association between drinking-water sodium and blood pressure. The implications of the results presented here, however, are not limited to low-arsenic areas: in high-arsenic coastal areas, the salinity problems described above would complicate the search for safe drinking water alternatives if people wanted to change from a high-arsenic water source to a safe, low-arsenic alternative.
Limitations of the study include the nonrandom selection of participants exposed to different concentrations of drinking water salinity and its open (unblinded) nature, which could have led to selection and other biases. Villages were selected on pragmatic grounds (see “Methods”) in locations with a broad range of (changing) drinking-water salinity. Participants drinking from water sources with relatively low sodium concentrations were more likely to be better educated, to have a higher socioeconomic status, and to perform less physical activity than participants drinking from high-saline drinking-water sources, which could have confounded the relationship between drinking-water salinity and blood pressure. However, all models were adjusted for these factors, and the results did not change significantly from the crude models. Diet is reasonably homogeneous in the study region, and neither socioeconomic status nor education nor physical activity was associated with estimated food salt intake. Furthermore, we found an effect of water sodium changes in within-year analyses, which are not subject to the same potential biases (for example, physical activity) as comparisons between years. This study did not control for the concentration of specific anions attached to sodium, such as chloride or bicarbonate; certain sodium–anion combinations have been hypothesized to have a smaller effect on blood pressure than sodium chloride (Hoque and Butler 2015), and the bicarbonate anion has even been hypothesized to have a blood pressure–lowering effect (Hildebrant et al. 1986; Luft et al. 1990; Morgan 1982; Santos et al. 2010). The concentrations of sodium bicarbonate were found to be higher in tube wells than in ponds (Hoque and Butler 2015); the influence of anions should therefore be explored in several different sources to more accurately quantify the association between drinking-water salinity and blood pressure.
BP measurements were not always taken on bare skin, which could have affected the accuracy of the measurements; however, several studies assessing this issue did not find a difference between bare-skin or sleeved measurements (e.g., Eder et al. 2008; Ma et al. 2008). Furthermore, it is unlikely that this led to bias in the association between salinity and blood pressure because nonbare skin measurements are not associated with drinking sources. Assessment of water intake was based on self-reporting and could have led to misclassification, but a cross-validation of self-reported and actual intake in a group of volunteers did not show important over- or under-reporting of volume intake (see “Methods”). Estimation of dietary sodium intake had limited accuracy because it was based on 24-h urinary levels imputed from spot urine samples using an algorithm developed in a subsample that had both spot and 24-h urine measurements (see “Methods”); however, this is not likely to have led to differences between groups. Drinking-water jars were commonly cleaned with potassium-rich wood ash, which led to greatly varying potassium concentrations in stored drinking water depending on cleaning frequency. Because water samples were only measured once per measurement period, it was not possible to estimate individual daily potassium intake. However, it is unlikely that drinking-water potassium would have played an important role in the study area because measurements in the area revealed median potassium levels of (Hoque and Butler 2015). This amount would form of the recommended daily intake of (WHO 2012a) when consuming 2.5 L drinking water/d).
The three comparison groups represent plausible scenarios of what may happen in coastal areas affected by climate change in the future. The group with stable sodium concentrations reflect the current situation. The other two groups show possible future scenarios: first, intervening and providing low-saline drinking water alternatives (dNa); and second, a “do-nothing” scenario (iNa), in which people will experience increases in drinking-water sodium levels over time. Based on future predictions (Hijioka et al. 2014; Singh et al. 2000), small-scale modeling (Hoque et al. 2016) has indicated that salinity levels in Khulna and in similar coastal areas in southeast Asia are likely to continue to increase, although the size of this increase is difficult to quantify.
According to Cook et al. (2007, 2014), an increase of 1.9 g of dietary salt is associated with a 32% increase in stroke risk. An increase in drinking water sodium in Bangladesh of ( salt) owing to exacerbation of salinity problems would lead to this additional 1.9 g of salt intake solely through drinking water. A systematic review (Aburto et al. 2013) indicated that reduction of dietary sodium intake would lead to a fall in systolic/diastolic BP of , associated with a 19% reduction in stroke risk, a 39% decrease in stroke mortality, and a 42% decrease in coronary heart disease mortality. Because we found a stronger effect on BP for sodium consumed through water than through food, this may translate into a larger sodium-related morbidity and mortality in salinity-affected areas than would be predicted from the abovementioned findings.
We also documented the limitations of currently available approaches to reducing drinking-water salinity. The MAR sites used in this study had variable effects across locations (Figure 2), in some cases resulting in higher sodium levels. This variability reflected the fact that in many instances pond water, in addition to rainwater, was used to recharge the aquifer. The higher salinities of the pond water, in turn, affected the performance of the MAR; MAR could therefore not be considered a reliable low-saline alternative to conventional sources. Assessment of salinity mechanisms in MAR systems and improvement of the construction, which is currently being performed by several research groups in Bangladesh, will guide further improvements of MAR for future implementation and use.
All measured private and communal rainwater harvesting sources were low in salinity; however, the effectiveness of rainwater harvesting as an adaptation strategy is limited by the capacity to safely store sufficient fresh water until the end of the dry season.
Conclusions
Drinking-water sodium is an important source of daily sodium intake and is therefore a risk factor for increased BP in salinity-prone coastal areas. This increased salinity adds to the cardiovascular health risks associated with food sodium intake in southeast Asian populations: in Bangladesh, 20% of all stroke deaths are attributable to high-sodium diets [Institute for Health Metrics and Evaluation (IHME) 2015]. Current predictions estimate an increase in drinking-water salinity in these areas for the future, and prompt action is required. Low-saline alternative drinking-water sources could effectively help prevent high BP and hypertension-related morbidity and mortality in these coastal populations, and new technologies for the supply of such alternative sources, including safeguarding the microbial quality, should be further studied.
Supplemental Material
Acknowledgements
The authors would like to thank A. Tanweer for his contributions to the study design and questionnaires; K. Hasan for his help with data management; M.A. Hasan for conducting the urine analyses; K.M. Ahmed and M. Akhtaruzzaman at Dhaka University for their great help in getting all water and food samples analyzed; S. Hossein and A. Hossein for the outstanding management of the field teams; and all data collectors for the excellent work in and around Dacope.
This study was funded by the Leverhulme Trust. P.S. was additionally supported by the MRC-PHE Centre for Environment and Health and, along with MH, the Wellcome Trust Institutional Strategic Support Fund (ISSF). P.E. is supported by the National Institute for Health Research (NIHR) Imperial College Healthcare NHS Trust (ICHNT) and Imperial College Biomedical Research Centre (BRC), the MRC-PHE Centre for Environment and Health, and the NIHR Health Protection Research Unit on Health Impact of Environmental Hazards. P.V. was supported by the MRC-PHE Centre for Environment and Health and Imperial College Healthcare NHS Trust (ICHNT).
References
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. 2012. Arsenic exposure and hypertension: A systematic review. Environ Health Perspect 120:494, PMID: 22138666, 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. 2013. Effect of lower sodium intake on health: Systematic review and meta-analyses. BMJ 346:f1326, PMID: 23558163, 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, et al. 2013. Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in western populations the Intersalt study. Am J Epidemiol 177:1180–1192, PMID: 23673246, 10.1093/aje/kwt066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Tuthill RW. 1985. The massachusetts blood pressure study, part 3. Experimental reduction of sodium in drinking water: Effects on blood pressure. Toxicol Ind Health 1:19–34, PMID: 3842544, 10.1177/074823378500100103. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Wang SL, Chiou JM, Tseng CH, Chiou HY, Hsueh YM, et al. 2007. Arsenic and diabetes and hypertension in human populations: A review. Toxicol Appl Pharmacol 222:298–304, PMID: 17307211, 10.1016/j.taap.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Chen Y, Factor-Litvak P, Howe GR, Graziano JH, Brandt-Rauf P, Parvez F, et al. 2007. Arsenic exposure from drinking water, dietary intakes of b vitamins and folate, and risk of high blood pressure in Bangladesh: A population-based, cross-sectional study. Am J Epidemiol 165:541–552, PMID: 17164464, 10.1093/aje/kwk037. [DOI] [PubMed] [Google Scholar]
- Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, et al. 2011. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 342:d2431, PMID: 21546419, 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Appel LJ, Whelton PK. 2014. Lower levels of sodium intake and reduced cardiovascular risk. Circulation 129:981–989, PMID: 24415713, 10.1161/CIRCULATIONAHA.113.006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. 2007. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334:885, PMID: 17449506, 10.1136/bmj.39147.604896.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DPHE (Department of Public Health Engineering, Government of the People's Republic of Bangladesh). 2016. Arsenic Contamination and Mitigation in Bangladesh. http://www.dphe.gov.bd/index.php?option=com_content&view=article&id=96&Itemid=104 [accessed 9 June 2016].
- DPHE/BGS (Department of Public Health Engineering and British Geological Survey). 2001. Bangladesh: DPHE/BGS National Hydrochemical Survey. http://www.bgs.ac.uk/research/groundwater/health/arsenic/Bangladesh/mapsnhs.html [accessed 10 June 2016].
- Eder M, Holzgreve H, Liebl M, Bogner J. 2008. Effect of clothing on sphygmomanometric and oscillometric blood pressure measurement in hypertensive subjects [in German]. Dtsch Med Wochenschr 133:1288–1292, 10.1055/s-2008-1077254. [DOI] [PubMed] [Google Scholar]
- Elliott P, Stamler J, Nichols R, Dyer AR, Stamler R, Kesteloot H, et al. 1996. Intersalt revisited: Further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ 312:1249–1253, PMID: 8634612, 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P, Stamler J. 2002. Commentary: Evidence on salt and blood pressure is consistent and persuasive. Int J Epidemiol 31:316–319, PMID: 11980788, 10.1093/ije/31.2.316. [DOI] [PubMed] [Google Scholar]
- Elliott P, Walker LL, Little MP, Blair-West JR, Shade RE, Lee DR, et al. 2007. Change in salt intake affects blood pressure of chimpanzees implications for human populations. Circulation 116:1563–1568, PMID: 17785625, 10.1161/CIRCULATIONAHA.106.675579. [DOI] [PubMed] [Google Scholar]
- Hasan M. 2012. Investigations on Groundwater Buffering in Khulna-Satkhira Coastal Belt using Managed Aquifer Recharge. Dhaka: University of Dhaka. [Google Scholar]
- He FJ, Li J, Macgregor GA. 2013. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 4:CD004937. [DOI] [PubMed] [Google Scholar]
- Hijioka Y, Lin E, Pereira JJ, Corlett R, Cui X, Insarov G, et al. 2014. Chapter 24: Asia In: Climate Change 2014: Impacts, Adaptation, and Vulnerability IPCC Working Group II Contribution to AR5. Field CB, Barros VR, Dokken DJ, Mach KJ, Mastandrea MD, Bilir TE, et al. , eds. Geneva, Switzerland:World Meteorological Organization; https://www.ipcc.ch/pdf/assessment-report/ar5/wg2/WGIIAR5-IntegrationBrochure_FINAL.pdf [accessed 10 June 2016]. [Google Scholar]
- Hildebrant G, Beudt M, Gutenbrunner C. 1986. The effects of sodium-containing mineral water on blood pressure. Z Phys Med 15:321–327. [Google Scholar]
- Hofman A, Valkenburg HA, Vaandrager GJ. 1980. Increased blood pressure in schoolchildren related to high sodium levels in drinking water. J Epidemiol Community Health 34:179–181, PMID: 7441137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque M, Scheelbeek P, Vineis P, Khan A, Ahmed K, Butler A. 2016. Drinking water vulnerability to climate change and alternatives for adaptation in coastal south and south east asia. Clim Change 136:247–263, PMID: 27471332, 10.1007/s10584-016-1617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque MA, Butler AP. 2015. Medical hydrogeology of asian deltas: Status of groundwater toxicants and nutrients, and implications for human health. Int J Environ Res Public Health 13:81, PMID: 26712780, 10.3390/ijerph13010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Health Metrics and Evaluation (IHME). 2015. Global burden of disease data - compare. http://vizhub.healthdata.org/gbd-compare [accessed 29 May 2016].
- IWM (Institute of Water Modelling). 2014. Annual Report 2013. Dhaka:IWM. [Google Scholar]
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014. 2014 Evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311:507–520, 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Khan AE, Scheelbeek PF, Shilpi AB, Chan Q, Mojumder SK, Rahman A, et al. 2014. Salinity in drinking water and the risk of (pre)eclampsia and gestational hypertension in coastal Bangladesh: A case-control study. PLoS One 9:e108715, PMID: 25268785, 10.1371/journal.pone.0108715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish L. 1949. A procedure for objective respondent selection within the household. JASA 44:380–387, 10.2307/2280236. [DOI] [Google Scholar]
- Law M, Frost C, Wald N. 1991. By how much does dietary salt reduction lower blood pressure? III–Analysis of data from trials of salt reduction. BMJ 302:819–824, PMID: 1827353, 10.1136/bmj.302.6780.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenel R, Katz L, Rodbard S. 1948. Arterial hypertension in the chicken. Am J Physiol 152:557–562, PMID: 18863154. [DOI] [PubMed] [Google Scholar]
- Lifshitz F, Wapnir RA. 1985. Oral hydration solutions: Experimental optimization of water and sodium absorption. J Pediatr 106:383–389, PMID: 3973775 , 10.1016/S0022-3476(85)80661-2. [DOI] [PubMed] [Google Scholar]
- Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH. 1990. Sodium bicarbonate and sodium chloride: Effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens 8:663–670, PMID: 2168457. [DOI] [PubMed] [Google Scholar]
- Ma G, Sabin N, Dawes M. 2008. A comparison of blood pressure measurement over a sleeved arm versus a bare arm. CMAJ 178:585–589, PMID: 18299548, 10.1503/cmaj.070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmuduzzaman M, Ahmed ZU, Nuruzzaman A, Ahmed FRS. 2014. Causes of salinity intrusion in coastal belt of Bangladesh. Int J Plant Res 4:8–13. [Google Scholar]
- Morgan T. 1982. The effect of potassium and bicarbonate ions on the rise in blood pressure caused by sodium chloride. Clin Sci 63:S407–S409, 10.1042/cs063407s. [DOI] [Google Scholar]
- Netherlands Embassy in Dhaka. 2014. Rural water supply, sanitation and hygiene in difficult and hard-to-reach areas of Bangladesh. http://bangladesh.nlembassy.org/binaries/content/assets/postenweb/b/bangladesh/netherlands-embassy-in-dhaka/import/water-management/project-factsheets/unicef-wash-project-factsheet.pdf [accessed 10 June 2016].
- Pietinen P, Uusitalo U, Nissinen A, Group ICR. 1988. Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297:319–328, PMID: 3416162, 10.1136/bmj.297.6644.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. 2001. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 344:3–10, PMID: 11136953, 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- Santos A, Martins MJ, Guimarães JT, Severo M, Azevedo I. 2010. Sodium-rich carbonated natural mineral water ingestion and blood pressure. Rev Port Cardiol 29:159–172. [PubMed] [Google Scholar]
- Sapirstein LA, Brandt WL, Drury DR. 1950. Production of hypertension in the rat by substituting hypertonic sodium chloride solutions for drinking water. Proc Soc Exp Biol Med 73:82–85, PMID: 15402528, 10.3181/00379727-73-17583. [DOI] [PubMed] [Google Scholar]
- Scheelbeek PFD. 2015. Hypertensive disorders in salinity prone coastal areas: Relevance to global climate change. [Dissertation]. London, UK:Imperial College London. [Google Scholar]
- Schorr U, Distler A, Sharma AM. 1996. Effect of sodium chloride-and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: A randomized double-blind crossover trial. J Hypertens 14:131–136, PMID: 12013486. [PubMed] [Google Scholar]
- Shirreffs SM, Taylor AJ, Leiper JB, Maughan RJ. 1996. Post-exercise rehydration in man: Effects of volume consumed and drink sodium content. Med Sci Sports Exerc 28:1260–1271, PMID: 8897383. [DOI] [PubMed] [Google Scholar]
- Singh O, Khan TA, Rahman MS. 2000. Changes in the frequency of tropical cyclones over the north indian ocean. Meteorol Atmospher Physics 75:11–20, 10.1007/s007030070011. [DOI] [Google Scholar]
- Skrondal A, Rabe-Hesketh S. 2003. Some applications of generalized linear latent and mixed models in epidemiology: Repeated measures, measurement error and multilevel modeling. Norsk Epidemiologi 13. [Google Scholar]
- Smith AH, Lingas EO, Rahman M. 2000. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bull World Health Organ 78:1093–1103, PMID: 11019458. [PMC free article] [PubMed] [Google Scholar]
- Sultana S, Ahmed K, Mahtab-Ul-Alam S, Hasan M, Tuinhof A, Ghosh S, et al. 2014. Low-cost aquifer storage and recovery: Implications for improving drinking water access for rural communities in coastal Bangladesh. J Hydrol Eng 20: B5014007, http://ascelibrary.org/doi/abs/10.1061/%28ASCE%29HE.1943-5584.0001100 [accessed 19 May 2017]. [Google Scholar]
- Tuthill RW, Calabrese EJ. 1989. Reducing drinking water sodium concentrations did not influence adolescent blood pressure. Journal of Environmental Science & Health Part A 24:711–729, 10.1080/10934528909375512. [DOI] [Google Scholar]
- UNICEF. 2014. Bangladesh country office. WES. September 2014. Factsheet. http://www.unicef.org/bangladesh/MAR_WASH.pdf [accessed 10 June 2016].
- Van Vliet B, Montani J. 2008. The time course of salt-induced hypertension, and why it matters. Int J Obes Relat Metab Disord 32:S35–S47, 10.1038/ijo.2008.205. [DOI] [PubMed] [Google Scholar]
- WHO. 2011a. Arsenic in drinking-water: Background document for development of WHO guidelines for drinking-water quality. Geneva, Switzerland:WHO; http://www.who.int/water_sanitation_health/dwq/chemicals/arsenic.pdf [accessed 10 June 2016]. [Google Scholar]
- WHO. 2011b. Non-communicable disease, risk factor survey - Bangladesh 2010. Dhaka, Bangladesh:WHO; http://www.who.int/chp/steps/2010_STEPS_Report_Bangladesh.pdf [accessed 10 June 2016]. [Google Scholar]
- WHO. 2012a. Guideline: Potassium intake for adults and children. Geneva, Switzerland:WHO; http://www.who.int/nutrition/publications/guidelines/potassium_intake_printversion.pdf [accessed 14 May 2017]. [Google Scholar]
- WHO. 2012b. Guideline: Sodium intake for adults and children. Geneva, Switzerland:WHO; http://www.who.int/nutrition/publications/guidelines/sodium_intake_printversion.pdf [accessed 10 June 2016]. [Google Scholar]
- WHO. 2017. WHO STEPS surveillance manual: The WHO STEPwise approach to noncommunicable disease risk factor surveillance. http://www.who.int/chp/steps/STEPS_Manual.pdf?ua=1 accessed on 14-May-2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.